Abstract
Invasive Pseudomonas aeruginosa (PA) can enter epithelial cells wherein they mediate formation of plasma membrane bleb-niches for intracellular compartmentalization. This phenotype, and capacity for intracellular replication, requires the ADP-ribosyltransferase (ADPr) activity of ExoS, a PA type III secretion system (T3SS) effector protein. Thus, PA T3SS mutants lack these capacities and instead traffic to perinuclear vacuoles. Here, we tested the hypothesis that the T3SS, via the ADPr activity of ExoS, allows PA to evade acidic vacuoles that otherwise suppress its intracellular viability. The acidification state of bacteria-occupied vacuoles within infected corneal epithelial cells was studied using LysoTracker to visualize acidic, lysosomal vacuoles. Steady state analysis showed that within cells wild-type PAO1 localized to both membrane bleb-niches and vacuoles, while both exsA (transcriptional activator) and popB (effector translocation) T3SS mutants were only found in vacuoles. The acidification state of occupied vacuoles suggested a relationship with ExoS expression, i.e. vacuoles occupied by the exsA mutant (unable to express ExoS) were more often acidified than either popB mutant or wild-type PAO1 occupied vacuoles (p < 0.001). An exoS-gfp reporter construct pJNE05 confirmed that high exoS transcriptional output coincided with low occupation of acidified vacuoles, and vice versa, for both popB mutants and wild-type bacteria. Complementation of a triple effector null mutant of PAO1 with exoS (pUCPexoS) reduced the number of acidified bacteria-occupied vacuoles per cell; pUCPexoSE381D which lacks ADPr activity did not. The H+-ATPase inhibitor bafilomycin rescued intracellular replication to wild-type levels for exsA mutants, showing its viability is suppressed by vacuolar acidification. Taken together, the data show that the mechanism by which ExoS ADPr activity allows intracellular replication by PA involves suppression of vacuolar acidification. They also show that variability in ExoS expression by wild-type PA inside cells can differentially influence the fate of individual intracellular bacteria, even within the same cell.
Introduction
Pseudomonas aeruginosa is a highly adaptable bacterial pathogen that plays a major role in nosocomial infections including pneumonia, septicemia, and urinary tract infections, as well as community-acquired opportunistic infections of the skin, soft tissue, and ocular surface [1-7]. P. aeruginosa adaptability is reflected by the diversity of genetic traits and large genome sizes seen among clinical isolates, suggesting it has a proclivity for acquiring new DNA through horizontal transfer and retaining traits that enable survival in different host tissues [8,9]. Part of P. aeruginosa’s success as a pathogen is derived from its ability to adapt to the in vivo environment, and express virulence traits that help the bacteria evade host defenses. In the latter regard, the type III secretion system (T3SS) plays a major role through the expression of one or more of four known effector proteins ExoS, ExoU, ExoT and ExoY which promote P. aeruginosa virulence by modulating bacterial interactions with epithelial cells, immune cells, and host tissues [10-16].
Phagocytes and some "non-professional" phagocytes, including epithelial cells, facilitate the destruction of internalized microbes by trafficking them through a series of intracellular vacuolar compartments starting in phagosomes (similar to early endosomes) and terminating in acidified bactericidal phagolysosomes [17]. Some microbes meet a similar fate via autophagy in which autophagosomes fuse with lysosomes to form acidified bactericidal autolysosomes [18]. Successful intracellular pathogens, however, either show intrinsic resistance to acidified phagolysosomes, e.g. Coxiella spp. or Mycobacterium spp. [19,20] and/or escape default trafficking to establish alternative intracellular survival niches. For example, Listeria monocytogenes uses listeriolysin O to destabilize vacuolar membranes and escape to the cytosol [21], and Streptococcus pyogenes uses streptolysin O to reduce lysosomal colocalization bacterial-occupied vacuoles [22]. Burkholderia cenocepacia containing vacuoles acquire late endosomal markers, but delay recruitment of the NADPH oxidase needed for vacuole acidification using type 6 secretion system-dependent interference with RhoGTPases [23,24]. Other Gram-negative bacteria utilize a T3SS to survive intracellularly. These include Salmonella enterica altering the maturation of early endosomes by manipulating Rab proteins involved in vacuolar fusion, allowing formation of a Salmonella -containing vacuole [25-27], and Shigella spp. using a T3SS effector IcsB to escape autophagy in the cytosol [28].
We previously reported that the ADPr activity of the P. aeruginosa T3SS effector ExoS promotes P. aeruginosa intracellular survival and is associated with the formation of membrane bleb-niches within human epithelial cells [16,29]. Mutants in the T3SS that cannot express ExoS, e.g. exsA (T3SS transcriptional activator) mutants and pscC (T3SS needle) mutants, or exoS mutants lacking ADPr activity, do not induce bleb formation, are defective in intracellular survival, and traffic to perinuclear vacuoles [16,29]. Using exsA mutants, we have shown that these perinuclear vacuoles are LAMP3+ [29], a feature of late endosomes. In contrast, popB mutants (which lack the T3SS translocon, but can secrete effectors) traffic to LAMP3- vacuoles and retain the capacity to replicate intracellularly. Like wild-type P. aeruginosa, replication of popB mutants is dependent on the ADPr activity of ExoS [30].
The aim of this study was to further our understanding of how ExoS ADPr activity enables P. aeruginosa to replicate intracellularly, and how epithelial cells suppress P. aeruginosa viability when ExoS activity is absent. Thus, we tested the hypothesis that ExoS-mediated intracellular survival involves evasion of acidified intracellular compartments, and that without ExoS, internalized bacteria are trafficked to acidified vacuolar compartments wherein they lose viability.
Materials and Methods
Bacterial Strains
P. aeruginosa strain PAO1, T3SS mutants, and plasmid-complemented strains used in this study are described in Table 1. For fluorescent imaging, bacteria were transformed by electroporation with plasmids encoding either green fluorescent protein (pSMC2) [31] or dTomato (p67T1) [32] and selectively cultured at 37°C overnight on tryptic soy agar (TSA) (BD Bioscience, CA) containing carbenicillin (200 µg/mL) (Sigma, MO). If antibiotic selection was not needed, bacteria were grown on TSA plates at 37°C overnight. Bacterial inocula were prepared by resuspending in warm keratinocyte growth medium (KGM) (no antibiotics) to an optical density of 0.1 at 650 nm (Spectronic 21D; Milton Roy, PA), and diluted 1:10 to yield ~1 x 107 CFU/mL. Inoculum sizes were confirmed by viable count. To study exoS transcription, PAO1 and the popB mutant were transformed by electroporation with a reporter plasmid, bearing gfp under control of the exoS promoter (pJNE05) [33], and cultured at 37°C overnight on TSA containing gentamicin (200 µg/mL) (Lonza, MD). Expression of the GFP-reporter was confirmed under T3SS-inducing conditions [Tryptic soy broth supplemented with 1% glycerol, 100 mM monosodium glutamate, and 2 mM EGTA (Sigma, MO)].
Table 1. Bacterial strains, mutants and recombinant plasmids used.
| Strain, mutant, and/or plasmid | T3SS Description | Replicates in Epithelial cells | Reference |
|---|---|---|---|
| PAO1 | Wild-type P. aeruginosa. Expresses ExoS, ExoT, ExoY | + | [13,29] |
| PAO1exsA::Ω | Lacks T3S transcriptional activator | - | [29,48] |
| (exsA mutant) | No T3S expression | ||
| PAO1ΔpopB | Lacks T3S translocon | + | [13,29] |
| (popB mutant) | Encodes ExoS, ExoT, ExoY | ||
| PAO1ΔexoSTY | No known T3S effectors | - | [13,29] |
| PAO1ΔexoSTY + pUCPexoS | Complementation with plasmid-expressed ExoS | + | [16,49] |
| PAO1ΔexoSTY + pUCPexoS (E381D) | Complementation with plasmid-expressed ExoS without ADPr activity | - | [16,49] |
| PAO1ΔexoSTY + pUCP18 | Plasmid control | - | [16] |
| pSMC2 | Plasmid encoding constitutively-expressed green fluorescent protein (GFP) | NA | [31] |
| pJNE05 | Plasmid encoding gfp fused the ExsA-dependent promoter of exoS | NA | [33] |
| p67T1 | Plasmid encoding constitutively expressed dTomato | NA | [32] |
Cell Culture
Telomerase-immortalized human corneal epithelial cells (hTCEpi) [34] were cultured in KGM containing the antibiotics gentamicin (30 µg/mL) and amphotericin B (15 ng/mL) (Lonza, MD) at 37 °C under 5% CO2 on sterile 25 mm glass coverslips until ~ 80% confluence. Prior to infection (24 h), cultures were washed with 3 equal volumes (2 mL) of warm phosphate buffered saline (PBS) and switched to KGM without antibiotics.
Confocal Microscopy
Epithelial cells were inoculated with ~107 CFU/mL of bacteria and incubated for 3 h at 37 °C (5% CO2). Viable extracellular bacteria were then eliminated by washing with 3 equivalent volumes (2 mL) of warm PBS and culturing in warm KGM (2 mL) containing amikacin (200 µg/mL) (Sigma, MO) for 1 h at 37 °C (5% CO2). Infected cultures were then stained with the acidophilic dye - LysoTracker DND-22 (Life Technologies, NY) as a 1 µM solution in warm, phenol red - free KGM (Promocell, Germany) containing amikacin (200 µg/mL) for 30 min as described above. Cultures were then immediately transferred to an attofluor chamber (Life Technologies, NY) and viewed with a Fluoview FV1000 laser scanning confocal microscope (Olympus, PA) equipped with 60 x magnification water-immersion objective, 100W halogen illumination (for Nomarski differential interference contrast - DIC), 405 nm and 559 nm diode lasers (used for the excitation of LysoTracker DND-22 and dTomato, respectively) and a multi-line argon laser (used to excite GFP at 488 nm). Fluorescent and transmitted light was collected simultaneously using spectral-based PMT detection and integrated DIC in 0.5 µm increments along the z-axis. Resulting images were processed and quantified with FV1000 ASW software (Olympus, PA) using ≥10 fields per condition. Each field contained an average of 10 infected epithelial cells. Thus, for each condition, ~100 infected cells and >300 bacteria-occupied vacuoles were counted or measured respectively. The diameter of each bacteria-occupied vacuole was also noted. Mean values of bacteria-occupied vacuoles per cell and relative percentage of total occupied vacuoles are reported along with standard error of the mean (SEM). In some instances, the mean value of all intracellular bacteria per cell, including bacteria within bleb niches, was also tabulated. Statistical significance was assessed with ANOVA followed by a Welch’s corrected t-Test based on the unequal variance of each normally distributed dataset.
Intracellular Survival Assays
Bacterial survival and intracellular replication was assessed using culture conditions slightly modified from those described above. Paired sets of epithelial cell cultures were grown in 12 well tissue culture plates to confluence in KGM containing antibiotics (gentamicin and amphotericin B as above) and switched to antibiotic-free media 24 h prior to infection. To block vacuolar acidification, a subset of cultures were treated with a vATPase inhibitor, bafilomycin A1 (Sigma, MO), suspended in KGM (final concentration of 200 nM). These treatments were initiated 1 h prior to infection and maintained throughout the assay. Epithelial cells were inoculated with ~106 CFU/mL of bacteria (in 1 mL) and incubated for 3 h at 37 °C (5% CO2). Viable extracellular bacteria were then removed by washing with 3 volumes (2 mL) of warm PBS, then incubating with warm KGM containing amikacin (200 µg/mL), as previously described, for 1 h (4 h time point) or 5 h (8 h time point) amongst paired cultures, to allow intracellular replication. Viable intracellular bacteria were recovered from PBS-washed cultures using a 0.25% Triton X-100 solution (0.5 mL/well) and enumerated by viable counting on TSA plates. Each sample was assessed in triplicate, and data were expressed as a mean +/- SEM per sample. Intracellular replication was reported as the increase in recovered CFU at 8 h post-infection as a percentage of a baseline measurement made after 4 h.
Statistical Analysis
Significance of differences between groups was assessed using ANOVA and Welch’s corrected t-Test (based on unequal variance among normally distributed datasets) or the Chi-square test. P values < 0.05 were considered significant. Experiments were repeated at least three times unless stated otherwise.
Results
P. aeruginosa Mutants Lacking Expression of Type III Secretion Traffic to Acidified Vacuoles
We have previously shown that T3SS (exsA) mutants of P. aeruginosa strain PAO1 traffic to perinuclear vacuoles that label with the late endosomal marker LAMP3 after they are internalization by epithelial cells, and that this correlates with an inability to thrive [29]. Here, we examined whether vacuoles occupied by exsA mutants of PAO1 were acidified. Confocal imaging of human corneal epithelial cells infected with GFP-expressing P. aeruginosa and labeled with LysoTracker (LT) DND-22 showed different intracellular localization for wild-type bacteria and T3SS mutants (exsA or popB) at 5 h post-infection (Figure 1). As expected, intracellular exsA mutants were confined to vacuoles, and the majority of these vacuoles were found to be LT-labeled (i.e. acidified) (Figure 1B, co-localization appears yellow). In contrast, translocon (popB) mutants and wild-type bacteria, which can both replicate intracellularly (due to their capacity to secrete ExoS), showed little or no co-localization with acidified vacuoles (Figure 1C and 1D, respectively). As expected, wild-type bacteria caused membrane bleb-niche formation in (~50%) of PAO1 infected cells (Figure 1D inset). Interestingly, LT (-) individual bleb niches occasionally contained LT (+) vacuoles containing bacteria (see Figure 1D inset). However, blebbing cells otherwise stained poorly with LT, displaying > 20-fold reduced total fluorescence intensity [174.5 +/- 78] compared to non-blebbing PAO1-infected cells [3737.0 +/- 708.8] [p < 0.001 Welch’s corrected t-Test]. Non-blebbing PAO1-infected cells showed similar intensity to cells infected with the exsA mutant [3537.7 +/- 205.9].
Figure 1. Colocalization of the P. aeruginosa exsA mutant with acidified vacuoles in epithelial cells compared to that of wild-type bacteria or a popB (translocon) mutant.
Confocal microscopy images of human corneal epithelial cells at 5 h post-infection with GFP-expressing P. aeruginosa (green). Prior to imaging, infected cultures were infused with LysoTracker (LT) DND-22 (pseudo-colored red). Panels depict (A) Uninfected control, (B) PAO1 exsA mutant (C) PAO1 popB (translocon) mutant and (D) wild-type PAO1. Uninfected cells appeared healthy. The intracellular exsA mutant appeared more frequently in LT (+) (acidified) vacuoles which co-localized yellow (arrows) than either the intracellular popB mutant or wild-type PAO1. PAO1-infected cells which displayed bleb-niche formation (1D inset) showed reduced fluorescence (< 10% fluorescence intensity of PAO1-infected non-blebbing cells, p < 0.001 Welch’s corrected t-Test). Occasional bleb-niches contained LT (+) vacuoles containing bacteria (1D inset, yellow). Representative images are shown. Magnification ~ 600 x.
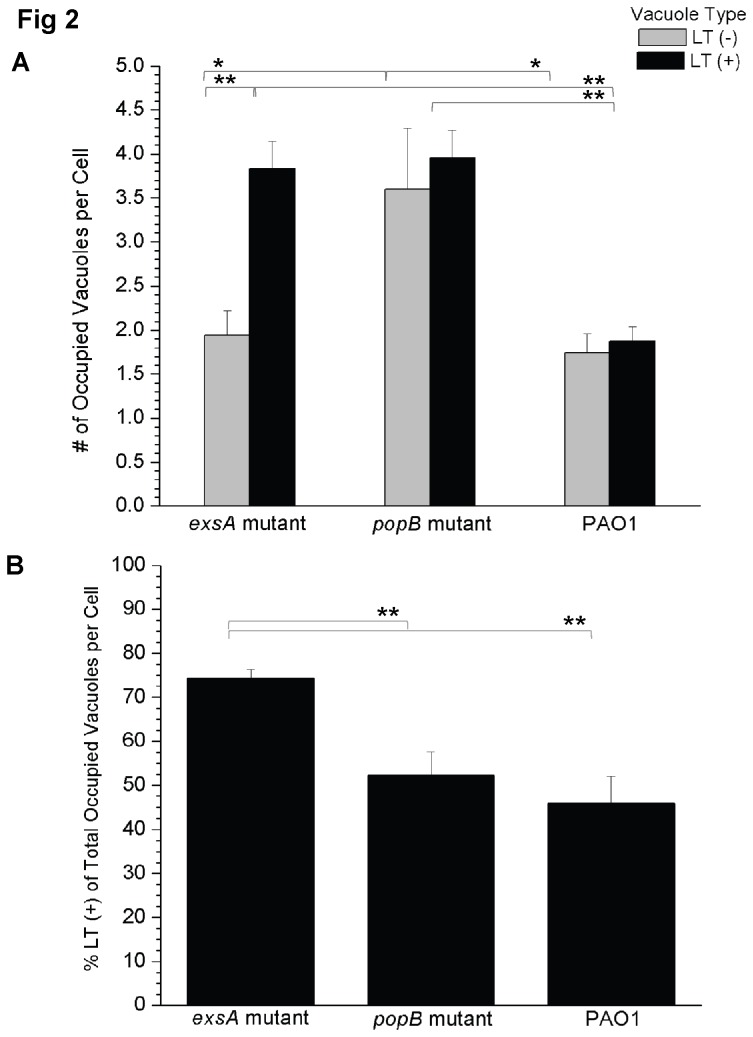
The number of vacuoles per cell occupied by exsA mutants, popB mutants or wild-type PAO1 with (LT+) or without (LT-) LysoTracker staining was quantified (Figure 2). For exsA mutants, the mean number of bacterial-occupied LT (+) vacuoles per cell was 3.8 +/- 0.3, significantly more than the number of bacterial-occupied LT (-) vacuoles at 1.9 +/- 0.3 [p < 0.001 Welch’s corrected t-Test] (Figure 2A). The popB mutant was more likely to occupy LT (-) vacuoles than the exsA mutant with 3.6 +/- 0.7 bacteria-occupied LT (-) vacuoles per cell versus 1.9 +/- 0.3 for the exsA mutant [p < 0.05, Welch’s corrected t-Test] (Figure 2A). Indeed, the popB mutant was located exclusively in LT (-) vacuoles in ~13% of all infected epithelial cells versus ~2% for the exsA mutant [p = 0.01, Chi-square test]. There was no significant difference in the numbers of LT (+) and LT (-) bacteria-occupied vacuoles per cell for the popB mutant compared to wild-type PAO1 infected cells (Figure 2A). As would be expected, considering that wild-type PAO1, but not the popB mutant, can form and traffic to bleb-niches, there were significantly fewer bacterial-occupied vacuoles per cell for PAO1 compared to popB mutant-infected cells, regardless of LysoTracker-staining [LT (-) = 1.7 +/- 0.2 versus 3.6 +/- 0.7, respectively, p < 0.05: LT (+) = 1.9 +/- 0.2 versus 3.95 +/-0.3, respectively, p < 0.001 Welch’s corrected t-Test] (Figure 2A). From counts of individual GFP-expressing wild-type bacteria, it appeared that epithelial cells with bleb-niches contained significantly more intracellular bacteria [mean value of 7.1 +/- 1.3 bacteria per cell] than non-blebbing cells [2.4 +/- 0.3 bacteria per cell, p = 0.002 Welch’s corrected t-Test].
Figure 2. Quantification of acidified versus non-acidified vacuole occupation by wild-type P. aeruginosa and its type III secretion mutants.
(A) Confocal microscopy images were used to classify bacteria-occupied vacuoles in human corneal epithelial cells as either LT (+) (acidified) or LT (-) (non-acidic) at 5 h post-infection with P. aeruginosa PAO1 or its type III secretion mutants (exsA or popB). The data are shown as the mean (+/- SEM) number of bacteria-occupied vacuoles per cell. Grey columns denote LT (-) vacuoles, black columns LT (+) vacuoles. The exsA and popB mutants were both associated with increased numbers of acidified LT (+) bacteria-occupied vacuoles per cell compared to wild-type PAO1 (p < 0.001, Welch’s corrected t-Test). The exsA mutant showed more acidified than non-acidified bacteria-occupied vacuoles per cell (p < 0.001, Welch’s corrected t-Test). (B) To normalize differences in internalization and replication, the percentage of LT (+) bacteria-occupied vacuoles was calculated as a function of the total number of bacteria-occupied vacuoles per cell. Mean percentage (+/- SEM) is shown. The exsA mutant was associated with more acidified bacteria-occupied vacuoles per cell than either the popB mutant or wild-type bacteria (p < 0.001, Welch’s corrected t-Test). A representative experiment of 3 independent experiments is shown in both panels (A) and (B). Calculations excluded cells showing bleb-niche formation. Significant differences between all groups were identified using ANOVA analysis (p < 0.0001), and characterized on a pairwise basis using Welch’s correct t-Test [*p < 0.05, **p < 0.001].
LT (+) vacuoles occupied by the popB and exsA mutants were found at a similar frequency [mean value of 3.95 +/- 0.3 per cell versus 3.8 +/- 0.3 per cell, respectively, p = 0.79, Welch’s corrected t-Test] (Figure 2A). To normalize for differences in bacterial internalization between these two mutants, the mean percentage of bacteria-occupied LT (+) vacuoles was calculated as a function of the total number of occupied vacuoles in a given cell (Figure 2B). For the exsA mutant most occupied vacuoles were LT (+) (73.9 +/- 2.1%), significantly more than either the popB mutant (52.4 +/- 5.4%, p = 0.001 Welch’s correct t-Test) or wild-type PAO1 (45.9 +/- 5.8%, p = 0.001 Welch’s corrected t-Test), which did not significantly differ from each other (p = 0.42 Welch’s corrected t-Test).
Using the same experimental conditions, P. aeruginosa infected epithelial cells were then examined for vacuole size and vacuole spatial localization, the latter classified as either perinuclear or otherwise (Table 2). LT (+) vacuoles occupied by the exsA mutant were significantly larger in diameter [1.15 +/- 0.04 µm] than the corresponding LT (-) group [0.99 +/- 0.06 µm, p < 0.05 Welch’s corrected t-Test]. The LT (+) occupied vacuoles were also more likely to be perinuclear (Table 2). PAO1 occupied LT (+) vacuoles were also significantly larger than their LT (-) counterparts, and more of those LT (+) occupied vacuoles were perinuclear, although that difference was not significant. LT (-) vacuoles occupied by the popB mutant were larger [1.07 +/- 0.06 µm] than those occupied by PAO1 [0.89 +/- 0.07 µm].
Table 2. Mean size and spatial distribution of bacteria-occupied vacuoles.
| Bacterial Strain | Mean Vacuole Size (+/- SEM) µm |
% Bacteria-Occupied Vacuoles which are Perinuclear [Mean (+/- SEM)] |
||
|---|---|---|---|---|
| Non-Acidic Vacuoles | Acidic Vacuoles | Non-Acidic Vacuoles | Acidic Vacuoles | |
| exsA mutant | 0.99 +/- 0.06 | 1.15 +/- 0.04† | 10.9 +/- 1.5 | 34.3 +/- 3.7† |
| popB mutant | 1.07 +/- 0.06†† | 1.18 +/- 0.05 | 12.8 +/- 2.0 | 24.4 +/- 5.4 |
| PAO1 wild-type | 0.89 +/- 0.07 | 1.10 +/- 0.05† | 19.2 +/- 4.8 | 32.9 +/- 5.3 |
Values compared using ANOVA (p = 0.004) and pairwise using Welch’s corrected t-Test
† Significant difference from non-acidic vacuoles of the same strain (p < 0.05, Welch’s corrected t-Test)
† † Significant difference from non-acidic vacuoles of PAO1 (p < 0.05, Welch’s corrected t-Test)
Together, the data show that without the T3SS, i.e. exsA mutants, the majority of intracellular P. aeruginosa are trafficked to acidified perinuclear vacuoles within epithelial cells, while wild-type and translocon (popB) mutants (both able to secrete T3SS effectors including ExoS) are less likely to occupy acidified vacuoles, even though the popB mutant cannot translocate effectors across host membranes.
Bafilomycin Rescues Intracellular Survival of the exsA Mutant
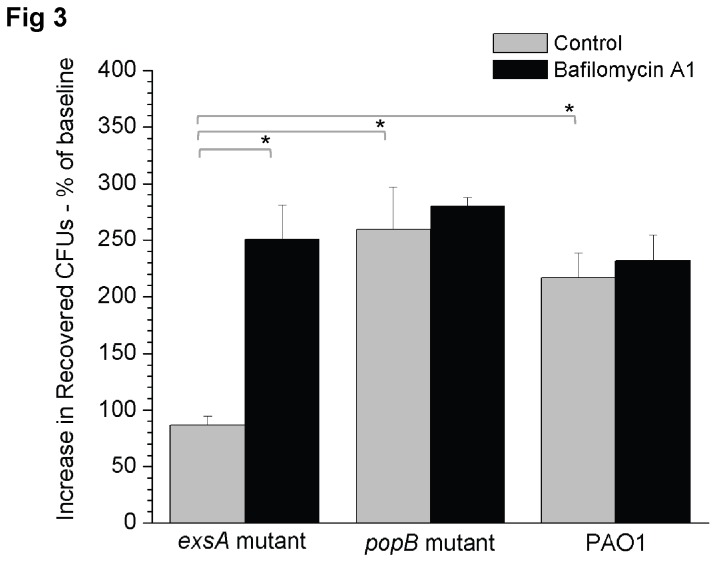
We next explored if association of the exsA mutant with acidified vacuoles was related to a reduced capacity to thrive intracellularly. For this purpose, intracellular survival assays were performed with and without bafilomycin A1, an inhibitor of vacuole acidification, using the exsA mutant. Wild-type PAO1 and the popB (translocon) mutant were included as controls (Figure 3). As expected, wild-type and translocon mutants replicated intracellularly, and their replication rate was unaffected by bafilomycin A1. Without bafilomycin, the exsA mutant was confirmed to be defective in intracellular replication [86.4 +/- 15% relative to baseline] compared to wild-type (216.8 +/- 40%) and popB mutant bacteria (259.6 +/- 65%, p < 0.05, Welch’s corrected t-Test). With bafilomycin A1 (200 nM) intracellular replication by the exsA mutant was rescued to levels similar to wild-type PAO1 (250.7 +/- 52.2%) (Figure 3). Control experiments (not shown), confirmed that 200 nM bafilomycin A1 blocked LysoTracker staining of epithelial cells and had no impact on bacterial viability. Thus, vacuolar acidification was required for cells to suppress intracellular replication by the exsA mutant.
Figure 3. Intracellular survival and replication of P. aeruginosa PAO1 and its type III secretion mutants in corneal epithelial cells in the presence bafilomycin A1 (200 nM) (black boxes) versus control cells treated with vehicle only (grey boxes).
Bafilomycin treatment restored intracellular survival of the exsA mutant to that of the popB mutant and wild-type PAO1. Bafilomycin A1 was added 1 h before infection and continued throughout the assay. Intracellular survival was expressed as the mean percentage increase in viable intracellular bacteria at 8 h versus 4 h post-infection (+/- SEM). A representative experiment of 3 independent experiments in shown above. ANOVA (p = 0.0002) and Welch’s corrected t-test were used for statistical analysis (* p < 0.05).
The ExoS ADP-ribosylation Domain Reduces Bacterial Occupation of Acidified Vacuoles
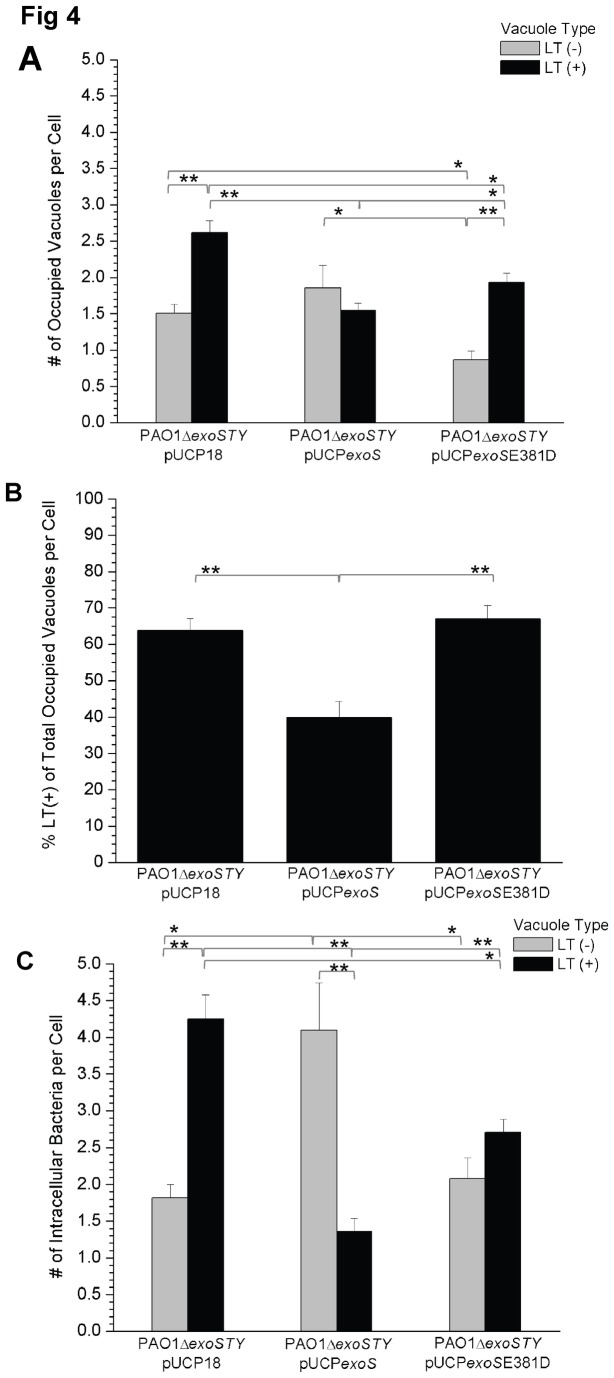
We previously reported that the ADPr domain of ExoS confers intracellular replication without the T3SS translocon or other known effectors [30]. Thus, we tested if this domain of ExoS impacts P. aeruginosa occupation of acidified vacuoles. A triple effector mutant of PAO1 (PAO1ΔexoSTY) complemented with exoS (pUCPexoS) was compared to the same mutant complemented with ADPr-inactive exoS (pUCPexoSE381D) and a vector control (pUCP18). The two controls occupied more LT (+) acidified vacuoles than LT (-) vacuoles (Figure 4A) [pUCP18 LT (+): 2.6 +/- 0.2 versus LT (-): 1.5 +/- 0.1, pUCPexoSE381D LT (+): 1.9 +/- 0.1 versus LT (-): 0.9 +/- 0.1, p < 0.001 Welch’s corrected t-Test], similar to the results for the exsA mutant (Figure 2A). Complementation with ADPr active ExoS reduced this bias towards bacteria-occupied LT (+) vacuoles relative to LT (-) vacuoles (Figure 4A) [LT (+): 1.5 +/- 0.1 versus LT (-): 1.6 +/- 0.2, p = 0.82 Welch’s corrected t-Test]. This was the case even after normalizing for differences in bacterial internalization, i.e. when the mean percentage of bacteria-occupied LT (+) vacuoles was calculated as a function of the total number of occupied vacuoles: PAO1ΔexoSTY + pUCPexoS (39.9 +/- 4.5%) versus PAO1ΔexoSTY + pUCPexoSE381D (67.7 +/- 3.7%) [p < 0.001 Welch’s correct t-Test], the latter was not significantly different from PAO1ΔexoSTY + pUCP18 (63.8 +/- 3.3%) (Figure 4B). It was noted that pUCPexoS-complemented bacteria partitioned exclusively to LT (-) vacuoles in 35% of the infected, non-blebbing cells versus only 10% of the cells infected with pUCP18 strain [p < 0.001 (chi-square)]. This would account for the lower overall percentage of LT (+) vacuoles per cell calculated for the exoS-expressing strain, despite the apparent overlap in the mean number of LT (+) versus LT (-) occupied vacuoles per cell.
Figure 4. Quantification of acidified versus non-acidified vacuole occupation by a triple effector type III secretion mutant of P. aeruginosa complemented with either exoS or exoS without ADPr activity.
(A) Confocal microscopy images were used to classify bacteria-occupied vacuoles as LysoTracker LT (+) (acidified) or LT (-) at 5 h post-infection with a triple effector mutant of P. aeruginosa (PAO1ΔexoSTY) complemented with exoS (pUCPexoS), exoS without ADPr activity (pUCPexoSE381D) or a vector control (pUCP18). Data are shown as the mean (+/- SEM) values of bacteria-occupied vacuoles per cell. Grey columns denote LT (-) vacuoles, black columns denote LT (+) vacuoles. Calculations excluded cells showing bleb-niche formation. Without ExoS ADPr activity (complementation with pUCP18 or pUCPexoSE381D), there were significantly more acidified bacteria-occupied vacuoles per cell (p < 0.05 Welch’s corrected t-Test). (B) The number of LT (+) bacteria-occupied vacuoles per cell was also calculated as a function of the total number of bacteria-occupied vacuoles per cell. Mean percentage (+/- SEM) is shown. Expression of ADPr active exoS was associated with reduced occupation of acidified vacuoles. Calculations also excluded cells showing bleb-niche formation. (C) Mean (+/- SEM) values of intracellular bacteria were determined to account for both the number of bacteria per vacuole and bacteria within blebbing cells in non-vacuolar niches. Complementation of the triple effector mutant PAO1ΔexoSTY with exoS (pUCPexoS) significantly reduced the number of intracellular bacteria per cell within acidified compartments. Grey columns denote LT (-) vacuoles, black columns LT (+) vacuoles. Each panel above is a representative experiment of 3 independent experiments. Significant differences were observed between groups by ANOVA (p < 0.0001). Welch’s corrected t-Test was used in pair-wise comparisons [*p < 0.05, **p < 0.001].
To account for variation in the number of bacteria per vacuole and the ability of some bacteria to traffic to non-vacuolar compartments, the mean number of intracellular bacteria per cell was also calculated, regardless of whether bacteria occupied vacuoles or bleb-niches, and their association with LysoTracker was recorded (Figure 4C). Complementation of PAO1ΔexoSTY with exoS was associated with significantly fewer intracellular bacteria in acidified compartments [+ pUCPexoS LT (+): 1.4 +/- 0.2, p < 0.001 Welch’s corrected t-Test] compared to either the control plasmid [pUCP18 LT (+): 4.2 +/- 0.3] or complementation with ADPr-inactive exoS [+ pUCPexoSE381D LT (+): 2.7 +/- 0.2]. Interestingly, complementation with ADPr-inactive exoS also reduced bacterial occupation of LT (+) compartments compared to the control plasmid complemented mutant, but not to the reduced levels achieved by pUCPexoS complementation (Figure 4C). These data show that the ADPr domain of exoS is important in P. aeruginosa evasion of acidified compartments in epithelial cells after internalization.
Transcription of exoS by Intracellular P. aeruginosa and Evasion of Acidified Vacuoles
Expression of ExoS by P. aeruginosa is activated in a low calcium environment or by contact with host cells [35,36]. ExoS also regulates contact-dependent T3SS expression [37]. Since our data showed that ExoS ADPr activity reduces bacterial occupation of acidified compartments, we used a transcriptional reporter to study both relative levels and spatial patterns of exoS expression by intracellular P. aeruginosa. To accomplish this, P. aeruginosa PAO1 was transformed with a reporter construct pJNE05 (Table 1) that expresses gfp under control of the exoS promoter [33]. They were also transformed with plasmid p67T1 (Table 1), such that they constitutively express dTomato, another fluorophore. Under non-inducing conditions, i.e. tissue culture media, nearly all of the plasmid-bearing bacteria produced detectable, but low levels of GFP [< 1000 total fluorescence intensity] (data not shown). Under T3SS-inducing conditions (i.e. low calcium media), ~ 50% of transformed PAO1 expressed GFP at levels > 1000 total fluorescence intensity. Consequently, these values were used as guidelines to classify intracellular bacteria as having a low or high exoS transcriptional output.
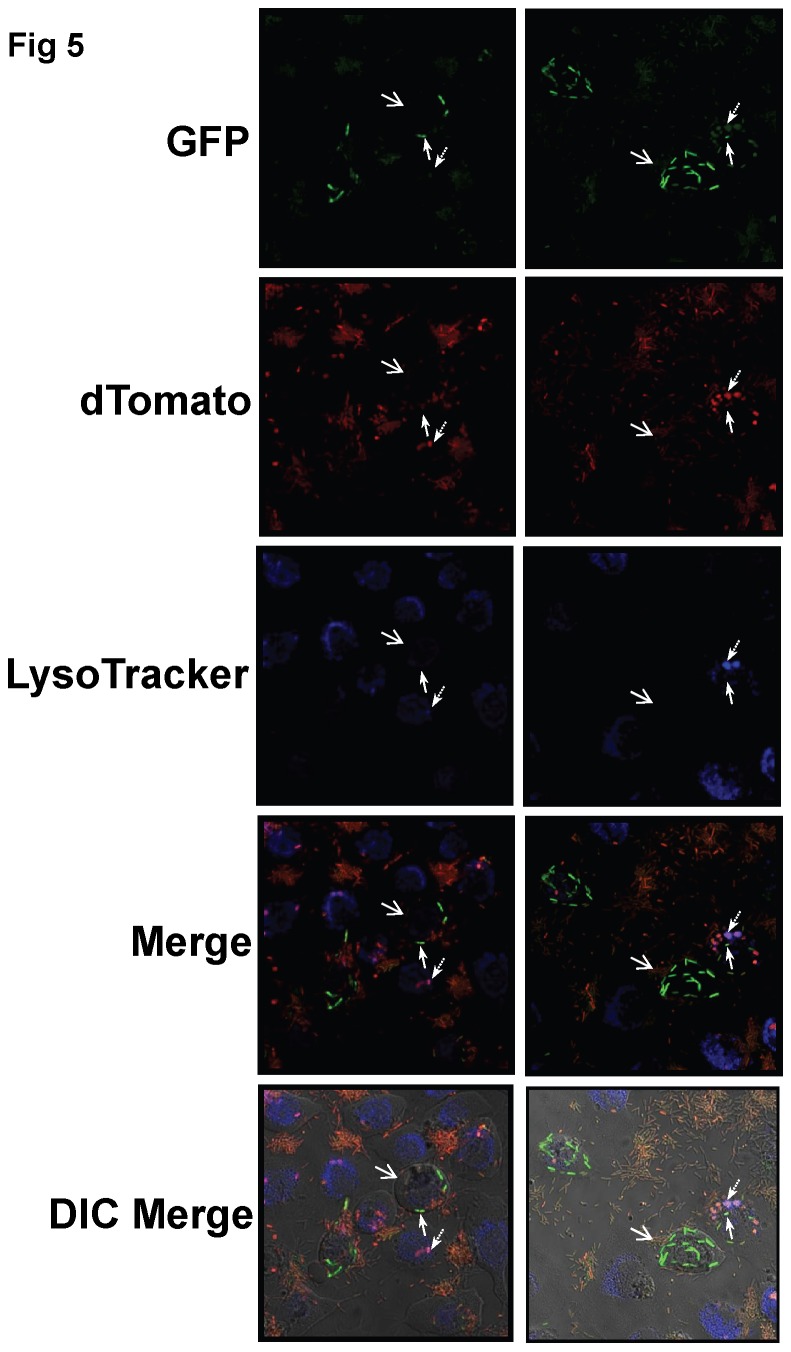
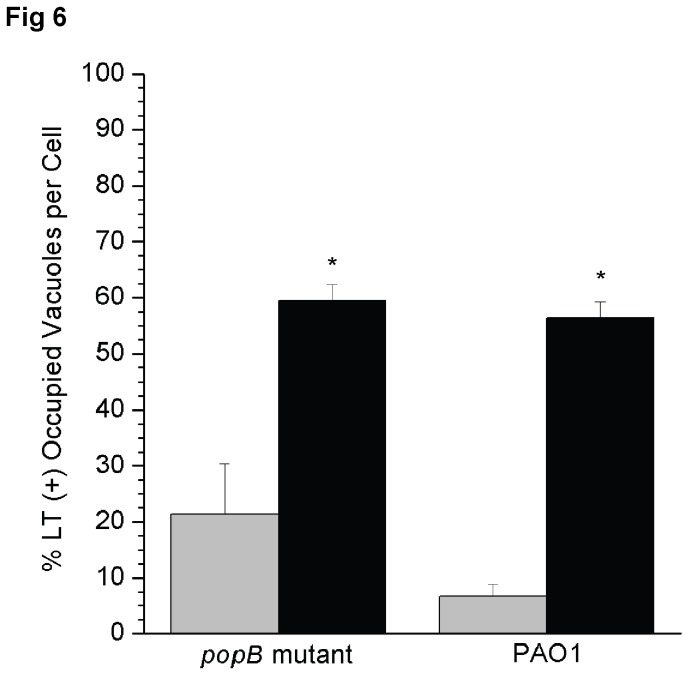
When this transcriptional reporter strain was studied in the context of epithelial cell infection, the results show a clear distinction between intracellular bacteria with high or low exoS output and occupation of LT (+) vacuoles (Figure 5). High exoS transcriptional output coincided with low occupation of LT (+) vacuoles and vice versa. For example, high exoS transcriptional output was observed in 26 +/- 6.7% of all intracellular PAO1, very few of which occupied LT (+) vacuoles (6.6 +/- 2.2%) (Figure 6), and most were within blebbing cells. The remaining intracellular PAO1 (74 +/- 6.7% of total bacteria) displayed low level exoS transcription and were more likely to occupy LT (+) vacuoles (56.4 +/- 3.5%) than the high transcriptional output group (p <0.001 Welch’s corrected t-Test) (Figure 6). Similar results were obtained with the popB mutant transformed with the same plasmids. For example, high exoS transcriptional output was seen in 10.6 +/- 1.6% of all intracellular translocon mutants, which were less likely to occupy LT (+) vacuoles (21.4 +/- 9.0%) as compared to bacteria with a low exoS transcriptional output (59.4 +/- 3.0%, p < 0.001 Welch’s corrected t-Test) (Figure 6). Together, the data show that exoS expression is associated with reduced occupation of acidified vacuoles by intracellular P. aeruginosa.
Figure 5. Colocalization of P. aeruginosa with acidified versus non-acidified vacuoles in relation to exoS transcriptional output.
Confocal and Differential Interference Contrast (DIC) microscopy of human corneal epithelial cells at 5 h post-infection with P. aeruginosa PAO1 complemented with a reporter construct pJNE05 encoding the exoS promoter fused to gfp (green), and p67T1 which constitutively expresses dTomato (red). Bacteria were classified as having a high exoS transcriptional output using a threshold value of 1000 units of GFP fluorescent intensity (green) based on expression levels observed under T3SS-inducing conditions (see Results). Prior to imaging, epithelial cells were infused with LysoTracker DND-22 (blue). ExoS-expressing bacteria (high output, green) [solid arrows] were located primarily outside of acidified (blue) intracellular compartments, which often contained bacteria with low exoS output [dashed arrows]. Blebs are indicated with open arrows. Representative images are shown from two independent experiments. Magnification ~ 600 x.
Figure 6. Quantification of acidified vacuole occupation by P. aeruginosa in relation to exoS transcriptional output.
Data show the mean (+/- SEM) percentage of bacteria-occupied acidified (LT+) vacuoles at 5 h post-infection for P. aeruginosa PAO1 and a popB (translocon) mutant. Bacteria were transformed with an exoS transcriptional reporter plasmid pJNE05 (exoS-gfp) and plasmid p67T1 (dTomato). Infected cells were also stained with LysoTracker. Bacteria with high exoS expression (grey columns) were significantly less likely to occupy acidified vacuoles than those with a low exoS expression (black columns) (* p < 0.001, Welch’s corrected t-Test). Data is representative of 3 independent experiments.
Discussion
The data presented in this study show that the T3SS, and specifically the ADPr activity of ExoS, redirects P. aeruginosa away from acidified compartments within epithelial cells that have internalized them, and that this enables intracellular replication. Thus, ExoS mutants lacking ADPr activity traffic more often to acidified compartments, where they fail to thrive.
The fact that inhibition of vacuolar acidification restored the ability of the T3SS defective exsA mutant to replicate intracellularly shows that the inability to thrive results from acidification of the vacuoles that they are confined within. This provides insights into the likely mechanism by which epithelial cells kill intracellular P. aeruginosa lacking ExoS ADPr activity; inhibiting acidification reduces the activity of acid-dependent antimicrobial factors, e.g. acid-hydrolases, within epithelial vacuoles by drug-induced elevation of vacuolar pH and/or the prevention of phagosome maturation by inhibition of lysosome fusion as shown previously for autophagosome maturation [38].
Whether ExoS inhibits vacuolar acidification directly, or by redirecting bacteria to other compartments within the cell is yet to be directly determined. Supporting the latter possibility, wild-type PAO1 traffics to membrane blebs, where they are free to replicate without suppression by intravacuolar factors. However, popB (translocon) mutants, which also replicate within cells in a ExoS ADPr activity-dependent fashion, do not traffic to blebs and instead replicate within vacuoles [30]. Supporting the likelihood that ExoS acts locally upon the vacuole to inhibit acidification/promote intracellular replication is our data showing that popB mutant infected cells, like cells infected with wild-type bacteria, harbor a larger percentage of bacterial-occupied vacuoles that are not acidified compared to cells infected with exsA mutants. Also supporting the probability of direct manipulation, rather than an escape mechanism, is that ExoS ADPr activity, when introduced into cells without bacteria, blocks endocytic vesicle trafficking [39].
ExoS ADPr activity acts upon multiple cellular targets [40]. For example, inhibition of endocytic vesicle trafficking and lysosomal degradation of the epidermal growth factor receptor results from ExoS ADP-ribosylation of Rab5 and Rab9 [39]. Thus, ExoS inhibition of phagosome maturation through ADP-ribosylation of Rab5, and perhaps ExoS ADPr effects on other Rab GTPases (e.g. Rab6 or Rab9) with which it is known to interact [41], could explain our results. Those effects of ExoS could help internalized bacteria remain in immature phagosomes rather than trafficking to inhibitory LAMP3+, acidified (mature) phagolysosomes. Arguing against Rabs being the critical target for this activity of ExoS ADPr activity, however, is that popB mutants inhibit vacuolar acidification and replicate intracellularly. These mutants remain in vacuoles and should be unable to translocate ExoS across host (vacuolar or plasma) membranes to access Rabs that are located in the cytoplasm outside of vacuoles. None of the other known targets of ExoS ADPr activity including Ras [42] and the ERM proteins (Ezrin, Radixin, Moesin) [43], however, are known to be located inside vacuoles. However, it remains possible that ExoS can escape vacuoles in a translocon-mutant background through an alternative mechanism to exert its effects on vacuole acidification.
The observation that acidified vacuoles were sometimes within membrane blebs suggests that vacuolar trafficking might also be impacted. If so, that may or may not involve ExoS. A potential mechanism could be lysosomal exocytosis, a process triggered by plasma membrane damage including that induced by a T3SS as shown for Salmonella spp. [44]. This results in upregulated production of vesicles/lysosomes that are then exported to the plasma membrane for repair purposes [45]. Such a mechanism could conceivably contribute to formation of membrane blebs, and might induce bacterial trafficking to them during P. aeruginosa infection of epithelial cells.
Some individual bacterial cells among populations of mutants lacking ExoS ADPr activity were found in LT- (non-acidified) vacuoles. While it is possible that P. aeruginosa uses virulence factors in addition to the T3SS to avoid trafficking to acidic compartments, it is more likely that these were bacteria in the process of being trafficked to acidic compartments and had not yet reached their destination. The data showing that bacterial-occupied LT- (non-acidified) vacuoles were located further away from the nucleus than those that were LT+ supports that there are earlier steps in trafficking, considering that mature lysosomes tend to be perinuclear. Further, the finding that LT-vacuoles containing exsA mutants (ExoS secretion incapable) were smaller than those containing popB mutants (ExoS secretion capable) suggests they represent different compartments, consistent with the fact that only the latter can replicate inside vacuoles.
Some bacteria in wild-type infected cells expressed low levels of exoS, as shown using an exoS transcriptional reporter, and as expected these tended to localize to acidic vacuoles. Low levels of ExoS expression by those bacteria might follow loss of viability because they had been trapped in acidic vacuoles. Alternatively, these could be low-level expressers among the viable population and that is why they were trafficked to acidic vacuoles. The T3SS of P. aeruginosa is known to be triggered by host cell contact or low calcium conditions and requires translocation of the negative regulator ExsE [36,46]. Thus, individual bacteria inside a cell could be exposed to environments with differential triggering potential. Differences in gene expression among individual bacterial cells can also occur even when the entire population is exposed to the same inducing conditions, a phenomenon referred to as bistability [47]. Bistable gene expression could provide a survival advantage considering that activities of some P. aeruginosa T3SS effectors (e.g. the GAP activity of ExoS and ExoT) can inhibit host cell invasion and that the desirability of being internalized by a cell can depend on the host cell type and the prevailing extracellular conditions. Whatever the case, the fact that not all bacteria within a population express the T3SS at a given time point could explain the paradox of how invasive P. aeruginosa invade cells when capable of expressing antiphagocytic factors, and why only some internalized bacteria escape acidic compartments.
In summary, this study shows that epithelial cells are capable of killing internalized bacteria through a process involving trafficking to acidic compartments. This includes P. aeruginosa when not expressing the ADPr activity of ExoS, one of its T3SS effectors. Evasion of this fate by ExoS ADPr activity correlates with suppression of acidification, a feat that it can accomplish even when bacteria are trapped inside vacuoles, and are without the translocon normally used to transport T3SS effectors across host membranes. How ExoS accomplishes this when known targets of ExoS are located within the cell cytoplasm is to be determined.
Acknowledgments
Thanks to Dr. Arne Rietsch (Case Western Reserve University) for generously providing PAO1ΔpopB, and Dr. Dara Frank (Medical College of Wisconsin) for providing PAO1exsA::Ω. Thanks also to Dr. John Singer (University of Maine) for providing the plasmid construct p67T1 (dTomato).
Funding Statement
This work was funded by the National Institutes of Health (NIH) AI079192 (SMJF), AI030162 (JTB) and AI055042 (TY). Funding from Allergan Inc. was provided under a research agreement with the University of California, Berkeley. The NIH had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. Dr. Stern is a paid employee of Allergan Inc., and contributed to conception of the project idea. No other external funding was received for this work.
References
- 1. Eber MR, Shardell M, Schweizer ML, Laxminarayan R, Perencevich EN (2011) Seasonal and temperature-associated increases in gram-negative bacterial bloodstream infections among hospitalized patients. PLOS ONE 6: e25298. doi:10.1371/journal.pone.0025298. PubMed: 21966489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McColley SA, Ren CL, Schechter MS, Regelmann WE, Pasta DJ et al. (2012) Risk factors for onset of persistent respiratory symptoms in children with cystic fibrosis. Pediatr Pulmonol 47: 966-972. doi:10.1002/ppul.22519. PubMed: 22359344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Koulenti D, Lisboa T, Brun-Buisson C, Krueger W, Macor A et al. (2009) Spectrum of practice in the diagnosis of nosocomial pneumonia in patients requiring mechanical ventilation in European intensive care units. Crit Care Med 37: 2360-2368. doi:10.1097/CCM.0b013e3181a037ac. PubMed: 19531951. [DOI] [PubMed] [Google Scholar]
- 4. Stapleton F, Carnt N (2012) Contact lens-related microbial keratitis: how have epidemiology and genetics helped us with pathogenesis and prophylaxis. Eye (Lond) 26: 185-193. doi:10.1038/eye.2011.288. PubMed: 22134592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Keen EF 3rd, Robinson BJ, Hospenthal DR, Aldous WK, Wolf SE et al. (2010) Prevalence of multidrug-resistant organisms recovered at a military burn center. Burns 36: 819-825. doi:10.1016/j.burns.2009.10.013. PubMed: 20080354. [DOI] [PubMed] [Google Scholar]
- 6. Itani KM, Merchant S, Lin SJ, Akhras K, Alandete JC et al. (2011) Outcomes and management costs in patients hospitalized for skin and skin-structure infections. Am J Infect Control 39: 42-49. doi:10.1016/j.ajic.2011.04.095. PubMed: 20673598. [DOI] [PubMed] [Google Scholar]
- 7. Agodi A, Auxilia F, Barchitta M, Brusaferro S, D’Alessandro D et al. (2010) Building a benchmark through active surveillance of intensive care unit-acquired infections: the Italian network SPIN-UTI. J Hosp Infect 74: 258-265. doi:10.1016/j.jhin.2009.08.015. PubMed: 19914739. [DOI] [PubMed] [Google Scholar]
- 8. Lee DG, Urbach JM, Wu G, Liberati NT, Feinbaum RL et al. (2006) Genomic analysis reveals that Pseudomonas aeruginosa virulence is combinatorial. Genome Biol 7: R90. doi:10.1186/gb-2006-7-10-r90. PubMed: 17038190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mathee K, Narasimhan G, Valdes C, Qiu X, Matewish JM et al. (2008) Dynamics of Pseudomonas aeruginosa genome evolution. Proc Natl Acad Sci U S A 105: 3100-3105. doi:10.1073/pnas.0711982105. PubMed: 18287045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zolfaghar I, Evans DJ, Ronaghi R, Fleiszig SM (2006) Type III secretion-dependent modulation of innate immunity as one of multiple factors regulated by Pseudomonas aeruginosa RetS. Infect Immun 74: 3880-3889. doi:10.1128/IAI.01891-05. PubMed: 16790760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fleiszig SM, Wiener-Kronish JP, Miyazaki H, Vallas V, Mostov KE et al. (1997) Pseudomonas aeruginosa-mediated cytotoxicity and invasion correlate with distinct genotypes at the loci encoding exoenzyme S. Infect Immun 65: 579-586. PubMed: 9009316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee EJ, Cowell BA, Evans DJ, Fleiszig SM (2003) Contribution of ExsA-regulated factors to corneal infection by cytotoxic and invasive Pseudomonas aeruginosa in a murine scarification model. Invest Ophthalmol Vis Sci 44: 3892-3898. doi:10.1167/iovs.02-1302. PubMed: 12939306. [DOI] [PubMed] [Google Scholar]
- 13. Vance RE, Rietsch A, Mekalanos JJ (2005) Role of the type III secreted exoenzymes S, T, and Y in systemic spread of Pseudomonas aeruginosa PAO1 in vivo. Infect Immun 73: 1706-1713. doi:10.1128/IAI.73.3.1706-1713.2005. PubMed: 15731071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee EJ, Evans DJ, Fleiszig SM (2003) Role of Pseudomonas aeruginosa ExsA in penetration through corneal epithelium in a novel in vivo model. Invest Ophthalmol Vis Sci 44: 5220-5227. doi:10.1167/iovs.03-0229. PubMed: 14638720. [DOI] [PubMed] [Google Scholar]
- 15. Shaver CM, Hauser AR (2004) Relative contributions of Pseudomonas aeruginosa ExoU, ExoS, and ExoT to virulence in the lung. Infect Immun 72: 6969-6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Angus AA, Evans DJ, Barbieri JT, Fleiszig SM (2010) The ADP-ribosylation domain of Pseudomonas aeruginosa ExoS is required for membrane bleb niche formation and bacterial survival within epithelial cells. Infect Immun 78: 4500-4510. doi:10.1128/IAI.00417-10. PubMed: 20732998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alix E, Mukherjee S, Roy CR (2011) Subversion of membrane transport pathways by vacuolar pathogens. J Cell Biol 195: 943-952. doi:10.1083/jcb.201105019. PubMed: 22123831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ogawa M, Sasakawa C (2006) Shigella and autophagy. Autophagy 2: 171-174. PubMed: 16874102. [DOI] [PubMed] [Google Scholar]
- 19. Gomes MS, Paul S, Moreira AL, Appelberg R, Rabinovitch M et al. (1999) Survival of Mycobacterium avium and Mycobacterium tuberculosis in acidified vacuoles of murine macrophages. Infect Immun 67: 3199-3206. PubMed: 10377091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ghigo E, Colombo MI, Heinzen RA (2012) The Coxiella burnetii parasitophorous vacuole. Adv Exp Med Biol 984: 141-169. doi:10.1007/978-94-007-4315-1_8. PubMed: 22711631. [DOI] [PubMed] [Google Scholar]
- 21. Beauregard KE, Lee KD, Collier RJ, Swanson JA (1997) pH-dependent perforation of macrophage phagosomes by listeriolysin O from Listeria monocytogenes . J Exp Med 186: 1159-1163. doi:10.1084/jem.186.7.1159. PubMed: 9314564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Logsdon LK, Håkansson AP, Cortés G, Wessels MR (2011) Streptolysin O inhibits clathrin-dependent internalization of group A Streptococcus. mBio 2: e00332-00310 PubMed: 21325037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lamothe J, Huynh KK, Grinstein S, Valvano MA (2007) Intracellular survival of Burkholderia cenocepacia in macrophages is associated with a delay in the maturation of bacteria-containing vacuoles. Cell Microbiol 9: 40-53. doi:10.1111/j.1462-5822.2006.00766.x. PubMed: 16869828. [DOI] [PubMed] [Google Scholar]
- 24. Keith KE, Hynes DW, Sholdice JE, Valvano MA (2009) Delayed association of the NADPH oxidase complex with macrophage vacuoles containing the opportunistic pathogen Burkholderia cenocepacia . Microbiology 155: 1004-1015. doi:10.1099/mic.0.026781-0. PubMed: 19332803. [DOI] [PubMed] [Google Scholar]
- 25. Smith AC, Heo WD, Braun V, Jiang X, Macrae C et al. (2007) A network of Rab GTPases controls phagosome maturation and is modulated by Salmonella enterica serovar Typhimurium. J Cell Biol 176: 263-268. doi:10.1083/jcb.200611056. PubMed: 17261845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Knodler LA, Steele-Mortimer O (2003) Taking possession: biogenesis of the Salmonella-containing vacuole. Traffic 4: 587-599. doi:10.1034/j.1600-0854.2003.00118.x. PubMed: 12911813. [DOI] [PubMed] [Google Scholar]
- 27. Holden DW (2002) Trafficking of the Salmonella vacuole in macrophages. Traffic 3: 161-169. doi:10.1034/j.1600-0854.2002.030301.x. PubMed: 11886586. [DOI] [PubMed] [Google Scholar]
- 28. Ogawa M, Yoshimori T, Suzuki T, Sagara H, Mizushima N et al. (2005) Escape of intracellular Shigella from autophagy. Science 307: 727-731. doi:10.1126/science.1106036. PubMed: 15576571. [DOI] [PubMed] [Google Scholar]
- 29. Angus AA, Lee AA, Augustin DK, Lee EJ, Evans DJ et al. (2008) Pseudomonas aeruginosa induces membrane blebs in epithelial cells, which are utilized as a niche for intracellular replication and motility. Infect Immun 76: 1992-2001. doi:10.1128/IAI.01221-07. PubMed: 18316391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hritonenko V, Evans DJ, Fleiszig SM (2012) Translocon-independent intracellular replication by Pseudomonas aeruginosa requires the ADP-ribosylation domain of ExoS. Microbes Infect, 14: 1366–73. PubMed: 22981600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bloemberg GV, O’Toole GA, Lugtenberg BJ, Kolter R (1997) Green fluorescent protein as a marker for Pseudomonas spp. Appl Environ Microbiol 63: 4543-4551. PubMed: 9361441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Singer JT, Phennicie RT, Sullivan MJ, Porter LA, Shaffer VJ et al. (2010) Broad-host-range plasmids for red fluorescent protein labeling of gram-negative bacteria for use in the zebrafish model system. Appl Environ Microbiol 76: 3467-3474. doi:10.1128/AEM.01679-09. PubMed: 20363780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brutinel ED, Vakulskas CA, Brady KM, Yahr TL (2008) Characterization of ExsA and of ExsA-dependent promoters required for expression of the Pseudomonas aeruginosa type III secretion system. Mol Microbiol 68: 657-671. doi:10.1111/j.1365-2958.2008.06179.x. PubMed: 18373522. [DOI] [PubMed] [Google Scholar]
- 34. Robertson DM, Li L, Fisher S, Pearce VP, Shay JW et al. (2005) Characterization of growth and differentiation in a telomerase-immortalized human corneal epithelial cell line. Invest Ophthalmol Vis Sci 46: 470-478. doi:10.1167/iovs.04-0528. PubMed: 15671271. [DOI] [PubMed] [Google Scholar]
- 35. Yahr TL, Mende-Mueller LM, Friese MB, Frank DW (1997) Identification of type III secreted products of the Pseudomonas aeruginosa exoenzyme S regulon. J Bacteriol 179: 7165-7168. PubMed: 9371466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vallis AJ, Yahr TL, Barbieri JT, Frank DW (1999) Regulation of ExoS production and secretion by Pseudomonas aeruginosa in response to tissue culture conditions. Infect Immun 67: 914-920. PubMed: 9916108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cisz M, Lee PC, Rietsch A (2008) ExoS controls the cell contact-mediated switch to effector secretion in Pseudomonas aeruginosa . J Bacteriol 190: 2726-2738. doi:10.1128/JB.01553-07. PubMed: 18039770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yamamoto A, Tagawa Y, Yoshimori T, Moriyama Y, Masaki R et al. (1998) Bafilomycin A1 prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E cells. Cell Struct Funct 23: 33-42. doi:10.1247/csf.23.33. PubMed: 9639028. [DOI] [PubMed] [Google Scholar]
- 39. Deng Q, Barbieri JT (2008) Modulation of host cell endocytosis by the type III cytotoxin, Pseudomonas ExoS. Traffic 9: 1948-1957. doi:10.1111/j.1600-0854.2008.00808.x. PubMed: 18778330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Barbieri JT, Sun J (2004) Pseudomonas aeruginosa ExoS and ExoT. Rev Physiol Biochem Pharmacol 152: 79-92. PubMed: 15375697. [DOI] [PubMed] [Google Scholar]
- 41. Zhang Y, Deng Q, Barbieri JT (2007) Intracellular localization of type III-delivered Pseudomonas ExoS with endosome vesicles. J Biol Chem 282: 13022-13032. doi:10.1074/jbc.M606305200. PubMed: 17311921. [DOI] [PubMed] [Google Scholar]
- 42. Ganesan AK, Frank DW, Misra RP, Schmidt G, Barbieri JT (1998) Pseudomonas aeruginosa exoenzyme S ADP-ribosylates Ras at multiple sites. J Biol Chem 273: 7332-7337. doi:10.1074/jbc.273.13.7332. PubMed: 9516428. [DOI] [PubMed] [Google Scholar]
- 43. Maresso AW, Baldwin MR, Barbieri JT (2004) Ezrin/radixin/moesin proteins are high affinity targets for ADP-ribosylation by Pseudomonas aeruginosa ExoS. J Biol Chem 279: 38402-38408. doi:10.1074/jbc.M405707200. PubMed: 15252013. [DOI] [PubMed] [Google Scholar]
- 44. Roy D, Liston DR, Idone VJ, Di A, Nelson DJ et al. (2004) A process for controlling intracellular bacterial infections induced by membrane injury. Science 304: 1515-1518. doi:10.1126/science.1098371. PubMed: 15178804. [DOI] [PubMed] [Google Scholar]
- 45. McNeil PL (2002) Repairing a torn cell surface: make way, lysosomes to the rescue. J Cell Sci 115: 873-879. PubMed: 11870206. [DOI] [PubMed] [Google Scholar]
- 46. Urbanowski ML, Brutinel ED, Yahr TL (2007) Translocation of ExsE into Chinese hamster ovary cells is required for transcriptional induction of the Pseudomonas aeruginosa type III secretion system. Infect Immun 75: 4432-4439. doi:10.1128/IAI.00664-07. PubMed: 17635873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rietsch A, Mekalanos JJ (2006) Metabolic regulation of type III secretion gene expression in Pseudomonas aeruginosa . Mol Microbiol 59: 807-820. doi:10.1111/j.1365-2958.2005.04990.x. PubMed: 16420353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yahr TL, Frank DW (1994) Transcriptional organization of the trans-regulatory locus which controls exoenzyme S synthesis in Pseudomonas aeruginosa . J Bacteriol 176: 3832-3838. PubMed: 8021164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pederson KJ, Pal S, Vallis AJ, Frank DW, Barbieri JT (2000) Intracellular localization and processing of Pseudomonas aeruginosa ExoS in eukaryotic cells. Mol Microbiol 37: 287-299. doi:10.1046/j.1365-2958.2000.01990.x. PubMed: 10931325. [DOI] [PubMed] [Google Scholar]