Abstract
Humanin (HN) is a novel 24-amino acid mitochondrial-derived peptide that has demonstrated diverse cytoprotective effects, including an emerging role in diabetes. The purpose of this study was to examine the pharmacokinetics of humanin analogues, which show great potential as therapeutic agents (HNG and the non-IGFBP-3 binding, HNGF6A). 11-week-old male IGFBP-3−/− and wild type (WT) mice were divided into 3 groups: WT mice treated with HNG, WT mice treated with HNGF6A, and IGFBP-3−/− mice treated with HNG. Plasma was obtained from mice following ip injection with HN analogues, and HN levels were measured with ELISA. WT mice treated with HNGF6A and IGFBP-3−/− mice treated with HNG displayed a longer half-life of HN compared with WT mice treated with HNG. Following HNG injection, both IGF-1 and IGFBP-3 levels decreased over time. Adult male Sprague Dawley rats were also ip injected with HNG, and HN levels were measured in various tissues (plasma, liver, heart, and brain) by ELISA. The half-life of HN was found to be longer in rats compared with mice. In rats, HN levels were found to be highest in plasma, present in liver, and undetectable in brain or heart. The current study provides evidence of HN and IGFBP-3 association in the circulation and suggests that native HN may modulate the distribution of IGF-1 and IGFBP-3. The results also demonstrate varying kinetic profiles of HN analogues and interspecies variation in rodents. Sustainable levels of circulating HN measured in plasma underline the potential value of HN analogues as a new therapeutic intervention in the treatment of diabetes.
Humanin (HN) is a novel 24-amino acid mitochondrial-derived peptide transcribed from an open reading frame within the mitochondrial 16S rRNA. Discovered in 2001, it was cloned independently by 3 separate groups. Hashimoto et al. (1) first identified HN by screening a cDNA library from the brain of an Alzheimer's disease patient. They found that HN exerts a neuroprotective effect upon neurons exposed to amyloid-β-induced cytotoxicity (2). Guo et al. (3) described HN as a Bax antagonist that increases survival in susceptible cells exposed to UV irradiation and serum deprivation. Using a yeast 2-hybrid screen, our group cloned HN as an IGF-binding protein 3 (IGFBP-3) binding partner that antagonizes the actions of IGFBP-3 on various cell types (4). Since the seminal discovery, this peptide has demonstrated diverse cytoprotective effects across multiple organ systems, including heart, kidney, testis, and brain (5–8). Furthermore, HN was reported to have metabolo-protective effects including a sensitizing role in peripheral insulin action (9). Our group has recently shown that HN can potently suppress hepatic glucose production (9)and delay the onset of diabetes in the nonobese diabetic mouse (10).
A potential mechanism of action for HN involves binding to a receptor and initiating a cascade of intracellular signaling to provide cytoprotection. HN was shown to protect neurons from amyloid β by binding to a cytokine-receptor complex composed of glycoprotein 130, ciliary neurotrophic factor receptor α, and IL-27 receptor (WSX-1), and subsequently activating the Janus family of tyrosine kinases 2/signal transducer and activator of transcription 3 pathway (11, 12). HN also binds with high affinity to IGFBP-3 (4). It is thought to interact with IGFBP-3 and other binding partners inside or outside the cell, thus potentially regulating their activity (4).
HN and its synthetic analogues are currently under investigation as potential treatment for a variety of conditions including Alzheimer's disease. These specific HN analogues have increased potency when compared with endogenous HN in vivo (9, 13). HNG, produced by amino acid substitution at position 14 (Gly for Ser), has substantially greater cytoprotective action than HN. HNGF6A, another variant produced by amino acid substitution at position 6 (Ala for Phe) and position 14 (Gly for Ser), is unique in that it does not bind to IGFBP-3 (14) (Figure 1). Despite this complete loss of IGFBP-3 binding, HN's cytoprotective effects remain preserved.
Figure 1.
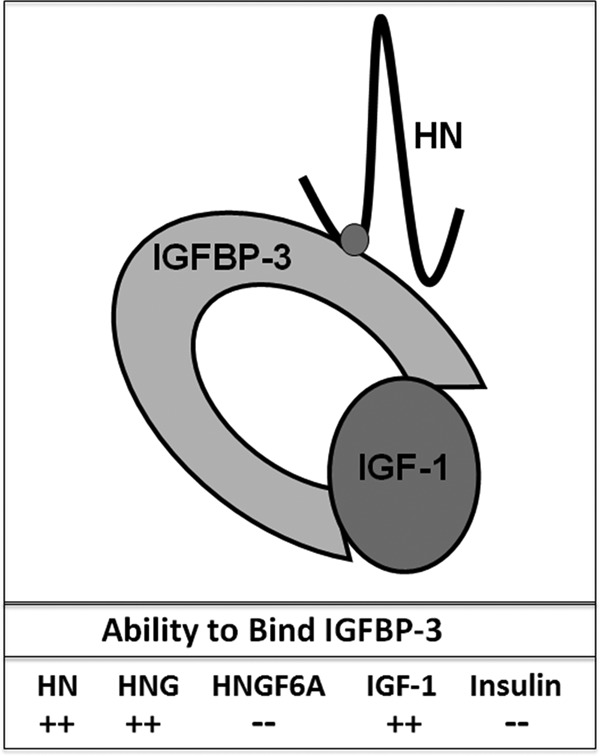
Ability of HN Analogues to Bind IGFBP-3 HN, HNG, and IGF-1 bind with high affinity (++, quantitatively unknown) to IGFBP-3, whereas HNGF6A and insulin do not (−).
Given the potent effects of HN and its analogues seen in type 1 and type 2 diabetic models, it appears that these peptides have great potential as therapeutic agents in diabetes control and prevention (9). Thus, understanding their pharmacokinetics is critical. In this study, we first compared the pharmacokinetic profiles of HN analogues, HNG and HNGF6A, in IGFBP-3−/− mice and their wild-type (WT) littermates, which we have previously described (15). Using a newly developed HN ELISA, we examined the effects of circulating IGF-1 and IGFBP-3 on HN pharmacokinetics. We also characterized the kinetics and distribution of HNG treatment in various tissues in a rat model.
Materials and Methods
Materials
Mouse recombinant IGF-1 (catalog no. 791), IGFBP-3 (catalog no. 775), ALS (catalog no. 1436), specific monoclonal antibodies (MABs) to these proteins (MAB 791, MAB 775, MAB 1436, respectively), and biotinylated polyclonal detection antibodies (BAF 791, BAF 775, BAF 1436, respectively) were obtained from R&D Systems. Microtiter plates were obtained from Nalgene Nunc. Streptavidin-horseradish peroxidase conjugate, o-phenylenediamine hydrochloride, and hydrogen peroxide substrate were purchased from Pierce Chemical Co Other reagents were obtained from Sigma-Aldrich.
Animals
C57/BL6 mice were purchased from Charles River Laboratories and acclimatized for 1 week at the University of California Los Angeles (UCLA) Animal Facility prior to any testing. IGFBP-3−/− mice and homozygous littermates were bred and propagated under approved ARC protocols at UCLA. Male and female pups were weaned, weighed, and genotyped at 3 weeks of age and maintained on normal chow (4.5% fat, Lab Diet 5001) until study initiation. Male mice (11 weeks of age) were divided into 3 treatment groups: WT treated with HNG, WT treated with HNGF6A, and IGFBP-3−/− treated with HNG. Each mouse was ip injected with 2 mg/kg peptide. The control mice received ip vehicle injection. Blood was collected from the IGFBP-3−/− mice and WT littermates at 10, 30, 60, 100, and 180 min (n = 5 per time point) following injection of HN analogues. Plasma was isolated by blood collection into BD Vacutainer tubes and centrifugation, with plasma subsequently removed and stored at −70°C until the assay was performed. The above studies were approved by the UCLA Animal Care and Use Committee.
Adult male Sprague Dawley (SD) rats (12–15 wk old) were purchased from Charles River Laboratories and housed in a standard animal facility under controlled temperature (22°C) and photoperiod of 12 hours of light and 12 hours of darkness with free access to food and water. Animal handling and experimentation were in accordance with recommendations from the American Veterinary Medical Association and were approved by the animal care and use review committee at the Los Angeles Biomedical Research Institute at Harbor-University of California, Los Angeles (Harbor-UCLA) Medical Center. In the treatment group, each rat was ip injected with 5 mg/kg of HNG at time 0. They were euthanized, and plasma samples were obtained at 30 minutes, 90 minutes, 4 hours, and 24 hours after HNG injection (n = 3 to 5 per time point). The control group received ip vehicle injection. Liver, heart, and brain tissue were collected after saline perfusion of the animals, then snap-frozen in liquid nitrogen and stored in −80°C.
Drug preparation and administration
HN analogues HNG and HNGF6A were synthesized by CPC Scientific Inc. Lyophilized HNG and HNGF6A were reconstituted with 1% trifluoroacetic acid, PBS, and 0.9% saline to a concentration of 0.2 mg/mL at pH 7.0.
Mouse IGF-1 and IGFBP-3 measurement
Mouse IGF-1 and IGFBP-3 ELISA were measured by a UCLA in-house ELISA as previously described (16).
HN measurement
HN levels of plasma and tissue extracts were measured by in-house HN ELISA developed at UCLA. The rabbit antihuman analogue HNG polyclonal antisera were produced at Harlan Laboratories. IgG subclasses purified with a protein A column chromatography (Pierce Chemical Co) were used as capture antibodies. IgG was further purified with an HNG-conjugated ligand affinity column and labeled with biotin. This biotinylated ligand affinity-purified anti-HNG IgG was used as a detection antibody. To measure endogenous HN levels, synthetic HN purchased from Bachem was used as a standard within the range of 0.1 ng/mL to 50 ng/mL. The intra- and interassay coefficient variations (CV) were less than 10%. Prior to assay, HN was extracted in 90% acetonitrile and 10% 1 N HCl. Briefly, 200 μL of extraction reagent was added to 100 μL of plasma or protein extracts, gently mixed, and incubated at room temperature for 30 minutes. The mixture was centrifuged and the supernatant was removed and dried. The dried extract was reconstituted with 200 μL of phosphate buffer (50 mM sodium phosphate, 150 mM sodium chloride, 0.5% Tween-20, pH 7.6) and then used for assay. Microtiter plates (96 well) were coated with capture antibody at 0.5 μg/well in 200 μL of 50 mM sodium bicarbonate buffer, pH 9.5, incubated 3–4 hours at room temperature on a shaker, and then washed with wash buffer followed by 2 washes with Superblock buffer (Pierce Chemical Co). Standards, controls or extracted samples, and pretitered detection antibody were added to the appropriate wells and incubated overnight. Wells were then added with streptavidin-horseradish peroxidase conjugate after wash and further incubated for 30 minutes at room temperature. After 4 washes with wash buffer, 200 μL/well of o-phenylenediamine hydrochloride solution (1 mg/mL in hydrogen peroxide substrate) was incubated for 10–20 minutes. The reaction was terminated by the addition of 50 μL/well 2 N H2SO4, and absorbance was measured on a plate spectrophotometer (Molecular Designs) at 490 nm.
This HN ELISA was also used to measure exogenous HNG or HNGF6A, and data are presented as nanograms/mL for plasma or nanograms/mg protein for tissue extracts. This anti-HNG antibody's difference in affinity between HN and its analogues HNG/HNGF6A was examined by comparing prepared HNG/HNGF6A concentrations and measuring them using HN as the standard. Our data showed that the measured HNG and HNGF6A were approximately 16-fold higher than their actual concentration (see Supplemental Table 1 published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). The HN ELISA does not have cross-reactivity with synthetic rattin (Phoenix Pharmaceuticals), the rat HN analogue, at 200 ng/mL.
Data analysis
For the HN data in rats, we used tobit regression for left censored data because readings of less than 1 ng/L were not detectable. Wald tests of 2 joint linear hypotheses were performed with the Bonferonni correction for 2 tests applied. Analyses were performed in R version 2.13.2 (http://www.r-project.org/) and STATA version 12.1 (Stata Corp).
The ELISA standard curves were analyzed using 4-parameter logistic curvefit. Results are reported as the mean ± SD. Differences between groups were analyzed by one-way ANOVA. For all other studies, data were expressed as mean ± SEM or mean ± SD. Results were analyzed with Student's t test (pair-wise comparisons) or ANOVA with post hoc correction (multiple comparisons). The level of significance was set for P < .05 for all tests. Nonlinear regression analysis was used to fit curves for different concentrations using Prism (GraphPad Software).
Results
Kinetics of HN analogues in WT and IGFBP-3−/− mice
Initial pharmacokinetic studies of HN analogues were performed in WT and IGFBP-3−/− mice. The high-potency analogues, HNG and HNGF6A (IGFBP-3 binding deficient) were used. All treatment groups showed peak plasma HN levels at 10 minutes after ip injection. WT mice treated with either HNG or HNGF6A had peak HN plasma levels of 139 ng/mL ± 3.8 and 146 ng/mL ± 27.3 respectively, whereas IGFBP-3−/− mice treated with HNG had significantly lower peak plasma HN levels of 63 ng/mL ± 11.5(P < .05). Clearance of HN analogues was slower in the 2 groups characterized by absent HN and IGFBP-3 interaction (WT mice injected with HNGF6A, and IGFBP-3−/− mice injected with HNG), compared with WT mice given HNG. Mean levels of HN and its analogues were plotted on a logarithmic scale and the half-life was calculated (Figure 2).
Figure 2.
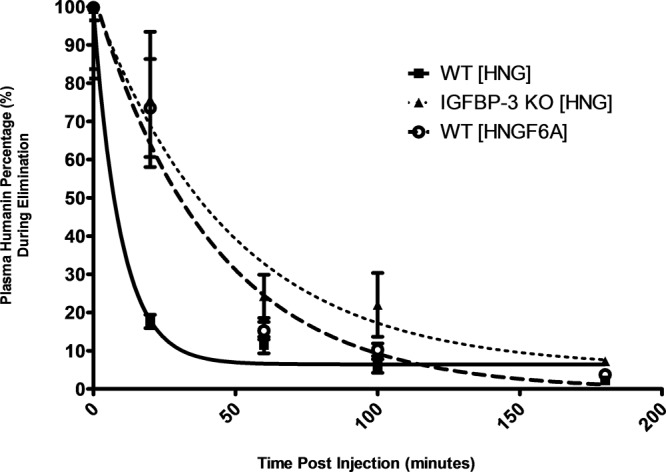
Kinetics of HN analogues in WT and IGFBP-3−/− mice. Following ip injection with HN analogues, HN levels were measured with ELISA in the 3 treatment groups: WT mice treated with HNG (solid line, squares), WT mice treated with HNGF6A (hatched line, circles), and IGFBP-3−/− mice treated with HNG (dotted line, triangles). The half-lives of HNGF6A in WT mice and of HNG inIGFBP-3−/− mice were longer than the half-life of HNG observed in WT mice.
HN analogue effects on IGFBP-3 levels
Next, the effects of HNG and HNGF6A injections on serum IGFBP-3 levels were examined. As published previously (15), IGFBP-3−/− mice had nondetectable levels of IGFBP-3, whereas WT mice had a baseline IGFBP-3 level of 1411 ng/mL ± 213. Following HNG injection, plasma IGFBP-3 levels in WT mice decreased over time, falling to 80% of baseline after 180 minutes (P < .01). As predicted, IGFBP-3 levels remained unchanged after HNGF6A injection (Figure 3).
Figure 3.
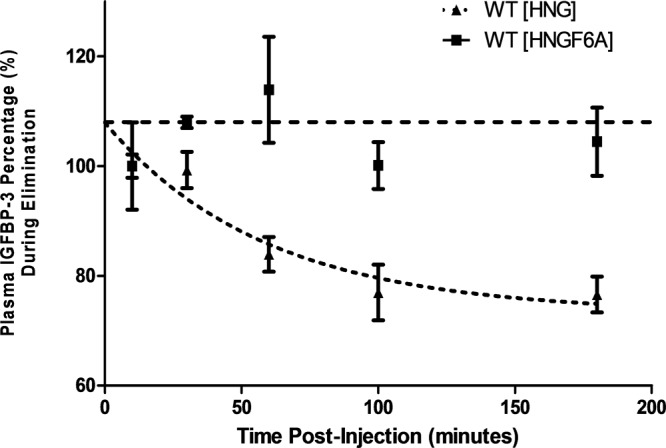
Modulation of IGFBP-3 by HN analogues in WT mice. WT mice received ip injection with either HNG or HNGF6A, and IGFBP-3 levels were subsequently measured (HNG, dotted line; HNGF6A, hatched line). Following injection with HNG, IGFBP-3 levels decreased to 80% of baseline after 180 minutes (dotted line) (P < .01). After injection with HNGF6A, IGFBP-3 levels remained unchanged in these mice (hatched line).
HN analogue effects on IGF-1 levels
IGF-1 levels were also assessed in the 3 treatment groups. In the IGFBP-3−/− mice, IGF-1 levels were observed to be 3–4 times lower than in WT mice (118.75 ng/mL ± 4.19 compared with 445.75 ng/mL ± 58.7) (P < .0001), consistent with previous observations (15). Similar to alterations seen in IGFBP-3 in WT mice after ip injection, IGF-1 levels decreased by approximately 30% in WT mice injected with HNG (P < .01). Conversely, IGF-I levels remained unchanged in the IGFBP-3−/− mice treated with HNG, and in WT mice injected with HNGF6A (Figure 4).
Figure 4.
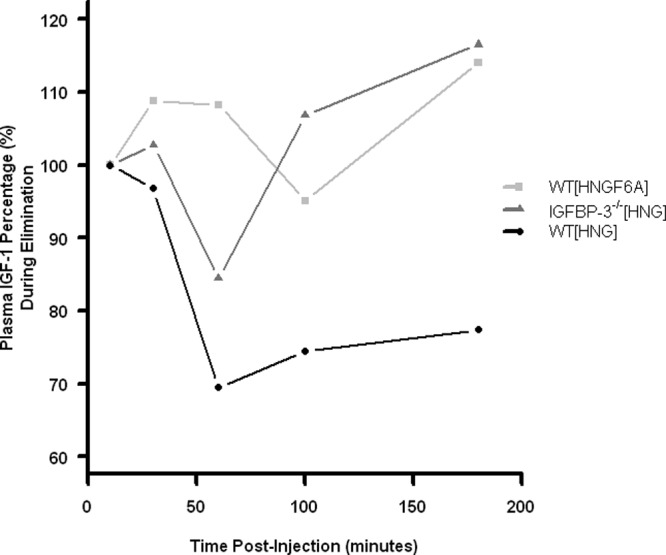
Modulation of IGF-1 by HN analogues in WT and IGFBP-3−/− mice. Following ip injection with HN analogues, IGF-1 levels were measured in the 3 treatment groups: WT mice treated with HNG (circles), WT mice treated with HNGF6A (squares), and IGFBP-3−/− mice treated with HNG (triangles). After treatment with HNG, IGF-1 levels in WT mice decreased by 23% (circles) (P < .01). In contrast, IGF-1 levels remained unchanged in the IGFBP-3−/− mice injected with HNG (triangles), and in WT mice injected with HNGF6A (squares).
Kinetics of HN in rats
To assess for any HN interspecies variability, pharmacokinetic studies were performed in rats. The peak plasma HN level was 14.6 ng/mL at 4 hours, whereas the mean plasma HN levels over time were 6.3 ng/mL ± 1.9 (30 minutes), 4.7 ng/mL ± 2.2(90 minutes), 8.5 ng/mL ± 2.4 (4 hours), and 2.7 ng/mL ± 0.7 (24 hours) (Figure 5). Shown is a best-fit curve of the kinetic data as well as mean values of HN levels at the first 4 hours and at 24 hours (P < .05).
Figure 5.
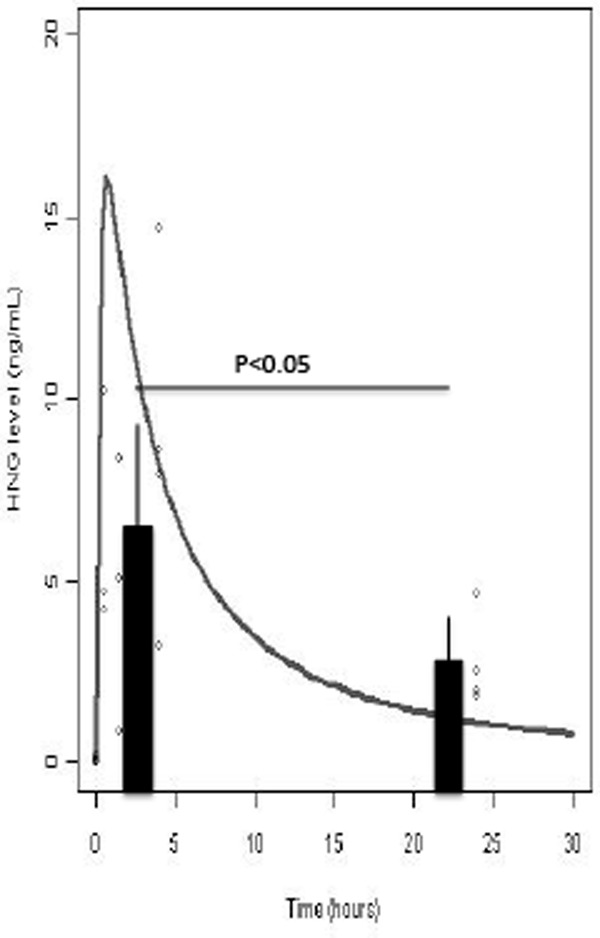
Plasma HN levels in sprague dawley rats. Error bars represent the 95% confidence intervals. Rats received an ip injection with HNG 4 mg/kg, and HN levels were measured in plasma with ELISA. Shown is a best-fit curve of the kinetic data as well as mean values of HN levels at the first 4 hours and at 24 hours (P < .05). Pooling the data from the first 3 time points, the average serum level is significantly higher than that of the averaged data at the 24th hour (6.8 ng/mL ± 1.3 compared with 2.7 ng/mL ± 1.3) (P < .05). The sample size for the first 3 time points group is 10 rats, and sample size for the 24th hour group is 4 rats. The half-life of HN appears to be greater than 4 hours, which is much longer than the half-life measured in mice (<1 hour).
Tissue distribution of HN in rats
HN levels were measured in various tissues of rats injected with HNG. Levels were found to be highest in plasma and present at lower levels in the liver, whereas HN was not detectable in brain or heart (Figure 6). Mean HN levels in liver were 0.9 ng/mg (30 minutes), 0.4 ng/mg (90 minutes), 1.0 ng/mg (4 hours), and 0.1 ng/mg (24 hours).
Figure 6.
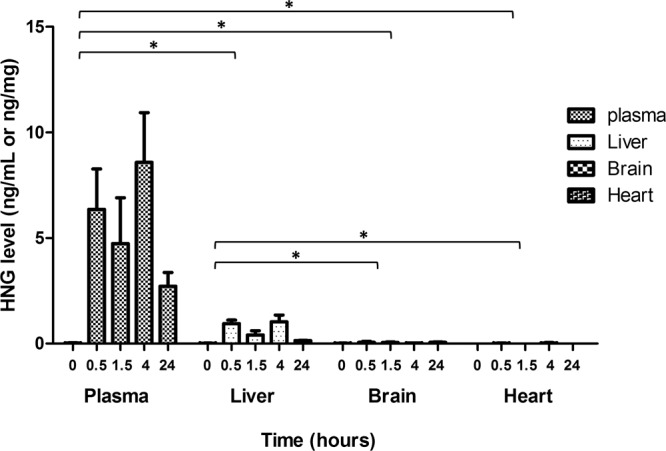
Distribution of HN in various tissues and plasma in sprague dawley rats. Rats received an ip injection with HNG 4 mg/kg, and HN levels were measured with ELISA in various organs including liver, brain, and heart. *, P < .0001. Means ± SEM are presented. Data from each organ are combined and then compared with the other groups. HN distribution in plasma overall is significant when compared with liver, brain, and heart. Also, HN distribution in liver as a whole is significant when compared with brain and heart.
Discussion
The pharmacokinetics of increased potency HN analogues HNG and HNGF6A were analyzed in WT and IGFBP-3−/− mice. HN analogues were found to have different half-lives, and kinetics was dependent on binding to IGFBP-3 (Figure 1). The kinetics of HNG after ip injection in WT mice was rapid, with a short half-life of approximately 30 minutes. At 10 minutes after ip injection, the peak plasma HN level of WT mice treated with HNG was 50% of the level seen in the other 2 groups with IGFBP-3-deficient binding (WT mice treated with HNGF6A, and IGFBP-3−/− mice treated with HNG). Possible reasons for this lower peak level include a larger volume of distribution or increased degradation due to lack of HNG binding and stabilization by IGFBP-3. HNG bound to IGFBP-3 resulted in a higher peak level, but more rapid clearance from plasma when compared with HNGF6A in WT mice or HNG in IGFBP-3−/− mice. These results suggest that the interaction of HN with IGFBP-3 promotes the clearance of HN from plasma.
To further understand the relationship between HN, IGF-1, and IGFBP-3, we examined the effects of HNG and HNGF6A on the levels of these proteins. Circulating IGFBP-3 appeared to modulate HN levels, including the duration, peak level, and clearance rate of injected HN analogues. We observed a decline in circulating IGFBP-3 following the rapid clearance of HN analogue, suggesting an effect of HN on the elimination of IGFBP-3. IGF-1 levels similarly decreased in WT mice treated with HNG. Based on our previous work (15), the presence of IGF-1 does not interfere with the ability to measure IGFBP-3. Because it is well known that IGFBP-3, IGF-1, and ALS form a ternary complex in the circulation (17), these data suggest that HN may have the ability to interfere with the ternary complex, possibly by altering the interaction between IGF-1 and IGFBP-3, and thus leading to enhanced clearance and reduced circulating levels of both factors. Because of these findings, HN analogues (eg, HNGF6A) that do not bind IGFBP-3 (and thus do not disrupt the ternary complex) appear to have a superior pharmacokinetic profile and may prove important in the development of targeted HN-related therapies without disturbing the GH-IGF system. HNGF6A has already been shown to improve both hepatic and peripheral insulin sensitivity, as well as blood glucose levels in ZDF rats with a single dose (9). Chronic administration of HNGF6A to apolipoprotein E-deficient mice on a high-cholesterol diet was shown to prevent endothelial dysfunction and the progression of atherosclerotic plaques (18).
We also performed studies in rats. This provided us with an ideal system to verify that the HN levels detected were indeed exogenous HN because our ELISA does not recognize the rat homolog of HN. Variability in HN kinetics was noted between mice and rats, and the etiology of these interspecies differences remains unclear. The half-life of circulating exogenous HN was found to be much longer in rats compared with mice, though the rats did receive a higher dose of HN treatment (5 mg/kg) compared with mice (2 mg/kg). Interestingly, at 30 minutes postinjection, the mean HN level in rats (6.3 ng/mL) was much lower than the mean HN levels measured in both the WT and IGFBP-3−/− mice (49.6 ng/mL and 48.3 ng/mL, respectively). The distribution of HN in rat plasma and various tissues was also examined. Based on the data, exogenous HN circulates in the plasma at high levels. However, it is present in low levels in the liver and does not appear to enter the brain or heart at the times tested. Perhaps HN acts on intermediary receptors or penetrates in low concentrations at a later time in these organs. In addition, the low levels found in liver could be reflective of circulating plasma levels in a highly vascularized organ.
There are currently 14 published papers describing HN analogues given to rodent disease models in vivo (5–7, 9, 10, 18–26). Routes of administration include ip or intracerebroventricular injection, iv, and intranasally. Treatment doses of HN have ranged from 4 μg/kg/d to 4 mg/kg/d, and they are typically given once daily or every other day. Given the HN distribution seen in our plasma data, it would appear that daily administration of HN for therapeutic purposes could result in substantial clinical effects. Based on the dramatic effects of daily injections of HN analogues in these animal models, it is also reasonable to assume that the biological half-life of HN is longer than the kinetic half-life.
In summary, the current study demonstrates various kinetic profiles of HN analogues in rodents, with HN dependence on IGFBP-3 binding, a longer half-life of HN in rats compared with mice, and sustainable HN levels in plasma. As the role of HN in diabetes and related diseases continues to emerge, these data will help provide the basis for the development of HN as a clinical intervention in metabolic diseases and yield important information in the assessment of therapy delivery and efficacy.
Acknowledgments
We thank UCLA statistician Ning Li and D. Hwang, M. Sycip, P.M. Yamada, H. Mizuno, and K. Miyako for their technical help (all from School of Medicine, University of California, Los Angeles, California 90095).
This work was supported by a Glenn Foundation Award and National Institute of Health Grants 1R01AG 034430, 1R01GM 090311, 1R01ES 020812, and 1R21DK089447 (to P.C.) and Clinical and Translational Science Institute (CTSI) Grant UL1TR000124. UCLA statistician Ning Li was supported by CTSI Grant UL1TR000124.
Disclosure Summary: Dr Cobb is a stock holder and employee of CohBar, Inc. Dr Cohen is a consultant and stock holder of CohBar, Inc.
Footnotes
- HN
- humanin
- IGFBP
- IGF-binding protein
- WT
- wild type.
References
- 1. Hashimoto Y, Niikura T, Tajima H, et al. A rescue factor abolishing neuronal cell death by a wide spectrum of familial Alzheimer's disease genes and Abeta. Proc Natl Acad Sci USA. 2001;98:6336–6341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Niikura T, Chiba T, Aiso S, Matsuoka M, Nishimoto I. Humanin: after the discovery. Mol Neurobiol. 2004;30:327–340 [DOI] [PubMed] [Google Scholar]
- 3. Guo B, Zhai D, Cabezas E, et al. Humanin peptide suppresses apoptosis by interfering with Bax activation. Nature. 2003;423:456–461 [DOI] [PubMed] [Google Scholar]
- 4. Ikonen M, Liu B, Hashimoto Y, et al. Interaction between the Alzheimer's survival peptide humanin and insulin-like growth factor-binding protein 3 regulates cell survival and apoptosis. Proc Natl Acad Sci USA. 2003;100:13042–13047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Muzumdar RH, Huffman DM, Calvert JW, et al. Acute humanin therapy attenuates myocardial ischemia and reperfusion injury in mice. Arterioscl Thromb Vasc Biol. 2010;30:1940–1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Singh BK, Mascarenhas DD. Bioactive peptides control receptor for advanced glycated end product-induced elevation of kidney insulin receptor substrate 2 and reduce albuminuria in diabetic mice. Am J Nephrol. 2008;28:890–899 [DOI] [PubMed] [Google Scholar]
- 7. Xu X, Chua CC, Gao J, Hamdy RC, Chua BH. Humanin is a novel neuroprotective agent against stroke. Stroke. 2006;37:2613–2619 [DOI] [PubMed] [Google Scholar]
- 8. Lue Y, Swerdloff R, Liu Q, et al. Opposing roles of insulin-like growth factor binding protein 3 and humanin in the regulation of testicular germ cell apoptosis. Endocrinology. 2010;151:350–357 [DOI] [PubMed] [Google Scholar]
- 9. Muzumdar RH, Huffman DM, Atzmon G, et al. Humanin: a novel central regulator of peripheral insulin action. PloS one. 2009;4:e6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hoang PT, Park P, Cobb LJ, et al. The neurosurvival factor Humanin inhibits β-cell apoptosis via signal transducer and activator of transcription 3 activation and delays and ameliorates diabetes in nonobese diabetic mice. Metabolism. 2010;59:343–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hashimoto Y, Kurita M, Aiso S, Nishimoto I, Matsuoka M. Humanin inhibits neuronal cell death by interacting with a cytokine receptor complex or complexes involving CNTF receptor α/WSX-1/gp130. Mol Biol Cell. 2009;20:2864–2873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hashimoto Y, Suzuki H, Aiso S, et al. Involvement of tyrosine kinases and STAT3 in humanin-mediated neuroprotection. Life Sci. 2005;77:3092–3104 [DOI] [PubMed] [Google Scholar]
- 13. Mamiya T, Ukai M. [Gly(14)]-Humanin improved the learning and memory impairment induced by scopolamine in vivo. Br J Pharmacol. 2001;134:1597–1599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yen K, Lee C, Metha H, et al. The emerging role of the mitochondrial-derived peptide humanin in stress resistance. J Mol Endocrinol. 2013;50(1):R11–R19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yamada PM, Mehta HH, Hwang D, Roos KP, Hevener AL, Lee KW. Evidence of a role for insulin-like growth factor binding protein (IGFBP)-3 in metabolic regulation. Endocrinology. 2010;151:5741–5750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hwang DL, Lee PD, Cohen P. Quantitative ontogeny of murine insulin-like growth factor (IGF)-I, IGF-binding protein-3 and the IGF-related acid-labile subunit. Growth Horm IGF Res. 2008;18:65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guler HP, Zapf J, Schmid C, Froesch ER. Insulin-like growth factors I and II in healthy man. Estimations of half-lives and production rates. Acta Endocrinol. 1989;121:753–758 [DOI] [PubMed] [Google Scholar]
- 18. Oh YK, Bachar AR, Zacharias DG, et al. Humanin preserves endothelial function and prevents atherosclerotic plaque progression in hypercholesterolemic ApoE deficient mice. Atherosclerosis. 2011;219:65–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guo F, Jing W, Ma CG, et al. [Gly(14)]-humanin rescues long-term potentiation from amyloid β protein-induced impairment in the rat hippocampal CA1 region in vivo. Synapse. 2010;64:83–91 [DOI] [PubMed] [Google Scholar]
- 20. Krejcova G, Patocka J, Slaninova J. Effect of humanin analogues on experimentally induced impairment of spatial memory in rats. J Pept Sci. 2004;10:636–639 [DOI] [PubMed] [Google Scholar]
- 21. Miao J, Zhang W, Yin R, et al. S14G-Humanin ameliorates Aβ25–35-induced behavioral deficits by reducing neuroinflammatory responses and apoptosis in mice. Neuropeptides. 2008;42:557–567 [DOI] [PubMed] [Google Scholar]
- 22. Niikura T, Sidahmed E, Hirata-Fukae C, Aisen PS, Matsuoka Y. A humanin derivative reduces amyloid β accumulation and ameliorates memory deficit in triple transgenic mice. PloS one. 2011;6:e16259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xu X, Chua CC, Gao J, et al. Neuroprotective effect of humanin on cerebral ischemia/reperfusion injury is mediated by a PI3K/Akt pathway. Brain Res. 2008;1227:12–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xu X, Chua KW, Chua CC, Liu CF, Hamdy RC, Chua BH. Synergistic protective effects of humanin and necrostatin-1 on hypoxia and ischemia/reperfusion injury. Brain Res. 2010;1355:189–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang W, Zhang W, Li Z, et al. S14G-humanin improves cognitive deficits and reduces amyloid pathology in the middle-aged APPswe/PS1dE9 mice. Pharmacol Biochem Behav. 2012;100:361–369 [DOI] [PubMed] [Google Scholar]
- 26. Zhang X, Urbieta-Caceres VH, Eirin A, et al. Humanin prevents intra-renal microvascular remodeling and inflammation in hypercholesterolemic ApoE deficient mice. Life Sci. 2012;91:199–206 [DOI] [PMC free article] [PubMed] [Google Scholar]


