Abstract
Background & Aims
The incidence of upper gastrointestinal bleeding (GIB) has not been reduced despite the decreasing incidence of peptic ulcers, strategies to eradicate Helicobacter pylori infection, and prophylaxis against ulceration from nonsteroidal anti-inflammatory drugs. Other factors might therefore be involved in the pathogenesis of GIB. Patients with GIB have increasing nongastrointestinal comorbidity, so we investigated whether comorbidity itself increased the risk of GIB.
Methods
We conducted a matched case-control study using linked primary and secondary care data collected in England from April 1, 1997 through August 31, 2010. Patients older than 15 years with nonvariceal GIB (n = 16,355) were matched to 5 controls by age, sex, year, and practice (n = 81,636). All available risk factors for GIB were extracted and modeled using conditional logistic regression. Adjusted associations with nongastrointestinal comorbidity, defined using the Charlson Index, were then tested and sequential population attributable fractions calculated.
Results
Comorbidity had a strong graded association with GIB; the adjusted odds ratio for a single comorbidity was 1.43 (95% confidence interval [CI]: 1.35–1.52) and for multiple or severe comorbidity was 2.26 (95% CI: 2.14%–2.38%). The additional population attributable fraction for comorbidity (19.8%; 95% CI: 18.4%–21.2%) was considerably larger than that for any other measured risk factor, including aspirin or nonsteroidal anti-inflammatory drug use (3.0% and 3.1%, respectively).
Conclusions
Nongastrointestinal comorbidity is an independent risk factor for GIB, and contributes to a greater proportion of patients with bleeding in the population than other recognized risk factors. These findings could help in the assessment of potential causes of GIB, and also explain why the incidence of GIB remains high in an aging population.
Keywords: Etiology, Gastrointestinal Bleeding, Stomach
Abbreviations used in this paper: GIB, gastrointestinal bleeding; GPRD, General Practice Research Database; ITU, intensive therapy unit; NSAID, nonsteroidal anti-inflammatory drug; OR, odds ratio; PAF, population attributable fraction; PPI, proton pump inhibitors
See Covering the Cover synopsis on page 1327.
Helicobacter pylori infection, nonsteroidal anti-inflammatory medications (NSAIDs), and aspirin are believed to be the main causes of nonvariceal upper gastrointestinal bleeding,1 and with the discovery of proton pump inhibitors (PPIs) and H pylori eradication therapy, the burden of peptic ulcer disease has been decreasing.2 Despite this, upper gastrointestinal hemorrhage remains the most common acute severe medical admission for gastroenterology,3,4 and its incidence in population-based studies remains virtually unchanged.5,6 This suggests that other (previously unidentified) risk factors are contributing to its population burden.
Historically, nongastrointestinal comorbidity was believed to be associated with stress ulceration7 but, currently, the role of comorbidity in the etiology of gastrointestinal bleeding (GIB) is not recognized apart from in severe illness; for example, sicker cirrhotic patients are known to have an increased risk of variceal bleeding,8 and sicker patients in intensive therapy units (ITUs) have an increased risk of nonvariceal bleeding.9 However, as the proportion of bleed patients with comorbidity has increased during the last decade,5 we wondered if exposure to less severe but chronic comorbidity could itself be responsible for the persisting incidence of bleeding. Outside of ITU though, the effect of comorbidity has only been assessed as a confounder in studies that focused on the effect of medications on gastrointestinal bleeds.10 Although these studies do support a role for comorbidity, they do not allow us to understand whether it is an important independent contributor to the persisting burden of upper GIB.
We have therefore conducted a study aimed primarily at assessing whether comorbidity might have an important role in the etiology of upper GIB. To do this we have conducted a case-control study and formed a model fully corrected for known measured risk factors of upper GIB. We have then calculated the additional explanatory effect of adding comorbidity to our model to understand its effect on bleeding incidence in the general population.
Methods
Study Design
We conducted a matched case control study.
Data
To provide the detailed longitudinal data and necessary power for this study, we have used the recently linked English Hospital Episodes Statistics data (secondary care data) and General Practice Research Database (GPRD) (primary care data). Because of the comprehensive English primary care system, the population registered to the GPRD is representative of the general English population.11 The data are subject to quality checks and a practice's data are only used when they are of high enough quality to be used in research, at these times the data are said to be “up to research standard.”12 The GPRD has been extensively validated for a wide range of diagnoses, with a mean positive predictive value of 89%.13 Ethical approval for this study was obtained from the Independent Scientific Advisory Committee for Medicines and Healthcare products Regulatory Agency database research. Fifty-one percent of English practices in GPRD have consented to record level linkage of their population to Hospital Episodes Statistics. This records all hospital admissions from the population registered to one of the linked primary care practices contributing to the GPRD. For this study, the linked dataset was available between April 1, 1997 and August 31, 2010.
Case Definition
We have previously published the codes and methods used to define upper gastrointestinal bleeds in this study.14 In brief, we selected as exposed all patients with a first nonvariceal upper gastrointestinal bleed. A bleed was defined by a specific code for an upper gastrointestinal nonvariceal bleed in either primary or secondary care who had a supporting code in the linked dataset (defined as a likely symptom, cause, therapy, investigation, or outcome of upper gastrointestinal hemorrhage). Variceal bleeds or nonspecific gastrointestinal bleed codes with either a lower gastrointestinal diagnosis or procedure were excluded. Further exclusions were temporary patients (patients not registered permanently at a GPRD primary care practice, who might just be visiting the area of the practice briefly, and who are therefore not part of the GPRD's underlying population), children younger than 16 years old, cases with invalid date codes, or cases outside the up-to-research-standard observed time periods. Patients were required to be registered with the primary care practice for at least 3 months before an upper gastrointestinal bleed event to avoid including prevalent cases that might have been coded at the initial registration consultation. Only the first event for each patient was included. We have previously demonstrated that this selection strategy minimizes selection bias in studies of upper GIB in these data.14 A secondary analysis was then stratified by whether the defining bleed code or supporting code specifically referred to a peptic ulcer (Read codes J11 to J14 or International Classification of Diseases, 10th Revision codes K25–K28). The Read codes had high positive predictive values (>95%) for peptic ulcers and upper gastrointestinal complications when validated in English primary care routine records.15,16
Matched Controls
Each case was age (±5 years) and sex matched without replacement to 5 controls selected randomly who were alive at the time of the gastrointestinal bleed and registered to the same primary care practice. Controls were required to have been registered with the primary care practice for at least 3 months before the match date to be consistent with the definition for cases.
Exposures
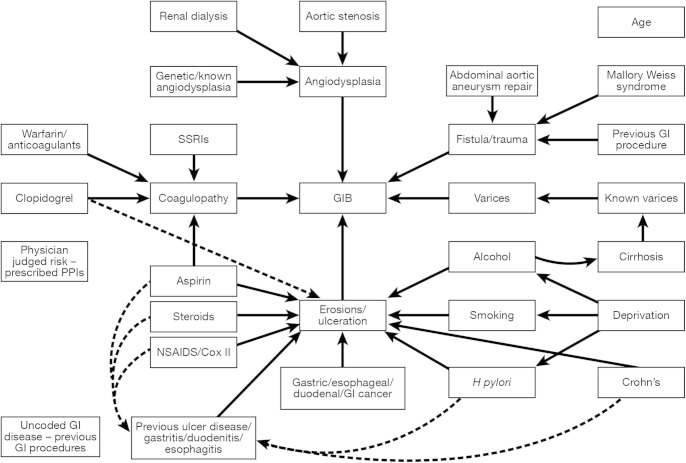
Potential final common causal pathways of an upper gastrointestinal bleed were defined a priori for erosions/ulceration, varices, angiodysplasia, fistula/trauma and coagulopathy, and code lists derived for diagnoses and medications that might be associated with each pathway based on published literature (Figure 1). Although variceal bleeds were excluded from the cases and controls, cirrhosis itself was included as a risk factor, as cirrhotic patients can have nonvariceal bleeds. Medication risk factors were included if there was a coded prescription within the year before the admission. Exposures coded within 2 months of the admission date were excluded to avoid identifying events and prescriptions related to the actual bleed event. PPIs were included as an indicator of physicians' judgement of the risk of upper gastrointestinal hemorrhage that was not captured by other measured risk factors. Alcohol consumption was classified as either nondrinker, alcohol mentioned, ex–alcohol dependency, alcohol excess, alcohol complications, and missing. Smoking was classified as never smoked, current smoker, ex-smoker, and missing. Cirrhosis was classified as uncomplicated, with varices, with ascites, or with encephalopathy or liver failure coded. All other exposures were binary variables.
Figure 1.
Risk factors for upper GIB. SSRI, selective serotonin reuptake inhibitor.
Comorbidity
Comorbidity was defined using the Charlson Index.17 This is a well-validated weighted comorbidity score derived from unselected hospital admissions that predicts 1-year mortality after hospital discharge. It has since been used in many contexts and has repeatedly measured the burden of comorbidity reliably. The original article demonstrated a graded increase in the risk in mortality associated with an increase in total score. The different comorbidities were assigned weights of 1, 2, 3, and 6, depending on their association with mortality. Where a graded effect was observed within a disease, for example, in diabetes or malignancy, these diseases were further stratified according to their severity. The conditions included in the original score (in order of weighting) were myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, connective tissue disease, peptic ulcer disease, mild liver disease, diabetes, hemiplegia, moderate or severe renal disease, diabetes with end organ damage, leukemia, lymphoma, moderate or severe liver disease, metastatic solid tumor, and acquired immunodeficiency syndrome. For our study, any codes already used to define risk factors of upper GIB in Figure 1 were excluded when calculating the index, ie, peptic ulcer and cirrhosis codes. For clarity in reporting in the tables, the index was summarized as no comorbidity (Charlson Index = 0), single comorbidity (Charlson Index = 1), and multiple or severe comorbidity (Charlson Index = 2).
Analysis
Unadjusted analysis
Unadjusted odds ratios (ORs) were calculated for each exposure using conditional logistic regression to allow for the matched study design.
Multivariable analysis
Adjusted ORs for each exposure of interest were calculated with conditional logistic regression adjusting for all exposures in addition to age, PPI use, and previous gastrointestinal procedures. As calendar year, sex, and primary care practice were precisely matched on in the controls, it was not necessary to include them in the model. Comorbidity was added last, and its association with bleeding tested using a likelihood ratio test. The variance inflation factor (a measure of the increase in model variance due to correlation between variables) was calculated for each exposure of interest to assess the effect of correlation between variables. All exposures with a variance inflation factor >5 were excluded from the final conditional logistic regression model.18 The final model was then stratified into cases with a recording of peptic ulcer and those without.
Sequential (or Extra) Population Attributable Fractions
Sequential (or extra) population attributable fractions (PAFs) were calculated for each exposure, using the prevalence among the cases and the respective coefficients from the conditional logistic regression model.19 Sequential PAFs differ from the standard adjusted PAFs that are usually presented. They are calculated by estimating the additional proportion of cases attributable to each exposure, after removing the proportion of cases already attributed to the combined effect of all other exposures in the model. The final model was then stratified into cases with a recording of peptic ulcer and those without. All analysis was performed using Stata software, version 12 (StataCorp LP, College Station, TX).
Sensitivity Analyses
Previous studies of risk factor medications, such as NSAIDs,20 have been conducted in study populations that excluded patients with known risk factors for GIB. To allow comparisons with these, we re-estimated the crude ORs for each of the risk factor medications after excluding any cases and their controls with nonmedication bleed risk factors. To assess the effect of the choice of the exposure exclusion time window before the bleed event on the effect of NSAIDs, we also re-estimated a model that included NSAID use up to 30 days before the index date.
Two additional sensitivity analyses were performed to assess the effect of potential under-reporting. First the analysis was restricted to those older than 65 years old and who were eligible for free prescriptions, to assess the effect of potential under-reporting of nonprescribed NSAID use. Secondly, multiple imputation was used to re-estimate the association with comorbidity by imputing missing values for alcohol and smoking status. Alcohol and smoking were categorised as binary exposures of excess alcohol or current smoking to fit the logistic regression imputation model. All previously extracted exposures were used in the imputation model with addition of the socioeconomic status, and 20 sets of imputations were calculated. Socioeconomic status was measured by the Index of Multiple Deprivation quintiles obtained from linked Office of National Statistics data.
Finally, to assess the effect of using the aggregated and weighted Charlson Index, the model was re-estimated to assess the effect of the individual component comorbidities from the Charlson Index.
Results
Cases and Matching
There were 16,355 unique cases identified with a first nonvariceal bleed; 13,372 with specific code in Hospital Episodes Statistics, 10,938 with a specific code in GPRD, and 7955 with a specific code in both datasets. There were 16,304 (99.7%) cases matched to 5 controls each and only 8 cases (0.05%) were not matched to any controls. Median observed time before admission for cases was 7.4 years (interquartile range, 3.4–11.5) compared with 7.5 years (interquartile range, 3.5–11.5) for controls.
Unadjusted Analysis
Table 1 shows the proportion of cases and controls with each exposure. As expected, aspirin and NSAIDs were the most frequently prescribed risk factor medications, and peptic ulcer and gastritis/duodenitis/esophagitis were the most frequent risk factor diagnoses. All a priori risk factors were associated with upper GIB. Peptic ulcers were coded as a diagnosis within the linked data in 4,823 patients (29% of cases). The exposures stratified by coding of peptic ulcer are shown in Supplementary Table 1.
Table 1.
Proportion of Cases and Controls Exposed 2 Months Before Bleed Date or Match Date
| Controls, n | Exposed, % | Cases, n | Exposed, % | |
|---|---|---|---|---|
| Charlson Indexa | ||||
| No comorbidity | 30,194 | 37.0 | 3440 | 21.0 |
| Single comorbidity | 18,714 | 22.9 | 3222 | 19.7 |
| Multiple or severe | 32,728 | 40.1 | 9693 | 59.3 |
| Gastrointestinal | ||||
| Cirrhosis—none coded | 81,385 | 99.7 | 16,004 | 97.9 |
| Cirrhosis only | 65 | 0.1 | 63 | 0.4 |
| Cirrhosis—varices | 62 | 0.1 | 65 | 0.4 |
| Cirrhosis—ascites | 86 | 0.1 | 172 | 1.1 |
| Cirrhosis—encephalopathy | 38 | 0.0 | 51 | 0.3 |
| Gastritis, duodenitis, or esophagitis | 7904 | 9.7 | 3051 | 18.7 |
| Peptic ulcer | 3830 | 4.7 | 1852 | 11.3 |
| Helicobacter pylori | 1964 | 2.4 | 609 | 3.7 |
| Angiodysplasia | 14 | 0.0 | 6 | 0.0 |
| Mallory-Weiss syndrome | 34 | 0.0 | 96 | 0.6 |
| Crohn's disease | 222 | 0.3 | 114 | 0.7 |
| GI cancer | 2494 | 3.1 | 1174 | 7.2 |
| Lifestyle | ||||
| Alcohol—not coded | 61,536 | 75.4 | 11,026 | 67.4 |
| Alcohol—nondrinker | 1485 | 1.8 | 375 | 2.3 |
| Alcohol—ex-drinker | 176 | 0.2 | 64 | 0.4 |
| Alcohol—mentioned | 4317 | 5.3 | 977 | 6.0 |
| Alcohol—over limits | 14,073 | 17.2 | 3763 | 23.0 |
| Alcohol—complications | 49 | 0.1 | 150 | 0.9 |
| Smoking—not coded | 51,751 | 63.4 | 9187 | 56.2 |
| Smoking—nonsmoker | 11,666 | 14.3 | 2332 | 14.3 |
| Smoking—ex-smoker | 4075 | 5.0 | 888 | 5.4 |
| Smoking—passive | 5574 | 6.8 | 1455 | 8.9 |
| Smoking—current | 8570 | 10.5 | 2493 | 15.2 |
| Medications | ||||
| Aspirin | 18,079 | 22.1 | 5392 | 33.0 |
| NSAIDs | 12,722 | 15.6 | 3820 | 23.4 |
| COX II inhibitors | 1687 | 2.1 | 605 | 3.7 |
| Clopidogrel | 1297 | 1.6 | 668 | 4.1 |
| Oral steroids | 4135 | 5.1 | 1578 | 9.6 |
| Anticoagulants | 3799 | 4.7 | 1617 | 9.9 |
| SSRIs | 4813 | 5.9 | 2025 | 12.4 |
| Other diagnoses | ||||
| Aortic stenosis | 782 | 1.0 | 350 | 2.1 |
| Repair of AAA | 307 | 0.4 | 115 | 0.7 |
| Dialysis | 70 | 0.1 | 88 | 0.5 |
| Confounders | ||||
| Previous upper GI procedure | 10,471 | 12.8 | 3438 | 21.0 |
| PPI | 10,909 | 13.4 | 4585 | 28.0 |
| Age (median and interquartile range) | 73 | 57–82 | 72 | 57–81 |
AAA, Abdominal aortic aneursym; SSRI, selective serotonin reuptake inhibitors.
Nongastrointestinal comorbidity is catogorized as: Charslon Index = 0 is no comorbidity, Charlson Index = 1 single or comorbidity, and Charlson Index = 2 is multiple or severe comorbidity.
Multivariable Analysis and PAFs
There was strong evidence for an association between the nongastrointestinal Charlson Index and upper GIB after adjusting for all measured risk factors (single comorbidity adjusted OR = 1.43; 95% CI: 1.35–1.52; multiple or severe comorbidity adjusted OR = 2.26; 95% CI: 2.14–2.38; P < .001 likelihood ratio test). Table 2 shows the adjusted ORs from the final model for each exposure. We found the largest association with a bleed was with a previous Mallory-Weiss syndrome, which reflects the inherent risk of bleeding in recurrent vomitters. The variables for angiodysplasia and dialysis had the highest variance inflation factors, 1.48 and 2.35, respectively. As both of these were less than the a priori threshold of 5, all exposures were included in the final conditional logistic regression model.
Table 2.
Adjusted Model for Nonvariceal Upper Gastrointestinal Bleeding All Cases (Age, Year, Practice, and Sex Matched)
| Adjusted OR | Lower 95% CI | Upper 95% CI | |
|---|---|---|---|
| Charlson Index | |||
| No comorbidity | 1.00 | 1.00 | 1.00 |
| Single comorbidity | 1.43 | 1.35 | 1.52 |
| Multiple or severe | 2.26 | 2.14 | 2.38 |
| Gastrointestinal | |||
| Cirrhosis—none | 1.00 | 1.00 | 1.00 |
| Cirrhosis only | 3.89 | 2.61 | 5.77 |
| Cirrhosis—varices | 3.75 | 2.51 | 5.61 |
| Cirrhosis—ascites | 5.96 | 4.46 | 7.96 |
| Cirrhosis—encephalopathy | 5.05 | 3.14 | 8.10 |
| Gastritis, duodenitis, or esophagitis | 1.46 | 1.39 | 1.55 |
| Peptic ulcer | 2.11 | 1.98 | 2.26 |
| Helicobacter pylori | 0.96 | 0.86 | 1.07 |
| Angiodysplasia | 1.67 | 0.58 | 4.80 |
| Mallory-Weiss syndrome | 12.39 | 8.16 | 18.82 |
| Crohn's disease | 2.19 | 1.71 | 2.81 |
| GI cancer | 2.13 | 1.97 | 2.31 |
| Lifestyle | |||
| Alcohol—not | 1.00 | 1.00 | 1.00 |
| Alcohol—nondrinker | 1.25 | 1.10 | 1.42 |
| Alcohol—ex-drinker | 1.39 | 1.01 | 1.92 |
| Alcohol—mentioned | 1.05 | 0.96 | 1.14 |
| Alcohol—over limits | 1.42 | 1.35 | 1.49 |
| Alcohol—complications | 9.33 | 6.48 | 13.44 |
| Smoking—not | 1.00 | 1.00 | 1.00 |
| Smoking—non-smoker | 0.97 | 0.92 | 1.04 |
| Smoking—ex-smoker | 0.94 | 0.86 | 1.02 |
| Smoking—passive | 1.03 | 0.95 | 1.11 |
| Smoking—current | 1.29 | 1.22 | 1.37 |
| Medications | |||
| Aspirin | 1.50 | 1.43 | 1.57 |
| NSAIDs | 1.59 | 1.52 | 1.66 |
| COX II inhibitors | 1.52 | 1.37 | 1.69 |
| Clopidogrel | 1.74 | 1.57 | 1.94 |
| Oral steroids | 1.38 | 1.29 | 1.48 |
| Anticoagulants | 1.94 | 1.81 | 2.08 |
| SSRIs | 1.72 | 1.62 | 1.83 |
| Other diagnoses | |||
| Aortic stenosis | 1.58 | 1.38 | 1.82 |
| Repair of AAA | 1.29 | 1.02 | 1.64 |
| Dialysis | 3.59 | 2.55 | 5.05 |
| Confounders | |||
| Previous upper GI procedure | 1.10 | 1.04 | 1.15 |
| PPI | 1.59 | 1.52 | 1.67 |
| Age | 1.09 | 1.08 | 1.10 |
AAA, Abdominal aortic aneurysm; SSRI, selective serotonin reuptake inhibitors.
Stratifying this model demonstrated similar associations with comorbidity, whether or not peptic ulcer coding was present, and slightly higher associations for a peptic ulcer with exposure to previous peptic ulcers, NSAID, or aspirin use (Table 3). Associations with other risk factors were higher in the nonpeptic ulcer cohort.
Table 3.
Adjusted Model for Nonvariceal Upper Gastrointestinal Bleeding Stratified by Coding of Peptic Ulcer (Age, Year, Practice, and Sex Matched)
| Peptic ulcer |
Nonpeptic ulcer |
|||||
|---|---|---|---|---|---|---|
| Adjusted OR | Lower 95% CI | Upper 95% CI | Adjusted OR | Lower 95% CI | Upper 95% CI | |
| Charlson Indexa | ||||||
| No comorbidity | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Single comorbidity | 1.45 | 1.30 | 1.62 | 1.42 | 1.33 | 1.52 |
| Multiple or severe | 2.28 | 2.06 | 2.52 | 2.27 | 2.13 | 2.42 |
| Gastrointestinal | ||||||
| Cirrhosis—none | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Cirrhosis only | 3.98 | 2.03 | 7.80 | 3.80 | 2.30 | 6.27 |
| Cirrhosis—varices | 2.33 | 0.92 | 5.94 | 4.15 | 2.63 | 6.54 |
| Cirrhosis—ascites | 4.67 | 2.63 | 8.29 | 6.85 | 4.85 | 9.65 |
| Cirrhosis—encephalopathy | 3.16 | 1.39 | 7.20 | 6.66 | 3.70 | 12.01 |
| Gastritis, duodenitis, or esophagitis | 1.22 | 1.10 | 1.36 | 1.58 | 1.48 | 1.68 |
| Peptic ulcer | 4.36 | 3.92 | 4.85 | 1.37 | 1.25 | 1.49 |
| Helicobacter pylori | 1.04 | 0.85 | 1.27 | 0.94 | 0.83 | 1.06 |
| Angiodysplasia | 1.71 | 0.16 | 18.64 | 1.49 | 0.44 | 5.00 |
| Mallory Weiss syndrome | 3.75 | 1.43 | 9.84 | 16.54 | 10.23 | 26.77 |
| Crohns disease | 1.18 | 0.68 | 2.05 | 2.65 | 1.99 | 3.54 |
| GI cancer | 1.45 | 1.23 | 1.69 | 2.45 | 2.23 | 2.70 |
| Lifestyle | ||||||
| Alcohol—not | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Alcohol—nondrinker | 1.14 | 0.89 | 1.47 | 1.30 | 1.11 | 1.51 |
| Alcohol—ex-drinker | 1.58 | 0.89 | 2.81 | 1.30 | 0.88 | 1.93 |
| Alcohol—mentioned | 1.02 | 0.87 | 1.20 | 1.04 | 0.94 | 1.16 |
| Alcohol—over limits | 1.34 | 1.22 | 1.47 | 1.45 | 1.36 | 1.54 |
| Alcohol—complications | 3.88 | 1.70 | 8.87 | 11.85 | 7.76 | 18.10 |
| Smoking—not | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Smoking—nonsmoker | 0.99 | 0.88 | 1.11 | 0.96 | 0.90 | 1.04 |
| Smoking—ex-smoker | 0.96 | 0.82 | 1.13 | 0.92 | 0.83 | 1.03 |
| Smoking—passive | 0.95 | 0.82 | 1.09 | 1.06 | 0.97 | 1.16 |
| Smoking—current | 1.35 | 1.21 | 1.51 | 1.28 | 1.19 | 1.37 |
| Medications | ||||||
| Aspirin | 1.69 | 1.56 | 1.82 | 1.42 | 1.34 | 1.50 |
| NSAIDs | 2.21 | 2.04 | 2.39 | 1.37 | 1.29 | 1.45 |
| COX II inhibitors | 1.81 | 1.51 | 2.17 | 1.42 | 1.24 | 1.62 |
| Clopidogrel | 2.04 | 1.68 | 2.48 | 1.70 | 1.49 | 1.93 |
| Oral steroids | 1.31 | 1.16 | 1.49 | 1.40 | 1.29 | 1.51 |
| Anticoagulants | 1.67 | 1.47 | 1.90 | 2.10 | 1.94 | 2.28 |
| SSRIs | 1.47 | 1.30 | 1.66 | 1.84 | 1.71 | 1.97 |
| Other diagnoses | ||||||
| Aortic stenosis | 1.79 | 1.41 | 2.26 | 1.46 | 1.23 | 1.75 |
| Repair of AAA | 1.33 | 0.87 | 2.04 | 1.27 | 0.95 | 1.68 |
| Dialysis | 5.56 | 2.95 | 10.48 | 2.92 | 1.94 | 4.41 |
| Confounders | ||||||
| Previous upper GI procedure | 0.88 | 0.80 | 0.98 | 1.20 | 1.13 | 1.28 |
| PPI | 0.82 | 0.74 | 0.91 | 2.01 | 1.90 | 2.13 |
| Age | 1.10 | 1.08 | 1.11 | 1.09 | 1.08 | 1.10 |
AAA, Abdominal aortic aneursym; SSRI, selective serotonin reuptake inhibitors.
Nongastrointestinal comorbidity is catogorized as: Charlon Index = 0 is no comorbidity, Charlson Index = 1 single or comorbidity, and Charlson Index = 2 is multiple or severe comorbidity.
The proportion of cases attributable in the population to the combined effect of all available measured exposures was 48%, not including the effect of nongastrointestinal comorbidity. The additional proportion of cases attributable to nongastrointestinal comorbidity (or the sequential PAF) was 20%, and this was higher in magnitude than for any other measured exposure (Table 4). The next largest PAFs were 3% for aspirin and NSAID use.
Table 4.
Sequential Population Attributable Fractions for Nonvariceal Upper Gastrointestinal Hemorrhage (All Cases)
| Sequential population attributable fractionsa,b |
||
|---|---|---|
| % | 95% CI | |
| Nongastrointestinal comorbidity | 19.80 | 18.43 to 21.18 |
| Gastrointestinal | ||
| Cirrhosis | 0.49 | 0.41 to 0.57 |
| Gastritis, duodenitis or esophagitis | 1.98 | 1.66 to 2.30 |
| Peptic ulcer | 2.05 | 1.81 to 2.28 |
| Helicobacter pylori | −0.04 | −0.15 to 0.08 |
| Angiodysplasia | 0.01 | −0.01 to 0.02 |
| Mallory-Weiss syndrome | 0.29 | 0.22 to 0.37 |
| Crohn's disease | 0.14 | 0.08 to 0.19 |
| GI cancer | 1.11 | 0.96 to 1.27 |
| Lifestyle | ||
| Alcohol use | 2.89 | 2.39 to 3.39 |
| Smoking | 0.83 | 0.27 to 3.42 |
| Medications | ||
| Aspirin | 2.95 | 2.54 to 3.36 |
| NSAIDs | 3.07 | 2.72 to 3.42 |
| COX II inhibitors | 0.33 | 0.23 to 0.44 |
| Clopidogrel | 0.34 | 0.26 to 0.43 |
| Oral steroids | 0.59 | 0.44 to 0.74 |
| Anticoagulants | 1.19 | 1.04 to 1.35 |
| SSRIs | 1.58 | 1.36 to 1.80 |
| Other diagnoses | ||
| Aortic stenosis | 0.16 | 0.10 to 0.22 |
| Repair of aorta | 0.03 | 0.00 to 0.06 |
| Dialysis | 0.07 | 0.04 to 0.09 |
SSRI, selective serotonin reuptake inhibitors.
Age, year, practice, and sex matched and adjusted for PPI use, previous upper gastrointestinal procedures, and age.
The estimate in each row are calculated separately conditional on all the other variables in the model. They should therefore not be interpreted as summing over the column to 100%. Sequential PAF estimates the additional proportion of nonvariceal bleeding cases attributable to each risk factor after cases attributable to all the other risk factors in the model have been removed.
The PAF for comorbidity associated with peptic ulcer bleeds was slightly lower than that for nonulcer bleeds (18% vs 21%), with a higher contribution from previous peptic ulcer bleeds and aspirin and NSAIDs (Table 5). In contrast, for nonulcer bleeds, the PAF was slightly increased for gastrointestinal cancer, alcohol, anticoagulants, and selective serotonin reuptake inhibitors.
Table 5.
Sequential Population Attributable Fractions for Nonvariceal Upper Gastrointestinal Hemorrhage Strafied by Coding for Peptic Ulcer
| Sequential population attributable fractions,a,b% |
||
|---|---|---|
| Peptic ulcer | Nonpeptic ulcer | |
| Nongastrointestinal comorbidity | 18.44 | 20.50 |
| Gastrointestinal | ||
| Cirrhosis | 0.32 | 0.57 |
| Gastritis, duodenitis, or esophagitis | 0.69 | 2.74 |
| Peptic ulcer | 5.31 | 0.69 |
| Helicobacter pylori | 0.05 | −0.07 |
| Angiodysplasia | 0.01 | 0.00 |
| Mallory-Weiss syndrome | 0.06 | 0.39 |
| Crohn's disease | 0.02 | 0.19 |
| GI cancer | 0.35 | 1.48 |
| Lifestyle | ||
| Alcohol use | 1.93 | 3.30 |
| Smoking | 0.80 | 0.81 |
| Medications | ||
| Aspirin | 3.99 | 2.42 |
| NSAIDs | 5.40 | 2.00 |
| COX II inhibitors | 0.47 | 0.28 |
| Clopidogrel | 0.38 | 0.35 |
| Oral steroids | 0.36 | 0.66 |
| Anticoagulants | 0.78 | 1.41 |
| SSRIs | 0.74 | 2.02 |
| Other diagnoses | ||
| Aortic stenosis | 0.22 | 0.12 |
| Repair of aorta | 0.02 | 0.03 |
| Dialysis | 0.09 | 0.05 |
SSRI, selective serotonin reuptake inhibitors.
Age, year, practice, and sex matched and adjusted for PPI use, previous upper gastrointestinal procedures and age.
The estimate in each row are calculated separately conditional on all the other variables in the model. They should therefore not be interpreted as summing over the column to 100%. Sequential PAF estimates the additional proportion of nonvariceal bleeding cases attributable to each risk factor after cases attributable to all the other risk factors in the model have been removed.
Sensitivity Analyses
The crude ORs were re-estimated for medications after excluding cases with nonmedication risk factors and these are shown in Supplementary Table 2. NSAID use was strongly associated with bleeding, with an OR of 1.67, and this increased to 2.80 with the exclusion of nonmedication risk factors. The corresponding adjusted ORs associated with NSAIDs were 1.59 with nonmedication risk factors included and 1.73 without. Altering the exposure exclusion window for NSAIDs to 30 days rather than 60 days before the bleed slightly increased the effect of NSAIDS, but had only a minimal effect on the other results, including comorbidity (see Supplementary Table 3).
Restricting the analysis to those older than 65 years old increased the proportion of cases attributable to the combined effect of all exposures from 48% to 63%, and reduced the additional proportion of cases attributable to nongastrointestinal comorbidity from 19.8% to 16.1%. Re-estimating the model using multiple imputation for missing alcohol and smoking status (modeled as binary exposures) slightly reduced the PAF associated with comorbidity from 22.9% to 22.4%, but when alcohol and smoking status were omitted from the model, the PAF was almost unaltered at 22.2%. Finally, the full model was re-estimated for each component of the Charlson Index (Table 6). The contribution of these individual comorbidities was minimal in comparison with their combined weighted effect in the Charlson Index in the main analysis.
Table 6.
The Adjusteda Association of the Component Comorbidities of Charlson Index With Nonvariceal Bleeding
| Proportion of cases exposed, % | ORa | Lower 95% CI | Upper 95% CI | PAF,b% | |
|---|---|---|---|---|---|
| Myocardial infarction | 13.98 | 1.04 | 0.97 | 1.10 | 0.12 |
| Congestive cardiac disease | 19.90 | 1.49 | 1.41 | 1.58 | 1.95 |
| Peripheral vascular disease | 11.17 | 1.31 | 1.23 | 1.41 | 0.70 |
| Dementia | 8.98 | 1.40 | 1.30 | 1.50 | 1.00 |
| Chronic pulmonary disease | 31.80 | 1.11 | 1.06 | 1.16 | 1.10 |
| Cerebrovascular disease | 23.1 | 1.13 | 1.08 | 1.19 | 0.80 |
| Rheumatological disease | 10.13 | 1.06 | 0.99 | 1.13 | 0.17 |
| Uncomplicated diabetes | 17.88 | 1.01 | 0.96 | 1.06 | 0.04 |
| Hemiplegia | 4.73 | 1.79 | 1.62 | 1.97 | 0.67 |
| Renal disease | 14.42 | 1.71 | 1.61 | 1.82 | 1.74 |
| Diabetes with complications | 12.30 | 1.00 | 0.94 | 1.06 | −0.01 |
| Any malignancy | 13.11 | 1.21 | 1.14 | 1.28 | 0.78 |
| Lymphoproliferative disorders | 2.21 | 1.95 | 1.70 | 2.24 | 0.43 |
| Metastatic solid tumour | 5.89 | 2.35 | 2.14 | 2.57 | 1.29 |
| HIV/AIDS | 0.06 | 0.69 | 0.31 | 1.55 | −0.00 |
HIV/AIDS, human immunodeficiency virus/acquired immunodeficiency syndrome.
Adjusted for all other variables in this Table and Table 2 and matched for year, practice, and sex.
Sequential population attributable fractions: The estimates in each row are calculated separately conditional on all the other variables in the model. They should therefore not be interpreted as summing over the column to 100%. Sequential PAF estimates the additional proportion of nonvariceal bleeding cases attributable to each risk factor after cases attributable to all the other risk factors in the model have been removed.
Discussion
This study has demonstrated that a combined measure of nongastrointestinal comorbidity is a significant independent predictor of upper GIB, even after accounting for all other recognized and measured risk factors. In addition, it explained a greater proportion of the burden of bleeding than any other risk factor in the population. The effect of this combined measure of nongastrointestinal comorbidity was far in excess of that which would be expected from its constituent diseases.
The association of comorbidities with upper GIB has been studied previously, but only in smaller secondary care surveys with comorbidity as a confounder and not as the primary exposure. We searched PubMed using variants of comorbidity, etiology, causality, risk factors, and gastrointestinal hemorrhage; however, no studies were identified that set out to address the question of our article. Studies were most frequently designed to measure the association of a single medication while adjusting for any confounding by comorbidity.21,22
Two studies assessed a larger range of medications in cross-sectional hospital-based surveys.10,23 First, Weil found that only 2 comorbidities, heart failure and diabetes, contributed to upper GIB with adjusted PAFs of 5% and 4%, respectively.23 However, the study was retrospective, and with <1000 cases limiting its power. In contrast to the “extra PAF” we calculated, the adjusted PAFs in their article calculated the effect of each exposure in a pseudo-population with no other risk factors present, potentially overestimating the effect in the general population, in which a case can be caused by many risk factors. The second comparable paper of Gallerani et al found an association with comorbidity and a similar 2-fold increase in risk in those exposed to NSAIDs to what we found in our peptic ulcer cohort.10 However, it was also a retrospective survey–based study potentially subject to recall bias, and had <1000 cases. Furthermore, the authors did not separate out gastrointestinal comorbidity from nongastrointestinal comorbidity and used hospital controls, therefore limiting comparisons with our population-based study.
Other studies assessed higher alcohol intake,24 H pylori,25 smoking,26 acute renal failure,27 and acute myocardial infarction28 and found associations with upper GIB. But these studies were in small selected hospitalised cohorts (n < 1000 bleeds) with limited assessments of individual comorbidity and no measure of their PAFs.
Our study has a number of important strengths when compared with these previous works because we set out specifically to assess the degree to which nongastrointestinal comorbidity predicts nonvariceal upper GIB after removing the effects of all the available known risk factors in a much larger general population. In addition, we used a method of defining cases and exposures that utilized information from both primary and secondary care, thereby maximizing the evidence supporting each case while not excluding severe events.14 Furthermore, due to the comprehensive coverage of the English primary care system, our study's results are likely to be generalizable to the whole English population and, we believe, further afield. The linked dataset used for our study remained representative of the GPRD overall, as whole practices rather than individual patients declined or consented to the linkage. Consequently, we were able to estimate the additional attributable fraction for comorbidity in the English population that was not already attributable to other risk factors.19
As our study was one of the first to assess the effect of the burden of comorbidity as a risk factor for upper GIB, no measure of comorbidity had been specifically validated for this purpose. We decided to use the Charlson Index because it is a well-validated score for measuring comorbidity in many different contexts. Other comorbidity scores that could be used, such as the Elixhauser Index or a simple counts of diagnoses, have been used and validated less frequently and in fewer contexts. In addition, some of the other scores also include other outcomes, such as financial cost, which are not necessarily a measure of the severity of disease. The Charlson Index was therefore selected as the most appropriate comorbidity score for our study.
We do need to consider alternative explanations for our observed association of comorbidity with upper GIB. A potential weakness of our study is the inevitably imperfect data on some recognized risk factors that might have caused us to underestimate their importance. The GPRD contains comprehensive recording of all available diagnoses and prescriptions. However, under-reporting is likely to have occurred for H pylori infection, NSAID use, alcohol, and smoking. In the case of H pylori, there was inevitable under-reporting because there was no population screening. However, if the under-reporting of H pylori infection was to explain our study's findings, it would have to be strongly associated with comorbidity, and the evidence for this is conflicting and underpowered.29,30 In studies of ischemic heart disease, for which there is the largest body of evidence, any significant association with H pylori was minimal after adjustments for confounding.31 In our study, the apparent protective effect of H pylori after adjustments for confounding was not surprising because H pylori will have been eradicated when found.
NSAID use might also have been under-reported, as NSAIDs can be bought over the counter from a pharmacy without a prescription, potentially explaining the low association between NSAIDs and bleeding in our study compared with a previous meta-analysis.20 However, we had higher recorded NSAID use than was reported in a recent national audit,32 and the studies used in the meta-analysis excluded patients with other known GIB risk factors.20 When we made the same exclusions in our study (Supplementary Table 2), or restricted to peptic ulcers, the association of bleeding with NSAIDs increased and became comparable with figures in the literature. With regard to over-the-counter use, nondifferential under-reporting has been shown to reduce the measured effect of prescribed medications.33 In our study, this would cause an underestimate of the effect of NSAIDs. However, in England, certain groups receive free prescriptions, such as patients older than 65 years or those with certain chronic diseases, and these groups have been shown to purchase far fewer medications over the counter than those who have to pay for prescriptions.34,35 When we restricted our analysis to those older than 65 years, thereby reducing confounding by over-the-counter medications, we found only a small reduction in the estimated PAF for comorbidity, but no change in PAF for NSAIDs. The final area of under-reporting that could affect our study was missing data for alcohol and smoking status, but these variables were not strong confounders of the association between comorbidity and bleeding and there was only a minimal effect on the PAF of comorbidity when missing data were imputed conditional on all available data and socioeconomic status.
We therefore believe that potential under-reporting of exposures does not explain the association we found between upper GIB and a general measure of comorbidity. This suggests that comorbidity itself, or other factors not included in our study that are associated with comorbidity, might be causing the association. It is possible that other medications not included in the study were responsible for some of this association, however, we are not aware of any additional prescribed or nonprescribed medication that would fulfill the requirements of common usage and a strong association with bleeding. Historically, nongastrointestinal comorbidity itself was commonly recognized as a risk factor for upper GIB.7 However, the concept of “stress ulceration” is no longer accepted, aside from patients on ITU who are exposed to severe acute physiological stresses from ventilation, coagulopathy, liver failure, renal failure, septic shock, or nutritional support.9 The physiological effects from chronic comorbidities in our study are unlikely to be as severe as those that occur on ITU and, therefore, what we are describing is likely to have a different mechanism than that seen in the ITU setting. Many potential mechanisms for our observed association can be hypothesized; for example, reduced epithelial microperfusion in cardiac failure,36 decreased oxygen levels in chronic obstructive pulmonary disease,37,38 poor nutritional status in many diseases, or the platelet and clotting dysfunction in end-stage renal failure.27,39 However, it is unlikely that there is a single mechanism that accounts for the association we found, but rather that multiple illnesses and mechanisms have a cumulative effect. This was shown by the graded effect of the Charlson Index and by Table 6, in which no individual disease accounted for the magnitude of the overall association with comorbidity.
Our findings contrast with current beliefs that the main burden of bleeding in the general population comes from known iatrogenic causes, such as NSAIDs prescribed for analgesia or antiplatelet agents prescribed for cardiac and cerebrovascular disease,40 and that this burden would be reduced by increasing PPI use.41 Instead, we have demonstrated that the extra contribution of these medications to bleeding cases was not large after considering the contributions of other risk factors present in the population. Therefore, simply increasing PPI prescriptions in patients on high-risk medications might not have as large an impact as previously thought.
In conclusion, the largest measurable burden of upper gastrointestinal hemorrhage in this study was attributed to nongastrointestinal comorbidity. In a proportion of patients, a bleed is an indicator of the burden of their comorbidity, and recognizing this will help guide management, particularly in the absence of modifiable gastrointestinal risk factors. However, our finding also explains why the incidence of nonvariceal bleeding is likely to remain high in an aging population, thereby necessitating continued acute gastroenterology service provision.
Footnotes
This article has an accompanying continuing medical education activity on page e18. Learning Objective: Upon completion of this CME exercise, and reading of the associated paper, successful learners will be able to appraise how multiple risk factors can contribute to upper gastrointestinal bleeding and recognize the large contribution of comorbidity.
Conflicts of interest This author discloses the following: Timothy Richard Card is married to an employee of Takaeda Pharmaceuticals (recently changed jobs from AstraZeneca). The remaining authors disclose no conflicts.
Funding This work was supported by personal fellowships: Colin Crooks is a research fellow and gastroenterology trainee supported by a Medical Research Council Population Health Scientist fellowship (grant number G0802427), Tim Card is a Clinical Associate Professor and Consultant Gastroenterologist supported by Nottingham University and the National Health Service, and Joe West is a Clinical Associate Professor, Reader in Epidemiology, and Consultant Gastroenterologist supported by a University of Nottingham/Nottingham University Hospitals NHS Trust Senior Clinical Research fellowship. These funding bodies had no role in the study design, or the collection, analysis, or interpretation of data.
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at http://dx.doi.org/10.1053/j.gastro.2013.02.040.
Supplementary Material
Supplementary Table 1.
Proportion of Cases Exposed 2 Months Before Bleed Date or Match Date Stratified by Coded Peptic Ulcer
| Peptic ulcer coded, n | Exposed, % | No peptic ulcer coded, n | Exposed, % | |
|---|---|---|---|---|
| Charlson index+ | ||||
| No comorbidity | 883 | 18.3 | 2557 | 22.2 |
| Single comorbidity | 916 | 19.0 | 2306 | 20.0 |
| Multiple or severe | 3024 | 62.7 | 6669 | 57.8 |
| Gastrointestinal | ||||
| Cirrhosis—none coded | 4753 | 98.5 | 11,251 | 97.6 |
| Cirrhosis only | 17 | 0.4 | 46 | 0.4 |
| Cirrhosis—varices | 8 | 0.2 | 57 | 0.5 |
| Cirrhosis—ascites | 32 | 0.7 | 140 | 1.2 |
| Cirrhosis—encephalopathy | 13 | 0.3 | 38 | 0.3 |
| Gastritis, duodenitis, or esophagitis | 710 | 14.7 | 2341 | 20.3 |
| Peptic ulcer | 864 | 17.9 | 988 | 8.6 |
| Helicobacter pylori | 162 | 3.4 | 447 | 3.9 |
| Angiodysplasia | 1 | 0.0 | 5 | 0.0 |
| Mallory-Weiss syndrome | 11 | 0.2 | 85 | 0.7 |
| Crohn's disease | 19 | 0.4 | 95 | 0.8 |
| GI cancer | 254 | 5.3 | 920 | 8.0 |
| Lifestyle | ||||
| Alcohol—not coded | 3299 | 68.4 | 7727 | 67.0 |
| Alcohol—non-drinker | 97 | 2.0 | 278 | 2.4 |
| Alcohol—ex-drinker | 20 | 0.4 | 44 | 0.4 |
| Alcohol—mentioned | 284 | 5.9 | 693 | 6.0 |
| Alcohol—over limits | 1105 | 22.9 | 2658 | 23.0 |
| Alcohol—complications | 18 | 0.4 | 132 | 1.1 |
| Smoking—not coded | 2753 | 57.1 | 6434 | 55.8 |
| Smoking—nonsmoker | 646 | 13.4 | 1686 | 14.6 |
| Smoking—ex-smoker | 288 | 6.0 | 600 | 5.2 |
| Smoking—passive | 405 | 8.4 | 1050 | 9.1 |
| Smoking—current | 731 | 15.2 | 1762 | 15.3 |
| Medications | ||||
| Aspirin | 1831 | 38.0 | 3561 | 30.9 |
| NSAIDs | 1431 | 29.7 | 2467 | 21.4 |
| COX II inhibitors | 222 | 4.6 | 383 | 3.3 |
| Clopidogrel | 198 | 4.1 | 470 | 4.1 |
| Oral steroids | 428 | 8.9 | 1150 | 10.0 |
| Anticoagulants | 427 | 8.9 | 1190 | 10.3 |
| SSRIs | 460 | 9.5 | 1565 | 13.6 |
| Other diagnoses | ||||
| Aortic stenosis | 125 | 2.6 | 225 | 2.0 |
| Repair of AAA | 36 | 0.7 | 79 | 0.7 |
| Dialysis | 30 | 0.6 | 58 | 0.5 |
| Confounders | ||||
| Previous upper GI procedure | 817 | 16.9 | 2621 | 22.7 |
| Previous PPI use | 906 | 18.8 | 3679 | 31.9 |
| Age (median and interquartile range) | 75 | 64–83 | 72 | 54–82 |
AAA, Abdominal aortic aneurysm; SSRI, selective serotonin reuptake inhibitors.
aNongastrointestinal comorbidity is catogorized as: Charlon Index = 0 is no comorbidity, Charlson Index = 1 single or comorbidity, and Charlson Index = 2 is multiple or severe comorbidity.
Supplementary Table 2.
The Association of Medications With Upper Gastrointestinal Bleeding After Excluding Patients With Nonmedication Risk Factors (Age, Year, Practice, and Sex Matched)
| Crude OR | Adjusteda OR | Lower 95% CI | Upper 95% CI | |
|---|---|---|---|---|
| Aspirin | 2.39 | 1.73 | 1.60 | 1.87 |
| NSAIDs | 2.80 | 1.78 | 1.64 | 1.93 |
| COX II inhibitors | 2.59 | 1.50 | 1.23 | 1.83 |
| Clopidogrel | 7.30 | 2.15 | 1.70 | 2.73 |
| Oral steroids | 1.23 | 1.23 | 1.08 | 1.41 |
| Anticoagulants | 4.83 | 2.26 | 1.99 | 2.57 |
| SSRIs | 2.78 | 1.52 | 1.34 | 1.71 |
SSRI, selective serotonin reuptake inhibitors.
Adjusted for all other variables in Table and in Figure 1.
Supplementary Table 3.
Sequential Population Attributable Fractions for Nonvariceal Upper Gastrointestinal Hemorrhage (NSAIDS Prescribed Between 1 Month and 1 Year Before Bleed)
| Sequential population attributable fractions,a,b% | |
|---|---|
| Nongastrointestinal comorbidity | 19.92 |
| Gastrointestinal | |
| Cirrhosis | 0.48 |
| Gastritis, duodenitis, or esophagitis | 1.86 |
| Peptic ulcer | 2.01 |
| Helicobacter pylori | −0.06 |
| Angiodysplasia | 0.01 |
| Mallory-Weiss syndrome | 0.28 |
| Crohn's disease | 0.14 |
| GI cancer | 1.09 |
| Lifestyle | |
| Alcohol use | 2.83 |
| Smoking | 0.93 |
| Medications | |
| Aspirin | 2.91 |
| NSAIDs | 3.18 |
| COX II inhibitors | 0.36 |
| Clopidogrel | 0.32 |
| Oral steroids | 0.58 |
| Anticoagulants | 1.11 |
| SSRIs | 1.60 |
| Other diagnoses | |
| Aortic stenosis | 0.16 |
| Repair of aorta | 0.02 |
| Dialysis | 0.06 |
SSRI, selective serotonin reuptake inhibitors.
Age, year, practice, and sex matched and adjusted for PPI use, previous upper gastrointestinal procedures, and age.
The estimate in each row are calculated separately conditional on all the other variables in the model. They should therefore not be interpreted as summing over the column to 100%. Sequential PAF estimates the additional proportion of nonvariceal bleeding cases attributable to each risk factor after cases attributable to all the other risk factors in the model have been removed.
References
- 1.Yeomans N.D. The ulcer sleuths: the search for the cause of peptic ulcers. J Gastroenterol Hepatol. 2011;26(Suppl 1):35–41. doi: 10.1111/j.1440-1746.2010.06537.x. [DOI] [PubMed] [Google Scholar]
- 2.Sung J.J.Y., Kuipers E.J., El-Serag H.B. Systematic review: the global incidence and prevalence of peptic ulcer disease. Aliment Pharmacol Ther. 2009;29:938–946. doi: 10.1111/j.1365-2036.2009.03960.x. [DOI] [PubMed] [Google Scholar]
- 3.Williams J.G., Roberts S.E., Ali M.F. Gastroenterology services in the UK: The burden of disease, and the organisation and delivery of services for gastrointestinal and liver disorders: a review of the evidence. Gut. 2007;56(Suppl 1):1–113. doi: 10.1136/gut.2006.117598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peery A.F., Dellon E.S., Lund J. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143:1179–1187.e3. doi: 10.1053/j.gastro.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewis J.D., Bilker W.B., Brensinger C. Hospitalization and mortality rates from peptic ulcer disease and GI bleeding in the 1990s: relationship to sales of nonsteroidal anti-inflammatory drugs and acid suppression medications. Am J Gastroenterol. 2002;97:2540–2549. doi: 10.1111/j.1572-0241.2002.06037.x. [DOI] [PubMed] [Google Scholar]
- 6.Crooks C.J., West J., Card T.R. Upper gastrointestinal haemorrhage and deprivation: a nationwide cohort study of health inequality in hospital admissions. Gut. 2012;61:514–520. doi: 10.1136/gutjnl-2011-300186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breckenridge I.M., Walton E.W., Walker W.F. Stress ulcers in the stomach. BMJ. 1959;2(5163) doi: 10.1136/bmj.2.5163.1362. 1362–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fleming K.M., Aithal G.P., Card T.R. The rate of decompensation and clinical progression of disease in people with cirrhosis: a cohort study. Aliment Pharmacol Ther. 2010;32:1343–1350. doi: 10.1111/j.1365-2036.2010.04473.x. [DOI] [PubMed] [Google Scholar]
- 9.Cook D.J., Fuller H.D., Guyatt G.H. Risk factors for gastrointestinal bleeding in critically ill patients: Canadian Critical Care Trials Group. N Engl J Med. 1994;330:377–381. doi: 10.1056/NEJM199402103300601. [DOI] [PubMed] [Google Scholar]
- 10.Gallerani M., Simonato M., Manfredini R. Risk of hospitalization for upper gastrointestinal tract bleeding. J Clin Epidemiol. 2004;57:103–110. doi: 10.1016/S0895-4356(03)00255-5. [DOI] [PubMed] [Google Scholar]
- 11.Hollowell J. The General Practice Research Database: quality of morbidity data. Popul Trends. 1997;87:36–40. [PubMed] [Google Scholar]
- 12.Jick H., Jick S.S., Derby L.E. Validation of information recorded on general practitioner based computerised data resource in the United Kingdom. BMJ. 1991;302(6779):766–768. doi: 10.1136/bmj.302.6779.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herrett E., Thomas S.L., Schoonen W.M. Validation and validity of diagnoses in the General Practice Research Database: a systematic review. Br J Clin Pharmacol. 2010;69:4–14. doi: 10.1111/j.1365-2125.2009.03537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crooks C.J., Card T.R., West J. Defining upper gastrointestinal bleeding from linked primary and secondary care data and the effect on occurrence and 28 day mortality. BMC Health Serv Res. 2012;12:392. doi: 10.1186/1472-6963-12-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.García Rodríguez L.A., Barreales Tolosa L. Risk of upper gastrointestinal complications among users of traditional NSAIDs and COXIBs in the general population. Gastroenterology. 2007;132:498–506. doi: 10.1053/j.gastro.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 16.Margulis A.V., García Rodríguez L.A., Hernández-Díaz S. Positive predictive value of computerized medical records for uncomplicated and complicated upper gastrointestinal ulcer. Pharmacoepidemiol Drug Saf. 2009;18:900–909. doi: 10.1002/pds.1787. [DOI] [PubMed] [Google Scholar]
- 17.Charlson M.E.E., Pompei P., Ales K.L.L. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 18.Stine R.A. Graphical interpretation of variance inflation factors. Am Stat. 1995;49:53. [Google Scholar]
- 19.Eide G. Sequential and average attributable fractions as aids in the selection of preventive strategies. J Clin Epidemiol. 1995;48:645–655. doi: 10.1016/0895-4356(94)00161-i. [DOI] [PubMed] [Google Scholar]
- 20.Hernandez-Diaz S., Rodriguez L.A.G. Association between nonsteroidal anti-inflammatory drugs and upper gastrointestinal tract bleeding/perforation: an overview of epidemiologic studies published in the 1990s. Arch Intern Med. 2000;160:2093–2099. doi: 10.1001/archinte.160.14.2093. [DOI] [PubMed] [Google Scholar]
- 21.Dalton S.O., Johansen C., Mellemkjaer L. Use of selective serotonin reuptake inhibitors and risk of upper gastrointestinal tract bleeding: a population-based cohort study. Arch Intern Med. 2003;163:59–64. doi: 10.1001/archinte.163.1.59. [DOI] [PubMed] [Google Scholar]
- 22.van Walraven C., Mamdani M.M., Wells P.S. Inhibition of serotonin reuptake by antidepressants and upper gastrointestinal bleeding in elderly patients: retrospective cohort study. BMJ. 2001;323(7314):655–658. doi: 10.1136/bmj.323.7314.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weil J. Peptic ulcer bleeding: accessory risk factors and interactions with non-steroidal anti-inflammatory drugs. Gut. 2000;46:27–31. doi: 10.1136/gut.46.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelly J.P., Kaufman D.W., Koff R.S. Alcohol consumption and the risk of major upper gastrointestinal bleeding. Am J Gastroenterol. 1995;90:1058–1064. [PubMed] [Google Scholar]
- 25.Lanas A., Fuentes J., Benito R. Helicobacter pylori increases the risk of upper gastrointestinal bleeding in patients taking low-dose aspirin. Aliment Pharmacol Ther. 2002;16:779–786. doi: 10.1046/j.1365-2036.2002.01230.x. [DOI] [PubMed] [Google Scholar]
- 26.Stack W.A., Atherton J.C., Hawkey G.M. Interactions between Helicobacter pylori and other risk factors for peptic ulcer bleeding. Aliment Pharmacol Ther. 2002;16:497–506. doi: 10.1046/j.1365-2036.2002.01197.x. [DOI] [PubMed] [Google Scholar]
- 27.Fiaccadori E., Maggiore U., Clima B. Incidence, risk factors, and prognosis of gastrointestinal hemorrhage complicating acute renal failure. Kidney Int. 2001;59:1510–1519. doi: 10.1046/j.1523-1755.2001.0590041510.x. [DOI] [PubMed] [Google Scholar]
- 28.Al-Mallah M., Bazari R., Jankowski M. Predictors and outcomes associated with gastrointestinal bleeding in patients with acute coronary syndromes. J Thromb Thrombolysis. 2007;23:51–55. doi: 10.1007/s11239-006-9005-8. [DOI] [PubMed] [Google Scholar]
- 29.Franceschi F., Gasbarrini A. Helicobacter pylori and extragastric diseases. Best Pract Res Clin Gastroenterol. 2007;21:325–334. doi: 10.1016/j.bpg.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Chen Y., Segers S., Blaser M.J. Association between Helicobacter pylori and mortality in the NHANES III study. Gut. 2013 Jan 16 doi: 10.1136/gutjnl-2012-303018. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wald N.J., Law M.R., Morris J.K. Helicobacter pylori infection and mortality from ischaemic heart disease: negative result from a large, prospective study. BMJ. 1997;315(7117):1199–1201. doi: 10.1136/bmj.315.7117.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hearnshaw S.A., Logan R.F.A., Lowe D. Acute upper gastrointestinal bleeding in the UK: patient characteristics, diagnoses and outcomes in the 2007 UK audit. Gut. 2011;60:1327–1335. doi: 10.1136/gut.2010.228437. [DOI] [PubMed] [Google Scholar]
- 33.Yood M.U., Campbell U.B., Rothman K.J. Using prescription claims data for drugs available over-the-counter (OTC) Pharmacoepidemiol Drug Saf. 2007;16:961–968. doi: 10.1002/pds.1454. [DOI] [PubMed] [Google Scholar]
- 34.Hopper S., Pierce M. Aspirin after myocardial infarction: the importance of over-the-counter use. Fam Pract. 1998;15(Suppl 1):S10–S13. [PubMed] [Google Scholar]
- 35.Bedson J., Whitehurst T., Lewis M., Croft P. Factors affecting over-the-counter use of aspirin in the secondary prophylaxis of cardiovascular disease. Br J Gen Pract. 2001;51(473):1001–1003. [PMC free article] [PubMed] [Google Scholar]
- 36.De Backer D., Creteur J., Dubois M.J. Microvascular alterations in patients with acute severe heart failure and cardiogenic shock. Am Heart J. 2004;147:91–99. doi: 10.1016/j.ahj.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 37.Christensen S., Thomsen R.W., Torring Impact of COPD on outcome among patients with complicated peptic ulcer. Chest. 2008;133:1360–1366. doi: 10.1378/chest.07-2543. [DOI] [PubMed] [Google Scholar]
- 38.Huang K.-W., Luo J.-C., Leu H.-B. Chronic obstructive pulmonary disease: an independent risk factor for peptic ulcer bleeding: a nationwide population-based study. Aliment Pharmacol Ther. 2012;35:796–802. doi: 10.1111/j.1365-2036.2012.05028.x. [DOI] [PubMed] [Google Scholar]
- 39.Luo J.-C., Leu H.-B., Huang K.-W. Incidence of bleeding from gastroduodenal ulcers in patients with end-stage renal disease receiving hemodialysis. CMAJ. 2011;183:E1345–E1351. doi: 10.1503/cmaj.110299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin K.J., Hernández-Díaz S., García Rodríguez L.A. Acid suppressants reduce risk of gastrointestinal bleeding in patients on antithrombotic or anti-inflammatory therapy. Gastroenterology. 2011;141:71–79. doi: 10.1053/j.gastro.2011.03.049. [DOI] [PubMed] [Google Scholar]
- 41.van Soest E.M., Valkhoff V.E., Mazzaglia G. Suboptimal gastroprotective coverage of NSAID use and the risk of upper gastrointestinal bleeding and ulcers: an observational study using three European databases. Gut. 2011;60:1650–1659. doi: 10.1136/gut.2011.239848. [DOI] [PubMed] [Google Scholar]