Abstract
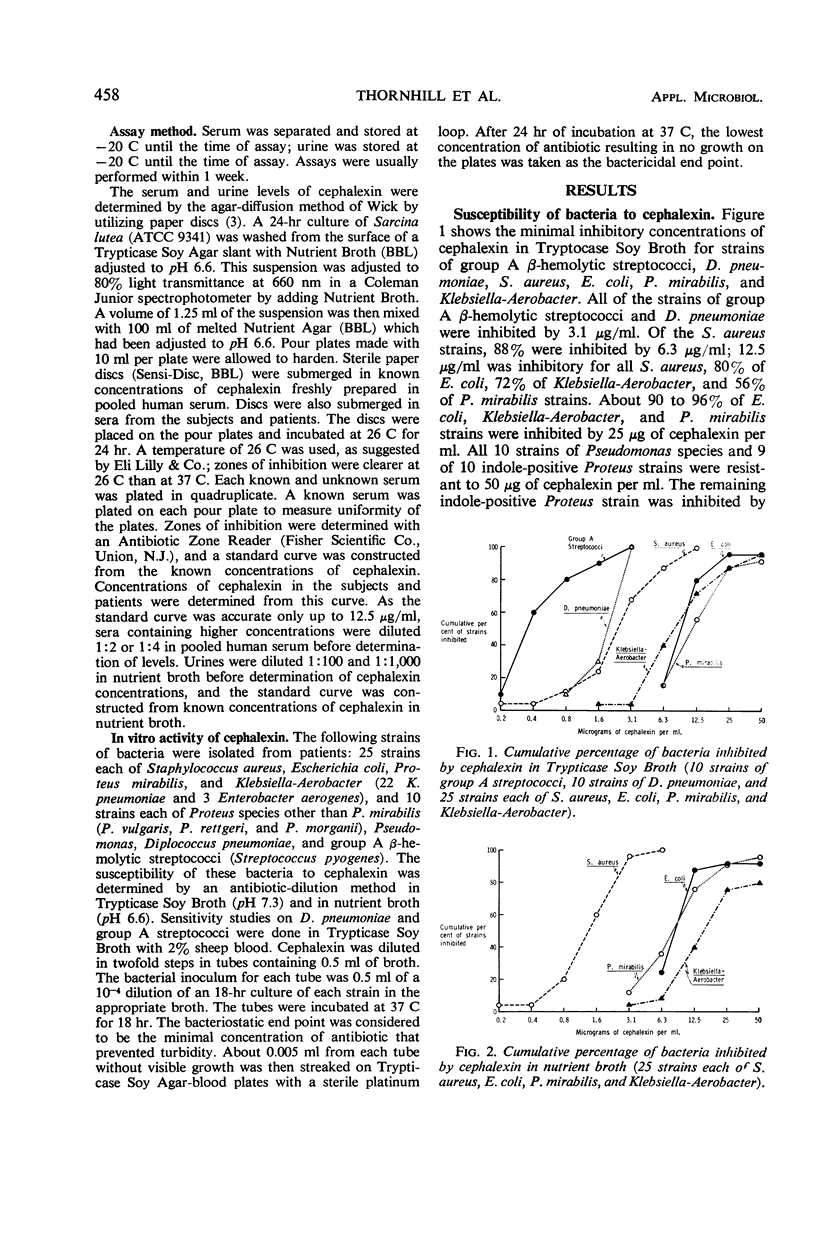
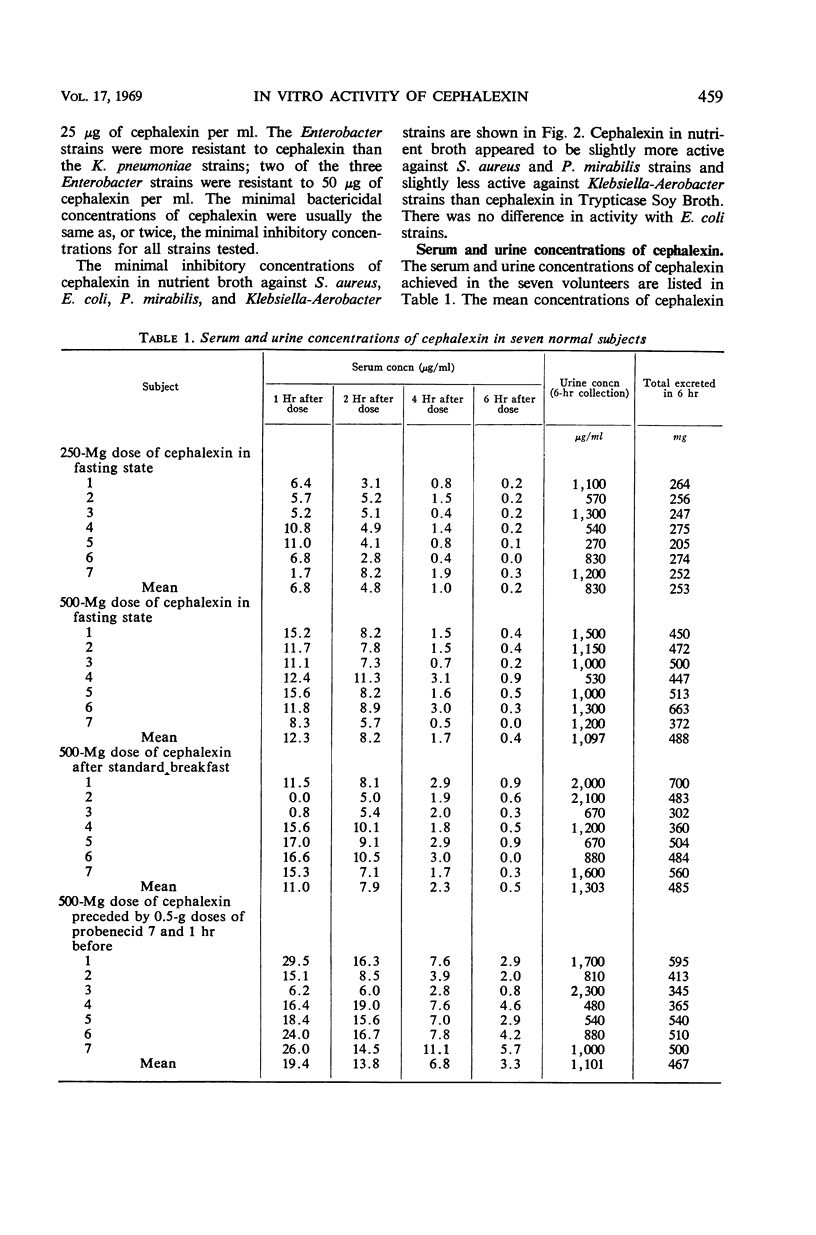
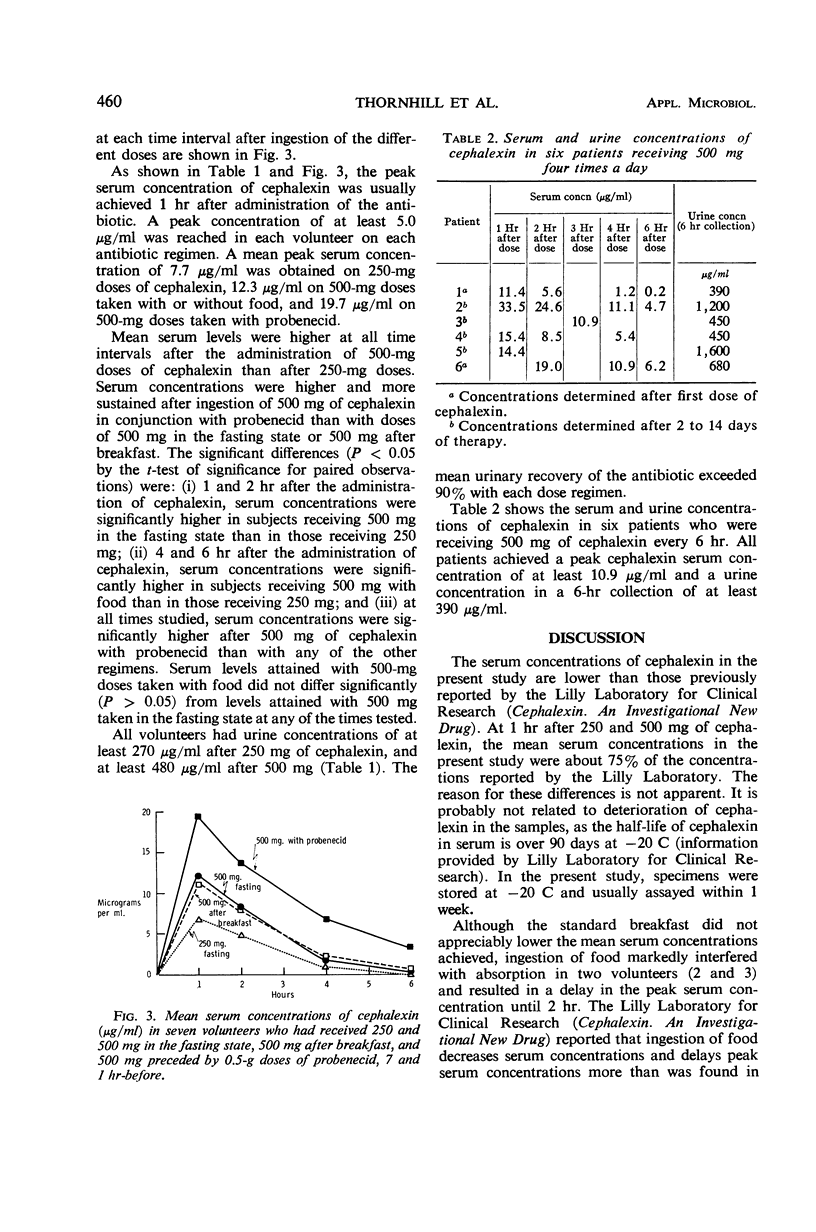
Concentrations of cephalexin (an orally absorbed derivative of cephalosporin C) in serum and urine were determined in normal volunteers and patients. The in vitro antibacterial activity was also studied. All strains of group A β-hemolytic streptococci and Diplococcus pneumoniae were inhibited by 3.1 μg/ml. Of the Staphylococcus aureus strains, 88% were inhibited by 6.3 μg/ml, and 12.5 μg/ml was inhibitory for all S. aureus, 80% of Escherichia coli, 72% of Klebsiella-Aerobacter, and 56% of Proteus mirabilis strains. About 90 to 96% of E. coli, Klebsiella Aerobacter, and P. mirabilis strains were inhibited by 25 μg of cephalexin per ml. Pseudomonas and indole-positive Proteus strains proved to be quite resistant to cephalexin. Cephalexin was well absorbed after oral administration. A peak serum concentration of cephalexin of at least 5 μg/ml was achieved in each volunteer with 250 and 500-mg doses. A mean peak serum concentration of 7.7 μg/ml was achieved with 250-mg doses; 12.3μg/ml was achieved with 500-mg doses of antibiotic. Food did not interfere with absorption. Probenecid enhanced both the peak serum concentration and the duration of antibiotic activity in the serum. Over 90% of the administered dose was excreted in the urine within 6 hr. The mean peak serum concentration of cephalexin after an oral dose of 500 mg was adequate to inhibit all group A streptococci, D. pneumoniae, and S. aureus, 85% of E. coli, and about 40 to 75% of Klebsiella-Aerobacter and P. mirabilis strains. Levels of cephalexin in urine were adequate to inhibit over 90% of E. coli, and P. mirabilis and 80 to 96% of Klebsiella-Aerobacter strains.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Applestein J. M., Crosby E. B., Johnson W. D., Kaye D. In vitro antimicrobial activity and human pharmacology of cephaloglycin. Appl Microbiol. 1968 Jul;16(7):1006–1010. doi: 10.1128/am.16.7.1006-1010.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WICK W. E., BONIECE W. S. IN VITRO AND IN VIVO LABORATORY EVALUATION OF CEPHALOGLYCIN AND CEPHALORIDINE. Appl Microbiol. 1965 Mar;13:248–253. doi: 10.1128/am.13.2.248-253.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick W. E. Cephalexin, a new orally absorbed cephalosporin antibiotic. Appl Microbiol. 1967 Jul;15(4):765–769. doi: 10.1128/am.15.4.765-769.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]


