Abstract
The synthesis of MII2 complexes (MII = Co, Mn) with terminal hydroxo ligands has been achieved utilizing a dinucleating ligand containing a bridging pyrazolate unit and appended (neopentyl)aminopyridyl groups. Structurally studies on the complexes revealed that the MII–OH units are positioned in a syn-configuration, placing the hydroxo ligands in close proximity (ca. 3 Å apart), which may be a prerequisite for water oxidation.
Multinuclear metal complexes with terminal hydroxo or oxo ligand(s) have been proposed to participate in a variety of different biochemical processes, including the catalytic cycle of water oxidation in photosystem II (PSII).1 Exploring the chemistry of related synthetic systems has provided information into the structural and mechanistic requirements necessary for catalysis, yet most artificial systems still lack the catalytic efficiency found in metalloproteins. One synthetic approach is to develop complexes that initially place two M–OHn (n = 1, 2) units in close proximity.2,3 This approach is often hampered because of the tendency for hydroxo and aquo ligands to bridge between metal ions. Nonetheless, there are structurally characterized examples of dinuclear complexes containing discrete M–OHn units. 4 Meyer reported that the [(bpy)2Ru-OH2]2(μ-O) complex contains a diruthenium core with two terminal aquo ligands5 and Ménage showed that the [(trpy)2Fe-OH]2(μ-O) has two FeIII–OH units;6 however, in these complexes the M–OHn units have an anti-configuration in the solid state with the two OHn groups separated by over 5 Å. Success in preparing dinuclear complexes with M–OHn unit in the syn-configuration has been achieved using dinucleating ligands.7 For instance, Llobet and Meyer have reported complexes containing Ru2-(H3O2),7a Zn2-(H3O2)7b,c and Ni-(H3O2)7d unit respectively, utilizing a pyrazolate bridging group that prevents the formation hydroxo or oxo bridges. In this report we describe the preparation of a new dinucleating ligand that supports the formation of [CoII–OH]2 and [MnII–OH]2 complexes. The MII–OH units in these complexes adopt syn-configurations, which are stabilized by intramolecular hydrogen bonding (H-bonding) networks.
We have been preparing dinucleating ligands that also utilize a bridging pyrazolate group to separate the metal centers. Using the structural concepts developed by Meyer,8 we reasoned that ligands containing a (3,5-diaminomethylene)-pyrazolate unit would provide sufficient spacing between the metal ions (ca 3.5-4.5 Å) to allow the binding of two terminal hydroxo ligands. In addition, our ligands include H-bond donors within the secondary coordination sphere to form intramolecular H-bonding networks with the M–OH units;9,7d these types of noncovalent interactions have previously been exploited in the isolation of mononuclear M–OH analogues.10 Two earlier versions of this design contained urea ([H4PRbuam]5-) 11 and carboxyamidopyridyl ([H3bppap]2-)12 groups (Fig. 1) but we were unable to prepare dinuclear M–OH complexes. For instance, the CoII2 complex of [H3bppap]2- had only one terminal CoII–OH center: the binding of a second hydroxo ligand appeared to be hindered by the coordination of the oxygen atoms of the appended carboxyamido groups.13 These results suggested that reduction of carboxyamido groups to neopentylamino moieties would produce 3,5-bis[bis(N-6-neopentylamino-2-pyridylmethyl)aminomethyl]-1H-pyrazole (H5bnppap), a compound containing H-bond donors that cannot readily coordinate to the metal ions.14,10c,d
Fig. 1.
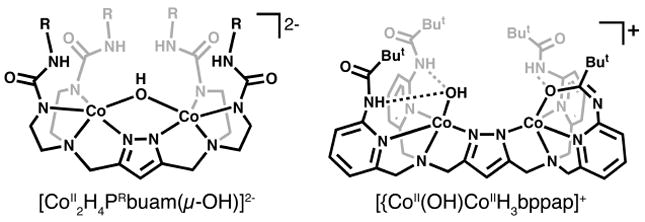
CoII–OH complexes of [H4PRbuam]5- and [H3bppap]2-.
H5bnppap was synthesized from H5bppap in nearly quantitative yield by reduction using LiAlH4 in THF (Scheme 1). The dinuclear CoII and MnII complexes of [H4bnppap]– were prepared according to the route outlined in Scheme 2. H5bnppap in MeOH was treated with 3 equiv of NaH under an argon atmosphere. After stirring for 30 min, the MII precursors (either CoII(NO3)2·6H2O or MnII(OTf)2·2MeCN) were added in one portion and stirred for an additional 30 min. The reaction mixtures were then treated with 2 equiv of H2O, followed by the addition of NaBPh4, which resulted in the immediate formation of precipitates. The solids were isolated via filtration and purified by recrystallization from THF/pentane.
Scheme I.
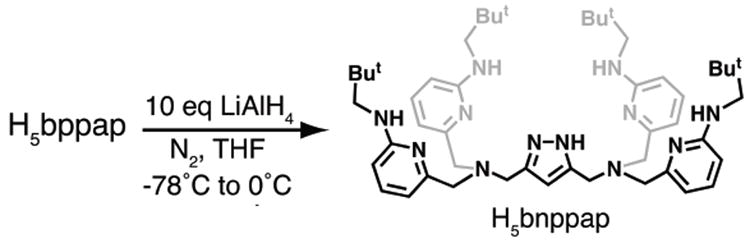
Preparative route of H5bnppap from H5bppap.
Scheme II.
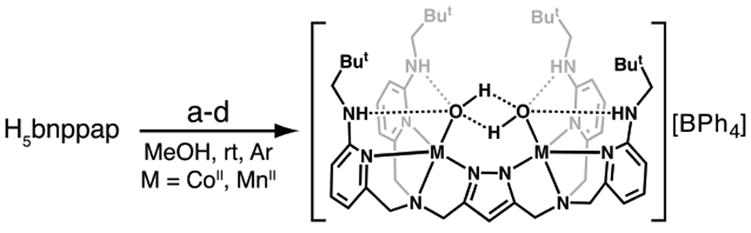
Synthetic route to [MII2H4bnppap(OH)2]+ (M = CoII, MnII). Conditions: (a) 3 equiv NaH, (b) 2 equiv Co(NO3)2·6H2O or Mn(OTf)2·2MeCN (c) 2 equiv of H2O (d) 1 equiv NaBPh4.
Analytical and spectroscopic investigations indicated that the salts contained the dinuclear metal complexes, [MII2H4bnppap(OH)2]+ (MII = Co, Mn). The electrospray ionization mass spectrum (ESI-MS) of [Co2IIH4bnppap(OH)2]+ contained a large ion peak at a charge-to-mass ratio (m/z) of 981.4, which matches the expected mass and isotopic distribution of a (CoII–OH)2 complex (calcd, 981.5). Similarly the ESI-MS spectrum for ([Mn2IIH4bnppap(OH)2]+ exhibited a peak at a m/z of 973.5 (calcd, 973.5). Each peak shifted by 4 mass-units when H2 18O was used in the synthesis, indicating that the source of the hydroxo ligands is water.¶ Effective magnetic moments (μeff) at 298 K of 8.01 and 5.23 μBM were obtained for [CoII2H4bnppap(OH)2]+ and [MnII2H4bnppap(OH)2]+ respectively,§ values that are close to the spin-only values for two individual high-spin MnII and CoII centers. 15 These preliminary findings suggest weak magnetic coupling, which is inconsistent with the presence of single atom bridge(s) between the metal centers.
The solid-state structures of the complexes were investigated using X-ray diffraction methods to reveal dinuclear species in which each metal center has a coordinated hydroxo ligand. In [CoII2H4bnppap(OH)2]+ (Fig. 2A) both CoII centers have trigonal bipyramidal coordination geometries as judged by index of trigonality parameter (τ) of 0.99 measured for both metal ions.16 An N4O primary coordination sphere exists about each CoII ion, consisting of pyrazolate and pyridyl nitrogen atoms defining the trigonal plane, and the tertiary amino nitrogen and hydroxo oxygen atoms in the axial positions. The Co1–O1 and Co2–O2 bond distances of 1.9379(2) Å and 1.9444(2) Å are similar to the Co–O(H) bond length of 1.931(2) Å observed in [{CoII(OH)}CoIIH3bppap]+, but are significantly shorter than those in [CoII2H4PRbuam(μ-OH)]2- (greater than 2.1 Å) (Figure 1).10b The pyrazolate unit bridges between the CoII centers with Co1–N4 and Co2–N10 bond distances of 2.034(2) and 2.046(2) Å, and a Co1⋯Co2 separation of 4.286 Å. The remaining Co–N bond distances and angles are unexceptional with avg. Co–Ntrig and Co–Naxial bond lengths of 2.108(2) Å and 2.194(2), Å and avg. Ntrig–Co–Ntrig angle and O–Co–Naxial angles of 115.11(8)° and 176.66(8)°
Fig. 2.
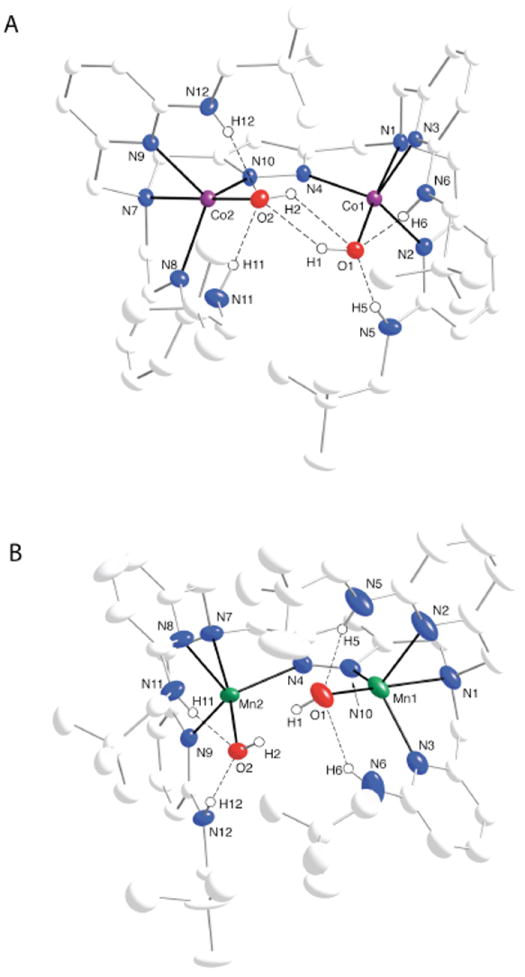
Thermal ellipsoid plots of [CoII2H4bnppap(OH)2]+ (A) and [MnII2H4bnppap(OH)2]+ (B). Thermal ellipsoids are drawn at the 50% probability level. Only hydroxo and amino hydrogen atoms are shown for clarity. Selected bond lengths (Å) and angles (°) for [CoII2H4bnppap(OH)2]+ and [MnII2H4bnppap(OH)2]+: Co1–O1, 1.938(2); Co1-N4, 2.034(2); Co1–N3, 2.102(2); Co1–N2, 2.107(2); Co1–N1, 2.192(2); Co2–O2, 1.944(2); Co2–N10, 2.046(2); Co2–N9, 2.119(2); Co2–N8, 2.104(2); Co2–N7, 2.195(2); O1–Co1–N4, 106.28(8); O1–Co1–N3, 101.94(8); N4–Co1–N3, 114.64(8); O1–Co1–N2, 100.48(2); N4–Co1–N2, 116.10(8); N3–Co1–N2, 114.73(8); O1–Co1–N1, 176.51(8); N4–Co1–N1, 76.99(8); N3–Co1–N1, 77.50(8); N2–Co1–N1, 76.78(8); O2–Co2–N10, 106.61(8); O2–Co2–N8, 102.23(8); N10–Co2–N8, 115.00(8); O2–Co2–N9, 100.11(8), N10–Co2–N9, 117.25(8), N8–Co2–N9, 112.95(8); O2–Co2–N9, 176.81(8); N10–Co2–N7, 76.25(8); N8–Co2–N7, 77.51(8); N9–Co2–N7, 77.17(8). Mn1–O1, 2.003(3); Mn1-N4, 2.128(4); Mn1–N3, 2.204(4); Mn1–N2, 2.234(4); Mn1–N1, 2.330(4); Mn2–O2, 2.006(4); Mn2–N10, 2.137(4); Mn2–N9, 2.230(5); Mn2–N8, 2.207(5); Mn2–N7, 2.285(5); O1–Mn1–N4, 111.20(2); O1–Mn1–N3, 101.67(2); N4–Mn1–N3, 112.52(2); O1–Mn1–N2, 100.60(1); N4–Mn1–N2, 114.75(2); N3–Mn1–N2, 114.56(2); O1–Mn1–N1, 174.00(2); N4–Mn1–N1, 74.57(2); N3–Mn1–N1, 76.94(2); N2–Mn1–N1, 74.98(2); O2–Mn2–N10, 113.07(2); O2–Mn2–N8, 102.03(2); N10–Mn2–N8, 114.23(2); O2–Mn2–N9, 99.19(2), N10–Mn2–N9, 115.2(2), N8–Mn2–N9, 111.3(2); O2–Mn2–N9, 172.13(2); N10–Mn2–N7, 74.46(2); N8–Mn2–N7, 75.94(2); N9–Mn2–N7, 74.84(2).
A striking feature of the molecular structure of [CoII2H4bnppap(OH)2]+ is the syn-configuration of the two Co–OH units. Their close promixity is reflected in the relatively short O1⋯O2 separation of 2.971(2) Å, a distance that is indicative of H-bonds being present between the two ligands. 17, ¶ In addition, the hydroxo ligands formed intramolecular H-bonds with the neopentylamino groups of [H4bnppap]–. All the N–H vectors are positioned toward the hydroxo ligands with N–H–O angles of greater than 164°. This alignment produced O⋯N distances that are less than 2.8 Å, which, taken together, are consistent with the formation of strong H-bonds. FTIR measurements are also consistent with intramolecular H-bonds being present in [CoII2H4bnppap(OH)2]+ with broad signals from the amino NH groups appearing at 3235 cm-1. We were unable to observed FTIR signals from the hydroxo ligand, presumably because they are significantly broadened because of the H-bonds.
The molecular structure of [MnII2H4bnppap(OH)2]+ was also determined and contains nearly the same structural features as the cobalt analogue (Fig. 2B). Disorder in the neopentyl groups limited the quality of the structure;‡ nevertheless, it is clear at the present resolution that each MnII center has trigonal bipyramidal coordination geometry with τMn1 = 0.99 and τMn2 = 0.95 and a Mn1⋯Mn2 separation of 4.303 Å. Note that this type of coordination also promotes the Mn1–O1 and Mn2–O2 vectors to assume a syn-configuration (O1⋯O2, 3.235 Å) that is supported by an extensive intramolecular H-bonding network.
In summary, a new dinucleating ligand, [H4bnppap]– has been developed that contains four appended amino groups connected via a pyrazolate bridge. The ligand allows for the preparation of new dinuclear complexes of cobalt and manganese, each of which has two M–OH units. The intramolecular H-bonding network and the rigidity of ligand framework successfully reinforces terminal hydroxide coordination and the orientation of the hydroxo groups. These attributes result in rare examples of discrete molecular species with terminal metal hydroxides arranged in a syn-fashion that are in close proximity (less than 3.2 Å). Systems of this type allow for futher investigations into the interactions between M–O(H) groups, including exploring their reactivities.
Supplementary Material
Footnotes
Electronic Supplementary Information (ESI) available: Crystallographic data, FT-IR spectra, absorption spectra, ESI-MS spectra. For ESI and crystallographic data in CIF see DOI: 10.1039/b000000x/
ESI-MS data: [Co2 IIH4bnppap(18OH)2]+: m/z = 985.8 (calcd, 985.5); ([Mn2 IIH4bnppap(18OH)2]+: m/z = 977.5 (calcd, 977.5).
Magnetic moments were determined in DMSO using the Evans’ method. The calculations were done relative to the shift in the solvent peak.
For [Co2 IIH4bnppap(OH)2]+ hydrogen atoms on the amino groups were located from a difference-Fourier map and refined (x,y,z, and Uiso), while those of the hydroxo ligands were included using a riding model.
Various attempts using different solvents, experimental conditions, and counter anions to obtain crystal that gave a better structure of [Mn2 IIH4bnppap(OH)2](BPh4) were unsuccessful. The freely rotating neopentyl groups impose high intrinsic disorder to the cationic complex, which likely explains the poor diffraction of the crystals.
Notes and references
- 1.(a) Umena Y, Kawakami K, Shen J-R, Kamiya N. Nature. 2011;473:55–61. doi: 10.1038/nature09913. [DOI] [PubMed] [Google Scholar]; (b) Surendranath Y, Kanan MW, Nocera DG. J Am Chem Soc. 2010;132:16501–16509. doi: 10.1021/ja106102b. [DOI] [PubMed] [Google Scholar]; (c) Romero I, Rodrígues M, Sens C, Mola J, Kolllpara MR, Francàs L, Mas-Marza E, Esrlche L, Llobet A. Inorg Chem. 2008;47:1824–1834. doi: 10.1021/ic700911y. [DOI] [PubMed] [Google Scholar]; (d) Betley TA, Wu Q, Voorhis TV, Nocera DG. Inorg Chem. 2008;47:1849–1861. doi: 10.1021/ic701972n. [DOI] [PubMed] [Google Scholar]
- 2.Mononuclear systems Alliger GE, Müller P, Cummins CC, Nocera DG. Inorg Chem. 2010;49:3697–3699. doi: 10.1021/ic100395a.. Que L, Jr, Tolman WB. Angew Chem Int Ed. 2002;41:1114. doi: 10.1002/1521-3773(20020402)41:7<1114::aid-anie1114>3.0.co;2-6.
- 3.For examples of mononuclear systems that form O–O bonds see: Mirica LM, Ottenwaelder X, Stack TDP. Chem Rev. 2004;104:1013–1046. doi: 10.1021/cr020632z.. Lewis EA, Tolman WB. Chem Rev. 2004;104:1047–1076. doi: 10.1021/cr020633r.
- 4.For examples of M–OHn in main group clusters see Roesky HW, Singh S, Jancik V, Chandrasekhar V. Acc Chem Res. 2004:969–981. doi: 10.1021/ar0402154.. Leeland JW, White FJ, Love JB. J Am Chem Soc. 2011;133:7320–7323. doi: 10.1021/ja201630b.
- 5.Gilbert JA, Eggleston DS, Murphy WR, Jr, Geselowitz DA, Gersten SW, Hodgson DJ, Meyer TJ. J Am Chem Soc. 1985;107:3855–3864. [Google Scholar]
- 6.Duboc-Toia C, Ménage S, Vincet J-M, Thérèse A-P, Fontecave M. Inorg Chem. 1997;36:6148–6149. [Google Scholar]
- 7.(a) Bozoglian F, Romain S, Ertem MZ, Todorova TK, Sens C, Mola J, Rodrígues M, Romero L, Benet-Buchholz J, Fontrodona X, Cramer CJ, Gagliardi L, Llobet A. J Am Chem Soc. 2009;131:15176–15187. doi: 10.1021/ja9036127. [DOI] [PubMed] [Google Scholar]; (b) Meyer F, Rutsch R. Chem Commun. 1998:1037–1038. [Google Scholar]; (c) Baner-Siebenlist B, Meyer F, Farkas E, Vidovic D, Dechert S. Chem–Eur J. 2005;11:4349–4360. doi: 10.1002/chem.200400932. [DOI] [PubMed] [Google Scholar]; (d) Graef T, Galezowska J, Dechert S, Meyer F. Eur J Inorg Chem. 2011;2011:4161–4167. [Google Scholar]; (e) Strautmann JBH, Walleck S, Strammler A, Glaser T. Chem Commun. 2010;47:695–697. doi: 10.1039/c0cc03098h. [DOI] [PubMed] [Google Scholar]
- 8.(a) Roder JC, Meyer F, Pritzkow H. Organometallics. 2001;20:811–817. [Google Scholar]; (b) Klingele J, Dechert S, Meyer F. Coord Chem Rev. 2009;253:2698–2741. [Google Scholar]
- 9.(a) Arii H, Funahashi Y, Jitsukawa K, Masuda H. Dalton Trans. 2003:2115–2116. [Google Scholar]; (b) Feng G, Natale D, Prabaharan R, Mareque-Rivas JC, Williams NH. Angew Chem Int Ed. 2006;118:7214–7217. doi: 10.1002/anie.200602532. [DOI] [PubMed] [Google Scholar]
- 10.Shook RL, Borovik AS. Inorg Chem. 2011;49:3646–3660. doi: 10.1021/ic901550k. and references therin. Borovik AS. Acc Chem Res. 2005;38:54–61. doi: 10.1021/ar030160q. and references therein. Ogo S, Wada S, Watanabe Y, Iwase M, Wada A, Harata M, Jitsukawa K, Masuda H, Einaga H. Amgew Chem Int Ed. 1998;37:2102–2104. doi: 10.1002/(SICI)1521-3773(19980817)37:15<2102::AID-ANIE2102>3.0.CO;2-A.. Marqes-Rivas JC, Prabaharan R, Parsons S. Dalton Trans. 2004:1648–1655. doi: 10.1039/b402084g.
- 11.(a) Zinn PJ, Powell DR, Day VW, Hendrich MP, Sorrell TN, Borovik AS. Inorg Chem. 2006;45:3484–3486. doi: 10.1021/ic060009n. [DOI] [PubMed] [Google Scholar]; (b) Zinn PJ, Sorrell TN, Powell DR, Day VW, Borovik AS. Inorg Chem. 2007;46:10120–10132. doi: 10.1021/ic700685g. [DOI] [PubMed] [Google Scholar]
- 12.Ng GK-Y, Ziller JW, Borovik AS. Inorg Chem. 2011;50:7922–7924. doi: 10.1021/ic200881t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.(a) Shook RL, Peterson SM, Greaves J, Moore C, Rheingold AL, Borovik AS. J Am Chem Soc. 2011;133:5810–5817. doi: 10.1021/ja106564a. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ingle GK, Makowska-Grzyka MM, Szajna-Fuller E, Sen I, Price JC, Arif AM, Berreau LM. Inorg Chem. 2007;46:1471–1480. doi: 10.1021/ic062020t. [DOI] [PubMed] [Google Scholar]; (c) Rudzka K, Arif AM, Berreau LM. J Am Chem Soc. 2006;128:17018–17023. doi: 10.1021/ja0601336. [DOI] [PubMed] [Google Scholar]; (d) Szajna E, Makowska-Grzyka MM, Wasden CC, Arif AM, Berreau LM. Inorg Chem. 2005;44:7595–7605. doi: 10.1021/ic050750f. [DOI] [PubMed] [Google Scholar]; (e) Marques-Rivas JC, Salvagni E, Parsons S. Dalton Trans. 2004:4185–4192. doi: 10.1039/b414223c. [DOI] [PubMed] [Google Scholar]; (g) Marques-Rivas JC, de Rosoles RTM, Parsons S. Dalton Trans. 2003:2156–2163. [Google Scholar]
- 14.Grubel L, Fuller AL, Chambers BM, Arif AM, Berreau LM. Inorg Chem. 2010;49:1071–1081. doi: 10.1021/ic901981y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drago RS. Physical Methods for Chemists. 2. Chapter 11. Surfside; Gainesville, FL: 1992. [Google Scholar]
- 16.Addison AW, Rao TN, Reedijk J, Van Rijin J, Verschoor GC. J C S Dalton Trans. 1984:1349–1356. [Google Scholar]
- 17.Parthasarathi R, Subramanian V. In: Hydrogen Bonding—New Insights. Grabowski SJ, editor. chapter 1. Springer; Dordrecht: 2006. pp. 1–50. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


