Abstract
Bacterial infections can have adverse effects on the efficacy, lifetime and safety of an implanted device and are the second most commonly attributed cause of orthopedic implant failure. We have previously shown the assembly of PEGylated titanium-binding peptides (TBPs) on Ti to obtain a bacteriophobic surface coating that can effectively resist protein adsorption and Staphylococcus aureus (S. aureus) adhesion. In the present study, we examine the effect of multiple TBP repeats on coating performance in vitro. Mono, di, and tetravalent peptides were synthesized and assessed for binding affinity and serum stability. PEGylated analogs were prepared and evaluated for their effect on S. aureus attachment and biofilm formation. Coating performance improved with the number of TBP repeats, with the tetravalent coating, TBP4–PEG, showing the best performance in all assays. In particular, TBP4–PEG forms a serum-resistant surface coating capable of preventing S. aureus colonization and subsequent biofilm formation. These results further support the role that multivalency can play in the development of improved surface coatings with enhanced stabilities and efficacy for in vivo clinical use.
Keywords: Peptide, Surface modification, Bacteria, Biofilm, Implant, Biomaterial
1. Introduction
Implant-associated infection is a significant healthcare problem and is a major cause of post-surgical morbidity and mortality. Over half of the two million cases of nosocomial infection that occur in the United States annually are associated with indwelling devices. Although less common than catheter-based bloodstream infection, infections established on surgical implants and devices are considerably harder to treat because they require longer antibiotic regiments and repeated surgical procedures [1,2]. In the case of orthopedic surgeries, up to 10% of fracture fixators and 85% of external fixators typically become infected and have to be managed with several weeks of systemic antibiotic therapy. Surgical treatments usually involve the removal of the infected nail or pin followed by insertion of a replacement. Infection of implanted devices with particularly virulent strains, such as methicillin-resistant Staphylococcus aureus (MRSA), may necessitate multiple, and often traumatic, surgical procedures.
The treatment of device-associated infections is far more complex than the simple administration of antibiotics [3]. When an implant is inserted into host tissue, small biomolecules including extracellular matrix (ECM) proteins (e.g., fibronectin, fibrinogen and collagen) adsorb onto the material surface to form a conditioned protein layer [4,5] conducive to the adherence of free-floating planktonic bacteria. The adhered bacteria then rapidly proliferate, recruit other cells, and produce sticky secretions to form dense three-dimensional communities of attached (sessile) cells called biofilms [1,6]. Antibiotic agents show greatly reduced anti-microbial activity against bacteria within biofilms for a number of reasons, including poor diffusion into the matrix or altered metabolic activity [7]. Consequently, bacterial biofilms are notoriously difficult to eradicate and often lead to persistent and recurrent infections, which can adversely affect the performance of an implanted device with devastating consequences for the patient. Bacteria detaching from the biofilm and entering the blood stream can also result in life-threatening systemic infections [8]. Urinary tract infections associated with an indwelling catheter (UTIc), for example, account for more than 40% of the total number of reported nosocomial infections and affect an estimated 600,000 patients annually. Although usually benign, UTIc leads to bacteremia (blood infection) in 2–4% of patients and have been associated with higher fatality rates [9]. Clearly, there is a pressing clinical need for new coatings and treatments to address the issues of implant infection and anti-microbial resistance, preferably using strategies that can be simply and robustly administered to implants.
Since bacterial colonization of an indwelling device is a prelude to infection, prevention of cellular attachment will result in a lower rate of clinical infection [10]. Surfaces modified with polyethylene glycol (PEG) present one of the most simple and well-studied systems for preparation of bacteriophobic, or bacteria repellent, interfaces. PEG is a hydrophilic polyether that when tethered to surfaces, forms a hydrated, steric barrier to surface fouling by micro-organisms and proteins [11,12]. Surface PEGylation strategies have been extensively explored, and include self-assembled monolayers (SAMs) [13-15], covalent grafting [16-18], plasma deposition [19], and polyelectrolyte assemblies [20,21] However, existing immobilization strategies often rely on complex chemistry or require extensive surface pre-treatment, which limit their ability to modify a broad range of materials. Hence, we turn to bio-adhesives and take advantage of the natural affinity of peptides and proteins for synthetic materials in the development of more versatile anchors for the PEGylation of implant materials. Increasingly, engineered polypeptides capable of recognizing synthetic materials are being investigated for use as adhesive moieties to immobilize various functionalities on surfaces [22-26]. This strategy also allows for rapid and facile modification of surfaces without the need for harsh reaction conditions, and is therefore amenable to simple, point-of-care application in a surgical setting.
We have previously reported the development and characterization of a PEGylated-peptide surface coating for titanium (Ti), an important orthopedic implant metal [27]. This coating consists of a hydrophilic PEG chain conjugated to a Ti-binding peptide (TBP) anchor. The TBP domain, selected from phage display, spontaneously assembles via adsorptive mechanisms onto Ti, with PEG extending into aqueous solution to form a complete surface adlayer resistant to protein and bacteria fouling in vitro. However, further optimization is still needed in order to obtain a coating with the durability necessary for extended in vivo clinical use.
In this work, we examine the use of multiple TBP domains as a potential approach to enhance coating affinity, integrity and function. The mechanism of binding for a TBP has been shown to be primarily adsorptive, and is dependent upon the extent of non-covalent interactions between amino acid side chains and surface groups. Previous AFM experiments by Kenan et al. and Lee et al. determined the pull-off forces of physisorbed polypeptides to be 675 pN and 800 pN, respectively [26,28]. In comparison, the force required to break a covalent bond is reported to be on the order of a few nanonewtons. A multiple attachment approach could therefore be a viable strategy to bring the pull- forces of adsorptive coatings closer to those of covalent attachments. Herein, we describe our recent progress in the development of divalent and tetravalent coatings comprising two and four Ti-binding peptide domains, respectively (Fig. 1), for the prevention of staphylococcal attachment and biofilm formation on Ti surfaces.
Fig. 1.
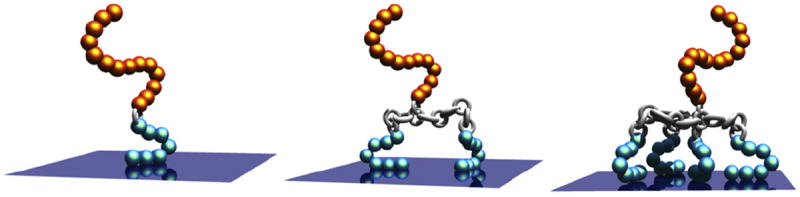
The bacteriophobic coatings possess one or more bottom TBPs (blue), a peptide linker (silver), and a top PEG domain (gold). Schematic of three bacteriophobic coatings under investigation where one (left), two (middle), or four (right) titanium-binding peptides (TBPs) are covalently attached to a polyethylene glycol (PEG).
2. Materials and methods
2.1. Phage display selection of titanium-binding peptides
Titanium-binding peptides (TBPs) were identified using a combinatorial phage display screening process described previously [27]. Briefly, fourteen different M13 phage libraries displaying random peptide sequences on their pIII coat proteins were screened for binding to Ti6Al4V. Implant grade Ti6Al4V beads approximately 4 mm in diameter (Dynamet, Washington, PA) were prepared for selections by washing extensively in series with 70% ethanol, 40% nitric acid, distilled water, 70% ethanol, and acetone to remove surface contaminants. For the panning procedure, one bead was placed per well of a 96-well polypropylene (PP) plate. (CoStar, Corning USA) Non-specific binding sites on the metal beads and the surface of the PP were blocked with 1% BSA in PBS for 1 h at room temperature (RT) with shaking at 50 rpm. The wells were then washed 5 times with 300 μl of PBS-Tween20 (PBS-T; 0.05% Tween20 in PBS). Each library was diluted in PBS-T and added at a concentration of 1010 PFU/ml in a total volume of 100 μL. After a 3 h incubation RT and shaking at 50 rpm, unbound phage were removed by 5 washes of PBS-T. To recover the phage bound to the Ti beads, the beads were added directly to exponential phase Escherichia coli (E. coli) DH5αF’ cells in 2xYT media. The mixture was incubated overnight at 37 °C in an incubator/shaker at 210 rpm. Phage supernatant was harvested after spinning at 8500 × g for 10 min. Second and third rounds of selection were performed using the amplified phage from the previous round as input. The procedure remained the same, except that the phage-bead incubation time was decreased to 20 min and the washing of unbound phage with PBS-T increased ten-fold. On-phage ELISAs were then performed to confirm the binding affinity of the selected sequences for Ti using a previously established protocol [29]. To define which amino acid residues or motifs were important for Ti-binding activity, a series of amino acid substitutions were designed, and the ‘HKH’ triad was found responsible for Ti-binding. Based on these results, a series of synthetic, second-generation peptides were designed, each containing repeats of the positively charged ‘KHK’ or ‘HKH’ tripeptides. Peptides were synthesized and again tested for binding to Ti beads. Peptides containing three KHK repeats showed a >150-fold improvement in binding strength over the original phage display selected peptide and was selected for further experimentation.
2.2. Synthesis of Ti-binding peptide and peptide multimers
Peptides and peptide multimers were synthesized using 9-fluorenylmethoxycarbonyl (Fmoc) based solid-phase peptide chemistry either manually in glass reaction vessels or on a Symphony multiplex automated peptide synthesizer (Protein Technologies Inc., Tucson AZ). A terminal biotin was attached through the epsilon amide of a lysine residue to the C-terminal of peptides for affinity constant determinations. Standard Fmoc/t-Bu chemistry using AA/HBTU/HOBt/NMM (1:1:1:2) as the coupling reagents was employed for the synthesis. Peptides were synthesized on H-Rink Amide Chem Matrix Resin (0.47 meq., Matrix Innovation, Montreal, Canada) or on Fmoc-PAL-Peg-PS (~0.20 mmol/g) (Applied Biosystems, Inc, CA) yielding C-terminal amides. Amino acids (Genzyme Corp, Cambridge, MA; Chem Impex International, Lousiville, KY; EMD Biosciences, San Diego, CA; Luxembourg Industries, Ltd., Tel Aviv, Israel; Sven Genetech Ltd., Cherapally, India and Senn Chemicals, Dielsdorf, Switzerland) were coupled in 5–10-fold excess in the synthesis cycles and all residues were doubly, triply or quadruply coupled for 1 h. The coupling reactions were monitored by Kaiser ninhydrin test or chloranil test. Removal of the Fmoc group was performed using 20% Piperidine (Mallinkrodt Baker, Phillipsburg, NJ) in DMF with 0.1 N HOBt – (15 min × 2) and the residual piperidine was removed by three consecutive DMF/MeOH/DMF washes. Peptide cleavage from the resin support was performed using 92% trifluoroacetic acid (TFA, Halocarbon, River Bend, NJ), 5%water, 2% triisoproplysilane (TIPS, Aldrich, St. Louis, MO) and 1% DODT (Aldrich) (10 mL/400 mg of resin) for 2 h at RT, followed by precipitation in cold ethyl ether. The precipitated peptide was centrifuged to pellet (1250 g, for 5 min, Beckman Allegra, Beckman Instrument, Palo Alto, CA) and washed three times with cold ethyl ether. Peptide pellets were dried under vacuum and redissolved in 50% acetonitrile (ACN, Mallinkrodt Baker, Phillipsburg, NJ) and lyophilized to obtain crude peptide as dry powder. The crude peptides were purified by preparative high performance liquid chromatography (HPLC) (Kromasil -100-10-C18 column; 250 × 21.2 mm) and the appropriate fractions lyophilized to give a white fluffy solid. Peptide identity was confirmed with electrospray ionization mass spectrometry (ESI-MS, Waters ZQ4000, Waters Corp., Woburn, MA) or MALDI. Pure peptides were stored at −20 °C under nitrogen atmosphere.
2.3. Synthesis of multivalent peptide–PEG conjugates
Cysteine groups were introduced on the C-terminus of the peptides. The maleimido group in the HO-PEG-Mal 3400 (Laysan Bio Inc, Alabama) was reacted specifically with the sulfhydryl group on the peptide in pH 7 PBS buffer to afford the peptide–PEG conjugates TBP1–PEG, TBP2–PEG and TBP4–PEG. Peptide multimers and peptide conjugates were purified by HPLC then characterized using gel electrophoresis to verify their molecular weights. (Fig. S1)
2.4. Gel electrophoresis characterization of peptide multimers and conjugates
Peptide multimers were resolved on a 16.5% Tris–Tricine polypeptide gel (Bio-Rad, Hercules, CA) at 100V for 1.5 h. Each peptide monomer or multimer was loaded at 2 μg per lane for silver staining and 3 μg per lane for Coomassie staining, in parallel with polypeptide standards. Individual dilutions of the peptide stocks were combined with the sample buffer containing β-mercaptoethanol and heated at 40 °C for 30 min before loading on the gel. After electrophoresis, the bands were visualized either by Silver staining or Coomassie blue (Invitrogen, Carlsbad, CA) following the manufacturer’s instructions.
2.5. Preparation of titanium substrates
Ti beads and Ti-coated glass slides were used for the subsequent studies. Implant grade 3/32″ Ti6Al4V beads (Abbott Ball; West Hartford, CT) were cleaned by sonication in acetone, methanol and water before use. To obtain Ti-coated slides, glass slides (Fisher Sci, Pittsburg, PA) were pre-cleaned in a 1:1 methanol/HCl mixture and then coated with 20 nm of c.p. Ti using electron beam evaporation (Sharon Vacuum, Brockton, MA). Ti-coated slides were cleaned by 10 min of UV/ozone treatment before use. Previous X-ray photo-electron spectroscopy (XPS) analysis revealed a similar surface composition for the Ti6Al4V and c.p. Ti substrates (Ti2p/O1s = 0.313 vs. 0.302), suggesting that coatings are exposed to similar TiO2 surfaces regardless of the underlying Ti composition [27].
2.6. Binding affinity measurements
The binding affinities for all peptides were calculated using a modified ELISA assay. The mono, di and tetravalent forms of the biotin-terminated peptides were serially diluted in Tris-buffered saline (TBS) to obtain a range of concentrations from 0.07 nm to 5 mm. 100 μL of each dilution was added over a clean Ti6Al4V beads in each well of a 96-well microtiter plate and incubated for 1 h at RT. Beads were washed three times in TBS-Tween 20 (TBS-T; 0.05% Tween; Sigma, St. Louis, MO) then blocked in 1% BSA for an hour. Following another wash, beads were incubated in 1:500 streptavidin-alkaline phosphatase (SA-AP) (USB Corporation, Cleveland, OH) for 30 min at RT. Washed beads were transferred into fresh wells and the chromogenic agent p-nitrophenyl phosphate (pNPP) in TBS (Sigma, St. Louis, MO) was added to wells. Supernatants were transferred to a fresh 96-well microtiter plate and the absorbance signal indicative of bound peptide was measured at 405 nm using a microplate reader (AD340C; Beckman Coulter; Fullerton, CA). The absorbance versus log peptide concentration was plotted to yield a sigmoidal binding curve for each of the peptides. The data was then fit to a four-parameter model using a data-fitting program (BioDataFit 1.02; Chang Bioscience, Inc.) and the concentration at the half point of the sigmoidal model curve was extracted as the apparent dissociation constant (or “relative Kd”).
2.7. AFM measurements
Solutions (2.6 μm) of TBP, TBP2 and TBP4 were prepared in DPBS. 1 mL of each peptide solution was added over freshly cleaved 12 mm mica discs in sterile 24-well tissue culture plates and incubated for 2 h at room temperature. Discs were rinsed three times with PBS and three times with ultrapure water and then dried with argon gas before imaging. Coated substrates were examined using a Nanoscope IIIa AFM (Digital Instruments, Santa Barbara, CA) in tapping mode. Samples were imaged in air using a silicon nitride cantilever (NSC15, MikroMasch, OR) at a scan rate of 1–2 Hz and a scan size of 5 μm. Images were flattened using a first or second order fit to correct for piezo bow and/or sample tilt during analysis.
2.8. In vitro serum stability
100 μL aliquots of biotinylated peptides TBP1, TBP2, and TBP4 (26 μm) were added over a Ti6Al4V bead in each well of a 96-well microtiter plate and incubated for 1 h at RT to obtain a saturated peptide coating. Beads were washed three times in TBS-T then transferred into 100% human serum (Innovative Research; Novi, MI) and kept at room temperature on an orbital shaker. After various serum exposure times (0 h, 2 h, 1 day, 1 week and 2 weeks), beads were removed and washed three times in TBS-T. Beads were incubated in 1:500 SA-AP for 30 min at RT. Following another three washes TBS-T, beads were transferred into fresh wells containing pNPP in TBS. The supernatants were transferred to a fresh 96-well microtiter plate and the absorbance signal indicative of bound peptide was measured at 405 nm using a microplate reader. Freshly coated beads that were not exposed to serum were used as a positive control in all cases.
2.9. Bacteria culture and medium
S. aureus strain MZ100 was used in this study. Bacterial cells for inoculum in the biofilm assays were grown in tryptic soy broth (TSB, 20 g/L, Difco Bacto Tryptic Soy Broth, Becton Dickinson, Sparks, MD) or on tryptic soy agar (TSA, comprised of TSB supplemented with 1.5% Difco agar). For all phenotypic assays, TSB + 0.2% glucose was used, as this medium promotes robust biofilm formation (data not shown).
2.10. In vitro biofilm formation
Press-to-seal adhesive silicone isolators (2 × 20 mm, Sigma–Aldrich) were used to create wells on Ti slides. 500 μL aliquots of TBP1–PEG, TBP2–PEG and TBP4–PEG coatings were applied to triplicate wells and incubated for 2 h at room temperature. The wells were washed twice with PBS before exposing to bacteria. Biofilm assays were performed largely as previously described [30,31]. Briefly, an overnight culture of S. aureus diluted to ~5 × 107 CFU/mL in TSB plus 0.2% glucose was applied to the Ti surface. Slides were incubated in a closed, humidified chamber at 37 °C for 5 h then stained with 0.1% crystal violet to visualize the biofilm. Phase-contrast micrographs were obtained with a Leica DM IRBE microscope (Leica Microsystems, Wetzlar, Germany) at 630× magnification. To quantify the biofilm, crystal violet was solubilized using 30% glacial acetic acid for 5 min, and then 100 μL aliquots of solubilized crystal violet was transferred to 96-well microtiter plates. Relative biofilm formation was assayed by reading the optical density at 550 nm using a Vmax kinetic microplate reader (Molecular Devices, Sunnyvale, CA).
3. Results and discussion
3.1. Selection and preparation of Ti-binding peptide coatings
Titanium-binding peptides (TBPs) were identified using a combinatorial phage display screening process described previously [27,29]. This iterative affinity selection process has been used to isolate peptides that recognize a variety of diverse targets such as GaAs nanocrystals, iron oxide, gold, polystyrene, and a variety of other materials [26,32-44]. Briefly, M13 phage libraries were screened for binding to 6Al-4V Ti, a lightweight and tough metal routinely used in orthopedic devices. Three successive screens yielded six unique peptide sequences 10 - 20 amino acids in length, which were then subjected to a series of additional focused library screens and amino acid substitution studies to assess the role of specific residues in binding (Data not shown). Based on these studies, we identified a second-generation peptide sequence containing three repeats of a positively charged KHK ‘triad’, SKHKGGKHKGGKHKG, with very high Ti affinity. This peptide, TBP1, was prepared using automated solid-phase peptide synthesis following standard protocols containing a spacer of SSG and terminal K(biotin) group for future assays. The corresponding PEGylated analog, TBP1–PEG, was prepared by conjugating amaleimido-PEG(Mw3400; Laysan Bio Inc., Alabama) to the C-terminus of the peptides via introduction of a terminal cysteine amino acid (Scheme 1). Other groups have reported the use of multiple binding domain repeats to increase the avidity of inorganic-binding peptides for their target substrates [45,46]. Likewise, we hypothesize that multiple TBP domains will result in enhanced coating affinity and stability, which will translate into improved performance in functional bacteria assays.
Scheme 1.
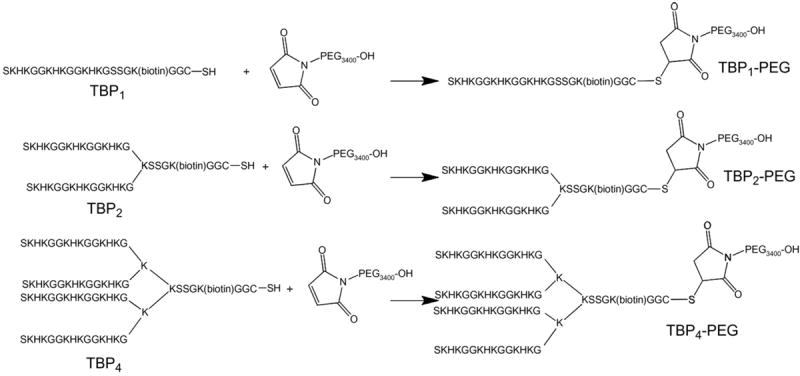
Synthetic route to the mono and multivalent titanium-binding peptides conjugated to a polyethylene glycol (Mw 3400 g/mol). Not to scale.
Multiple TBP1 domains were covalently linked through a branched lysine peptide core to afford the divalent and tetravalent peptides TBP2 and TBP4, respectively. As before, a terminal K (biotin) group was introduced into the sequence. Multivalent peptide–PEG conjugates containing either two or four repeats of the titanium-binding peptide (TBP2–PEG and TBP4–PEG) were also synthesized as shown in Scheme 1. Tables 1 and 2 list various peptide and peptide–PEG conjugates that were prepared. All peptides were purified by HPLC and their identities confirmed with MS. Peptide multimers and PEG conjugates were also characterized using gel electrophoresis to verify their molecular weights (Supplementary information).
Table 1.
Peptide multimers and their dissociation constants on Ti as measured by ELISA.
| Peptide | Sequence | Relative Kd (nM) |
|---|---|---|
| TBP1 | SKHKGGKHKGGKHKGSSGK(Biotin) | 163 |
| TBP2 | (SKHKGGKHKGGKHKG)2KSSGK(Biotin) | 118 |
| TBP4 | (SKHKGGKHKGGKHKG)2K)2KSSGK(Biotin) | 16.5 |
Table 2.
Peptide–PEG conjugates.
| Conjugate | Sequence |
|---|---|
| TBP1–PEG | SKHKGGKHKGGKHKGSSGK(Biotin) –GGC–PEG |
| TBP2–PEG | (SKHKGGKHKGGKHKG)2KSSGK(Biotin) –GGC–PEG |
| TBP4–PEG | (SKHKGGKHKGGKHKG)2K)2KSSGK(Biotin) –GGC–PEG |
3.2. Characterization of peptide coatings
Our previous work with a similar Lys and His rich peptide suggests that the mechanism for assembly of TBPs onto Ti is predominantly electrostatic adsorption, with hydrogen bonding and surface topology likely playing a minor effect [27]. We have previously confirmed, using a variety of surface techniques (e.g., XPS, QCM-D etc.), that a 2 h immersion of Ti substrates in a dilute TBP solution is sufficient for the formation of a saturated adherent peptide adlayer. AFM was used to elucidate the physical characteristics of the multivalent peptides on treated surfaces. Freshly cleaved mica was used in place of Ti substrates due to its uniformity and atomic level flatness. Surfaces exposed to the peptides showed distinct changes in surface profile with the presence of globular structures. As expected, macromolecule size increased with multivalency, with the largest structures observed on TBP4 coated surfaces (Supplementary information).
Next, in vitro titration experiments were carried out to determine the binding affinities of the multivalent peptide coatings for Ti. The mono, di and tetravalent forms of the biotin-terminated peptides were serially diluted in TBS to obtain a range of concentrations from 0.07 nm to 5 mm and then added over clean implant grade Ti beads. Using a modified ELISA, we obtained a representative binding curve for each peptide (Fig. 2). The data were fit to a four-parameter model using a data-fitting program (BioDataFit 1.02; Chang Bioscience, Inc.) and the concentration corresponding to the half-maximal signal of each model curve was extracted as the apparent dissociation constant (or “relative Kd”) for each peptide. (Table 1) As expected, the binding affinity for Ti increases significantly with the number of TBP repeat units. The monovalent peptide, TBP4, showed a reasonably high affinity for Ti with a Kd value of 163 nm. The tetravalent peptide TBP4, with a Kd value of 16.5 nm, binds with a 10-fold greater affinity. Kd values in the low nanomolar range correspond to very strong interactions comparable to those in monoclonal antibody–peptide epitope binding. Additionally, peptide binding was observed to saturate at a concentration around 10 μm. In this work, we typically coat surfaces using a 26 μm peptide solution to ensure the formation of a saturated peptide adlayer.
Fig. 2.
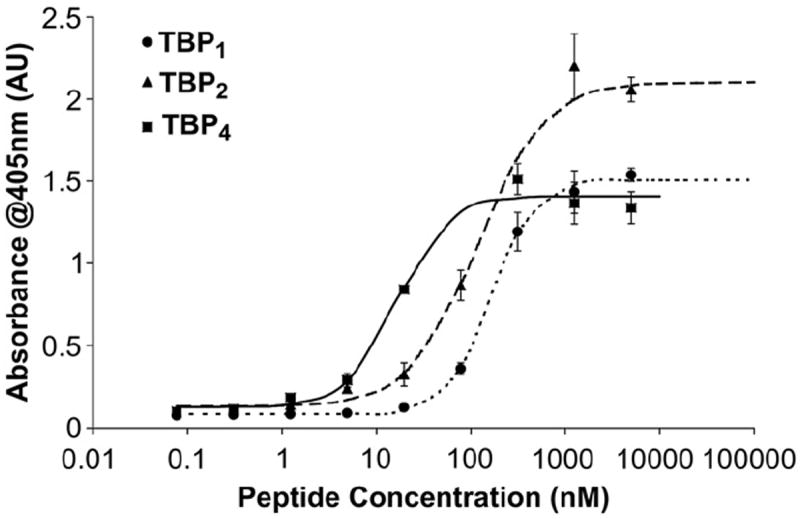
Binding affinity curves for the various multivalent peptides on Ti substrates. Peptides show increasing affinity with the number of TBP domains, highlighting its importance for Ti adsorption (N = 3).
To assess the effect of the improved binding affinity on coating stability, peptide-coated Ti beads were exposed to serum and the amount of peptide remaining on the Ti surface was monitored over 2 weeks. Antibiotics are typically administered to patients for the first 24 h after surgical implantation of a device. As a result, established infections often occur during the next couple of weeks. It is therefore essential that peptide coatings are stable even when exposed to physiological fluids containing a large excess of biomolecules capable of interacting with the surface. Beads were treated with biotinylated peptides TBP1, TBP2, and TBP4 for 2 h to obtain a saturated peptide coating. Coated beads were then transferred into 100% human serum (Innovative Research; Novi, MI) and kept at room temperature (RT) on an orbital shaker. Beads were removed at various time points ranging from 2 h to 2 weeks, washed, and assayed for peptide coating remaining on the surface using the SAAP/pNPP system. Freshly coated beads that were not exposed to serum were used as a positive control in all cases. The percentage of peptide remaining on the beads with serum exposure time is illustrated in Fig. 3. The monovalent TBP1 peptide showed extremely poor stability in serum. After 2 h of exposure to serum, only ~50% remained on the surface, and by the end of 2 weeks, more than 95% had been displaced. Dimeric TBP2 showed improved stability, with approximately 50% remaining at 2 weeks. The tetrameric TBP4 coating clearly exhibited the best serum stability, with more than 90% remaining on beads by the end of the experiment. Importantly, these studies highlight the benefits of using multiple TBP repeat units to significantly improve long-term coating performance under more physiologically relevant conditions.
Fig. 3.
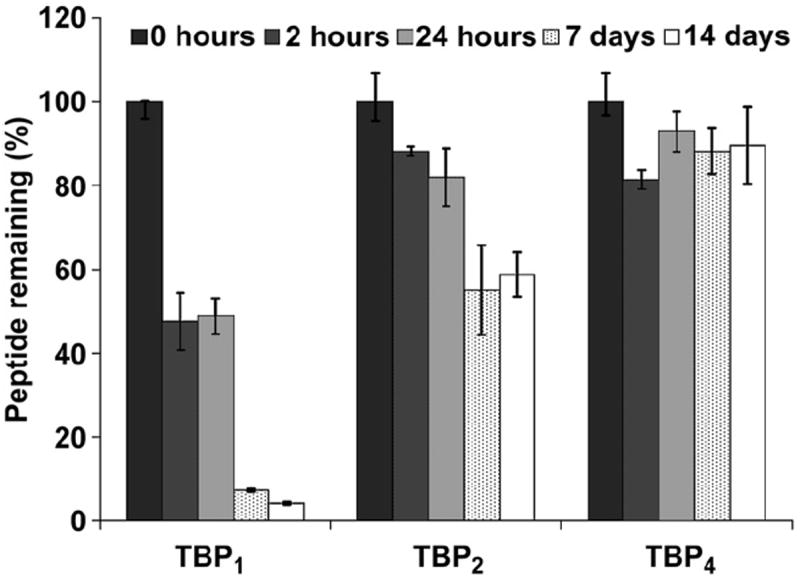
Serum stability of multivalent coatings on Ti beads. Peptide-treated beads were exposed to 100% human serum over two weeks and assayed for the amount of coating remaining at different serum exposure times (N = 3).
3.3. In vitro bacteria attachment and biofilm formation
Finally, the PEGylated-peptide analogs were subject to a series of in vitro S. aureus biofilm assays to evaluate the effect of multivalency on functional coating performance. S. aureus is one of the most common causes of hospital-acquired infections and postsurgical wound infections. It is often transferred to the implanted devices during handling [47-49]. Ti-coated slides were used as model substratum in these assays. The thin 20 nm Ti coating allowed for substantial light transmission and enabled the tracking of bacteria adhesion via phase-contrast microscopy. Aliquots of TBP1–PEG, TBP2–PEG, and TBP4–PEG were applied to wells on Ti slides in triplicate and incubated for 2 h at room temperature. A pathogenic strain of S. aureus (MZ100) was then added to wells at a starting concentration of ~5 × 107 CFU/well, about 10,000 times typical counts on human skin. It is important to note that for these assays we have optimized the growth conditions of S. aureus to obtain extremely robust cell proliferation in order to present a stringent bacterial challenge.
After 5 h of incubation, surfaces were assayed for biofilm formation using a modified version of the standard microtiter plate assay as previously described [30,31]. All surfaces treated with the PEGylated-peptides showed a significantly lower biofilm density compared to uncoated Ti surfaces, as determined by spectrophotometric absorbance of crystal violet (CV) staining measured at 550 nm (Fig. 4) The tetravalent TBP4–PEG coating demonstrated a 90% reduction in staphylococcal biofilm formation as compared to 32% and 47% with the monomer and dimer, respectively.
Fig. 4.
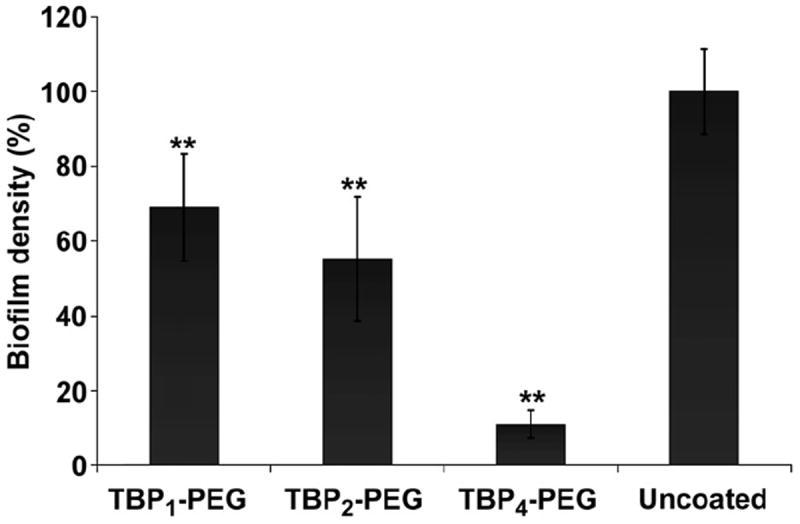
Biofilm formation on coated and uncoated Ti surfaces following a 5 h exposure to S. aureus (starting inoculum of ~5 × 107 CFU/mL) cultures (N = 3, **P < 0.01).
Additionally, biofilms were stained with 0.1% CV and observed directly using phase-contrast microscopy. At 5 h, a thick, dense biofilm could be macroscopically observed in uncoated Ti, TBP1–PEG, and TBP2–PEG coated wells (Fig. 5, top). In contrast, the TBP4–PEG coated well remained largely clear with small, disperse patches of staining. Phase-contrast micrographs revealed substantial cell attachment on uncoated Ti and TBP1–PEG surfaces, and the presence of multilayered clumps of cells. A slight decrease in cell density was seen with the TBP2–PEG coating, although large bacteria clusters were still observed. TBP4–PEG surfaces, on the other hand, showed significantly fewer adhered bacteria, with most present as individual cells. It has been reported that the host immune defense is threatened with a high risk of infection only beyond a critical bacteria inoculum of 105 organisms per gram of tissue [50]. We expect that the few bacterial cells that remain adhered on the TBP4–PEG coated surface to be easily eradicated by the host immune system and not develop into a serious infection. We plan to test this hypothesis in upcoming in vivo studies using an animal infection model. These results demonstrate the utility of using a multivalent peptide coating to enhance the resistance of Ti to S. aureus colonization and subsequent biofilm formation, even when exposed to a robust bacterial challenge.
Fig. 5.
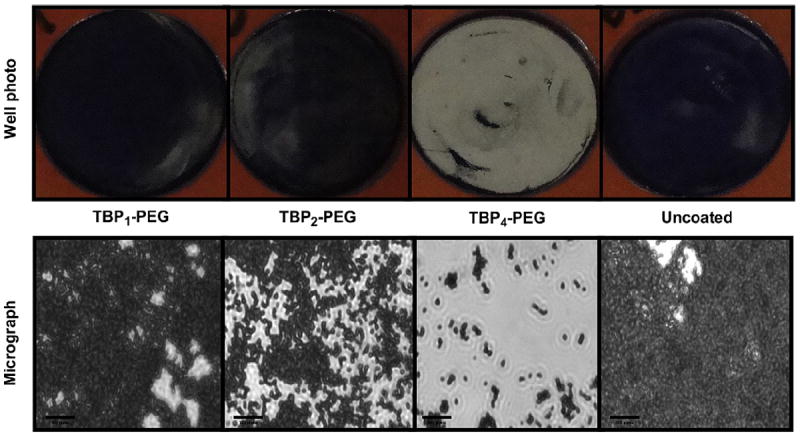
(top) Digital photographs and (bottom) phase-contrast micrographs (Magnification = 630×) of coated and uncoated Ti wells following a 5 h exposure to S. aureus cultures (starting inoculum of ~5 × 107 CFU/mL). Bacteria were stained with 0.1% crystal violet to aid visualization. Scale bars = 20 μm.
4. Conclusions
In summary, we have designed, synthesized and characterized mono, di and tetravalent peptides and PEGylated-peptide conjugates that rapidly and spontaneously adsorb onto Ti to form a thin adherent surface coating. We further examined the effect of multivalency on coating affinity, stability and bacteria resistance. Our results support the use of TBP4–PEG as a candidate bacteriophobic coating to prevent the attachment, proliferation and establishment of biofilm-based infections on Ti devices, thus improving their long-term efficacy and performance. The tetravalent peptide, with its increased binding affinity, forms a robust non-fouling surface coating that is highly resistant to displacement by serum proteins and resilient against S. aureus colonization. These results highlight how strong the cooperative multivalent attachment strategy can be in the establishment of robust coatings with affinities that lie somewhere between those of ideal covalent and non-covalent bonds and demonstrate the potential of these multivalent coatings for in vivo inhibition of orthopedic implant infections. We will subsequently assess the effectiveness of these coatings on biofilm formation in vivo.
This adsorptive approach to modification also offers several other advantages for use in the clinic, including the capacity to apply coatings to materials of complex shapes and sizes by a facile one-step immersion process. Furthermore, the modular nature of this platform presents a general approach for the directed assembly and organization of various other biological and chemical mediators on any target material, from metal to plastic to ceramic [29,51-53].
Supplementary Material
Acknowledgments
The authors thank the team at Affinergy Inc including Drs. Wayne Beyer, Sathya Ganesan Paul Hamilton, Bruce Lamb, and Guy Orgambide as well as Mr. Michael Doligalski for their contributions to the isolation, design of the Ti-binding peptides, peptide synthesis, purification, and discussion of interfacial biomaterials. This work was financially supported by the National Institutes of Health (Grant No. AR054872-01).
Footnotes
Conflict of interest
MWG and DJK are co-founders of Affinergy Inc. GAO was a consultant for Affinergy Inc.
Appendix. Supplementary data
Supplementary data related to this article can be found online at doi:10.1016/j.biomaterials.2010.08.031.
References
- 1.Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med. 2004;350(14):1422–9. doi: 10.1056/NEJMra035415. [DOI] [PubMed] [Google Scholar]
- 2.Whitehouse JD, Friedman ND, Kirkland KB, Richardson WJ, Sexton DJ. The impact of surgical site infections following orthopedic surgery at a community hospital and a university hospital: adverse quality of life, excess length of stay, and extra cost. Infect Control Hosp Epidemiol. 2002;23(4):183–9. doi: 10.1086/502033. [DOI] [PubMed] [Google Scholar]
- 3.Williams DF. Introduction: implantable materials and infection. Injury. 1996;27(Suppl. 3):SC1–4. doi: 10.1016/0020-1383(96)89024-3. [DOI] [PubMed] [Google Scholar]
- 4.Ratner BD, Hoffman AS, Schoen FJ, Lemons JE. Biomaterials science: an introduction to materials in medicine. San Diego: Academic Press; 2000. [Google Scholar]
- 5.Williams DF. On the mechanisms of biocompatibility. Biomaterials. 2008;29(20):2941–53. doi: 10.1016/j.biomaterials.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 6.Vinh DC, Embil JM. Device-related infections: a review. J Long Term Eff Med Implants. 2005;15(5):467–88. doi: 10.1615/jlongtermeffmedimplants.v15.i5.20. [DOI] [PubMed] [Google Scholar]
- 7.Anderson GG, O’Toole GA. Innate and induced resistance mechanisms of bacterial biofilms. Curr Top Microbiol Immunol. 2008;322:85–105. doi: 10.1007/978-3-540-75418-3_5. [DOI] [PubMed] [Google Scholar]
- 8.Hart E, Azzopardi K, Taing H, Graichen F, Jeffery J, Mayadunne R, et al. Efficacy of antimicrobial polymer coatings in an animal model of bacterial infection associated with foreign body implants. J Antimicrob Chemother. 2010;65(5):974–80. doi: 10.1093/jac/dkq057. [DOI] [PubMed] [Google Scholar]
- 9.Stamm WE. Catheter-associated urinary tract infections: epidemiology, pathogenesis, and prevention. Am J Med. 1991;91(3, Suppl. 2):S65–71. doi: 10.1016/0002-9343(91)90345-x. [DOI] [PubMed] [Google Scholar]
- 10.Darouiche RO. Antimicrobial coating of devices for prevention of infection: principles and protection. Int J Artif Organs. 2007;30(9):820–7. doi: 10.1177/039139880703000912. [DOI] [PubMed] [Google Scholar]
- 11.Currie EP, Norde W, Cohen Stuart MA. Tethered polymer chains: surface chemistry and their impact on colloidal and surface properties. Adv Colloid Interface Sci. 2003;100–102:205–65. doi: 10.1016/s0001-8686(02)00061-1. [DOI] [PubMed] [Google Scholar]
- 12.Saldarriaga Fernandez IC, van der Mei HC, Lochhead MJ, Grainger DW, Busscher HJ. The inhibition of the adhesion of clinically isolated bacterial strains on multi-component cross-linked poly(ethylene glycol)-based polymer coatings. Biomaterials. 2007;28(28):4105–12. doi: 10.1016/j.biomaterials.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 13.Ostuni EO, Chapman RG, Liang MN, Meluleni G, Pier G, Ingber DE, et al. Selfassembled monolayers that resist the adsorption of proteins and the adhesion of bacterial and mammalian cells. Langmuir. 2001;17:6336–43. [Google Scholar]
- 14.Hou S, Burton EA, Simon KA, Blodgett D, Luk YY, Ren D. Inhibition of Escherichia coli biofilm formation by self-assembled monolayers of functional alkanethiols on gold. Appl Environ Microbiol. 2007;73(13):4300–7. doi: 10.1128/AEM.02633-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xia N, Hu Y-H, Grainger DW, Castner DG. Functionalized poly(ethylene glycol)-grafted polysiloxane monolayers for control of protein binding. Langmuir. 2002;18:3225–62. [Google Scholar]
- 16.Kingshott P, Wei J, Bagge-Ravn D, Gadegaard N, Gram L. Covalent attachment of poly(ethylene glycol) to surfaces, critical for reducing bacterial adhesion. Langmuir. 2003;19:6912–21. [Google Scholar]
- 17.Scott EA, Nichols MD, Cordova LH, George BJ, Jun YS, Elbert DL. Protein adsorption and cell adhesion on nanoscale bioactive coatings formed from poly (ethylene glycol) and albumin microgels. Biomaterials. 2008;29(34):4481–93. doi: 10.1016/j.biomaterials.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barber TA, Gamble LJ, Castner DG, Healy KE. In vitro characterization of peptide-modified p(AAm-co-EG/AAc) IPN-coated titanium implants. J Orthop Res. 2006;24(7):1366–76. doi: 10.1002/jor.20165. [DOI] [PubMed] [Google Scholar]
- 19.Lopez GP, Ratner BD, Tidwell CD, Haycox CL, Rapoza RJ, Horbett TA. Glow discharge plasma deposition of tetraethylene glycol dimethyl ether for fouling-resistant biomaterial surfaces. J Biomed Mater Res. 1992;26(4):415–39. doi: 10.1002/jbm.820260402. [DOI] [PubMed] [Google Scholar]
- 20.Tosatti S, De Paul SM, Askenda LA, Vande-Vondele S, Hubbell JA, Tengvall P, et al. Peptide functionalized poly(l-lysine)-g-poly(ethylene glycol) on titanium: resistance to protein adsorption in full heparinized human blood plasma. Biomaterials. 2003;27:4949–58. doi: 10.1016/s0142-9612(03)00420-4. [DOI] [PubMed] [Google Scholar]
- 21.VandeVondele S, Voros J, Hubbell JA. RGD-grafted poly-l-lysine-graft-(polyethylene glycol) copolymers block non-specific protein adsorption while promoting cell adhesion. Biotechnol Bioeng. 2003;82(7):784–90. doi: 10.1002/bit.10625. [DOI] [PubMed] [Google Scholar]
- 22.Subbiahdoss G, Pidhatika B, Coullerez G, Charnley M, Kuijer R, van der Mei HC, et al. Bacterial biofilm formation versus mammalian cell growth on titaniumbased mono- and bi-functional coating. Eur Cells Mater. 2010;19:205–13. doi: 10.22203/ecm.v019a20. [DOI] [PubMed] [Google Scholar]
- 23.Lee H, Dellatore SM, Miller WM, Messersmith PB. Mussel-inspired surface chemistry for multifunctional coatings. Science. 2007;318(5849):426–30. doi: 10.1126/science.1147241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wach JY, Malisova B, Bonazzi S, Tosatti S, Textor M, Zurcher S, et al. Proteinresistant surfaces through mild dopamine surface functionalization. Chemistry. 2008;14(34):10579–84. doi: 10.1002/chem.200801134. [DOI] [PubMed] [Google Scholar]
- 25.Dalsin JL, Hu BH, Lee BP, Messersmith PB. Mussel adhesive protein mimetic polymers for the preparation of nonfouling surfaces. J Am Chem Soc. 2003;125:4253–8. doi: 10.1021/ja0284963. [DOI] [PubMed] [Google Scholar]
- 26.Kenan DJ, Walsh EB, Meyers SR, O’Toole GA, Carruthers EG, Lee WK, et al. Peptide-PEG amphiphiles as cytophobic coatings for mammalian and bacterial cells. Chem Biol. 2006;13:695–700. doi: 10.1016/j.chembiol.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 27.Khoo XJ, Hamilton P, O’Toole GA, Snyder BD, Kenan DJ, Grinstaff MW. Directed assembly of PEGylatedepeptide coatings for infection-resistant titanium metal. J Am Chem Soc. 2009;131(31):10992–7. doi: 10.1021/ja9020827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee H, Scherer NF, Messersmith PB. Single-molecule mechanics of mussel adhesion. Proc Natl Acad Sci U S A. 2006;103(35):12999–3003. doi: 10.1073/pnas.0605552103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meyers SR, Hamilton PT, Walsh EB, Kenan DJ, Grinstaff MW. Endothelialization of titanium surfaces. Adv Mater (Weinheim, Ger) 2007;19(18):2492–8. [Google Scholar]
- 30.Caiazza NC, O’Toole GA. Alpha-toxin is required for biofilm formation by Staphylococcus aureus. J Bacteriol. 2003;185:3214–7. doi: 10.1128/JB.185.10.3214-3217.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shanks RM, Sargent JL, Martinez RM, Graber ML, O’Toole GA. Catheter lock solutions influence staphylococcal biofilm formation on abiotic surfaces. Nephrol Dial Transplant. 2006;21(8):2247–55. doi: 10.1093/ndt/gfl170. [DOI] [PubMed] [Google Scholar]
- 32.Brown S. Metal-recognition by repeating polypeptides. Nat Biotechnol. 1997;15:269–72. doi: 10.1038/nbt0397-269. [DOI] [PubMed] [Google Scholar]
- 33.Pasqualini R, Ruoslahti E. Organ targeting in vivo using phage display peptide libraries. Nature. 1996;380(6572):364–6. doi: 10.1038/380364a0. [DOI] [PubMed] [Google Scholar]
- 34.Kay BK, Winter J, McCafferty J. Phage display of peptides and proteins. San Diego: Academic Press; 1996. [Google Scholar]
- 35.Burritt JB, Bond CW, Doss KW, Jesaitis AJ. Filamentous phage display of oligopeptide libraries. Anal Biochem. 1996;238(1):1–13. doi: 10.1006/abio.1996.0241. [DOI] [PubMed] [Google Scholar]
- 36.Gebhardt K, Lauvrak V, Babaie E, Eijsink V, Lindquist BH. Adhesive peptides selected by phage display: characterization, applications, and similarities with fibrinogen. Pept Res. 1996;9(6):269–78. [PubMed] [Google Scholar]
- 37.Sparks AB, Adey NB, Cwirla S, Kay BK. Screening phage-displayed random peptide libraries. In: Kay BK, Winter J, McCafferty J, editors. In phage display of peptides and proteins a laboratory manual. San Diego: Academic Press; 1996. pp. 227–53. [Google Scholar]
- 38.Healy JM, Murayama O, Maeda T, Yoshino K, Sekiguchi K, Kikuchi M. Peptide ligands for integrin alpha v beta 3 selected from random phage display libraries. Biochemistry (Mosc) 1995;34:3948–55. doi: 10.1021/bi00012a012. [DOI] [PubMed] [Google Scholar]
- 39.Murase K, Morrison KL, Tam PY, Stafford RL, Jurnak F, Weiss GA. EF-Tu binding peptides identified, dissected, and affinity optimized by phage display. Chem Biol. 2003;10:161–8. doi: 10.1016/s1074-5521(03)00025-5. [DOI] [PubMed] [Google Scholar]
- 40.Dwyer MA, Lu W, Dwyer JL, Kossiakoff AA. Biosynthetic phage display: a novel protein engineering tool combining chemical and genetic diversity. Chem Biol. 2000;7:263–74. doi: 10.1016/s1074-5521(00)00102-2. [DOI] [PubMed] [Google Scholar]
- 41.Adey NB, Mataragnon AH, Rider JE, Carter JM, Kay BK. Characterization of phage that bind plastic from phage-displayed random peptide libraries. Gene. 1995;156:27–31. doi: 10.1016/0378-1119(95)00058-e. [DOI] [PubMed] [Google Scholar]
- 42.Nowakowski GS, Dooner MS, Valinski HM, Mihaliak AM, Quesenberry PJ, Becker PS. A apecific heptapeptide from a phage display peptide library homes to bone marrow and binds to primitive hematopoietic stem cells. Stem Cells. 2004;22:1030–8. doi: 10.1634/stemcells.22-6-1030. [DOI] [PubMed] [Google Scholar]
- 43.Sano KI, Shiba K. A hexapeptide motif that electrostatically binds to the surface of titanium. J Am Chem Soc. 2003;125:14234–5. doi: 10.1021/ja038414q. [DOI] [PubMed] [Google Scholar]
- 44.Tamerler C, Sarikaya M. Molecular biomimetics: nanotechnology and bionanotechnology using genetically engineered peptides. Philos Transact A Math Phys Eng Sci. 2009;367(1894):1705–26. doi: 10.1098/rsta.2009.0018. [DOI] [PubMed] [Google Scholar]
- 45.Brown S, Sarikaya S, Johnson E. A genetic analysis of crystal growth. J Mol Biol. 2000;299:725–35. doi: 10.1006/jmbi.2000.3682. [DOI] [PubMed] [Google Scholar]
- 46.Seker UO, Wilson B, Sahin D, Tamerler C, Sarikaya M. Quantitative affinity of genetically engineered repeating polypeptides to inorganic surfaces. Biomacromolecules. 2009;10(2):250–7. doi: 10.1021/bm8009895. [DOI] [PubMed] [Google Scholar]
- 47.Gristina AG. Biomaterial-centered infection: microbial adhesion versus tissue integration. Science. 1987;237(4822):1588–95. doi: 10.1126/science.3629258. [DOI] [PubMed] [Google Scholar]
- 48.Arciola CR, Campoccia D, Montanaro L. Detection of biofilm-forming strains of Staphylococcus epidermidis and S. aureus. Expert RevMol Diagn. 2002;2:478–84. doi: 10.1586/14737159.2.5.478. [DOI] [PubMed] [Google Scholar]
- 49.Harris LG, Richards RG. Staphylococci and implant surfaces: a review. Injury. 2006;37(Suppl. 2):S3–14. doi: 10.1016/j.injury.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 50.Clifford RP. Open fractures. In: Rüedi TP, Murphy WM, editors. AO principles of fracture management. Stuttgart: AO Publishing; 2000. pp. 617–38. [Google Scholar]
- 51.Meyers SR, Kenan DJ, Grinstaff MW. Enzymatic release of a surface-adsorbed RGD therapeutic from a cleavable peptide anchor. Chemmedchem. 2008;3:1645–8. doi: 10.1002/cmdc.200800205. [DOI] [PubMed] [Google Scholar]
- 52.Meyers SR, Khoo X, Huang X, Walsh EB, Grinstaff MW, Kenan DJ. The development of peptide-based interfacial biomaterials for generating biological functionality on the surface of bioinert materials. Biomaterials. 2009;30(3):277–86. doi: 10.1016/j.biomaterials.2008.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang X, Zauscher S, Klitzman B, Truskey GA, Reichert WM, Kenan DJ, et al. Peptide interfacial biomaterials improve endothelial cell adhesion and spreading on synthetic polyglycolic acid materials. Ann Biomed Eng. 2010;38(6):1965–76. doi: 10.1007/s10439-010-9986-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


