Abstract
PI3K plays key roles in cell growth, differentiation, and survival by generating the second messenger phosphatidylinositol-(3,4,5)-trisphosphate (PIP3). PIP3 activates numerous enzymes, in part by recruiting them from the cytosol to the plasma membrane. We find that in immature B lymphocytes carrying a nonautoreactive Ag receptor, PI3K signaling suppresses RAG expression and promotes developmental progression. Inhibitors of PI3K signaling abrogate this positive selection. Furthermore, immature primary B cells from mice lacking the p85α regulatory subunit of PI3K suppress poorly RAG expression, undergo an exaggerated receptor editing response, and, as in BCR-ligated cells, fail to progress into the G1 phase of cell cycle. Moreover, immature B cells carrying an innocuous receptor have sustained elevation of PIP3 levels and activation of the downstream effectors phospholipase C (PLC)γ2, Akt, and Bruton's tyrosine kinase. Of these, PLCγ2 appears to play the most significant role in down-regulating RAG expression. It therefore appears that when the BCR of an immature B cell is ligated, PIP3 levels are reduced, PLCγ2 activation is diminished, and receptor editing is promoted by sustained RAG expression. Taken together, our results provide evidence that PI3K signaling is an important cue required for fostering development of B cells carrying a useful BCR.
Antigen recognition by the BCR may lead to distinct, and often diametrically opposed, functional consequences compared with the basal BCR signal. For example, basal BCR signals are essential for continued survival of naive mature B cells, which are uniformly nondividing (1, 2). In contrast, when the BCRs of these same cells are ligated either by self or foreign Ags, they undergo apoptosis or activation (3–6). Similarly, in immature B cells, BCR ligation promotes receptor editing and developmental arrest, whereas basal surface Ig (sIg)4 signals promote recombinase down-regulation and developmental progression (7, 8).
Ligated and unligated sIg are sensed through the signaling capacity of the BCR complex, which consists of sIg and the associated signal transducers Ig-αβ, which are required for plasma membrane expression of sIg and for signaling through the pre-BCR and BCR (9, 10). BCR ligation leads to phosphorylation of tyrosines within conserved ITAMs carried on the cytoplasmic portions of Ig-α and β (11), which in turn recruit tyrosine kinases to their cytoplasmic tails through other motifs (12). BCR signaling is initiated by tyrosine kinases of the Src family and by Syk, which mediate ITAM phosphorylation and transphosphorylation upon BCR aggregation (13). It is unclear whether the signals transmitted by unligated receptors are qualitatively distinct from those of ligated receptors or merely represent a quantitative difference.
An important step in BCR signaling is the phosphorylation of the coreceptor CD19 (14). CD19 physically associates with the BCR through intracellular and extracellular motifs (15–17). (CD19 has also been shown to facilitate pre-BCR signaling (18, 19)). BCR ligation leads to the recruitment by CD19 of PI3K via its p85α regulatory subunit, the generation of lipid products such as phosphatidylinositol-(3,4,5)-trisphosphate (PIP3), and the attendant recruitment to the plasma membrane of pleckstrin homology (PH) domain-containing proteins, such as phospholipase C (PLC)γ2 and cytoplasmic kinases, such as Bruton's tyrosine kinase (Btk) and Akt (20). PLCγ2 activation in turn promotes phosphatidylinositol-(4,5)-bisphosphate hydrolysis to inositol trisphosphate and diacylglycerol, mobilizing Ca2+ stores and activating protein kinase Cs. In mature B cells, the BCR signaling complex is rapidly recruited to cholesterol-rich lipid rafts on the plasma membrane, where several tyrosine kinases, including Lyn, are abundant (21).
Genetic experiments have shown that basal signaling through an unligated BCR is critical for peripheral B cell survival and/or immature B cell development (1, 22–24). This physiological signal can be mimicked by a membrane-localized ITAM derived from an artificial chimeric Igα protein (25), the EBV LMP2A protein (26, 27), or an engineered sIg molecule devoid of Ag specificity (22). During bone marrow development, transgene-enforced expression of prerearranged IgH or IgH/L combinations can suppress endogenous rearrangements, demonstrating a feedback regulation process; however, autoreactive receptors that presumably generate a distinct BCR-mediated signal fail to suppress recombination and promote instead ongoing rearrangement that often leads to receptor editing (reviewed in Ref. 28).
These results suggest that in immature B cells an unligated BCR promotes a signal that regulates V(D)J recombination, whereas a cross-linked receptor promotes a distinct signal. Furthermore, studies in which the sIg is inducibly lost from immature B cells suggest that this suppression of recombination, along with the loss of expression of maturation markers, is reversible for some time (24).
An important aspect of the regulation of L chain recombination involves the transcription rate of RAG1 and RAG2. But it is not clear how signals from an unligated BCR are distinguished from those of autoreactive BCRs. We found previously that one aspect of BCR-regulated RAG expression control involved the activation of NF-κB (29). Other studies have implicated effects on basal sIg signaling through the CD19 coreceptor as important in the signal to turn off V(D)J recombination (30, 31).
In this study, we have sought to identify signaling pathways that may regulate B cell-positive selection, using as a readout the down-regulation of RAG expression by an innocuous, i.e., nonli-gated, BCR, and to determine how ligated and unligated BCRs signal differentially at the immature B cell stage.
Materials and Methods
Mice
The 3-83 mice (4) on the B10.D2 genetic background were bred to HYG RAG2-GFP (32) and/or p85α-deficient (33) backgrounds. The HYG RAG2-GFP reporter transgene is derived from a large bac clone that appears to carry transcriptional control regions for both RAG1 and RAG2 (32). Mice were maintained in The Scripps Research Institute Animal Resources facility; all of these studies have been reviewed and approved by the relevant The Scripps Research Institute institutional animal care and use review committee.
Cell culture and stimulation
Immature B cells were either isolated directly from the bone marrow of 3-83 mice, or expanded in IL-7 cultures, and stimulated with BCR and control mAbs at 10 μg/ml, as described (34). S23 is a mouse IgG2b anti-3-83 Id raised in a JCκ-deficient mouse (D. Nemazee, unpublished observation); Y3 (American Type Culture Collection designation HB-176) is an IgG2b anti-H-2Kb Ab (35). Wortmannin, LY294002, U73122, m-3M3-FBS, Akt inhibitor (1L-6-hydroxymethyl-chiro-inositol 2-(R)-2-O-methyl-3-O-octadecylcarbonate), rapamycin, SB203580, PD98059, cyclosporin A, and FK506 were obtained from Calbiochem.
Protein extracts, immunoblotting, fusion proteins with transduction domains of HIV TAT, and EMSA
Nuclear and cytoplasmic extract fractions were prepared, as described (36). Whole cell extracts were prepared by resuspending 107 cells in 100 μl of lysis buffer (0.1% Triton X-100 in 1× PBS) supplemented with 20 mM NaF, 1 mM sodium orthovanadate, and protease inhibitors (Roche). After incubating for 15 min on ice, samples were cleared by 10,000 × g centrifugation for 10 min at 4°C, and supernatants were stored at –70°C. After reducing PAGE, transfer to nylon membranes was conducted using the X Cell II Blot Module (Invitrogen Life Technologies). Primary Abs used were: p50/p105 (sc-114), p65 (sc-372), c-rel (sc-71), IκBα (sc-371), p-IκBα (sc-8404), Akt1 (sc-1618), Btk (sc-1696), and cyclin D2 (sc-593) from Santa Cruz Biotechnology; phospho-Btk (Tyr223), phospho-Akt (Thr308), phospho-PLCγ2 (Tyr1217), and PLCγ2 from Cell Signaling Technology; GAPDH (mAb374) from Chemicon International; and fibrillarin, provided by Pollard (The Scripps Research Institute, La Jolla, CA). Secondary Abs used were goat anti-rabbit IgG HRP and goat anti-mouse IgG HRP (Jackson ImmunoResearch Laboratories). Expression and purification of TAT-superrepressor IκBα (37) and TAT-β-gal (38) were as described (29). EMSA was performed essentially as described (29).
Northern blotting and L chain rearrangement assays
Northern blots and L chain gene rearrangement assays were essentially as described (29). Probes and PCR conditions/primers are described for the following rearrangements: Vκ-Jκ1 (39), recombining sequence/κ-deleting element-intronic recombination sequence (40), and Vλ1-Jλ1 (7).
PIP3 assay
Assay was based on the work of Anzelon et al. (41); however, similar assays have been described by other investigators (e.g., 42). Cells were fixed and permeabilized using a kit (BD Biosciences), incubated in FACS buffer with biotinylated anti-PI-(3,4,5)-P3 (Echelon, Z-B345), followed by streptavidin-PerCP Cy5.5. Samples were analyzed on a FACSCalibur cytometer (BD Biosciences) using the FlowJo software package.
Results
CD19 phosphorylation
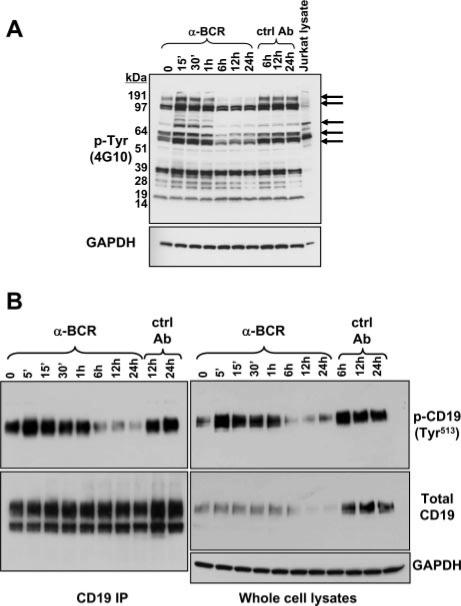
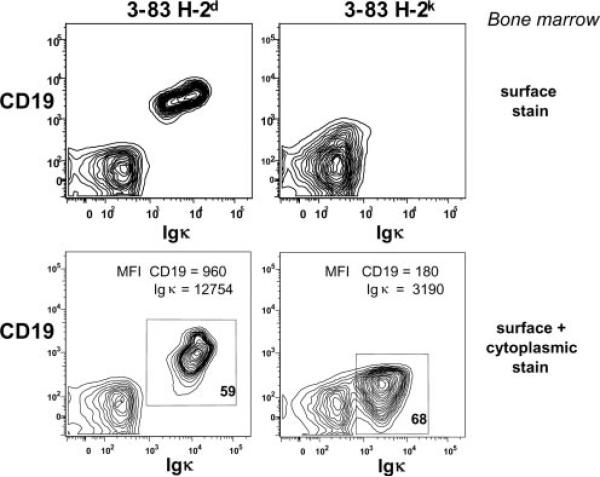
Evidence for active signaling through an innocuous BCR in immature B cells was sought by analysis of protein tyrosine phosphorylation in primary immature sIgM+ B cells, which were generated from bone marrow of 3-83 BCR transgenic (Tg) mice, as described (29, 34). Positive selection in this context involves the suppression of RAG gene expression and the up-regulation of cell surface maturation markers. BCR ligation of the maturing cells prevents or reverses maturation and promotes RAG gene expression and receptor editing. Thus, a comparison of BCR-ligated or unligated cells provides a way to compare signals promoting editing and positive selection, respectively. As shown in Fig. 1A, several prominent tyrosine-phosphorylated bands are detected in cells carrying innocuous receptors that are lost upon BCR ligation. One band appeared to be ~95 kDa, the molecular mass of CD19. To test this, we assessed CD19 tyrosine phosphorylation at amino acid position Y513 using a specific phosphopeptide Ab. As shown in Fig. 1B, strong CD19 phosphorylation was observed in cells carrying an unligated receptor and the phosphorylation signal was lost in B cells treated with anti-BCR Ab. Control experiments with a pan-reactive CD19-blotting Ab showed that the level of CD19 was also reduced in BCR-ligated cells compared with control cells (Fig. 1B) (the lower band in the lower left blot may be an under-glycosylated CD19 biosynthetic intermediate). To extend this analysis in vivo, we assessed CD19 levels in freshly isolated bone marrow B cells that were either innocuous (3-83 Tg on a nondeleting H-2d background) or autoreactive (3-83 Tg central-deleting H-2k background). In B cells on the autoreactive background, levels of both surface CD19 and intracellular CD19 were down-modulated significantly (Fig. 2). We conclude that CD19 tyrosine phosphorylation at Y513 correlates with B cell-positive selection, whereas in the context of negative selection, developing B cells carry reduced levels of CD19 that are hypophosphorylated.
FIGURE 1.
Innocuous BCR signal promotes protein tyrosine phosphorylations that are inhibited by prolonged BCR cross-linking. IL-7-cultured 3-83 Tg bone marrow B cells were treated after IL-7 withdrawal for the indicated times with either anti-BCR or control Abs. A, Total tyrosinephosphorylated proteins assessed in Western blot with 4G10 Ab. Lower panel, Shows signal of stripped blot reprobed with GAPDH Ab. B, Analysis of CD19 tyrosine phosphorylation and total CD19 levels of immunoprecipitated CD19 (left panels) compared with whole cell lysates (right panels).
FIGURE 2.
In vivo analysis of BCR and CD19 expression in bone marrow B cells undergoing positive and negative selection. The 3-83 (anti-H-2Kk,b) BCR Tg mice were bred to H-2d (B10.D2) or H-2k (B10.BR) backgrounds, and their bone marrow cells were analyzed by flow cytometry for the indicated markers. Viable (upper panels) or fixed and permeabilized (lower panels) cells were stained for expression of CD19 and Igκ. Lower panels, Show relative mean fluorescence intensity (MFI) for the two markers within the indicated boxes. Contour plots show data from bone marrow cells using a lymphocyte gate.
Effects of PI3K inhibitors
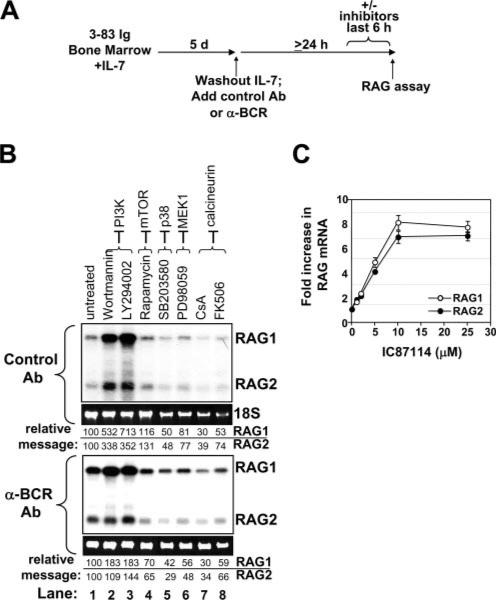
Because the major function of CD19 is believed to be the recruitment of PI3K upon phosphorylations of Y513 and Y482 (43, 44), we tested the possibility that positive selection was PI3K dependent. A first approach involved treating immature B cells with signaling inhibitors (Fig. 3A). As shown in Fig. 3B (top panel), in immature B cells carrying an innocuous BCR, PI3K inhibitors wortmannin and LY294002, but not a wide variety of other signaling inhibitors, were capable of eliciting a robust expression of RAG1 and RAG2. This effect was similar to that of BCR ligation itself, only more robust (Fig. 3B, lower panel). Because wortmannin and LY294002 may affect several PI3K, we also tested a p110δ inhibitor, IC87114 (45); it too promoted RAG expression in a dose-dependent manner (Fig. 3C). It therefore appears that chemical inhibitors of PI3K activity suppress B cell-positive selection in a manner comparable to BCR ligation.
FIGURE 3.
Effect of PI3K inhibitors on RAG expression in immature B cells carrying ligated or unligated BCR. A, Experimental design. B, Effect of inhibitors on RAG1 and RAG2 expression as assessed by Northern blot. The 3-83 Tg bone marrow B cells cultured for 24 h in the presence of control Ab (upper panel) or anti-BCR (lower panel) alone (untreated, lane 1) or for the last 6 h with 50 nM wortmannin, 7.5 μM LY294002, 100 nM rapamycin, 10 μM SB203580, 20 μM PD98059, 1 μg/ml cyclosporin A, and 100 ng/ml FK506 (lanes 2–8, respectively). Molecular targets of each inhibitor (—) are indicated above each lane. For both groups (control Ab and anti-BCR), relative RAG1 and RAG2 mRNA signal (calculated by normalizing RAG signal to 18S RNA content and setting untreated samples at 100%) is shown under each lane. Data are representative of at least three experiments. C, Dose/Response analysis of the effect of a specific inhibitor of the PI3K p110δ catalytic component (IC87114) on RAG1 and RAG2 expression.
Effect of p85α deficiency on RAG expression in vivo
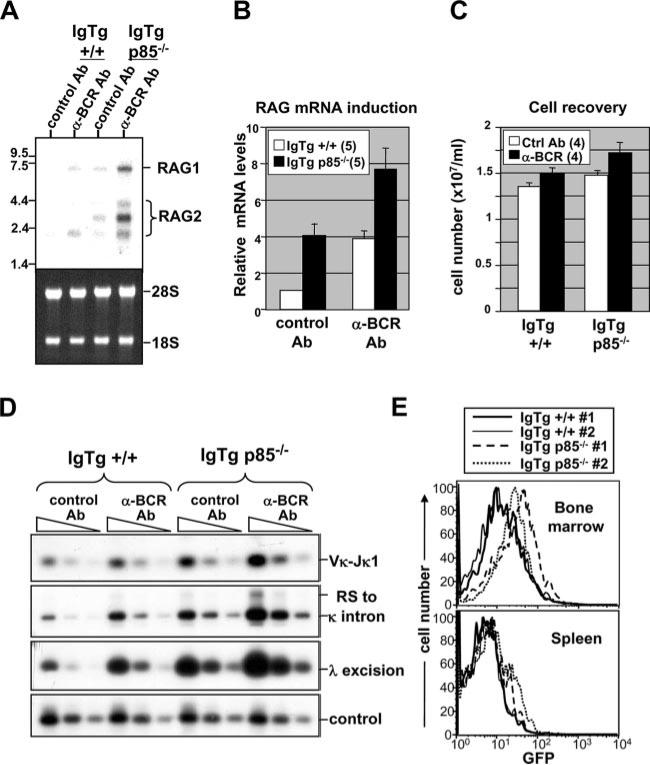
To further probe the role of PI3K in B cell positive-selection, 3-83 BCR Tg mice were bred to p85α-deficient mice (33), and RAG expression and V(D)J recombination were monitored in immature B cells in the context of innocuous and ligated Ag receptors (Fig. 4). As shown in Fig. 4A, p85-deficient cells carrying innocuous receptors failed to strongly suppress RAG1 and RAG2 expression as assessed by Northern blot. (In samples expressing high overall RAG transcript levels, we sometimes observed additional bands for RAG2, which may represent partially spliced nuclear intermediates.) Furthermore, BCR-induced RAG expression was further augmented in the absence of p85. Quantitation of these changes for RAG2 is summarized in Fig. 4B. As shown in Fig. 4C, minimal differences in cell recovery were observed between p85α-deficient and -sufficient cells, ruling out preferential survival of RAG-expressing cells with p85α deficiency as an explanation for these results. Consistent with the elevated RAG expression of the p85α-deficient cells, these cells manifested elevated L chain gene recombination as assessed by semiquantitative PCR assays (Fig. 4D). Analysis of ex vivo bone marrow B cells from p85α-deficient mice carrying an innocuous BCR was consistent with the above mentioned results using IL-7-cultured B cells. As shown in Fig. 4E, p85α-deficient cells clearly overexpressed RAG2 compared with p85α-sufficient controls, as measured using a RAG2/GFP reporter transgene (32). Hence, p85α function appears to be important for the innocuous BCR-mediated suppression of RAG expression in vivo.
FIGURE 4.
Analysis of RAG expression and L chain gene recombinations in p85-deficient 3-83 bone marrow B cell cultures and primary ex vivo cells. A, Northern analysis of RAG expression in BCR-stimulated and control cells that were either p85 deficient or sufficient. Lower panel, Shows ethidium bromide-stained gel before transfer to assess RNA loading. B, Statistical summary of changes in RAG2 mRNA expression over multiple experiments. C, Assessment of cell recovery in 3-83/p85–/– and 3-83/p85+/+ cultured cells at time of harvest for mRNA analysis (24 h post-IL-7 withdrawal). D, PCR analysis of L chain and RS recombinations in genomic DNA of B cells cultured with and without BCR ligation for 2 days post-IL-7 withdrawal. E, Elevated RAG expression in p85–/– B cells in vivo as assessed using a GFP reporter. B cells were from 3-83 Tg/RAG2-GFP background that either were p85+/+ or p85–/–. B220-gated cells from bone marrow or spleen of the indicated mice were assessed for GFP expression by flow cytometry. Note that cells from two independent mice/group were analyzed.
Effects of p85 deficiency on NF-κB activity in immature B cells
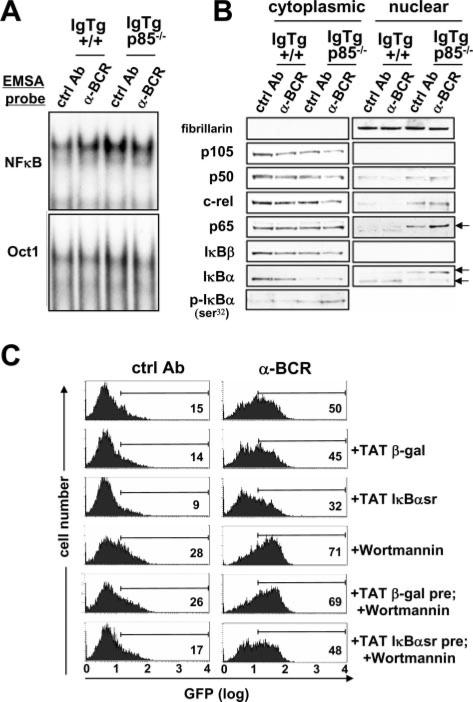
Because RAG expression was up-regulated in p85-deficient cells, and we previously observed that NF-κB/rel transcription factors regulate RAG expression, we tested the effects of p85α deficiency on the spontaneous and sustained BCR-induced activation of NF-κB. As shown in Fig. 5A, p85α deficiency was indeed correlated with elevated NF-κB nuclear activity, as detected by EMSA. BCR ligation increased activity in wild-type cells relative to control (lanes 1 and 2), whereas in p85α-deficient cells NF-κB/rel activity was high even in the absence of BCR ligation. Elevated NF-κB/rel was generally correlated with reduced levels of cytoplasmic IκBα and increases of p65 levels in the nucleus (Fig. 5B). However, in p85α–/– cells, the nuclear levels of p50, c-rel, and p65 were all substantially increased, and the p65 and IκBα components found in the nucleus had a distinctly slower electrophoretic mobility (Fig. 5B, lanes 7 and 8).
FIGURE 5.
Immunoblot and EMSA analysis of NF-κB activation in p85–/– and p85+/+ immature B cells. IL-7-expanded bone marrow B cells were treated for 24 h in the absence of IL-7 with control or anti-BCR Ab before assay. A, Nuclear extracts were incubated with the indicated radio-labeled probes and mobility shift monitored on polyacrylamide gels. B, Western blots of nuclear and cytoplasmic protein fractions of the indicated cell populations were probed with Abs to the indicated epitopes. Immunoblotting for the nuclear-localized protein fibrillarin was performed to confirm purity of preparations. C, Effect of membrane-permeable, super-repressive IκBα (TAT-IκBαsr) on wortmannin- and BCR-induced RAG expression. Immature 3-83/RAG2-GFP B cells obtained after IL-7 culture were treated with control or anti-BCR Ab for 48 h. IκBα-TAT or control β-gal-TAT fusion proteins were added at 22 h and wortmannin at 24 h. Flow cytometry analysis for GFP reporter expression was conducted at 48 h. At the time points surveyed under these conditions, survival in the presence of TAT-IκBαsr did not differ >10% compared with cultures not receiving TAT fusion protein.
We previously showed that the BCR ligation-induced RAG expression in immature B cells was regulated by NF-κB components and could be inhibited by a dominant-negative IκBα superrepressor protein (29). Consistent with the prediction that suppression of NF-κB-induced RAG activity was downstream of BCR-induced PI3K activity, IκBα superrepressor protein-TAT fusion protein was able to suppress RAG expression in cells treated with wortmannin, BCR Ab, or both (Fig. 5C). A control TAT-fusion protein had no effect in this RAG2/GFP reporter assay. These results suggested that a BCR-regulated and p85α-dependent PI3K activity suppresses activation of NF-κB in immature B cells. Furthermore, virtually all markers of B cell maturity were reduced in p85α-deficient B cells, including up-regulation of MHC class II, IgD, CD21, PirA/B, and CD23, and down-modulation of CD93, IgM, and CD24 (data not shown), consistent with prior studies of splenocytes of p85α-deficient mice (46, 47). Collectively, these data indicate that p85α-deficient immature B cells progress poorly, or slowly, in development in vivo, and that they have reduced ability to undergo (innocuous) BCR-mediated positive selection.
PIP3 levels are increased in primary B cells carrying innocuous receptors
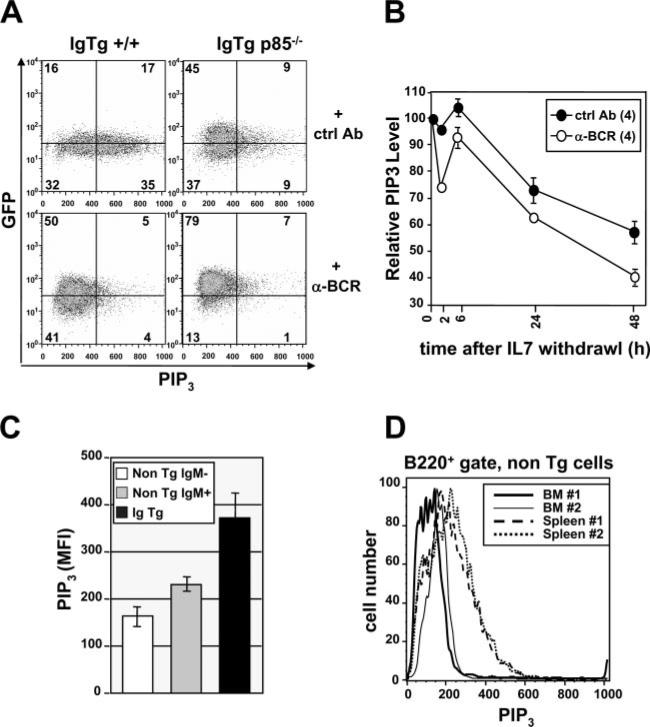
Because the foregoing evidence suggested a strong correlation between PI3K activity and BCR-mediated regulation of RAG expression, we directly assessed p85α-deficient and sufficient primary B cells, with and without BCR ligation, for PIP3 levels and RAG gene activity. As measured by intracellular staining with anti-PIP3 Ab (41), p85-sufficient B cells carrying unligated receptors obtained from IL-7-cultured bone marrow had significantly higher levels of PIP3 24 h after IL-7 withdrawal than those cultured with BCR ligand (Fig. 6A, cf left panels). In contrast, and as predicted, p85-deficient cells had significantly lower PIP3 levels. (As expected, wortmannin-treated cells had reduced immunoreactivity with the PIP3 Ab (data not shown)). In addition, low levels of PIP3 immunoreactivity correlated with high RAG2 expression, as measured at the level of single cells using the GFP reporter gene. A time course experiment showed that at all time points tested, BCR-ligated cells had consistently lower PIP3 levels than cells carrying unligated sIg (Fig. 6B). To see whether PIP3 levels were increased in newly formed B cells carrying innocuous BCR in vivo, we compared B220int bone marrow B cells from wild-type mice with those from 3-83 Tg mice carrying a bone fide innocuous receptor (Fig. 6C). Increasing PIP3 levels correlated with the expression of the innocuous BCR, and were lowest in non-Tg cells that were sIg negative. This result was further confirmed in a comparison of B220+ cells from bone marrow and spleen of B10.D2 mice, in which mature sIg+ cells of the spleen had significantly higher PIP3 levels than sIg– bone marrow pre-B cells (Fig. 6D). These results suggest that PIP3, a product of PI3K, is increased in cells carrying a sIg, including those undergoing positive selection. By contrast, PIP3 levels are reduced not only in pre-B cells lacking a BCR, but also in immature B cells expressing a ligated BCR, and in cells lacking the PI3K regulatory component p85.
FIGURE 6.
Flow cytometry analysis of PIP3 levels in immature B cells. A, IL-7-cultured 3-83 Ig/RAG2-GFP B cells of the indicated p85 genotypes were treated for 48 h after IL-7 withdrawal with control or anti-BCR Abs, then fixed, permeabilized, and assayed by flow cytometry for intracellular PIP3 levels and GFP reporter expression (as a measure of RAG2 transcription). Data are representative of three separate experiments. B, Time course analysis of PIP3 levels plotted as mean fluorescence intensity (MFI) values, relative to samples at time zero of IL-7 withdrawal, plotted as a function of time. Shown are means and SDs from four independent experiments. C, PIP3 levels in B220int-gated ex vivo bone marrow cells from non-Tg or 3-83 IgTg mice, as analyzed by flow cytometry. Data represent results from three independent mice/group. D, Intra-cellular staining for PIP3 levels from ex vivo wild-type splenic B cells compared with bone marrow pre-B cells taken from the same animals, showing increase in PIP3 levels in sIg+ cells.
Enzyme activation
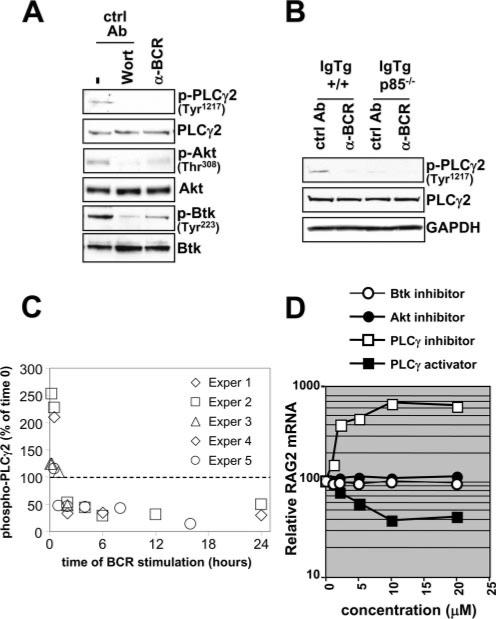
Given the clear evidence for a role for PIP3 in regulating B cell-positive selection, we considered the next possible steps in a signal transduction cascade, in particular cytosolic enzymes that are recruited to the plasma membrane through PH domains, which recognize and bind to PIP3. Among the major candidates in B cells are three enzymes, as follows: the protein tyrosine kinase Btk, the serine/threonine kinase Akt, and PLCγ2 (48). The activation of these enzymes is conveniently monitored by the use of well-defined phosphopeptide Abs. As shown in Fig. 7A, all three enzymes are activated in immature B cells carrying innocuous receptors, reduced in activity in anti-BCR-treated cells, and suppressed in wortmannin-treated cells. Consistent with these results, in p85α–/– cells, PLCγ2 activation, as detected by Tyr1217 phosphorylation, was impaired (Fig. 7B), but surprisingly Btk and Akt phosphorylation was retained (data not shown). Results entirely consistent with these were obtained using ex vivo bone marrow B cells because innocuous B cells had high-level Tyr1217 phosphor-ylation that was suppressed by either BCR ligation (Fig. 7C) or treatment with IC87114 (data not shown). These results indicated that in immature B cells Btk, Akt, and PLCγ2 may transmit PI3K-dependent positive selection signals from unligated BCRs, and that PLCγ2 is particularly dependent on p85α for its activation.
FIGURE 7.
Analysis of phosphorylations of proteins recruited by PIP3 in positively selecting and BCR-treated immature B cells and relationship to regulation of RAG expression. A, Immunoblot analysis of activating phosphorylations of PLCγ2, Akt, and Btk. B, Assessment of altered phosphorylation patterns in p85α-deficient B cells compared with wild-type controls. C, PLCγ2 Tyr1217 phosphorylation levels in ex vivo bone marrow B cells at various times post-BCR stimulation. The y-axis indicates levels relative to that obtained at time zero of stimulation. D, Effects of pharmacological manipulation of PIP3-recruited enzymes on RAG expression in immature B cells. RAG expression was determined by Northern blot. Similar results were seen in two additional experiments.
Linkage of PLCγ2 to RAG expression
To probe the functional relevance of Btk, Akt, and PLCγ2 downstream pathways to B cell-positive selection, the effects of inhibitors of these enzymes were assessed in a readout of RAG mRNA induction or suppression. As shown in Fig. 7D, an inhibitor of PLCγ (U73122), but not inhibitors of Btk or Akt, promoted a dose-dependent, and strikingly increased, expression of RAG2 mRNA. Furthermore, a specific chemical activator of PLCγ (m-3M3-FBS) had the opposite effect. These results strongly suggest that PLCγ2 is critical for the efficient RAG gene down-regulation associated with B cell-positive selection in immature B cells.
Cell size/cell cycle
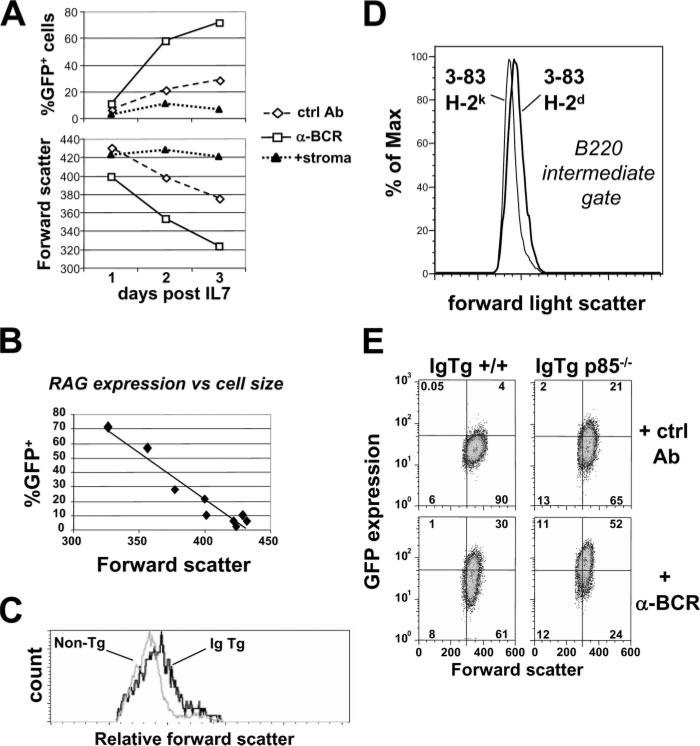
Because p85α has been reported to regulate cell cycle progression in mature B cells (49), we assessed parameters of cell cycle and cell size in relation to the effects of basal or ligated BCR signaling in immature B cells. Several lines of evidence suggested that B cell-positive selection is associated with an increase in cell size relative to unselected B cells that lack an innocuous BCR (Fig. 8). First, in bone marrow cultures, BCR-stimulated cells had a consistently reduced forward light scatter compared with cells treated with control Ab (Fig. 8A). The time- and BCR ligation-dependent RAG expression in these cells was inversely proportional to cell size (Fig. 8B). Second, newly formed B220int bone marrow B cells in vivo that carry innocuous BCRs are larger than comparable sIgM– pre-B cells from wild-type mice (Fig. 8C). Furthermore, in a comparison of innocuous and autoreactive bone marrow B cells isolated directly from mice, autoreactive cells had ~5% lower forward light scatter than innocuous ones at a similar developmental stage (B220int) (Fig. 8D). Supporting a role for PI3K activity in this process, p85α-deficient 3-83/RAG2-GFP-cultured cells had reduced size and increased GFP expression relative to p85α-sufficient controls (Fig. 8E). These data clearly establish the correlation in bone marrow B cells between positive selection and cell size as measured by forward scatter (or side scatter; data not shown), whereas negative selection is associated with lower cell size and increased recombinase expression.
FIGURE 8.
Relationship between cell size and RAG expression in immature B cells. A, Inverse correlation between RAG expression and cell size in immature B cells treated with control Ab or anti-BCR. Bone marrow B cells of 3-83 IgTg/RAG2-GFP mice were stimulated by BCR Abs, control Abs, or a stromal cell line lacking BCR ligand for the indicated times after IL-7 withdrawal. Cells were assayed for GFP expression (upper panel) and forward scatter (lower panel). B, Data from A replotted to show relationship between %GFP+ cells and forward scatter. C, Comparison of forward scatter in ex vivo analyzed immature B cells carrying an innocuous receptor (B220int/IgM+ bone marrow cells from IgTg mice) and pre-B cells (B220int/IgM– bone marrow cells from wild-type mice). D, Comparison of forward light scatter in negatively selecting (3-83/H-2k) and positively selecting (3-83/H-2d) ex vivo bone marrow B cells gated on B220int/sIgκ+ cells. E, Dot plot analysis of GFP expression vs cell size in p85–/– and p85+/+ IgTg B cells. Relative geometric means of fluorescence intensity were as follows: IgTg ctrl (GFP 23; forward scatter 378); IgTg anti-BCR (GFP 34; forward scatter 331); IgTg p85–/– ctrl (GFP 31; forward scatter 337); IgTg p85–/– anti-BCR (GFP 77; forward scatter 316).
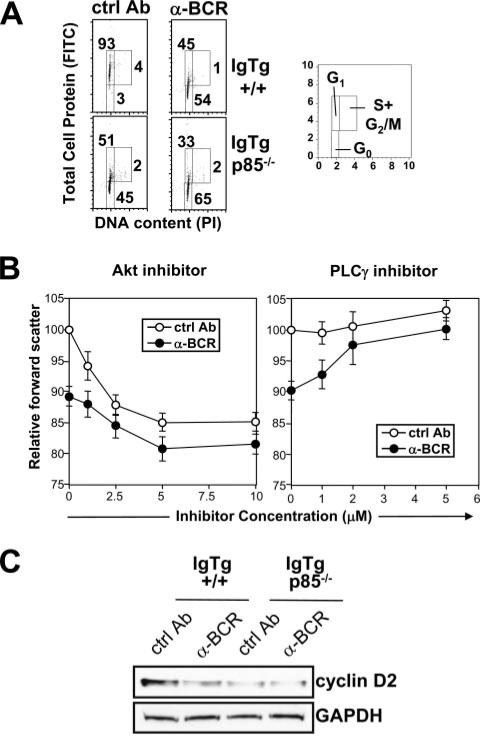
In support of the forward light scatter data, evidence from cell cycle analysis measuring DNA and protein content indicated that BCR-treated and p85α-deficient cells had 2N DNA, but significantly reduced protein content relative to control Ig-treated, p85α-sufficient cells, consistent with a block in G0 to G1 progression (Fig. 9A). Analysis with chemical inhibitors suggested that, with respect to cell size, an Akt inhibitor, but not a PLCγ inhibitor, could suppress the effect of an innocuous receptor (Fig. 9B). Furthermore, immature B cells carrying an innocuous, unligated BCR expressed significantly more cyclin D2 than BCR-ligated cells (Fig. 9C). Cyclin D proteins are known to be important in G0 to G1 progression, and in splenic B cells are regulated by p85α-mediated signals (46, 50). Compared with wild-type B cells, p85α-deficient cells had reduced cyclin D2 expression, cell size, and protein content. These data suggest that, unlike the RAG response, the PI3K-mediated signal-controlling cell size most likely requires Akt and is independent of PLCγ.
FIGURE 9.
A, Cell cycle analysis of BCR-ligated and control Ig-treated immature B cells from p85-deficient and -sufficient IgTg mice. Two-color analysis of total cell protein and DNA content, conducted as described (64). Right panel, Indicates cell cycle compartments distinguished in the analysis. B, Effect of Akt inhibitor (Akt inhibitor) and PLCγ inhibitor (U73122) on forward scatter characteristics of BCR-treated and control Ig-treated immature B cells. C, Western blot analysis of cyclin D levels in immature B cells taken at 24 h post-IL-7 withdrawal in the presence of the indicated Abs.
Discussion
Developmental progression in newly formed B cells involves a positive selection step that stops V(D)J recombination, down-regulates RAG gene expression, and up-regulates cell surface markers of maturation. Positive selection addressed in the present study probably applies to the major B cell lineage fated to populate the lymphoid follicles. We find that, in addition, positive selection involves a significant change in cell size and cyclin D2 expression indicative of partial G0 to G1 cell cycle progression. We show in this study that these pathways are most likely initiated by the innocuous BCR-dependent phosphorylation of CD19, leading to the recruitment of PI3K p85 subunit adjacent to the plasma membrane, allowing subsequent activation of PI3K and PIP3 generation. PIP3 in turn probably recruits Btk, Akt, and PLCγ2 to the plasma membrane, leading to their activation. These effectors are most likely critical in regulating different manifestations of BCR-induced positive selection.
The present study has revealed that the PI3K signaling pathway plays an important role in sIg-mediated B cell-positive selection at the immature B cell stage. Data supporting this conclusion include the following findings. First, innocuous sIg expression was associated with tyrosine phosphorylation of a restricted set of proteins, including CD19 at Y513, a site of p85 recruitment, whereas immature B cells whose sIg was cross-linked lost progressively this phosphorylation, and both in vivo and in vitro overall CD19 levels were reduced. Second, several chemical inhibitors of PI3K activity, including a highly specific p110δ inhibitor, led to a striking increase in RAG expression, whereas a large number of inhibitors of other biochemical pathways failed to promote RAG expression. Loss of RAG expression in developing sIgM+ cells is a cardinal indicator of B cell-positive selection. The ability of the less specific PI3K inhibitors wortmannin and LY294002 to stimulate RAG expression in IL-7-precultured B cells had been noted in earlier studies (24, 29). Third, mice deficient in the PI3K regulatory subunit p85α displayed elevated RAG levels, excess L chain gene recombination, and reduced expression of cell surface differentiation markers in vivo and in vitro. Fourth, cells lacking p85α or those treated with anti-BCR ligands manifested reduced PIP3 levels compared with wild-type cells carrying unligated BCRs. Fifth, in vivo small pre-B cells had lower PIP3 levels than newly formed immature B cells. Sixth, the phosphorylation of Akt, PLCγ2, and Btk, three key signaling molecules carrying PH domains recruited by PI3K, was elevated in cells carrying innocuous BCRs. In contrast, these phosphorylations were reduced in cells treated with PI3K inhibitors and in cells whose BCRs were ligated by Ab.
Our data suggest that PLCγ2 in particular may be important in transmitting the PI3K signal suppressing RAG expression. In immature B cells, a chemical inhibitor of PLCγ led to increased RAG expression, whereas a PLCγ activator had the opposite effect. PLCγ converts phosphatidylinositol-(4,5)-bisphosphate to the second messengers inositol-(1,4,5)-triphosphate and diacylglycerol, promoting Ca2+ flux and PKC activation, respectively. In immature B cells genetically lacking p85α, PLCγ2 phosphorylation was impaired, whereas Btk and Akt phosphorylation was substantially retained. These results are consistent with recent data indicating that in splenic B cells from mice deficient in p85α, BCR-mediated Btk and Akt activation is intact (46, 51).
Our findings are consistent with previous experiments showing that CD19–/– B cells carrying an innocuous Ab transgene fail to suppress efficiently RAG expression and endogenous Ig gene rearrangements, and fail to mature in the periphery (30, 52). The impaired BCR-signaling phenotype of CD19–/– mice, which abrogated feedback suppression of endogenous Ig L chain gene rearrangements, could be rescued by rendering the Ab transgene homozygous, suggesting that in the absence of CD19 the basal signaling of the BCR was subthreshold (30). Additional pathways undoubtedly contribute to the positive selection signal. BCAP, which has been shown to play a role in recruiting p85 and promoting PI3K activation downstream of BCR signaling, may function independently of CD19 (53).
Our data indicate that interactions between CD19 and the innocuous BCR are essential for optimal sustained activation of PI3K and PLCγ2, and the down-regulation of RAG expression. The simplest model would be that positive selection is inhibited (and editing by default promoted) simply by separating the intracytoplasmic tails of BCR and CD19 and their associated signaling molecules from one another, preventing in particular BCR-mediated tyrosine phosphorylation of CD19, p85 recruitment, and activation of PI3K. This model would be consistent with recent data suggesting that induced deletion of the IgH locus in immature B cells can activate RAG gene expression in immature B cells (24). The model would also be consistent with the elevated RAG levels and inefficient positive selection seen in CD19 deficiency (30). Moreover, our finding that BCR ligation of autoreactive receptors leads to a loss of CD19 in immature bone marrow B cells provides an explanation for how cells carrying self-reactive receptors are hindered in their developmental progression. The ability of BCR ligation to down-regulate CD19 has been noted before (15), but to our knowledge has not been studied in primary bone marrow B cells.
The B cell type studied in this work represents an early sIgM+ stage that appears to differ fundamentally in its signaling pathways from mature splenic B cells. In this study, we find that an unligated, innocuous receptor stimulates at steady-state PI3K activity and PLCγ2 phosphorylation correlating with reduced NF-κB activation and RAG expression. PI3K inhibitors or p85 deficiency result in nuclear NF-κB up-regulation. In contrast, in mature B cells, PI3K activity is associated with elevated NF-κB activation (46, 50).
The relative contributions of Akt and PLCγ to the regulation of RAG expression and editing have not been addressed directly in previous studies. Recently, however, PLCγ2–/– B cells were shown to express high levels of RAG, although these mice also developed tumors with a mixed pre-B/B cell phenotype to which RAG overexpression might have contributed (54). The adapter molecule B cell linker protein (BLNK), which is critical for PLCγ2 activation, has been suggested to be required for editing (55). However, because RAG levels were not assessed in these mice, it is difficult to determine whether the mutation affects another aspect of the developmental arrest process. In T cells, the related adapter molecule, murine SH2 domain-containing leukocyte protein (SLP-76), is required for tonic signaling and suppression of RAG (56).
Innocuous BCR expression on immature B cells promotes differentiation, including the expression of cell surface molecules associated with peripheral B cell maturation, including CD2, CD22, CD23, MHCII, etc. In this study, PI3K signaling may play a role through the recruitment of Btk, which is believed to promote expression of several of these markers (57). In Btk-deficient mice, neither receptor editing nor positive selection appears to be impaired (57, 58). Indeed, it has been argued that B cell-positive selection is accelerated in Btk-deficient xid mice, as judged by emigration from bone marrow (58).
Cell size regulation by innocuous BCR was suggested by several lines of evidence. In vivo, pre-B cells, which lack sIg, were found to be smaller (as measured by forward light scatter) than immature B cells carrying a transgene-encoded innocuous BCR. Furthermore, BCR cross-linking in vitro reduced immature B cell size relative to unligated control. Similarly, BCR-ligated or p85α-deficient cells had reduced forward scatter and total protein content compared with wild-type B cells with unligated receptors. However, in contrast to PI3K-mediated RAG expression, in transmitting signals regulating cell size, PLCγ is apparently not important. Rather, analysis with chemical inhibitors suggested that Akt, a different PI3K-recruited enzyme, most likely is important. Indeed, Akt is known to regulate cell size in many organisms and contexts, in part by regulating glycolysis and nutrient transport (59, 60).
The significance of tying cell size to BCR signaling in immature B cells is unknown; however, it is tempting to speculate that it has an important physiological role. BCR ligation leads to a combination of elevated L chain gene recombination, a block in cell cycle progression, and reduced Akt activation, whereas innocuous BCR signaling promotes increased cell size and cessation of recombination. We would like to propose that, at the pre-B/B cell transition, absence of an Ig L chain (or expression of an autoreactive BCR) leads to loss of Akt activity and attendant glucose uptake and metabolism. The net effect is to progressively reduce cell size and ultimately survival, placing a limit on the amount of time that a B cell has to generate an appropriate L chain. This limits therefore the number of recombination or editing attempts that can be conducted, a phenomenon that has been referred to as the crash factor (61). According to this model, expression of an appropriate, presumably innocuous BCR supports Akt activity, rescuing the cell from death by nutrient starvation (as has been proposed for cytokine withdrawal-induced cell death, autophagy (60)). This model is supported by previous studies from our laboratory showing that artificially extending B cell lifespan through expression of Bcl2 promotes receptor editing (6), presumably by allowing cells lacking in Akt activity to survive longer and to undergo additional rearrangements.
One can view the promotion of immature B cell development by the innocuous BCR as a transition in cell cycle from G0 to G1, with attendant increase in cell size and cyclin D expression. Self Ag ligation of the BCR disrupts this signal, promoting continued V(D)J recombination and receptor editing. A time limit is placed on this G0 editing state by the restriction in Akt activity, condemning cells to death by neglect. Rescue occurs when successful editing generates an innocuous receptor. It remains to be seen whether BCR ligands work merely by receptor down-modulation, denuding the B cell of sIg or CD19, and terminating the positive selection signal. If this simple model is correct, it predicts that in cells coexpressing two receptors, one autoreactive and one innocuous, receptor editing could be terminated. This would seem to be a dangerous possibility that is to be avoided; however, such a phenotype has been observed in some autoantibody Tg mice (e.g., 62, 63).
Acknowledgments
We thank Drs. R. Rickert, A. Anzelon, and D. Fruman for advice; Drs. Kabouridis and Dowdy for TAT-fusion protein constructs; Dr. A. Feeney for 3-83/H-2k mice; Dr. M. Nussenzweig for RAG2/GFP reporter mice; Dr. J. Cambier for CD19 blotting Ab; and Dr. S. Koyasu for providing access to p85α-deficient mice.
Footnotes
This work was supported by research and training grants from the National Institutes of Health (RO1AI33608 to D.N., T32HL07195 to D.A.-A., and Graduate Training Grant F31AI52484).
Abbreviations used in this paper: sIg, surface Ig; Btk, Bruton's tyrosine kinase; int, intermediate; PH, pleckstrin homology; PIP3, phosphatidylinositol-(3,4,5)-trisphosphate; PLC, phospholipase C; Tg, transgenic.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Lam KP, Kuhn R, Rajewsky K. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 1997;90:1073–1083. doi: 10.1016/s0092-8674(00)80373-6. [DOI] [PubMed] [Google Scholar]
- 2.Kraus M, Alimzhanov MB, Rajewsky N, Rajewsky K. Survival of resting mature B lymphocytes depends on BCR signaling via the Igαβ heterodimer. Cell. 2004;117:787–800. doi: 10.1016/j.cell.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 3.Goodnow CC, Crosbie J, Adelstein S, Lavoie TB, Smith-Gill SJ, Brink RA, Pritchard-Briscoe H, Wotherspoon JS, Loblay RH, Raphael K, et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988;334:676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- 4.Russell DM, Dembic Z, Morahan G, Miller JF, Burki K, Nemazee D. Peripheral deletion of self-reactive B cells. Nature. 1991;354:308–311. doi: 10.1038/354308a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fulcher DA, Basten A. Reduced life span of anergic self-reactive B cells in a double-transgenic model. J. Exp. Med. 1994;179:125–134. doi: 10.1084/jem.179.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lang J, Arnold B, Hammerling G, Harris AW, Korsmeyer S, Russell D, Strasser A, Nemazee D. Enforced Bcl-2 expression inhibits antigen-mediated clonal elimination of peripheral B cells in an antigen dose-dependent manner and promotes receptor editing in autoreactive, immature B cells. J. Exp. Med. 1997;186:1513–1522. doi: 10.1084/jem.186.9.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tiegs SL, Russell DM, Nemazee D. Receptor editing in self-reactive bone marrow B cells. J. Exp. Med. 1993;177:1009–1020. doi: 10.1084/jem.177.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Melamed D, Nemazee D. Self-antigen does not accelerate immature B cell apoptosis, but stimulates receptor editing as a consequence of developmental arrest. Proc. Natl. Acad. Sci. USA. 1997;94:9267–9272. doi: 10.1073/pnas.94.17.9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Papavasiliou F, Jankovic M, Suh H, Nussenzweig MC. The cytoplasmic domains of immunoglobulin (Ig) α and Igβ can independently induce the precursor B cell transition and allelic exclusion. J. Exp. Med. 1995;182:1389–1394. doi: 10.1084/jem.182.5.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neuberger MS, Patel KJ, Dariavach P, Nelms K, Peaker CJ, Williams GT. The mouse B-cell antigen receptor: definition and assembly of the core receptor of the five immunoglobulin isotypes. Immunol. Rev. 1993;132:147–161. doi: 10.1111/j.1600-065x.1993.tb00841.x. [DOI] [PubMed] [Google Scholar]
- 11.Rolli V, Gallwitz M, Wossning T, Flemming A, Schamel WW, Zurn C, Reth M. Amplification of B cell antigen receptor signaling by a Syk/ITAM positive feedback loop. Mol. Cell. 2002;10:1057–1069. doi: 10.1016/s1097-2765(02)00739-6. [DOI] [PubMed] [Google Scholar]
- 12.Pleiman CM, Abrams C, Gauen LT, Bedzyk W, Jongstra J, Shaw AS, Cambier JC. Distinct p53/56lyn and p59fyn domains associate with non-phosphorylated and phosphorylated Ig-α. Proc. Natl. Acad. Sci. USA. 1994;91:4268–4272. doi: 10.1073/pnas.91.10.4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reth M, Wienands J. Initiation and processing of signals from the B cell antigen receptor. Annu. Rev. Immunol. 1997;15:453–479. doi: 10.1146/annurev.immunol.15.1.453. [DOI] [PubMed] [Google Scholar]
- 14.Tedder TF, Poe JC, Fujimoto M, Haas KM, Sato S. The CD19-CD21 signal transduction complex of B lymphocytes regulates the balance between health and autoimmune disease: systemic sclerosis as a model system. Curr. Dir. Autoimmun. 2005;8:55–90. doi: 10.1159/000082087. [DOI] [PubMed] [Google Scholar]
- 15.Pesando JM, Bouchard LS, McMaster BE. CD19 is functionally and physically associated with surface immunoglobulin. J. Exp. Med. 1989;170:2159–2164. doi: 10.1084/jem.170.6.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Fougerolles AR, Batista F, Johnsson E, Fearon DT. IgM and stromal cell-associated heparan sulfate/heparin as complement-independent ligands for CD19. Eur. J. Immunol. 2001;31:2189–2199. doi: 10.1002/1521-4141(200107)31:7<2189::aid-immu2189>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 17.Carter RH, Doody GM, Bolen JB, Fearon DT. Membrane IgM-induced tyrosine phosphorylation of CD19 requires a CD19 domain that mediates association with components of the B cell antigen receptor complex. J. Immunol. 1997;158:3062–3069. [PubMed] [Google Scholar]
- 18.Krop I, Shaffer AL, Fearon DT, Schlissel MS. The signaling activity of murine CD19 is regulated during cell development. J. Immunol. 1996;157:48–56. [PubMed] [Google Scholar]
- 19.Otero DC, Rickert RC. CD19 function in early and late B cell development. II. CD19 facilitates the pro-B/pre-B transition. J. Immunol. 2003;171:5921–5930. doi: 10.4049/jimmunol.171.11.5921. [DOI] [PubMed] [Google Scholar]
- 20.Deane JA, Fruman DA. Phosphoinositide 3-kinase: diverse roles in immune cell activation. Annu. Rev. Immunol. 2004;22:563–598. doi: 10.1146/annurev.immunol.22.012703.104721. [DOI] [PubMed] [Google Scholar]
- 21.Dykstra M, Cherukuri A, Sohn HW, Tzeng SJ, Pierce SK. Location is everything: lipid rafts and immune cell signaling. Annu. Rev. Immunol. 2003;21:457–481. doi: 10.1146/annurev.immunol.21.120601.141021. [DOI] [PubMed] [Google Scholar]
- 22.Shaffer AL, Schlissel MS. A truncated heavy chain protein relieves the requirement for surrogate light chains in early B cell development. J. Immunol. 1997;159:1265–1275. [PubMed] [Google Scholar]
- 23.Meffre E, Nussenzweig MC. Deletion of immunoglobulin β in developing B cells leads to cell death. Proc. Natl. Acad. Sci. USA. 2002;99:11334–11339. doi: 10.1073/pnas.172369999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tze LE, Schram BR, Lam KP, Hogquist KA, Hippen KL, Liu J, Shinton SA, Otipoby KL, Rodine PR, Vegoe AL, et al. Basal immunoglobulin signaling actively maintains developmental stage in immature B cells. PLoS Biol. 2005;3:e82. doi: 10.1371/journal.pbio.0030082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bannish G, Fuentes-Panana EM, Cambier JC, Pear WS, Monroe JG. Ligand-independent signaling functions for the B lymphocyte antigen receptor and their role in positive selection during B lymphopoiesis. J. Exp. Med. 2001;194:1583–1596. doi: 10.1084/jem.194.11.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caldwell RG, Wilson JB, Anderson SJ, Longnecker R. Epstein-Barr virus LMP2A drives B cell development and survival in the absence of normal B cell receptor signals. Immunity. 1998;9:405–411. doi: 10.1016/s1074-7613(00)80623-8. [DOI] [PubMed] [Google Scholar]
- 27.Casola S, Otipoby KL, Alimzhanov M, Humme S, Uyttersprot N, Kutok JL, Carroll MC, Rajewsky K. B cell receptor signal strength determines B cell fate. Nat. Immunol. 2004;5:317–327. doi: 10.1038/ni1036. [DOI] [PubMed] [Google Scholar]
- 28.Nemazee D. Receptor selection in B and T lymphocytes. Annu. Rev. Immunol. 2000;18:19–51. doi: 10.1146/annurev.immunol.18.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verkoczy L, Ait-Azzouzene D, Skog P, Martensson A, Lang J, Duong B, Nemazee D. A role for nuclear factor κB/rel transcription factors in the regulation of the recombinase activator genes. Immunity. 2005;22:519–531. doi: 10.1016/j.immuni.2005.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shivtiel S, Leider N, Sadeh O, Kraiem Z, Melamed D. Impaired light chain allelic exclusion and lack of positive selection in immature B cells expressing incompetent receptor deficient of CD19. J. Immunol. 2002;168:5596–5604. doi: 10.4049/jimmunol.168.11.5596. [DOI] [PubMed] [Google Scholar]
- 31.Diamant E, Keren Z, Melamed D. CD19 regulates positive selection and maturation in B lymphopoiesis: lack of CD19 imposes developmental arrest of immature B cells and consequential stimulation of receptor editing. Blood. 2005;105:3247–3254. doi: 10.1182/blood-2004-08-3165. [DOI] [PubMed] [Google Scholar]
- 32.Yu W, Misulovin Z, Suh H, Hardy RR, Jankovic M, Yao XR, Nussenzweig MC. Coordinate regulation of RAG1 and RAG2 by cell type-specific DNA elements 5′ of RAG2. Science. 1999;285:1080–1084. doi: 10.1126/science.285.5430.1080. [DOI] [PubMed] [Google Scholar]
- 33.Terauchi Y, Tsuji Y, Satoh S, Minoura H, Murakami K, Okuno A, Inukai K, Asano T, Kaburagi Y, Ueki K, et al. Increased insulin sensitivity and hypoglycaemia in mice lacking the p85α subunit of phosphoinositide 3-kinase. Nat. Genet. 1999;21:230–235. doi: 10.1038/6023. [DOI] [PubMed] [Google Scholar]
- 34.Melamed D, Kench JA, Grabstein K, Rolink A, Nemazee D. A functional B cell receptor transgene allows efficient IL-7-independent maturation of B cell precursors. J. Immunol. 1997;159:1233–1239. [PubMed] [Google Scholar]
- 35.Hammerling GJ, Rusch E, Tada N, Kimura S, Hammerling U. Localization of allodeterminants on H-2Kb antigens determined with monoclonal antibodies and H-2 mutant mice. Proc. Natl. Acad. Sci. USA. 1982;79:4737–4741. doi: 10.1073/pnas.79.15.4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dyer RB, Herzog NK. Isolation of intact nuclei for nuclear extract preparation from a fragile B-lymphocyte cell line. BioTechniques. 1995;19:192–195. [PubMed] [Google Scholar]
- 37.Kabouridis PS, Hasan M, Newson J, Gilroy DW, Lawrence T. Inhibition of NF-κB activity by a membrane-transducing mutant of IκBα. J. Immunol. 2002;169:2587–2593. doi: 10.4049/jimmunol.169.5.2587. [DOI] [PubMed] [Google Scholar]
- 38.Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science. 1999;285:1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- 39.Hertz M, Nemazee D. BCR ligation induces receptor editing in IgM+IgD– bone marrow B cells in vitro. Immunity. 1997;6:429–436. doi: 10.1016/s1074-7613(00)80286-1. [DOI] [PubMed] [Google Scholar]
- 40.Retter MW, Nemazee D. Receptor editing occurs frequently during normal B cell development. J. Exp. Med. 1998;188:1231–1238. doi: 10.1084/jem.188.7.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anzelon AN, Wu H, Rickert RC. Pten inactivation alters peripheral B lymphocyte fate and reconstitutes CD19 function. Nat. Immunol. 2003;4:287–294. doi: 10.1038/ni892. [DOI] [PubMed] [Google Scholar]
- 42.Perez OD, Kinoshita S, Hitoshi Y, Payan DG, Kitamura T, Nolan GP, Lorens JB. Activation of the PKB/AKT pathway by ICAM-2. Immunity. 2002;16:51–65. doi: 10.1016/s1074-7613(02)00266-2. [DOI] [PubMed] [Google Scholar]
- 43.Tuveson DA, Carter RH, Soltoff SP, Fearon DT. CD19 of B cells as a surrogate kinase insert region to bind phosphatidylinositol 3-kinase. Science. 1993;260:986–989. doi: 10.1126/science.7684160. [DOI] [PubMed] [Google Scholar]
- 44.Wang Y, Brooks SR, Li X, Anzelon AN, Rickert RC, Carter RH. The physiologic role of CD19 cytoplasmic tyrosines. Immunity. 2002;17:501–514. doi: 10.1016/s1074-7613(02)00426-0. [DOI] [PubMed] [Google Scholar]
- 45.Sadhu C, Masinovsky B, Dick K, Sowell CG, Staunton DE. Essential role of phosphoinositide 3-kinase δ in neutrophil directional movement. J. Immunol. 2003;170:2647–2654. doi: 10.4049/jimmunol.170.5.2647. [DOI] [PubMed] [Google Scholar]
- 46.Suzuki H, Matsuda S, Terauchi Y, Fujiwara M, Ohteki T, Asano T, Behrens TW, Kouro T, Takatsu K, Kadowaki T, Koyasu S. PI3K and Btk differentially regulate B cell antigen receptor-mediated signal transduction. Nat. Immunol. 2003;4:280–286. doi: 10.1038/ni890. [DOI] [PubMed] [Google Scholar]
- 47.Donahue AC, Hess KL, Ng KL, Fruman DA. Altered splenic B cell subset development in mice lacking phosphoinositide 3-kinase p85α. Int. Immunol. 2004;16:1789–1798. doi: 10.1093/intimm/dxh180. [DOI] [PubMed] [Google Scholar]
- 48.Fruman DA, Satterthwaite AB, Witte ON. Xid-like phenotypes: a B cell signalosome takes shape. Immunity. 2000;13:1–3. doi: 10.1016/s1074-7613(00)00002-9. [DOI] [PubMed] [Google Scholar]
- 49.Glassford J, Vigorito E, Soeiro I, Madureira PA, Zoumpoulidou G, Brosens JJ, Turner M, Lam EW. Phosphatidylinositol 3-kinase is required for the transcriptional activation of cyclin D2 in BCR activated primary mouse B lymphocytes. Eur. J. Immunol. 2005;35:2748–2761. doi: 10.1002/eji.200425812. [DOI] [PubMed] [Google Scholar]
- 50.Piatelli MJ, Wardle C, Blois J, Doughty C, Schram BR, Rothstein TL, Chiles TC. Phosphatidylinositol 3-kinase-dependent mitogen-activated protein/extracellular signal-regulated kinase kinase 1/2 and NF-κB signaling pathways are required for B cell antigen receptor-mediated cyclin D2 induction in mature B cells. J. Immunol. 2004;172:2753–2762. doi: 10.4049/jimmunol.172.5.2753. [DOI] [PubMed] [Google Scholar]
- 51.Hess KL, Donahue AC, Ng KL, Moore TI, Oak J, Fruman DA. Frontline: the p85α isoform of phosphoinositide 3-kinase is essential for a subset of B cell receptor-initiated signaling responses. Eur. J. Immunol. 2004;34:2968–2976. doi: 10.1002/eji.200425326. [DOI] [PubMed] [Google Scholar]
- 52.Buhl AM, Nemazee D, Cambier JC, Rickert R, Hertz M. B-cell antigen receptor competence regulates B-lymphocyte selection and survival. Immunol. Rev. 2000;176:141–153. doi: 10.1034/j.1600-065x.2000.00613.x. [DOI] [PubMed] [Google Scholar]
- 53.Yamazaki T, Takeda K, Gotoh K, Takeshima H, Akira S, Kurosaki T. Essential immunoregulatory role for BCAP in B cell development and function. J. Exp. Med. 2002;195:535–545. doi: 10.1084/jem.20011751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wen R, Chen Y, Bai L, Fu G, Schuman J, Dai X, Zeng H, Yang C, Stephan RP, Cleveland JL, Wang D. Essential role of phospholipase Cγ2 in early B-cell development and Myc-mediated lymphomagenesis. Mol. Cell. Biol. 2006;26:9364–9376. doi: 10.1128/MCB.00839-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hayashi K, Nojima T, Goitsuka R, Kitamura D. Impaired receptor editing in the primary B cell repertoire of BASH-deficient mice. J. Immunol. 2004;173:5980–5988. doi: 10.4049/jimmunol.173.10.5980. [DOI] [PubMed] [Google Scholar]
- 56.Roose JP, Diehn M, Tomlinson MG, Lin J, Alizadeh AA, Botstein D, Brown PO, Weiss A. T cell receptor-independent basal signaling via Erk and Abl kinases suppresses RAG gene expression. PLoS Biol. 2003;1:E53. doi: 10.1371/journal.pbio.0000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Middendorp S, Hendriks RW. Cellular maturation defects in Bruton's tyrosine kinase-deficient immature B cells are amplified by premature B cell receptor expression and reduced by receptor editing. J. Immunol. 2004;172:1371–1379. doi: 10.4049/jimmunol.172.3.1371. [DOI] [PubMed] [Google Scholar]
- 58.Cariappa A, Kim TJ, Pillai S. Accelerated emigration of B lymphocytes in the Xid mouse. J. Immunol. 1999;162:4417–4423. [PubMed] [Google Scholar]
- 59.Kozma SC, Thomas G. Regulation of cell size in growth, development and human disease: PI3K, PKB and S6K. BioEssays. 2002;24:65–71. doi: 10.1002/bies.10031. [DOI] [PubMed] [Google Scholar]
- 60.Edinger AL, Thompson CB. Akt maintains cell size and survival by increasing mTOR-dependent nutrient uptake. Mol. Biol. Cell. 2002;13:2276–2288. doi: 10.1091/mbc.01-12-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Coleclough C. Chance, necessity and antibody gene dynamics. Nature. 1983;303:23–26. doi: 10.1038/303023a0. [DOI] [PubMed] [Google Scholar]
- 62.Li Y, Li H, Weigert M. Autoreactive B cells in the marginal zone that express dual receptors. J. Exp. Med. 2002;195:181–188. doi: 10.1084/jem.20011453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gerdes T, Wabl M. Autoreactivity and allelic inclusion in a B cell nuclear transfer mouse. Nat. Immunol. 2004;5:1282–1287. doi: 10.1038/ni1133. [DOI] [PubMed] [Google Scholar]
- 64.Williams CD, Linch DC, Watts MJ, Thomas NS. Characterization of cell cycle status and E2F complexes in mobilized CD34+ cells before and after cytokine stimulation. Blood. 1997;90:194–203. [PubMed] [Google Scholar]