Abstract
Clinical target volume (CTV) delineation is crucial for tumor control and normal tissue protection. This study aimed to define the locoregional extension patterns of nasopharyngeal carcinoma (NPC) and to improve CTV delineation. Magnetic resonance imaging scans of 2366 newly diagnosed NPC patients were reviewed. According to incidence rates of tumor invasion, the anatomic sites surrounding the nasopharynx were classified into high-risk (>30%), medium-risk (5%–30%), and low-risk (<5%) groups. The lymph node (LN) level was determined according to the Radiation Therapy Oncology Group guidelines, which were further categorized into the upper neck (retropharyngeal region and level II), middle neck (levels III and Va), and lower neck (levels IV and Vb and the supraclavicular fossa). The high-risk anatomic sites were adjacent to the nasopharynx, whereas those at medium- or low-risk were separated from the nasopharynx. If the high-risk anatomic sites were involved, the rates of tumor invasion into the adjacent medium-risk sites increased; if not, the rates were significantly lower (P < 0.01). Among the 1920 (81.1%) patients with positive LN, the incidence rates of LN metastasis in the upper, middle, and lower neck were 99.6%, 30.2%, and 7.2%, respectively, and skip metastasis happened in only 1.2% of patients. In the 929 patients who had unilateral upper neck involvement, the rates of contralateral middle neck and lower neck involvement were 1.8% and 0.4%, respectively. Thus, local disease spreads stepwise from proximal sites to distal sites, and LN metastasis spreads from the upper neck to the lower neck. Individualized CTV delineation for NPC may be feasible.
Keywords: Nasopharyngeal carcinoma, magnetic resonance imaging, local extension, lymph node spread, clinical target volume
Nasopharyngeal carcinoma (NPC) has an extremely unbalanced endemic distribution, and it is prevalent in southern China, with Chinese accounting for 40% of all incident NPC cases worldwide[1]; thus, NPC is also known as the “Cantonese cancer”. Radiotherapy is the mainstay treatment modality for NPC. Intensity-modulated radiotherapy (IMRT) has gradually replaced two-dimensional conventional radiotherapy as the primary means of radiotherapy because of better tumor target coverage and normal tissue sparing. With the application of IMRT, the treatment outcome and quality of life for NPC patients have been greatly improved[2]–[6]. IMRT requires the delineation of target volumes on cross-sectional imaging; precise target volume delineation is crucial for tumor control and normal tissue protection because of the highly conformal radiation dose distribution in IMRT. According to the International Commission on Radiation Units and Measurements (ICRU) reports 50[7] and 62[8], the gross tumor volume (GTV) consists of primary tumor and metastatic lympha-denopathy. In addition, clinical tumor volume (CTV) can be subdivided into CTV1 (high-risk subclinical disease) and CTV2 (low-risk subclinical disease), though the optimal delineation has not been determined. Better understanding the locoregional extension patterns of NPC will directly impact individualized CTV delineation. Therefore, we reviewed magnetic resonance imaging (MRI) scans of a large sample of NPC patients and documented the patterns of locoregional extension of NPC, aiming to improve CTV delineation.
Materials and Methods
Patients
A total of 2366 patients with non-distant metastatic and histologically proven NPC newly diagnosed between January 2003 and December 2008 were included in our study. All patients completed a pre-treatment evaluation that included physical examination, nasopharyngeal fiberoptic endoscopy, MRI scan of the nasopharynx and neck, chest radiography, abdominal sonography, and whole body bone scan. Additionally, 184(7.8%) patients underwent 18F-fluorodeoxyglucose-positron emission tomography/computed tomography (FDG-PET/CT). Medical records and imaging studies were analyzed, and all patients were restaged according to the 7th edition of the Union for International Cancer Control/American Joint Committee on Cancer (UICC/AJCC) staging system[9]. Patient clinicopathologic characteristics are shown in Table 1.
Table 1. Clinicopathologic characteristics of the 2366 nasopharyngeal carcinoma (NPC) patients.
| Characteristic | No. of patients (%) |
| Gender | |
| Male | 1775 (75.0) |
| Female | 591 (25.0) |
| Age (years) | |
| >50 | 766 (32.4) |
| ≤ 50 | 1600 (67.6) |
| Histology | |
| WHO type I | 4 (0.2) |
| WHO type II/III | 2362 (99.8) |
| Radiotherapy | |
| Two-dimensional conventional radiotherapy | 1540 (65.1) |
| Three-dimensional conformal radiotherapy | 17 (0.7) |
| Intensity-modulated radiotherapy | 809 (34.2) |
| Chemotherapy | |
| Yes | 1615 (68.3) |
| Concurrent chemoradiotherapy | 621 (26.2) |
| Neoadjuvant + concurrent chemoradiotherapy | 442 (18.7) |
| Concurrent chemoradiotherapy + adjuvant | 117 (4.9) |
| Others | 435 (18.4) |
| No | 751 (31.7) |
| T category | |
| T1 | 481 (20.3) |
| T2 | 525 (22.2) |
| T3 | 868 (36.7) |
| T4 | 492 (20.8) |
| N category | |
| N0 | 446 (18.9) |
| N1 | 1288 (54.4) |
| N2 | 452 (19.1) |
| N3 | 180 (7.6) |
| Stage | |
| I | 161 (6.8) |
| II | 580 (24.5) |
| III | 978 (41.3) |
| IVa | 467 (19.7) |
| IVb | 180 (7.6) |
Imaging protocol
All patients underwent MRI using a 1.5-T system (Signa CV/i; General Electric Healthcare, Chalfont St. Giles, United Kingdom) to examine the region from the suprasellar cistern to the inferior margin at the sternal end of the clavicle with a head-and-neck combined coil. T1-weighted fast spin-echo images in the axial, coronal, and sagittal planes (repetition time 500–600 ms, echo time 10–20 ms, 22-cm field of view) and T2-weighted fast spin-echo images in the axial plane (repetition time 4000–6000 ms, echo time 95–110 ms, 22-cm field of view) were obtained before injection of contrast material. After intravenous administration of gadopentetate dimeglumine (Gd-DTPA; Magnevist, Schering, Berlin, Germany) at a dose of 0.1 mmol/kg, T1-weighted spin-echo axial and sagittal sequences and T1-weighted spin-echo fat-suppressed coronal sequences were performed sequentially with the same parameters prior to Gd-DTPA injection, using a section thickness of 5 mm and a matrix size of 512 × 512.
Image assessment and diagnostic criteria
Two radiologists specializing in head-and-neck cancers evaluated all scans separately, and any disagreements were resolved by consensus. The anatomic sites surrounding the nasopharynx are listed in Table 2. Nasal cavity invasion was defined as the tumor invading the bony nasal septum, turbinate or exceeding beyond the line connecting bilateral pterygopalatine fossae[9]–[12]. Oropharyngeal involvement was defined as tumor involvement below the plane of the superior surface of the soft palate or the lower margin of C1[9],[12]–[14]. Hypopharyn geal involvement was defined as tumor detection below the plane of the superior border of the hyoid bone or the lower margin of C3[9],[10],[12]. Infratemporal fossa involvement was defined as extension beyond the anterior surface of the lateral pterygoid muscle or lateral extension beyond the posterolateral wall of the maxillary sinus or pterygomaxillary fissure[15]. The criterion for orbit invasion was the tumor extending to the orbital apex, the inferior orbital fissure, or the superior orbital fissure[16]. Bilateral NPC was defined as the primary tumor extending across the midline of the nasopharynx[17]. According to incidence rates of tumor invasion, the anatomic sites surrounding the nasopharynx were classified into high-risk (>30%), medium-risk (5%–30%), and low-risk (<5%) groups.
Table 2. Rates of tumor invasion into anatomic sites surrounding the nasopharynx.
| Anatomic site | No. of patients (%) |
| High-risk | |
| Tensor veli palatine muscle | 1570 (66.4) |
| Nasal cavity | 1224 (51.7) |
| Basis of sphenoid bone | 1105 (46.7) |
| Pterygoid process | 1063 (44.9) |
| Clivus | 934 (39.5) |
| Petrous apex | 932 (39.4) |
| Prevertebral muscle | 876 (37.0) |
| Foramen lacerum | 826 (34.9) |
| Medium-risk | |
| Foramen ovale | 557 (23.5) |
| Great wing of sphenoid bone | 554 (23.4) |
| Oropharynx | 509 (21.5) |
| Medial pterygoid muscle | 449 (19.0) |
| Cavernous sinus | 424 (17.9) |
| Pterygopalatine fossa | 407 (17.2) |
| Sphenoidal sinus | 374 (15.8) |
| Hypoglossal canal | 256 (10.8) |
| Lateral pterygoid muscle | 219 (9.3) |
| Ethmoid sinus | 124 (5.2) |
| Jugular foramen | 120 (5.1) |
| Low-risk | |
| Orbit | 93 (3.9) |
| Infratemporal fossa | 73 (3.1) |
| Cervical vertebrae | 54 (2.3) |
| Maxillary sinus | 51 (2.2) |
| Hypopharynx | 21 (0.9) |
| Frontal sinus | 5 (0.2) |
The diagnostic criteria for lymph node (LN) metastases[18],[19] included (a) lateral retropharyngeal lymph node (RLN) with a minimal axial diameter (MID) of ≥5 mm in the largest plane or any node in the median retropharyngeal group; (b) cervical lymph node (CLN) in the jugulodigastric region with a MID ≥11 mm or all other CLNs ≥10 mm; (c) LNs of any size with central necrosis or a contrast-enhanced rim; (d) nodal grouping, the presence of 3 or more aggregated LNs, each having an MID of 8 to 10 mm; and (e) LNs of any size with extracapsular spread as characterized by irregular LN capsular enhancement, obliterated fat space between the node and adjacent tissues, and/or confluent LNs. The assignment of LN location was made according to the Radiation Therapy Oncology Group (RTOG) guidelines[20],[21]. Using the superior border of the hyoid bone and the lower border of the cricoid cartilage as two separators, we further categorized the neck node levels into three volumes: upper neck, which included retropharyngeal region (RP) and level II; middle neck, which included levels III and Va; and lower neck, which included levels IV and Vb and the supraclavicular fossa (SCF).
Statistical analysis
Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS) 13.0 (Chicago, IL, USA). The Chi-square test was used to examine differences between categorical variables, and two-tailed P values < 0.05 were considered significant.
Results
Characteristics and pattern of local tumor extension
The cumulative rates of tumor invasion into the anatomic sites surrounding the nasopharynx are shown in Table 2. The tensor veli palatine muscle (TVPM) had the highest involvement rate (66.4%), followed by the nasal cavity (51.7%). The anatomic sites at high risk included the TVPM, nasal cavity, basis of sphenoid bone, pterygoid process, clivus, petrous apex, prevertebral muscle, and foramen lacerum. The medium-risk anatomic sites included the foramen ovale, great wing of the sphenoid bone, oropharynx, medial pterygoid muscle, cavernous sinus, pterygopalatine fossa, sphenoidal sinus, hypoglossal canal, lateral pterygoid muscle, ethmoid sinus, and jugular foramen. The low-risk sites included the orbit, infratemporal fossa, cervical vertebrae, maxillary sinus, hypopharynx, and frontal sinus. Thus, the high-risk anatomic sites were adjacent to the nasopharynx, whereas those at medium or low risk were separated from the nasopharynx (except the oropharynx).
If high-risk anatomic sites were involved, the rates of tumor invasion into the adjacent medium-risk sites increased; if not, the rates were significantly lower (P < 0.01) (Table 3). For example, when the tumor invaded the TVPM, 28.5% of patients had medial pterygoid muscle involvement; in contrast, when the TVPM was not involved, only 0.3% of patients had medial pterygoid muscle involvement. Similarly, the rate of tumor invasion into the cavernous sinus reached more than 40% if the clivus, petrous apex, and/or foramen lacerum were involved; if not, the rate was lower than 3%.
Table 3. Relationship between tumor invasion into anatomic sites at high risk and invasion into the adjacent medium-risk anatomic sites.
| Medium-risk site | Tumor invasion | P | |
| Tensor veli palatine muscle | |||
| Invasion | Non-invasion | ||
| Foramen ovale | 33.9% (533/1570) | 3.0% (24/796) | < 0.01 |
| Great wing of sphenoid bone | 33.8% (531/1570) | 2.9% (23/796) | < 0.01 |
| Medial pterygoid muscle | 28.5% (447/1570) | 0.3% (2/796) | < 0.01 |
| Oropharynx | 28.3% (445/1570) | 8.0% (64/796) | < 0.01 |
| Pterygopalatine fossa | 24.1% (378/1570) | 3.6% (29/796) | < 0.01 |
| Nasal cavity | |||
| Invasion | Non-invasion | ||
| Pterygopalatine fossa | 29.4% (360/1224) | 4.1% (47/1142) | < 0.01 |
| Ethmoid sinus | 9.2% (113/1224) | 1.0% (11/1142) | < 0.01 |
| Basis of sphenoid bone | |||
| Invasion | Non-invasion | ||
| Great wing of sphenoid bone | 48.8% (539/1105) | 1.2% (15/1261) | < 0.01 |
| Foramen ovale | 47.5% (525/1105) | 2.5% (32/1261) | < 0.01 |
| Sphenoidal sinus | 33.1% (366/1105) | 0.6% (8/1261) | < 0.01 |
| Pterygoid process | |||
| Invasion | Non-invasion | ||
| Great wing of sphenoid bone | 49.1% (522/1063) | 2.5% (32/1303) | < 0.01 |
| Foramen ovale | 48.4% (515/1063) | 3.2% (42/1303) | < 0.01 |
| Medial pterygoid muscle | 37.7% (401/1063) | 3.7% (48/1303) | < 0.01 |
| Pterygopalatine fossa | 36.9% (392/1063) | 1.2% (15/1303) | < 0.01 |
| Lateral pterygoid muscle | 19.4% (206/1063) | 1.0% (13/1303) | < 0.01 |
| Clivus | |||
| Invasion | Non-invasion | ||
| Great wing of sphenoid bone | 54.0% (504/934) | 3.5% (50/1432) | < 0.01 |
| Foramen ovale | 52.5% (490/934) | 4.7% (67/1432) | < 0.01 |
| Cavernous sinus | 42.4% (396/934) | 2.0% (28/1432) | < 0.01 |
| Sphenoidal sinus | 36.3% (339/934) | 2.4% (35/1432) | < 0.01 |
| Hypoglossal canal | 26.6% (248/934) | 0.6% (8/1432) | < 0.01 |
| Jugular foramen | 12.5% (117/934) | 0.2% (3/1432) | < 0.01 |
| Petrous apex | |||
| Invasion | Non-invasion | ||
| Foramen ovale | 54.8% (511/932) | 3.2% (46/1434) | < 0.01 |
| Great wing of sphenoid bone | 54.4% (507/932) | 3.3% (47/1434) | < 0.01 |
| Cavernous sinus | 42.8% (399/932) | 1.7% (25/1434) | < 0.01 |
| Hypoglossal canal | 27.0% (252/932) | 0.3% (4/1434) | < 0.01 |
| Jugular foramen | 12.8% (119/932) | 0.1% (1/1434) | < 0.01 |
| Prevertebral muscle | |||
| Invasion | Non-invasion | ||
| Oropharynx | 32.6% (286/876) | 15.0% (223/1490) | < 0.01 |
| Hypoglossal canal | 26.9% (236/876) | 1.3% (20/1490) | < 0.01 |
| Jugular foramen | 11.9% (104/876) | 1.1% (16/1490) | < 0.01 |
| Foramen lacerum | |||
| Invasion | Non-invasion | ||
| Foramen ovale | 57.9% (478/826) | 5.1% (79/1540) | < 0.01 |
| Great wing of sphenoid bone | 56.7% (468/826) | 5.6% (86/1540) | < 0.01 |
| Cavernous sinus | 46.4% (383/826) | 2.7% (41/1540) | < 0.01 |
Among all patients, 94% had bilateral NPC, and most had tumor invasion of the superior-posterior wall and extending across the midline of the nasopharynx. However, most anatomic sites were at low risk of concurrent bilateral tumor invasion (<10%) except the TVPM (13.8%) and prevertebral muscle (13%).
Characteristics and pattern of LN metastasis
In this cohort of patients, the rate of LN metastasis was 81.1% (1920/2366), and bilateral LN involvement was observed in 52.3% (1004/1920) of patients. Among the neck node levels according to the RTOG guidelines, RP (84.8%, 1628/1920) and level IIb (61.5%, 1181/1920) were the most frequently involved regions, followed in order by levels IIa, III, Va, IV, Vb, SCF, and Ib. No LN metastasis was found in the retrostyloid space or in levels Ia or VI (Table 4).
Table 4. Characteristics of nodal spread of the 1920 patients with node-positive NPC.
| Level | No. of patients (%) |
| Retropharyngeal region | 1628 (84.8) |
| Level IIb | 1181 (61.5) |
| Level IIa | 706 (36.8) |
| Level III | 545 (28.4) |
| Level Va | 155 (8.1) |
| Level IV | 102 (5.3) |
| Level Vb | 50 (2.6) |
| Supraclavicular fossa | 46 (2.4) |
| Level Ib | 39 (2.0) |
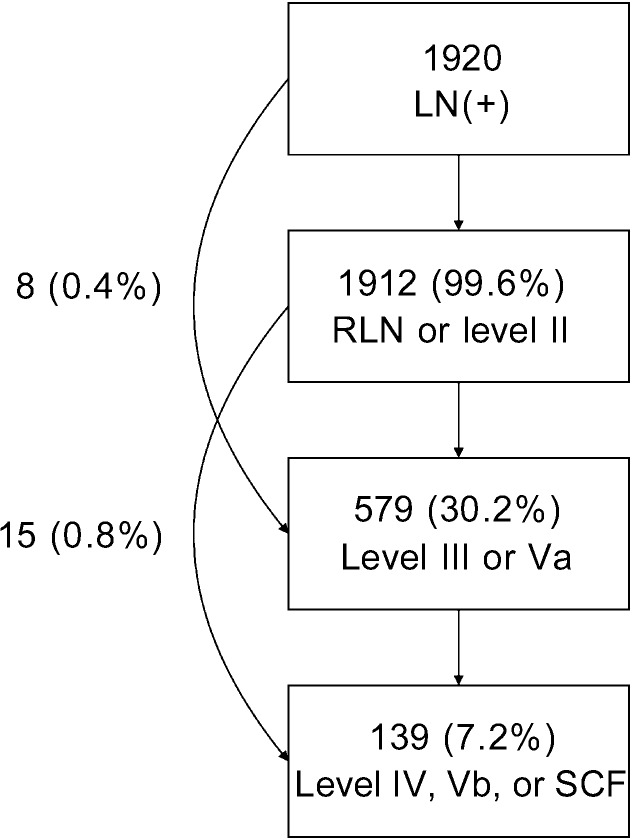
In the 1920 patients with positive LNs, the involvement rates of upper neck (RP and level II), middle neck (levels III and Va) and lower neck (levels IV, Vb, and SCF) were 99.6% (1912/1920), 30.2% (579/1920) and 7.2% (139/1920), respectively, and skip metastasis occurred in only 1.2% (23/1920) of patients (Figure 1). In the 929 patients who had unilateral upper neck involvement, the involvement rates of ipsilateral middle neck and lower neck were 19.5% (181/929) and 4.3% (40/929), respectively, whereas the involvement rates of contralateral middle neck and lower neck were only 1.8% (17/929) and 0.4% (4/929), respectively. However, in the 983 patients with bilateral upper neck involvement, the rates of LN metastasis to the middle neck and lower neck increased to 39.2% (385/983) and 9.6% (94/983), respectively.
Figure 1. The pattern of lymph node metastasis.
RLN, retropharyngeal lymph node; SCF, supraclavicular fossa.
Discussion
The CTV in IMRT planning for NPC includes CTV1 and CTV2. CTV1 is defined as high-risk regions including GTV (the primary tumor and RLN) plus a 5- to 10-mm margin and the entire nasopharyngeal mucosa plus a 5-mm submucosal volume. CTV2 includes the low-risk areas of the nasopharynx and neck that need prophylactic irradiation, but the optimal region remains controversial[3]–[6],[22],[23]. In some treatment centers, there is no CTV2 but CTV1 in NPC treatment planning, and the region of CTV1 is similar to that of CTV2. At treatment centers in North America[23], Hong Kong[3], and Singapore[4], CTV2 includes the entire nasopharynx, posterior 1/4 to 1/3 of the nasal cavity and maxillary sinuses, parapharyngeal space, pterygoid fossae, anterior 1/2 to 2/3 of the clivus (entire clivus, if involved), skull base (foramen ovale bilaterally), inferior sphenoid sinus, cavernous sinus, and bilateral RP, and levels II–IV (Table 5). Although locoregional control rates greater than 90% have been reported in these centers, the method of CTV2 delineation was largely derived from the experience of conventional radiotherapy, which encompassed most anatomic sites surrounding the nasopharynx bilaterally and did not differ according to the clinical stage. Thus, this delineation method is suboptimal and lacks individualization. Ng et al.[24] reported the patterns of failure after IMRT in 193 NPC patients and found that most of the locoregional failure occurred “in field” (within the 95% isodose lines), whereas marginal or outside-field failure was uncommon. Therefore, whether the volume of CTV2 can be selectively reduced to better protect normal tissue without affecting local tumor control has become a research focus in the field of IMRT planning for NPC.
Table 5. Differences of delineation of clinical target volume (CTV, for CTV2) in intensity-modulated radiotherapy for NPC in different cancer centers.
| Region | RTOG0615[23] | Singapore[4] | Hong Kong[3] | Fuzhou[5] | Chinese guideline[22] |
| Nasal cavity | Posterior 1/4–1/3 | Posterior 1/3 | Posterior 1/3 | 5 mm anterior to the posterior nasal aperture | Posterior part |
| Maxillary sinus | Posterior 1/4–1/3 | Posterior 1/3 | Posterior 1/3 | 5 mm anterior to the maxillary mucosa | 5 mm anterior to the maxillary mucosa |
| Clivus | Anterior 1/2–2/3 (entire if involved) | Anterior 1/2 (entire if involved) | Anterior 1/2 (entire if involved) | Anterior 1/3 | Anterior 1/3 |
| Foramen ovale | Bilaterally | Bilaterally | Bilaterally | – | Bilaterally |
| Sphenoid sinus | Inferior part (entire in T3–T4 disease) | Included | Included | Inferior part (entire if involved) | Inferior wall/basis |
| Cavernous sinus | Included in high-risk patients (T3, T4, bulky disease involving the roof of the nasopharynx) | Included | Included | Inferior part | – |
| Retropharyngeal lymph node | Bilaterally (skull base to cranial edge of the hyoid) | Bilaterally (skull base to the level of the hyoid) | Bilaterally (skull base to the bottom of the hyoid) | Bilaterally (skull base to cranial edge of C2) | Bilaterally (skull base to caudal edge of C2) |
| Levels II–V | Bilaterally | Bilaterally | Electively irradiated | Bilaterally | Electively irradiated |
| Level Ib | Bilaterally included in node-positive patients | Included if the ipsilateral neck involved | Electively irradiated | – | Electively irradiated |
Recently, Lin et al.[5] reported that IMRT using a reduced-CTV2 technique (Table 5) for NPC provided favorable 3-year local control, regional control, and overall survival rates of 95%, 98%, and 90%, respectively. In total, 10 of the 12 cases with local recurrence occurred within the GTV, and 2 additional cases recurred locally with a component out of the GTV. No isolated recurrence was found at the margin of the reduced CTV2. Among the 6 patients with regional recurrence, 4 recurred within the CTV2, and 2 additional cases recurred within the region of the spared parotid gland. Tang et al.[25] compared the prognosis of 138 NPC patients with N0 disease with or without prophylactic lower neck irradiation. None of the patients in either group experienced regional failure, and the risks of distant metastasis did not differ statistically. Gao et al.[26] retrospectively analyzed the clinical data of 410 patients with LN-negative NPC. CTV2 in the neck only included bilateral RP and levels II, III, and Va, instead of whole-neck irradiation. In a median follow-up of 54 months, 4 patients developed LN recurrence: 3 had nodal recurrences occurred in level II, and only 1 had outside-field LN recurrence in level IV. The above results suggest that individualized radiotherapy with reduced CTV2 in treating NPC is feasible.
The delineation of CTV2 should be designed individually based on the GTV, the locoregional extension patterns, and the biological nature of NPC, so that unnecessary or missed irradiation can be avoided[17]. For the local extension pattern, we classified the anatomic sites surrounding the nasopharynx into high-risk, medium-risk, and low-risk groups, and found that the risk of tumor invasion into various anatomic sites was closely correlated with the distance to the nasopharynx and whether the adjacent anatomic sites were involved. The anatomic sites at high risk, such as the TVPM, nasal cavity, and basis of sphenoid bone, were adjacent to the nasopharynx, whereas the anatomic sites at medium or low risk (except oropharynx) were distant from the nasopharynx and separated from it by other anatomic sites. If the high-risk anatomic sites were involved, the rates of tumor invasion into the adjacent medium-risk sites increased; if not, the rates were significantly lower (P < 0.01). The results of this study indicate that local disease spreads stepwise from proximal sites to distal sites, and a skip pattern of local extension is unusual, which further confirms the findings of Liang et al.[17].
Notably, in some cases, the rates of tumor invasion into the anatomic sites at medium risk still reached more than 5%, even when the adjacent high-risk sites were not involved. For example, oropharyngeal involvement had a rate of 8%–15% without TVPM and/or prevertebral muscle involvement, which indicated that NPC could directly invade the oropharynx through submucosal infiltration. There is a complicated relationship between the various anatomic sites s urrounding the nasopharynx, and an anatomic site is usually adjacent to various structures and connected with other distant sites through natural cavity and neural foramina. Dubrulle et al.[27] and Liang et al.[17] reported several common pathways of local NPC extension. The well-known routes included tumor invasion into the cavernous sinus through the foramen lacerum and/or foramen ovale, involvement of the infratemporal fossa through pterygopalatine fossa, and others. Thus, whether the anatomic site should be included in CTV2 may not only depend on if adjacent sites are involved. Because NPC has multiple focus origins and usually involves both sides of the nasopharynx, we recommend that bilateral anatomic sites at high risk (i.e., the parapharyngeal space, posterior part of nasal cavity, pterygoid process, prevertebral muscle, clivus, petrous apex, foramen lacerum, and basis of the sphenoid bone), as well as other high-risk neural foramina (i.e., the pterygopalatine fossa and foramen ovale bilaterally) should be included in CTV2, which is similar to the definition of CTV2 in the 2010 Chinese Consensus Guidelines for NPC IMRT planning (Table 5)[22]. However, the decision to include anatomic sites at medium or low risk in the definition of CTV2 should be based on multiple factors, including the distance from GTV, involvement of the ipsilateral adjacent anatomic sites, and the common routes of tumor spread, to maximize the tumor-killing and normal tissue-protecting effects and achieve individualized treatment.
For the pattern of LN metastasis, Tang et al.[25] showed that RP and level II were the first echelon of LN metastasis in NPC, with levels III and V being the second echelon, and levels IV and SCF being the third echelon. In the current study, we used the superior border of the hyoid bone and lower border of the cricoid cartilage as two separators and categorized the RTOG guidelines into upper neck (RP and level II), middle neck (levels III and Va), and lower neck (levels IV, Vb, and SCF). We found that the rates of LN metastasis to the upper, middle, and lower neck decreased successively, and skip metastasis happened only in 1.2% of patients. Moreover, in patients with unilateral upper neck involvement, the involvement rates of ipsilateral middle neck and lower neck were 19.5% and 4.3% respectively, whereas the involvement rates of contralateral middle or lower neck were less than 2%. However, when the bilateral upper neck was involved, the rates of LN metastasis to the middle or lower neck markedly increased. Our results confirm that LN metastasis spreads from upper neck to lower neck, and skip metastasis is unusual, which concurs with the findings of Tang et al.[25]. Tomita et al.[28] investigated the pattern of LN metastasis in unilateral NPC and found that the involvement rates of contralateral RLN and level II were 10% and 16%, respectively, whereas less than 3% of patients had involvement of contralateral levels III, IV, and/or V. Thus, the authors indicated that contralateral LN areas other than RLN and level II could be omitted in patients with unilateral NPC. In this study, 94% of patients had bilateral NPC, and the relationship between the laterality of local disease and LN metastasis was not analyzed. Therefore, we recommend that for patients with N0 disease, CTV2 should only include bilateral RP and levels II, III, and Va. For patients with unilateral LN involvement, CTV2 can include whole ipsilateral neck and contralateral RP and levels II, III, and Va; for patients with bilateral LN involvement, bilateral whole neck should be included.
The 2010 Chinese Consensus Guidelines were similar to our recommendations on the setting of nodal CTV2. However, they separated RLNs from CLNs and proposed that the CTV2 of RLN should refer to that of the primary disease because of their close relation. Furthermore, for patients with RLN metastasis alone, CTV2 can only include bilateral RP and levels II, III, and Va[22]. Several studies have indicated that both RLNs and level II nodes are the first-echelon nodes in NPC[25],[29], and RLN metastasis has been classified as N1 disease[9],[10]. Thus, the nodal CTV2 for patients with RLN metastasis should be the same as that for patients with level II involvement. Most recently, Ou et al.[30] reviewed records of 119 NPC patients with RLN metastasis alone and found that the treatment outcome of elective irradiation to RP and levels II, III, and Va was not inferior to that of whole neck irradiation; however, these results need to be confirmed with prospective data.
In conclusion, we showed in this large sample size, retrospective study that local disease spread stepwise from proximal sites to distal sites, that LN metastasis spread from upper neck to lower neck in NPC, and that a skip pattern of local extension and LN metastasis was unusual. Elective radiation of the anatomic sites surrounding the nasopharynx and the RTOG guidelines for neck levels may be feasible. However, our recommendations for individualized CTV delineation for NPC should be considered suggestive and not definitive until these results can be confirmed by large prospective studies.
References
- 1.Parkin DM, Whelan SL, Ferlay J, et al. Vol. VIII Lyon: IARC Press; 2002. Cancer incidence in five continents. [Google Scholar]
- 2.Lee N, Xia P, Quivey JM, et al. Intensity-modulated radiotherapy in the treatment of nasopharyngeal carcinoma: an update of the UCSF experience. Int J Radiat Oncol Biol Phys. 2002;53:12–22. doi: 10.1016/s0360-3016(02)02724-4. [DOI] [PubMed] [Google Scholar]
- 3.Kam MK, Teo PM, Chau RM, et al. Treatment of nasopharyngeal carcinoma with intensity-modulated radiotherapy: the Hong Kong experience. Int J Radiat Oncol Biol Phys. 2004;60:1440–1450. doi: 10.1016/j.ijrobp.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 4.Tham IW, Hee SW, Yeo RM, et al. Treatment of nasopharyngeal carcinoma using intensity-modulated radiotherapy—the national cancer centre Singapore experience. Int J Radiat Oncol Biol Phys. 2009;75:1481–1486. doi: 10.1016/j.ijrobp.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 5.Lin S, Pan J, Han L, et al. Nasopharyngeal carcinoma treated with reduced-volume intensity-modulated radiation therapy: report on the 3-year outcome of a prospective series. Int J Radiat Oncol Biol Phys. 2009;75:1071–1078. doi: 10.1016/j.ijrobp.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 6.Lai SZ, Li WF, Chen L, et al. How does intensity-modulated radiotherapy versus conventional two-dimensional radiotherapy influence the treatment results in nasopharyngeal carcinoma patients? Int J Radiat Oncol Biol Phys. 2011;80:661–668. doi: 10.1016/j.ijrobp.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 7.International Commission of Radiation Units and Measurements . Bethesda: International Commission on Radiation Units and Measurements; 1993. ICRU report 50: prescribing, recording, and reporting photon beam therapy. [Google Scholar]
- 8.International Commission of Radiation Units and Measurements . Bethesda: International Commission on Radiation Units and Measurements; 1999. ICRU report 62: prescribing, recording, and reporting photon beam therapy (supplement to ICRU report 50) [Google Scholar]
- 9.Edge SB, Byrd DR, Compton CC, et al. 7th edition. New York: Springer; 2010. AJCC cancer staging manual. [Google Scholar]
- 10.Committee of Chinese Clinical Staging of Nasopharyngeal Carcinoma Report on the revision of nasopharyngeal carcinoma '92 staging. Chin J Radiat Oncol. 2009;18:2–6. [in Chinese] [Google Scholar]
- 11.Sun Y, Ma J. Comment for the Chinese 2008 staging system for nasopharyngeal carcinoma. Chin J Cancer. 2009;28:1016–1021. doi: 10.5732/cjc.009.10448. [DOI] [PubMed] [Google Scholar]
- 12.Liao XB, Mao YP, Liu LZ, et al. How does magnetic resonance imaging influence staging according to AJCC staging system for nasopharyngeal carcinoma compared with computed tomography? Int J Radiat Oncol Biol Phys. 2008;72:1368–1377. doi: 10.1016/j.ijrobp.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 13.Chong VF, Mukherji SK, Ng SH, et al. Nasopharyngeal carcinoma: review of how imaging affects staging. J Comput Assist Tomogr. 1999;23:984–993. doi: 10.1097/00004728-199911000-00032. [DOI] [PubMed] [Google Scholar]
- 14.Heng DM, Wee J, Fong KW, et al. Prognostic factors in 677 patients in Singapore with nondisseminated nasopharyngeal carcinoma. Cancer. 1999;86:1912–1920. [PubMed] [Google Scholar]
- 15.Cooper J, Fleming ID, Henson DE. 6th edition. Philadelphia: JB Lippincott; 2002. AJCC cancer staging manual. [Google Scholar]
- 16.Hsu WM, Wang AG. Nasopharyngeal carcinoma with orbital invasion. Eye (Lond) 2004;18:833–838. doi: 10.1038/sj.eye.6701358. [DOI] [PubMed] [Google Scholar]
- 17.Liang SB, Sun Y, Liu LZ, et al. Extension of local disease in nasopharyngeal carcinoma detected by magnetic resonance imaging: improvement of clinical target volume delineation. Int J Radiat Oncol Biol Phys. 2009;75:742–750. doi: 10.1016/j.ijrobp.2008.11.053. [DOI] [PubMed] [Google Scholar]
- 18.van den Brekel MW, Stel HV, Castelijns JA, et al. Cervical lymph node metastasis: assessment of radiologic criteria. Radiology. 1990;177:379–384. doi: 10.1148/radiology.177.2.2217772. [DOI] [PubMed] [Google Scholar]
- 19.Tang L, Li L, Mao Y, et al. Retropharyngeal lymph node metastasis in nasopharyngeal carcinoma detected by magnetic resonance imaging: prognostic value and staging categories. Cancer. 2008;113:347–354. doi: 10.1002/cncr.23555. [DOI] [PubMed] [Google Scholar]
- 20.Gregoire V, Levendag P, Ang KK, et al. CT-based delineation of lymph node levels and related CTVs in the node-negative neck: DAHANCA, EORTC, GORTEC, NCIC, RTOG consensus guidelines. Radiother Oncol. 2003;69:227–236. doi: 10.1016/j.radonc.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 21.Gregoire V, Eisbruch A, Hamoir M, et al. Proposal for the delineation of the nodal CTV in the node-positive and the postoperative neck. Radiother Oncol. 2006;79:15–20. doi: 10.1016/j.radonc.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 22.Committee of Chinese Clinical Staging of Nasopharyngeal Carcinoma 2010 Consensus Guidelines for intensity-modulated radiotherapy target volume and dosimetric planning in nasopharyngeal carcinoma. Chin J Radiat Oncol. 2011;20:267–269. [in Chinese] [Google Scholar]
- 23.Lee NY, Zhang Q, Pfister DG, et al. Addition of bevacizumab to standard chemoradiation for locoregionally advanced nasopharyngeal carcinoma (RTOG 0615): a phase 2 multi-institutional trial. Lancet Oncol. 2012;13:172–180. doi: 10.1016/S1470-2045(11)70303-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ng WT, Lee MC, Hung WM, et al. Clinical outcomes and patterns of failure after intensity-modulated radiotherapy for nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2011;79:420–428. doi: 10.1016/j.ijrobp.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 25.Tang L, Mao Y, Liu L, et al. The volume to be irradiated during selective neck irradiation in nasopharyngeal carcinoma: analysis of the spread patterns in lymph nodes by magnetic resonance imaging. Cancer. 2009;115:680–688. doi: 10.1002/cncr.24049. [DOI] [PubMed] [Google Scholar]
- 26.Gao Y, Zhu G, Lu J, et al. Is elective irradiation to the lower neck necessary for N0 nasopharyngeal carcinoma? Int J Radiat Oncol Biol Phys. 2010;77:1397–1402. doi: 10.1016/j.ijrobp.2009.06.062. [DOI] [PubMed] [Google Scholar]
- 27.Dubrulle F, Souillard R, Hermans R. Extension patterns of nasopharyngeal carcinoma. Eur Radiol. 2007;17:2622–2630. doi: 10.1007/s00330-007-0616-z. [DOI] [PubMed] [Google Scholar]
- 28.Tomita N, Fuwa N, Ariji Y, et al. Factors associated with nodal metastasis in nasopharyngeal cancer: an approach to reduce the radiation field in selected patients. Br J Radiol. 2011;84:265–270. doi: 10.1259/bjr/47164832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu LZ, Zhang GY, Xie CM, et al. Magnetic resonance imaging of retropharyngeal lymph node metastasis in nasopharyngeal carcinoma: patterns of spread. Int J Radiat Oncol Biol Phys. 2006;66:721–730. doi: 10.1016/j.ijrobp.2006.05.054. [DOI] [PubMed] [Google Scholar]
- 30.Ou X, Shen C, Kong L, et al. Treatment outcome of nasopharyngeal carcinoma with retropharyngeal lymph nodes metastasis only and the feasibility of elective neck irradiation. Oral Oncol. 2012 doi: 10.1016/j.oraloncology.2012.04.011. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]