Abstract
Objective
To determine whether death and/or neurodevelopmental impairment (NDI) after severe intracranial hemorrhage (ICH; grade 3 or 4) differs by gestational age (GA) at birth in extremely low birth weight (ELBW) infants.
Study Design
Demographic, perinatal and neonatal factors potentially contributing to NDI for ELBW infants (23 to 28 weeks gestation) were obtained retrospectively; outcome data came from the ELBW Follow-up Study. NDI was defined at 18 to 22 months corrected age as moderate/severe cerebral palsy, Bayley Scales of Infant Development II cognitive or motor score <70, and/or blindness or deafness. Characteristics of younger versus older infants with no versus severe ICH associated with death or NDI were compared. Generalized linear mixed models predicted death or NDI in each GA cohort.
Result
Of the 6638 infants, 61.8% had no ICH and 13.6% had severe ICH; 39% of survivors had NDI. Risk-adjusted odds of death or NDI and death were higher in the lower GA group. Lower GA increased the odds of death before 30 days for infants with severe ICH. Necrotizing enterocolitis (particularly surgical NEC), late onset infection, cystic periventricular leukomalacia and post-natal steroids contributed to mortality risk. NDI differed by GA in infants without ICH and grade 3, but not grade 4 ICH. Contributors to NDI in infants with severe ICH included male gender, surgical NEC and post-hemorrhagic hydrocephalus requiring a shunt.
Conclusion
GA contributes to the risk of death in ELBW infants, but not NDI among survivors with severe ICH. Male gender, surgical NEC and need for a shunt add additional risk for NDI.
Keywords: infant, premature, extremely low birth weight, death, neurodevelopmental impairment
Introduction
Severe intracranial hemorrhage (ICH), which primarily occurs in the first week of life, worsens the neurodevelopmental prognosis of premature infants.1–8 Previous studies have not investigated an independent effect of gestational age (GA) on outcome after ICH. Infants born at the lower limit of viability have extremely immature undifferentiated brains. It is not known whether to expect better or worse outcome after ICH at these GA’s compared with infants born later in the second trimester of pregnancy. This information could aid clinicians in counseling parents during the early post-natal weeks concerning aggressiveness of care at various GA’s after a severe ICH has occurred. The objective of this study was to determine if death and/or neurodevelopmental outcome after severe ICH differs by GA at birth in extremely low birth weight (ELBW) infants born <29 weeks gestation. Specific aims were (1) to determine if the primary outcome, death or neurodevelopmental impairment (NDI), after severe ICH is more likely in infants born at 23 to 25 compared with 26 to 28 weeks gestation and (2) to determine, among surviving infants, if the prevalence of NDI, after comparable severity of ICH, is influenced by GA. We hypothesized that lower GA is associated with higher risk of death or NDI in infants with a severe ICH.
Methods
Population
This is a retrospective cohort analysis of infants born between 23 and 28 completed weeks of gestation with a birth weight 401 to 1000 g (ELBW) born at or transferred before 2 weeks of age to one of 18 NICHD NRN (Neonatal Research Network) centers during a 5-year period. The presence and severity of ICH was determined from a head ultrasound in the first 28 post-natal days.
Data collection
Demographic, perinatal and neonatal morbidity variables were obtained from the NRN Registry of Morbidity and Mortality (GDB (Generic Data Base)) and neurodevelopmental outcome data were obtained from the ELBW Follow-up Study by trained staff using standard definitions listed in the study’s Manual of Operations. Both the neurologic examination and administration of the Bayley Scales of Infant Development II (BSID)9 were performed by certified examiners who were blinded to the grade of ICH and trained to reliability at each participating Network center. NDI was defined as including at least one of the following: moderate/severe cerebral palsy (CP) with GMF (Gross Motor Function) score 3 to 5 (ref. 10), Mental Developmental Index (MDI) or Psychomotor Developmental Index (PDI) <70 on the BSID-II at 18 to 22 months CA (corrected age), blindness (no functional vision in both eyes) or deafness (requiring amplification in both ears). ICH was described by the worst grade noted on HUS within the first 28 days of life. Severe ICH included either grade 3 or 4. Grade 3 ICH referred to the presence of blood in one or both ventricles with ventricular dilatation and grade 4 ICH required the presence of blood (echodensity) in the brain parenchyma.11
Statistical analyses
Bivariate analyses were used to compare characteristics of infants with and without the outcomes of death and death or NDI, and for survivors with and without NDI for infants with no ICH and severe ICH. Student’s t-tests for continuous variables and chi-square tests for categorical variables were used in bivariate analyses. Generalized linear mixed models were created to predict the primary outcome, death or NDI, as well as death and NDI alone among survivors at follow-up. Death was also stratified as before or after 30 days because some infants diagnosed early with severe ICH could have died during the first weeks after withdrawal of support. Although data were collected for infants with all grades of ICH, models were only created for infants without ICH and those with grade 3 or 4 ICH. Predictors for the generalized linear models included demographic, perinatal and early neonatal morbidity variables, and Network center was controlled for as a random effect. Variables in the models to predict death before 30 days included GA at birth (23 to 25 weeks versus 26 to 28 weeks) Apgar score at 5 min, antenatal steroids (ANS) and early infection (positive culture and initial antibiotics >5 days). Additional variables included in models to predict death after 30 days were post-natal steroids (PNS), necrotizing enterocolitis treated medically (NEC medical) or requiring surgery (NEC surgical), late onset infection (culture proven or clinical), cystic PVL (periventricular leukomalacia) and VP (ventriculoperitoneal) shunt for posthemorrhagic hydrocephalus. In addition to the above, models to predict NDI among survivors included maternal education, Medicaid status and bronchopulmonary dysplasia (BPD; O2 at 36 weeks). Models were also created to predict NDI components (CP, MDI<70 and PDI<70), which included the same variables as those to predict NDI. Models to predict blindness or deafness contained only the GA group as a fixed effect and study center as a random effect because there were insufficient numbers of infants with these outcomes to include additional predictors. The generalized linear mixed models produced adjusted odds ratios (ORs) and 95% confidence intervals (CIs) for GA group and for the other covariates in the models.
Results
In all, 6638 infants were enrolled in the GDB during the study period and had a head ultrasound in the first 28 post-natal days. For this study only those infants with no ICH (4104 infants) and those with severe ICH (1352 infants) are included in the analyses (5456 infants total). The distribution of ICH among the different GA groups in the study population is shown in Table 1. The lower total number of infants enrolled at 27 and 28 weeks is because this Follow-up Study cohort was limited to ELBW (<1000 g BW) infants so those infants at these GA’s with BW>1000 g were not included. In all, 72% were known to have survived to 18 months adjusted age. Of the survivors, NDI outcome was determined for 87 and 39% of those had NDI. The demographic and medical characteristics of all infants followed at 18 to 22 months CA can be viewed in Supplementary Table 1. These infants differed from those lost to follow-up in only a few characteristics including race (4% more were African American), use of ANS (3% fewer) and use of PNS (4% more).
Table 1.
Distribution of ELBW study population (grade of ICH, survival and follow-up) for infants born at different gestational ages
| Gestational age (weeks) | |||||||
|---|---|---|---|---|---|---|---|
| Total | 23 | 24 | 25 | 26 | 27 | 28 | |
| Infants included in analyses | 5456 | 457 | 1052 | 1212 | 1260 | 915 | 560 |
| ICH grade 0 | 4104 | 230 | 675 | 866 | 1026 | 794 | 513 |
| ICH grade 3 | 592 | 84 | 158 | 153 | 112 | 59 | 26 |
| ICH grade 4 | 760 | 143 | 219 | 193 | 122 | 62 | 21 |
| Survived to 30 days (%) | 4561 (84) | 249 (54) | 774 (74) | 1022 (84) | 1156 (92) | 834 (91) | 526 (94) |
| Survived NICU staya (%) | 4179 (77) | 197 (43) | 673 (64) | 930 (77) | 1095 (87) | 785 (86) | 499 (89) |
| Survived to 18 –22 monthsb (%) | 3941 (72) | 188 (41) | 637 (61) | 875 (72) | 1028 (82) | 741 (81) | 472 (84) |
| Lost to follow-up (%) | 435 (8) | 18 (4) | 69 (7) | 99 (8) | 103 (8) | 87 (10) | 59 (11) |
| 18– 22 monthsb follow-up (%)c | 3672 (93) | 175 (93) | 594 (93) | 814 (93) | 970 (94) | 684 (92) | 435 (92) |
| NDI outcome obtained (%)d | 3445 (87) | 164 (87) | 553 (87) | 778 (89) | 901 (88) | 653 (88) | 396 (84) |
| NDI (%)e | 1327 (39) | 107 (65) | 295 (53) | 319 (41) | 324 (36) | 168 (26) | 114 (29) |
Abbreviations: ELBW, extremely low birth weight; ICH, intracranial hemorrhage; NDI, neurodevelopmental impairment; NICU, neonatal intensive care unit.
Survived to discharge, transfer or 1 year of age.
Corrected age (CA).
% Survivors evaluated at 18 –22 months CA.
% Infants successfully evaluated for NDI at 18 –22 months CA.
% Infants who had NDI status determined.
In bivariate analysis, the primary outcome, death (at any time) or NDI, and death alone were more prevalent in the lower GA group. NDI alone was also more prevalent among the lower GA group with no ICH or grade 3 ICH. GA did not influence odds of NDI in infants with grade 4 ICH (Figure 1).
Figure 1.
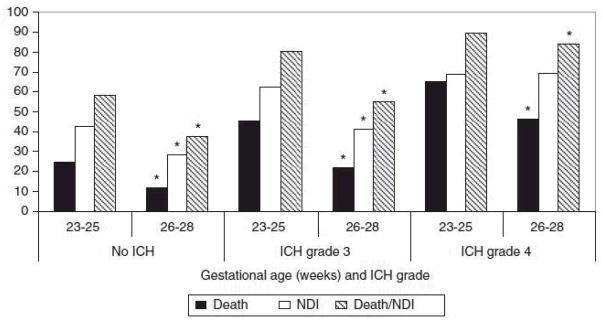
Percentage of infants with primary outcome, death or neurodevelopmental impairment (NDI), and its components by gestational age (GA) at birth and intracranial hemorrhage (ICH) grade. Values are from bivariate analyses. *P<0.05. Note: NDI (% of survivors), death and death/NDI (% of all infants).
The risk-adjusted odds of the composite (any death or NDI), as well as death before 30 days, was higher in the lower GA group for all infants. The adjusted odds of death after 30 days or NDI were significantly higher in infants born at 23 to 25 weeks for infants with no ICH or grade 3 ICH. The adjusted odds of death after 30 days or NDI alone were only higher in the lower GA group for infants with no ICH. GA group was not associated with death after 30 days or NDI or with NDI alone for infants with grade 4 (Supplementary Table 2). Infants with grade 4 ICH in both GA groups had an incidence of NDI near 70% and combined death after 30 days or NDI near 80%.
More deaths occurred before 30 post-natal days compared with after 30 days in the lower GA infants with no or grade 3 ICH. In infants with grade 4 ICH, this was true at all GAs except 28 weeks. Survival without NDI ranged from 22% at 23 weeks to 60% at 28 weeks for infants with no ICH. For infants with severe ICH, this percentage was much lower ranging from approximately 5 to 40% with increasing GA in infants with grade 3 ICH and from 5 to 20% in infants with grade 4 ICH (Figure 2). Besides lower GA, a few additional factors increased the odds of death in the first 30 days for infants with severe ICH. For grade 3 ICH these included SGA (small for gestational age) (OR: 2.56; CI: 1.78, 3.70), low 5 min APGAR (OR: 1.68; CI: 1.07, 2.66) and no ANS (OR: 3.10; CI: 1.97, 4.87). GA was the only significant factor for grade 4 ICH. Both surgical NEC and late onset infection increased the risk of death after 30 days in infants with severe ICH. Other significant morbidities contributing to later death were cystic PVL for grade 3 and medical NEC for grade 4 (Table 2).
Figure 2.
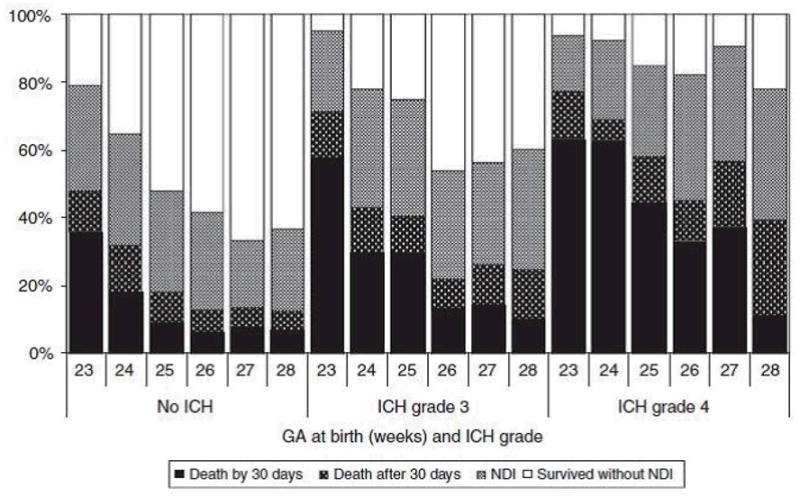
Death (before and after 30 days) and neurodevelopmental impairment (NDI) by gestational age (GA) at birth and intracranial hemorrhage (ICH) grade among infants with known outcomes
Table 2.
Risk-adjusted odds of death after 30 days in infants with severe ICH and other serious morbidities
| Grade 3 ICH | Grade 4 ICH | |
|---|---|---|
| NEC medical | 0.97 (0.25, 3.69) | 3.32 (1.11, 9.94)*** |
| NEC surgical | 5.78 (2.78, 12.04)* | 3.81 (1.66, 8.78)** |
| Late infection | 5.80 (1.33, 25.2)*** | 3.43 (1.10, 10.74)*** |
| PNS | 1.63 (0.86, 3.08) | 0.95 (0.52, 1.74) |
| Cystic PVL | 2.62 (1.14, 6.02)*** | 1.52 (0.81, 2.87) |
Abbreviations: CI, confidence interval; ICH, intracranial hemorrhage; NEC, necrotizing enterocolitis; OR, odds ratio; PNS, post-natal steroids; PVL, periventricular leukomalacia. Values represent OR (95% CI) from generalized linear mixed models.
P<0.0001.
P<0.01.
P<0.05.
Bold values indicate significant values.
In a mixed linear model, lower GA was not significantly associated with NDI among survivors with grade 3 ICH (OR: 1.64; 95% CI: 0.93, 2.89). Factors that predicted NDI among survivors with grade 3 ICH included less maternal education, black non-Hispanic race (versus white non-Hispanic), and male gender, as well as late onset infection, PNS and post-hemorrhagic hydrocephalus requiring a shunt. Need for a shunt had the greatest OR (3.33; 95% CI: 1.48, 7.48) in these infants (Supplementary Figure 1).
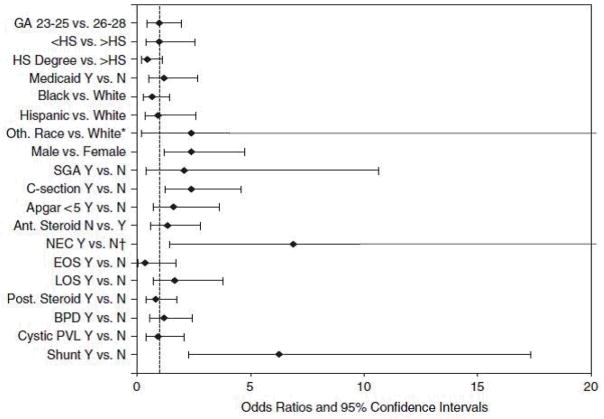
Among surviving infants with grade 4 ICH, lower GA was also not associated with NDI (OR: 0.98; 95% CI: 0.48, 1.99). Neonatal variables that contributed to NDI were male gender, C-section, medical or surgical NEC and need for a shunt. NEC and shunt had large OR (6.89; 95% CI: 1.44, 32.88 for NEC and OR: 6.29; 95% CI: 2.28, 17.32 for shunt) (Figure 3).
Figure 3.
Factors predicting neurodevelopmental impairment (NDI) in infants with grade 4 intracranial hemorrhage (ICH)
In models to predict the components of NDI among survivors with severe ICH, only odds of MDI<70 was greater in younger infants. Risk of deafness was also greater in younger infants with grade 3 ICH, but not grade 4 (Table 3).
Table 3.
Adjusted odds of NDI components for infants with gestational ages of 23–25 versus 26–28 weeks by grade of ICH
| Grade 0 ICH | Grade 3 ICH | Grade 4 ICH | |
|---|---|---|---|
| CP | 1.57 (1.01, 2.44)*** | 1.48 (0.64, 3.41) | 0.84 (0.42, 1.68) |
| MDI<70 | 1.41 (1.16, 1.72)* | 1.85 (1.06, 3.25)*** | 2.01 (1.01, 4.02)*** |
| PDI<70 | 1.38 (1.10, 1.75)** | 1.06 (0.56, 2.01) | 0.77 (0.38, 1.57) |
| Blindness | 4.66 (1.5, 14.52)** | a | a |
| Deafness | 2.67 (1.37, 5.20)** | 3.36 (1.08, 10.44)*** | 1.16 (0.42, 3.24) |
Abbreviations: CI, confidence interval; ICH, intracranial hemorrhage; MDI, Mental Developmental Index; NDI, neurodevelopmental impairment; OR, odds ratio; PDI, Psychomotor Developmental Index.
Number of infants with blindness are too small to obtain OR. Values represent OR (95% CI) from generalized linear mixed models.
P<0.001,
P<0.01,
P<0.05.
Bold values indicate significant values.
In the same models, when other demographic (in addition to GA), perinatal and neonatal variables were tested to determine which factors independently contribute to 2 or more of the most prevalent individual components of NDI (CP, MDI<70 or PDI<70), male gender and need for a shunt for post-hemorrhagic hydrocephalus was significant in infants with grades 3 and 4 ICH. BPD and PNS were also significant in infants with grade 3 and NEC in infants with grade 4 ICH (Table 4).
Table 4.
Demographic and neonatal factors that increase risk-adjusted odds of 2 or more components of NDI in infants with severe ICH
| Factors | Grade 3 ICH | Grade 4 ICH | ||||
|---|---|---|---|---|---|---|
| CP | MDI<70 | PDI<70 | CP | MDI<70 | PDI<70 | |
| Male gender | 1.48 (0.64, 3.41) | 1.77 (1.04, 3.00)**** | 2.23 (1.20, 4.13)**** | 1.31 (0.69, 2.49) | 3.19 (1.65, 6.15)** | 2.21 (1.16, 4.21)**** |
| NEC surgical | 1.73 (0.49, 6.10) | 0.73 (0.27, 1.97) | 3.14 (1.00, 9.82)**** | 4.13 (1.32, 12.89)**** | 7.13 (1.41, 36.06)**** | 4.99 (1.21, 20.61)**** |
| BPD | 3.43 (1.33, 8.84)**** | 2.10 (1.16, 3.79)**** | 1.91 (0.97, 3.77) | 1.36 (0.67, 2.77) | 1.37 (0.69, 2.73) | 1.08 (0.55, 2.15) |
| PNS | 3.93 (1.63, 9.50)*** | 2.33 (1.20, 4.51)**** | 3.33 (1.60, 6.95)*** | 1.23 (0.58, 2.62) | 1.02 (0.48, 2.19) | 1.15 (0.56, 2.38) |
| Shunt | 6.30 (2.54, 15.62)* | 1.87 (0.88, 3.96) | 7.99 (3.34, 19.14)* | 5.07 (2.49, 10.31)* | 4.06 (1.72, 9.58)*** | 7.61 (3.05, 18.99)* |
Abbreviations: BPD, bronchopulmonary dysplasia; CI, confidence interval; ICH, intracranial hemorrhage; MDI, Mental Developmental Index; NDI, neurodevelopmental impairment; NEC, necrotizing enterocolitis; OR, odds ratio; PDI, Psychomotor Developmental Index; PNS, post-natal steroids. Values are from generalized linear mixed models and represent OR (95% CI).
P<0.0001,
P<0.001,
P<0.01,
P<0.05.
Bold values indicate significant values.
Discussion
Reports of CP and cognitive delay after severe ICH, in general, have grouped ELBW infants of different GAs together. Our study sought to answer whether the most immature infants do better or worse with comparable severity of ICH. In the multicenter cohort studied, we found that death was associated with lower GA in infants with and without severe ICH. However, NDI and most of its components were not associated with GA in infants with severe ICH. This information will help inform clinicians when discussing prognosis, level of support and appropriate subsequent care in infants at different GA’s diagnosed with severe ICH in the first weeks of life.
Multiple factors contribute to the early death of premature infants.12–15 In our study, lower GA contributed significantly to early death for all infants studied. For infants with grade 3 ICH, in addition to lower GA, SGA, low APGAR and lack of ANS contributed significantly to early death. There were no additional significant factors, besides lower GA, for infants with grade 4 ICH. Possible explanations for this would be that infants with grade 4 ICH also had the most severe respiratory failure leading to early death or that parents chose to withdraw support in those infants with the most severe ICH.
Tyson et al.16 demonstrated that factors other than GA must be considered when predicting a favorable outcome in extremely premature infants at the time of delivery. These are sex, exposure to ANS, singleton or multiple and birth weight. However, once a baby is delivered and admitted to the intensive care nursery, clinicians need to know the relative importance of multiple factors in the context of the evolving clinical picture, including the occurrence of serious neonatal morbidities such as ICH. We also found that gender and exposure to ANS were important factors for survival.
Several reports from Network centers demonstrate that late onset infection and NEC contribute to later death and higher risk for NDI in all groups of premature infants.17–21 We found this to be true in infants with all grades of ICH. As reported by Blakely et al.,18 infants requiring a surgical procedure for NEC have a high risk of not surviving to discharge, independent of degree of ICH or GA.
Many reports of outcome in the neonatal literature have death or NDI as the primary outcome. In most studies, incidence of death includes both early and late deaths. Early death can also have various definitions (<12 h, <7 days, <30 days, etc). When examining which perinatal and neonatal factors are significant contributors to death, it is important to separate early and late death (as we did in this study) since later occurring factors such as late onset infection, NEC, use of PNS, diagnosis of BPD (O2 at 36 weeks), need for a shunt and development of cystic PVL may appear as protective for any death since they could not be diagnosed if the child died early. In our study, any death or NDI was significantly associated with GA for infants without ICH as well as those with severe ICH, but death after 30 days or NDI did not differ by GA group in those infants with grade 4 ICH. Survival without NDI progressively increased with increasing GA from 23 to 26 weeks.
The lack of increase, or even slight decline, in the percentage of infants who survived without NDI in the 27- and 28-week infants is probably due to the increased percentage of infants with BW at the lower end of normal (or even SGA infants) enrolled at these ages. Male gender has been found to increase risk of death, pulmonary complications and NDI in premature infants.22,23 The higher incidence of RDS and BPD in male infants may account for some of the male disadvantage but other factors, as yet not identified, also likely play a role. PNS have been associated with an increased incidence of CP and poorer cognitive and motor development.24 While the use of PNS has decreased greatly, it is still used in infants with the highest risk of severe BPD. Whether being more selective in choosing appropriate patients and using lower doses of steroids than in earlier trials will abolish this association remains to be seen.25,26
In generalized linear mixed models, GA group was not a significant factor in determining overall NDI in infants with severe ICH. However, gender and need for a shunt were both significantly associated with NDI in infants with either grade 3 or 4 ICH and NEC and C-section was associated with NDI in infants with grade 4 ICH. The finding that C-section is associated with worse outcome probably is due to the likelihood that a C-section occurred either due to significant maternal illness (for example, severe preeclampsia) or significant fetal distress (for example, placental abruption). Infants born prematurely to moms with preterm labor secondary to conditions such as premature cervical dilatation would more likely have been born vaginally. In addition, when some of the components of NDI (CP, MDI<70, PDI<70) were examined to determine what factors contributed to their occurrence, male gender, NEC and need for a shunt were significant for some but not all components.
Grade 4 ICH, also referred to as intraparenchymal echodensity or periventricular hemorrhagic infarction, is defined in this study by the presence of blood (echodensity) in the brain parenchyma. This may have been present in the periventricular white matter or other areas of the brain and is not necessarily associated with blood in the ipsilateral ventricle. In this study, no distinction is made between unilateral and bilateral lesions. This definition and lack of information on laterality is different than that used in other reports of outcome after grade 4 ICH and is the main limitation of this study.2,4–6,8
A recent study by Maitre et al.4 showed better outcome after unilateral versus bilateral grade 4 ICH. In fact, one group of infants with unilateral hemorrhage had normal or near normal cognitive development with a spectrum in severity of contralateral motor impairment.4 It has also been suggested that size and location of parenchymal blood, as well as the presence or absence of midline shift, may be used to score a periventricular hemorrhagic infarction and predict NDI.1,27 The need for a shunt for post-hemorrhagic hydrocephalus has also been found to be associated with poorer outcome, including both cognitive and motor performance on the Bayley, CP and visual impairment in infants with grades 3 or 4 ICH.28 Possible reasons for this finding include (1) persistent hydrocephalus causing further damage to the white matter surrounding the ventricle leading to injury of the motor neurons and (2) shunt failure and infection, which are common in some infants, leading to further neuronal loss.
Maitre et al.29 also found an increased incidence of NEC in extremely premature infants with grade 4 IVH. In that study, NEC also worsened the developmental outcome of infants with severe IVH. The main strength of this study is the large number of infants enrolled from multiple centers participating in the NICHD NRN. However, it has been shown that there are center differences that need to be accounted for when reporting multicenter results in death and neurodevelopmental outcome.30 Some of these center differences are in defining viability that impacts the incidence of early death, treatment practices including parameters for supplemental oxygen that impacts the diagnosis of BPD, use of PNS, incidence and types of late onset infections and NEC, and demographic variables such as race and maternal education as well as distribution of private versus Medicaid insurance.31 We controlled for correlation between infants at the same center by including center as a random effect in the models.
Conclusion
When all common risk factors for NDI are considered, lower GA is important in predicting early death in premature infants, but not NDI among survivors with the most severe ICH. Male infants with a grade IV ICH who subsequently develop NEC and/or require a VP shunt for post-hemorrhagic hydrocephalus have the worst longterm developmental prognosis. Important factors that contribute to death and/or NDI that are potentially preventable with further advances in neonatal care are late onset infection, severe NEC, BPD and the use of PNS. Factors that are not preventable, but also significantly contribute to poorer outcome, include male gender and the need for a VP shunt. Decreasing incidence of BPD, NEC, late onset infection and the use of PNS in infants with severe ICH may improve outcome. In addition, being better able to predict, in the first post-natal week, which infants with severe ICH will develop post-hemorrhagic hydrocephalus requiring shunt placement would also be helpful for clinicians when counseling parents regarding care options early in the hospital course.
Supplementary Material
Acknowledgments
The National Institutes of Health and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) provided grant support for the Neonatal Research Network’s Generic Database and Follow-up Studies. Data collected at participating sites of the NICHD Neonatal Research Network (NRN) were transmitted to RTI International, the data coordinating center (DCC) for the network, which stored, managed and analyzed the data for this study. On behalf of the NRN, Dr Abhik Das (DCC Principal Investigator) and Mr Douglas Kendrick (DCC Statistician) had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis.
We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study. The following investigators, in addition to those listed as authors, participated in this study: NRN Chair: Alan H Jobe, MD, PhD, University of Cincinnati. Alpert Medical School of Brown University and Women & Infants Hospital of Rhode Island (U10 HD27904)—William Oh, MD; Abbot R Laptook, MD; Betty R Vohr, MD; Barbara Alksninis, PNP; Angelita M Hensman, RN, BSN; Teresa M Leach, MEd, CAES; Martha R Leonard, BA, BS; Lucy Noel; Rachel A Vogt, MD; Victoria E Watson, MS, CAS. Case Western Reserve University Rainbow Babies & Children’s Hospital (GCRC M01 RR80, U10 HD21364)—Michele C Walsh, MD, MS; Avroy A Fanaroff, MD; Nancy S Newman, RN; Bonnie S Siner, RN; Harriet G Friedman, MA. Cincinnati Children’s Hospital Medical Center University of Cincinnati Hospital and Good Samaritan Hospital (GCRC M01 RR8084, U10 HD27853)—Edward F Donovan, MD; Jean J Steichen, MD; Barbara Alexander, RN; Cathy Grisby, BSN, CCRC; Marcia Worley Mersmann, RN; Holly L Mincey, RN, BSN; Jody Hessling, RN; Teresa L Gratton, PA. Duke University School of Medicine University Hospital, Alamance Regional Medical Center, and Durham Regional Hospital (GCRC M01 RR30, U10 HD40492)—Ronald N Goldberg, MD; Kathy J Auten, MSHS; Kathryn E Gustafson, PhD; Melody B Lohmeyer, RN, MSN. Emory University Children’s Healthcare of Atlanta, Grady Memorial Hospital, and Emory University Hospital Midtown (GCRC M01 RR39, U10 HD27851)—Barbara J Stoll, MD; Ira Adams-Chapman, MD; Sheena Carter, PhD; Elisabeth Dinkins, PNP; Ellen C Hale, RN, BS, CCRC; Maureen Mulligan LaRossa, RN; Gloria V Smikle, PNP, MSN. Eunice Kennedy Shriver National Institute of Child Health and Human Development—Linda L Wright, MD; Elizabeth M McClure, MEd. Indiana University Indiana University Hospital, Methodist Hospital, Riley Hospital for Children and Wishard Health Services (GCRC M01 RR750, U10HD27856)—Brenda B Poindexter, MD, MS; James A Lemons, MD; Marilyn Bull, MD; Anna M Dusick, MD, FAAP; Darlene Kardatzke, MD; Carolyn Lytle, MD, MPH; Diana D Appel, RN, BSN; Lon G Bohnke, MS; Greg Eaken, PhD; Dianne E Herron, RN; Lucy C Miller, RN, BSN, CCRC; Heike M Minnich, PsyD, HSPP; Leslie Richard, RN; Leslie Dawn Wilson, BSN, CCRC. RTI International (U01 HD36790)—Abhik Das, PhD; W Kenneth Poole, PhD; Betty Hastings; Elizabeth M McClure, MEd; Jeanette O’Donnell Auman, BS; Carolyn M Petrie Huitema, MS; Scott E Schaefer, MS. Stanford University Lucile Packard Children’s Hospital and California Pacific Medical Center (GCRC M01 RR70, U10 HD27880)—David K Stevenson, MD; Krisa P Van Meurs, MD; Charles E Ahlfors, MD; Jean G Kohn, MD, MPH; Dharshi Sivakumar, MD, MRCP; M Bethany Ball, BS, CCRC; Robert D Stebbins, MD; Carol G Kuelper, PhD; Julie C Lee-Ancajas, PhD; Joan M Baran, PhD; Lori E Bond, PhD; Ginger K Brudos, PhD; Anne M DeBattista, RN, PNP; Renee P Pyle, PhD; Nicholas H St John, PhD. University of Alabama at Birmingham Health System and Children’s Hospital of Alabama (GCRC M01 RR32, U10 HD34216)—Waldemar A Carlo, MD; Myriam Peralta-Carcelen, MD, MPH; Kathleen G Nelson, MD; Kirstin J Bailey, PhD; Fred J Biasini, PhD; Stephanie A Chopko, PhD; Monica V Collins, RN, BSN MaEd; Shirley S Cosby, RN, BSN; Mary Beth Moses, PT, MS, PCS; Vivien A Phillips, RN, BSN; Julie Preskitt, MSOT, MPH; Richard V Rector, PhD; Sally Whitley, MA, OTR-L, FAOTA. University of California–San Diego Medical Center and Sharp Mary Birch Hospital for Women and Newborns (U10HD40461)—Neil N Finer, MD; Yvonne E Vaucher, MD, MPH; Maynard R Rasmussen MD; Paul R Wozniak, MD; Kathy Arnell, RNC; Renee Bridge, RN; Clarence Demetrio, RN; Martha G Fuller, RN, MSN; Donna Posin, OTR/L, MPA; Wade Rich, BSHS, RRT. University of Miami Holtz Children’s Hospital (GCRC M01 RR16587, U10 HD21397)—Shahnaz Duara, MD; Charles R Bauer, MD; Mary Allison, RN; Ruth Everett-Thomas, RN, MSN; Alexis N Diaz, BA; Elaine O Mathews, RN; Kasey Hamlin-Smith, PhD; Lisa Jean-Gilles, BA; Maria Calejo, MS; Silvia M Frade Eguaras, BA; Silvia Hiriart-Fajardo, MD; Yamiley C Gideon, BA. University of New Mexico Health Sciences Center (GCRC M01 RR997, U10 HD27881)—Lu-Ann Papile, MD; Conra Backstrom Lacy, RN; Jean R Lowe, PhD. University of Rochester Golisano Children’s Hospital, University of Rochester Medical Center (U10 HD40521, GCRC M01 RR44)—Dale L Phelps, MD; Gary Myers, MD; Erica Burnell, RN; Mary Rowan, RN; Julie Babish Johnson, MSW; Diane Hust, RN, PNP; Rosemary L Jensen; Emily Kushner, MA; Joan Merzbach, LMSW; Linda Reubens, RN; Lauren Zwetsch, RN, MS, PNP. University of Tennessee (U10 HD21415)—Sheldon B Korones, MD; Henrietta S Bada, MD; Tina Hudson, RN, BSN; Marilyn Williams, LCSW; Kimberly Yolton, PhD. University of Texas Southwestern Medical Center at Dallas Parkland Health & Hospital System and Children’s Medical Center Dallas (GCRC M01 RR633, U10 HD40689)—Abbot R Laptook, MD; Charles R Rosenfeld, MD; Walid A Salhab, MD; R Sue Broyles, MD; Roy J Heyne, MD; Sally S Adams, PNP; Cathy Twell Boatman, MS; Cristin Dooley, PhD, LSSP; Alicia Guzman; Elizabeth Heyne, PA-C; Jackie F Hickman, RN; Linda A Madden, BSN, RN, CPNP; Nancy A Miller, RN; Janet S Morgan, RN; Susie Madison, RN; Gaynelle Hensley, RN. University of Texas Health Science Center at Houston Medical School, Children’s Memorial Hermann Hospital, and Lyndon B Johnson General Hospital (U10 HD21373)—Kathleen Kennedy, MD, MPH; Jon E Tyson, MD, MPH; Pamela J Bradt, MD, MPH; Terri Major-Kincade, MD, MPH; Brenda H Morris, MD; Laura L Whitely, MD; Esther G Akpa, RN, BSN; Nora I Alaniz, BS; Magda Cedillo; Patty A Cluff, RN; Susan Dieterich, PhD; Claudia I Franco, RNC, MSN; Anna E Lis, RN, BSN; Georgia E McDavid, RN; Patti L Pierce Tate, RCP. Wake Forest University Baptist Medical Center, Forsyth Medical Center, and Brenner Children’s Hospital (GCRC M01 RR7122, U10 HD40498)—T Michael O’Shea, MD, MPH; Robert G Dillard, MD; Nancy J Peters, RN, CCRP; Korinne Chiu, MA; Deborah Evans Allred, MA, LPA; Donald J Goldstein, PhD; Raquel Halfond, MA; Barbara G Jackson, RN, BSN; Carroll Peterson, MA; Ellen L Waldrep, MS; Melissa Whalen Morris, MA; Gail Wiley Hounshell, PhD. Wayne State University Hutzel Women’s Hospital and Children’s Hospital of Michigan (U10 HD21385)—Yvette R Johnson, MD; Rebecca Bara, RN, BSN; Debra Driscoll, RN, BSN; Laura Goldston, MA; Deborah Kennedy, RN, BSN; Geraldine Muran, RN, BSN. Yale University Yale-New Haven Children’s Hospital (GCRC M01 RR6022, U10 HD27871)—Richard A Ehrenkranz, MD; Patricia Gettner, RN; Nancy Close, PhD; Walter Gilliam, PhD; Monica Konstantino, RN, BSN; JoAnn Poulsen, RN; Elaine Romano, MSN; Janet Taft, RN, BSN; Joanne Williams, RN, BSN.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Bassan H, Limperopoulos C, Visconti K, Mayer DL, Feldman HA, Avery L, et al. Neurodevelopmental outcome in survivors of periventricular hemorrhagic infarction. Pediatrics. 2007;120(4):785–792. doi: 10.1542/peds.2007-0211. [DOI] [PubMed] [Google Scholar]
- 2.de Vries LS, Rademaker KJ, Groenendaal F, Eken P, van Haastert IC, Vandertop WP, et al. Correlation between neonatal cranial ultrasound, MRI in infancy and neurodevelopmental outcome in infants with a large intraventricular haemorrhage with or without unilateral parenchymal involvement. Neuropediatrics. 1998;29(4):180–188. doi: 10.1055/s-2007-973558. [DOI] [PubMed] [Google Scholar]
- 3.Hack M, Taylor HG. Perinatal brain injury in preterm infants and later neurobehavioral function. JAMA. 2000;284(15):1973–1974. doi: 10.1001/jama.284.15.1973. [DOI] [PubMed] [Google Scholar]
- 4.Maitre NL, Marshall DD, Price WA, Slaughter JC, O’Shea TM, Maxfield C, et al. Neurodevelopmental outcome of infants with unilateral or bilateral periventricular hemorrhagic infarction. Pediatrics. 2009;124(6):e1153–e1160. doi: 10.1542/peds.2009-0953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Shea TM, Kuban KC, Allred EN, Paneth N, Pagano M, Dammann O, et al. Neonatal cranial ultrasound lesions and developmental delays at 2 years of age among extremely low gestational age children. Pediatrics. 2008;122(3):e662–e669. doi: 10.1542/peds.2008-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roelants-van Rijn AM, Groenendaal F, Beek FJ, Eken P, van Haastert IC, de Vries LS. Parenchymal brain injury in the preterm infant: comparison of cranial ultrasound, MRI and neurodevelopmental outcome. Neuropediatrics. 2001;32(2):80–89. doi: 10.1055/s-2001-13875. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt B, Roberts RS, Fanaroff A, Davis P, Kirpalani HM, Nwaesei C, et al. Indomethacin prophylaxis, patent ductus arteriosus, and the risk of bronchopulmonary dysplasia: further analyses from the Trial of Indomethacin Prophylaxis in Preterms (TIPP) J Pediatr. 2006;148(6):730–734. doi: 10.1016/j.jpeds.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 8.Sherlock RL, Synnes AR, Grunau RE, Holsti L, Hubber-Richard P, Johannesen D, et al. Long-term outcome after neonatal intraparenchymal echodensities with porencephaly. Arch Dis Child Fetal Neonatal Ed. 2008;93(2):F127–F131. doi: 10.1136/adc.2006.110726. [DOI] [PubMed] [Google Scholar]
- 9.Bayley N. Bayley scales of infant development. 2. The Psychological Corporation; San Antonio, TX: 1993. [Google Scholar]
- 10.Palisano RJ, Hanna SE, Rosenbaum PL, Russell DJ, Walter SD, Wood EP, et al. Validation of a model of gross motor function for children with cerebral palsy. Phys Ther. 2000;80(10):974–985. [PubMed] [Google Scholar]
- 11.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92(4):529–534. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 12.Ambalavanan N, Carlo WA, Bobashev G, Mathias E, Liu B, Poole K, et al. Prediction of death for extremely low birth weight neonates. Pediatrics. 2005;116(6):1367–1373. doi: 10.1542/peds.2004-2099. [DOI] [PubMed] [Google Scholar]
- 13.Cotten CM, Oh W, McDonald S, Carlo W, Fanaroff AA, Duara S, et al. Prolonged hospital stay for extremely premature infants: risk factors, center differences, and the impact of mortality on selecting a best-performing center. J Perinatol. 2005;25(10):650–655. doi: 10.1038/sj.jp.7211369. [DOI] [PubMed] [Google Scholar]
- 14.Hintz SR, Poole WK, Wright LL, Fanaroff AA, Kendrick DE, Laptook AR, et al. Changes in mortality and morbidities among infants born at less than 25 weeks during the postsurfactant era. Arch Dis Child Fetal Neonatal Ed. 2005;90(2):F128–F133. doi: 10.1136/adc.2003.046268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shankaran S, Fanaroff AA, Wright LL, Stevenson DK, Donovan EF, Ehrenkranz RA, et al. Risk factors for early death among extremely low-birth-weight infants. Am J Obstet Gynecol. 2002;186(4):796–802. doi: 10.1067/mob.2002.121652. [DOI] [PubMed] [Google Scholar]
- 16.Tyson JE, Parikh NA, Langer J, Green C, Higgins RD. Intensive care for extreme prematurity—moving beyond gestational age. N Engl J Med. 2008;358(16):1672–1681. doi: 10.1056/NEJMoa073059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bassler D, Stoll BJ, Schmidt B, Asztalos EV, Roberts RS, Robertson CM, et al. Using a count of neonatal morbidities to predict poor outcome in extremely low birth weight infants: added role of neonatal infection. Pediatrics. 2009;123(1):313–318. doi: 10.1542/peds.2008-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blakely ML, Lally KP, McDonald S, Brown RL, Barnhart DC, Ricketts RR, et al. Postoperative outcomes of extremely low birth-weight infants with necrotizing enterocolitis or isolated intestinal perforation: a prospective cohort study by the NICHD Neonatal Research Network. Ann Surg. 2005;241(6):984–989. doi: 10.1097/01.sla.0000164181.67862.7f. discussion 989–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hintz SR, Kendrick DE, Stoll BJ, Vohr BR, Fanaroff AA, Donovan EF, et al. Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolitis. Pediatrics. 2005;115(3):696–703. doi: 10.1542/peds.2004-0569. [DOI] [PubMed] [Google Scholar]
- 20.Jen HC, Graber JJ, Hill JL, Alaish SM, Voigt RW, Strauch ED. Surgical necrotizing enterocolitis and intraventricular hemorrhage in premature infants below 1000 g. J Pediatr Surg. 2006;41(8):1425–1430. doi: 10.1016/j.jpedsurg.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 21.Stoll BJ, Hansen NI, Adams-Chapman I, Fanaroff AA, Hintz SR, Vohr B, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292(19):2357–2365. doi: 10.1001/jama.292.19.2357. [DOI] [PubMed] [Google Scholar]
- 22.Hintz SR, Kendrick DE, Vohr BR, Kenneth Poole W, Higgins RD. Gender differences in neurodevelopmental outcomes among extremely preterm, extremely-low-birthweight infants. Acta Paediatr. 2006;95(10):1239–1248. doi: 10.1080/08035250600599727. [DOI] [PubMed] [Google Scholar]
- 23.Stevenson DK, Verter J, Fanaroff AA, Oh W, Ehrenkranz RA, Shankaran S, et al. Sex differences in outcomes of very low birthweight infants: the newborn male disadvantage. Arch Dis Child Fetal Neonatal Ed. 2000;83(3):F182–F185. doi: 10.1136/fn.83.3.F182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson-Costello D, Walsh MC, Langer JC, Guillet R, Laptook AR, Stoll BJ, et al. Impact of postnatal corticosteroid use on neurodevelopment at 18 to 22 months’ adjusted age: effects of dose, timing, and risk of bronchopulmonary dysplasia in extremely low birth weight infants. Pediatrics. 2009;123(3):e430–e437. doi: 10.1542/peds.2008-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doyle LW, Halliday HL, Ehrenkranz RA, Davis PG, Sinclair JC. Impact of postnatal systemic corticosteroids on mortality and cerebral palsy in preterm infants: effect modification by risk for chronic lung disease. Pediatrics. 2005;115(3):655–661. doi: 10.1542/peds.2004-1238. [DOI] [PubMed] [Google Scholar]
- 26.Doyle LW, Davis PG, Morley CJ, McPhee A, Carlin JB. Low-dose dexamethasone facilitates extubation among chronically ventilator-dependent infants: a multicenter, international, randomized, controlled trial. Pediatrics. 2006;117(1):75–83. doi: 10.1542/peds.2004-2843. [DOI] [PubMed] [Google Scholar]
- 27.Bassan H, Feldman HA, Limperopoulos C, Benson CB, Ringer SA, Veracruz E, et al. Periventricular hemorrhagic infarction: risk factors and neonatal outcome. Pediatr Neurol. 2006;35(2):85–92. doi: 10.1016/j.pediatrneurol.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 28.Adams-Chapman I, Hansen NI, Stoll BJ, Higgins R. Neurodevelopmental outcome of extremely low birth weight infants with posthemorrhagic hydrocephalus requiring shunt insertion. Pediatrics. 2008;121(5):e1167–e1177. doi: 10.1542/peds.2007-0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maitre N, Marshall D, Goldstein RF, Slaughter JC, Price W. Necrotizing enterocolitis in infants with periventricular hemorrhagic infarction: associations and outcomes. Neonatology. 2011;99(2):97–103. doi: 10.1159/000313960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vohr BR, Wright LL, Dusick AM, Perritt R, Poole WK, Tyson JE, et al. Center differences and outcomes of extremely low birth weight infants. Pediatrics. 2004;113(4):781–789. doi: 10.1542/peds.113.4.781. [DOI] [PubMed] [Google Scholar]
- 31.Fanaroff AA, Stoll BJ, Wright LL, Carlo WA, Ehrenkranz RA, Stark AR, et al. Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol. 2007;196(2):147, e141–148. doi: 10.1016/j.ajog.2006.09.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.