Abstract
Objectives
To compare the changes in risk factors for cardiovascular disease (CVD) leading up to and following hysterectomy with or without bilateral oophorectomy with the changes observed up to and following natural menopause.
Background
Evidence suggests that hysterectomy status with or without bilateral oophorectomy may increase risk for CVD but most studies retrospectively assess menopausal status.
Methods
Study of Women’s Health across the Nation enrolled 3,302 premenopausal women not using hormone therapy between the ages of 42–52 years of age and followed them annually for over 11 years for sociodemographic characteristics, menopausal status, surgeries, body mass index (BMI), medication use, lifestyle factors, lipids, blood pressure, insulin resistance, and hemostatic and inflammatory factors. By 2008, 1,769 women had reached natural menopause, 77 women had a hysterectomy with ovarian conservation, and 106 women had a hysterectomy with bilateral oophorectomy. Piecewise hierarchical growth models compared these groups on annual changes in CVD risk factors prior to and following final menstrual period (FMP) or surgery.
Results
Multivariable analyses showed that annual changes in CVD risk factors did not vary by group with few exceptions, and the significant group differences that did emerge were not in the anticipated direction.
Conclusions
Hysterectomy with or without ovarian conservation is not a key determinant of CVD risk factor status either before or after elective surgery in mid-life. These results should provide reassurance to women and their clinicians that hysterectomy in mid-life is unlikely to accelerate women’s CVD risk.
Keywords: hysterectomy, metabolic factors, inflammation, blood pressure, epidemiology
Introduction
Elective hysterectomy is a common surgical procedure to improve quality of life among symptomatic women approaching the menopause (1–3). The clear benefits of surgery for reducing debilitating symptoms must be considered in light of potential long-term health consequences, including cardiovascular disease (CVD). The cardiovascular risk associated with hysterectomy, especially accompanied by bilateral oophorectomy, is not yet clear. In the Framingham Heart Study, women who had a hysterectomy, especially with bilateral oophorectomy, were later at elevated risk for CVD, adjusting for age group and smoking status (4). In the Nurse’s Health Study, women who had a bilateral oophorectomy, usually occurring in the fifth decade, were at greater risk for incident coronary heart disease (CHD) and total mortality than were women who had a hysterectomy without oophorectomy (5). On the other hand, in a large registry of Swedish women, the risk for incident CHD, stroke, and heart failure during the follow-up was not confined to women with hysterectomy accompanied by bilateral oophorectomy; both surgical groups were at elevated risk (6). However, this relationship was only observed among women 50 years of age or less and smoking was not statistically controlled. In an older cohort of women enrolled in the Women’s Health Initiative (WHI), similar to the results from the Swedish registry, women with bilateral oophorectomy and hysterectomy did not have a greater incidence of CVD, adjusting for a large number of CVD risk factors (7), compared to women with hysterectomy alone. In the same study, women who had hysterectomy regardless of ovarian conservation had elevated levels of CVD risk factors and were more often diabetic and hypertensive (8), compared to postmenopausal women who had not had a hysterectomy. Further, hysterectomy, regardless of ovarian conservation, was associated with incident CVD, with associations largely attenuated after introducing a wide array of cardiovascular risk factors and sociodemographic characteristics.
Taken together, the findings raise a number of important issues. Because these studies only assessed CVD risk factors years after hysterectomy and/or oophorectomy, without assessment of pre-surgery CVD risk factors levels, it is unknown if elevated CVD risk led to the conditions warranting a surgical menopause or if CVD risk was accelerated post-surgery. For example, obesity in premenopausal women increases the likelihood of abnormal bleeding and fibroids, which are common indications for these gynecologic surgeries (9–12). It is also not clear if hysterectomy with versus without ovarian conservation have similar or different effects on CVD risk factors, compared to not having surgery and experiencing a natural menopause.
One approach to addressing these issues is to describe the prospective changes in cardiovascular risk factors prior to and following elective hysterectomy with or without bilateral oophorectomy in relation to changes in CVD risk factors that occurred in a comparable period of time prior to and following final menstrual period (FMP) in women who experienced a natural menopause. This report is based on cardiovascular risk factor data from the Study of Women’s Health across the Nation (SWAN), a study of a multi-ethnic sample of women early in the menopausal transition who were subsequently followed annually. In a prior SWAN report, we found that the time interval around FMP due to natural menopause was associated with substantial increases in low density lipoprotein cholesterol (LDL-C) and apolipoprotein (Apo) B (13). We address in this report whether even larger increases in lipids and other risk factors, including blood pressure, insulin resistance, and hemostatic factors, occur in women who had a hysterectomy with or without bilateral oophorectomy compared those who had a natural menopause. We also examined these patterns with and without consideration of obesity, given that body mass index increased following bilateral oophorectomy relative to natural menopause in the present sample (14).
Methods
Participants
SWAN is a multi-site community-based prospective study designed to examine the physical and psychological health of women as they undergo the menopausal transition. Details of the SWAN design and recruitment procedures have been reported elsewhere (15). At baseline, all SWAN participants had an intact uterus and at least one ovary, and met the additional eligibility criteria: aged 42–52 years, not pregnant, not using reproductive hormones, and having 1 or more menstrual cycles in the 3 months prior to the interview. Each site recruited non-Hispanic Caucasian women as well as women belonging to a predetermined racial/ethnic minority group: African American women in Pittsburgh, Pennsylvania; Boston, Massachusetts; Detroit, Michigan; and Chicago, Illinois; Japanese women in Los Angeles, California; Hispanic women in Newark, New Jersey; and Chinese women in the Oakland area of California. Participants were recruited using established sampling techniques, random digit dialing, and random sampling from lists of names or household addresses. Select sites supplemented primary sampling frames to obtain adequate numbers of racial/ethnic minority women. Seventy-three percent of the women selected were contacted and provided information to determine eligibility; 51% (n = 3,302) of eligible women enrolled.
Participants returned to their local site facility annually for interviewer- and self-administered questionnaires, a fasting blood draw, and assessments of physical measures. Data collection for this analysis spanned from 1996–2008. SWAN was approved by the institutional review boards at each site, and each participant provided written, informed consent. Data collection ceased at the New Jersey SWAN site after 2001 for reasons unrelated to scientific aspects of the project. Because this resulted in an average length of follow-up for this site being systematically shorter than that of any other site, data from this site were excluded from the current analysis.
Measures and procedures
Menopausal and hysterectomy status
Menopausal status and the occurrence of hysterectomy and/or oophorectomy were assessed annually in SWAN. Participants were asked if they had a “hysterectomy (an operation to remove your uterus or womb)” and whether they had one or both ovaries removed since the last study visit. Those who indicated hysterectomy were further divided into those with and without bilateral oophorectomy, provided their hysterectomy occurred prior to becoming naturally postmenopausal. Medical records were sought for all women who reported hysterectomy; of the 166 obtained, all but 1 confirmed hysterectomy and/or oophorectomy. Women were categorized as naturally postmenopausal if they reported a complete absence of menstrual bleeding in the previous 12 months and no hysterectomy. FMP date or surgery date was based on participant self-report; if FMP date was unknown, it was set as 12 months prior to the date of the annual visit participants were first categorized postmenopausal.
Menopausal status at the annual visit immediately prior to each participant’s FMP/surgery date was defined as premenopausal (bleeding in the last 3 months with no cycle irregularity in the previous 12 months), early perimenopausal (bleeding in the last 3 months with some change in cycle regularity in the last 12 months), late perimenopausal (bleeding >3 months ago but within the last 12 months), and unknown (hormone therapy or other circumstance interfering with ability to characterize bleeding patterns), and collapsed for this analysis into four categories: premenopausal (premenopausal and early perimenopausal), perimenopausal (late perimenopausal), unknown, and missing (no menopausal status available for participant, generally due to nonattendance in the previous year’s annual visit).
By 2008, a total of 1,952 women, including 1,769 women who reached natural menopause, 77 women who had a hysterectomy with ovarian conservation, and 106 women who had a hysterectomy with bilateral oophorectomy comprised the analytic sample. Excluded from the analytic sample were 1,097 women who did not report hysterectomy or reach natural menopause during their participation in SWAN, 32 who had a hysterectomy after having been categorized naturally postmenopausal, 21 who reported hysterectomy in the presence of known or suspected endometrial, uterine, or ovarian cancer, 69 without body mass index (BMI) data from at least one annual visit post-FMP/surgery (because of its importance as a covariate for all outcomes and it increased post surgery (14)), and 131 women from the New Jersey site. The New Jersey site women did not complete in person clinic visits after the sixth annual followup, and resumed clinic visits at follow-up 12. Thus, this site had an average length of follow-up that is systematically shorter than that of any other site.
Cardiovascular Risk Factors
At each annual visit, blood was drawn in the morning after fasting. All lipid and lipoproteins were analyzed on EDTA-treated plasma. Total cholesterol and triglycerides were analyzed by enzymatic methods on a Hitachi 747 analyzer (Boehringer Mannheim Diagnostics, Indianapolis, IN), and high-density lipoprotein cholesterol (HDL-C) was isolated using heparin-2M manganese chloride. LDL-C was calculated using the Friedewald equation (16–18). Triglyceride levels and insulin resistance estimated by Homeostasis Model of Assessment -Insulin Resistance (HOMA-IR) scores were logged prior to analysis. Serum insulin was measured using radioimmunoassay (DPC Coat-a-count, Los Angeles, CA) procedure and monitored as part of the monthly quality assurance program by the Diabetes Diagnostic Laboratory at the University of Missouri. Glucose was measured using a hexokinase-coupled reaction on a Hitachi 747–200 (Boehringer Mannheim Diagnostics, Indianapolis, IN). Fibrinogen and Factor VII were measured in frozen citrated plasma using a clot-based turbidometric detection system, with Factor VII assay using Factor VII deficient plasma in preparing the standard curve. Tissue plasminogen activator- antigen (tPA-ag) was measured in plasma using a double antibody in an enzyme-linked immunosorbant assay (American Diagnostica, Greenwich, CT), with a human single chain tPA-ag as a standard calibrated against an international standard (Hertfordshire, England). Plasminogen activator inhibitor-1 (PAI-1) was measured using a solid phased monoclonal antibody and an enzyme labeled goal second antiserum for detection (American Diagnostica, Greenwich, CT). C-reactive protein-high sensitivity (CRP-hs) was measured using an ultra-sensitive rate immunoephelometry (Dade-Behring, Marburg, Germany).
Systolic blood pressure (SBP) was manually measured twice with a minimum two-minute rest period between measures, with readings taken on the right arm, with the respondent seated and feet flat on the floor for at least 5 minutes prior to the measurement. Respondents had not smoked or consumed any caffeinated beverage within 30 minutes of blood pressure measurement. Appropriate cuff size was determined based on arm circumference. The two sequential blood pressure values were averaged.
Covariates
Covariates included race/ethnicity, educational attainment, menopausal status and age the year prior to FMP or surgery, as well as annual measurements of physical activity, smoking status, self-rated health, myocardial infarction, stroke, hormone therapy use, BMI, and antidepressant, insulin, antihypertensive, lipid lowering and heart medication use. Race/ethnicity and educational level were self-reported in the screening interview. Age was calculated from the participant’s date of birth and date of FMP or surgery. Physical activity was assessed with the Kaiser Physical Activity Survey (19), an adaptation of the Baecke physical activity questionnaire (20) at baseline and annual follow-up visits 3, 5, and 6. A sum score was derived from responses to questions about physical activity during sports/exercise, household/caregiving tasks, and daily routine in the previous year. Current smoking status was self-reported at each annual visit. Self-rated health was assessed at each annual visit by response to the following question: “In general, would you say your health is excellent, very good, good, fair or poor? (21)” Responses were categorized into two categories, with responses of “excellent” and “very good” collapsed into one category, and “good”, “fair”, and “poor” collapsed into another category. Medication use and new medical diagnoses were self-reported at each annual visit. BMI was measured at each visit by a trained technician.
Statistical analyses
Site, race/ethnicity, age at FMP/surgery, educational attainment, menopausal status the year prior to FMP/surgery, and reported myocardial infarction or stroke were included as covariates in final models based on a priori decisions. Annual insulin use for outcomes related to glucose metabolism, annual hypertensive medication use for outcomes related to blood pressure, annual lipid-lowering medication use for outcomes related to dyslipidemia, and annual heart medications related to hemostatic and inflammatory factors and blood pressure were also included a priori. Preliminary analyses were conducted to assess independent associations between annual observations of antidepressant use, hormone therapy use, self-rated health, smoking status, and physical activity with outcomes using hierarchical linear regression. These time-varying covariates were retained in final models if they were associated with the outcomes in otherwise unadjusted models at p<.05.
Baseline characteristics of women with natural menopause, women with hysterectomy with ovarian conservation, and women with hysterectomy with bilateral oophorectomy were compared using chi-square or Fisher exact test for categorical variables, and analysis of variance for continuous variables (without covariates) using SPSS v.17. (SPSS for Windows, Rel 17.0.0, 2008.)
Piecewise hierarchical linear growth models were used to estimate the mean annual rate of change in CVD risk factors from baseline to the index visit, which was the first annual visit after FMP or surgery, and from the index visit to end of follow-up, with annual observations nested within women (HLM for Windows, version 6.08, 2010). Note that the time between FMP or surgery and the index visit was a covariate. Hierarchical linear modeling was used due to its utility in accounting for the dependence of repeated, correlated observations within individuals. Piecewise hierarchical linear growth models allowed for the possibility of different mean growth trajectories before and after FMP or surgery (22). The intercept was set at the index visit, and modeled as a function of group status with covariates: site, race/ethnicity, educational attainment, age at FMP or surgery, menopausal status at the visit prior to FMP or surgery, and elapsed time between FMP or surgery and index visit and elapsed time between index visit and end of observations. An interaction between hysterectomy status (hysterectomy with ovarian conservation or hysterectomy with bilateral oophorectomy, with natural menopause set as the referent) and each time variable examined whether either hysterectomy status group differed significantly from the natural menopause group at the index visit or the annual changes before or after. For the referent group, the estimate refers to whether the level differs from zero. All predictors and covariates were entered simultaneously in the final multivariable models presented in the tables below. For all analyses, p-values less than 0.05 (two-tailed) were considered statistically significant. Tables present the results without adjustment for BMI. When adjustments for BMI changed the pattern of results, it is noted in the results section.
To assess potential interactions between hysterectomy status and race/ethnicity, a secondary analysis was performed in a sample restricted to only African American and Caucasian women to determine whether potential increases in these CVD risk factors following hysterectomy with or without oophorectomy were greater among African American compared to Caucasian women. Other groups represented in SWAN were not included due to their very low numbers of hysterectomy with or without bilateral oophorectomy. The sample for this secondary analysis included 1,422 African American and Caucasian women who reached natural menopause, 71 women who had a hysterectomy with ovarian conservation and 88 women who had a hysterectomy with bilateral oophorectomy. Models were otherwise identical to those described above.
Results
Characteristics of analytic sample
Compared to the women who were excluded from the analysis, women in the analytic sample were older (M (standard deviation)(SD)) = 46.3 (2.6) vs. 45.2 (2.7), p<.001), more educated (46.5% v. 37.3% college or post-college, p<.001), reported better self-rated health (61.3% v. 52.6% excellent/very good health, p<.001), had higher HDL-C (M (SD) = 61.0 (16.5) vs. 54.7 (16.0), p<.001), and had lower BMI (Ms (SD) = 28.0 (7.3) vs. 28.8 (7.1), p<.01), HOMA-IR scores (M (SD) = 3.3 (4.0) vs. 5.0 (7.0), p <.01 based on logged values), and SBP (M (SD) = 117.8 (17.3) vs. 124.0 (15.6), p <.001) at baseline. Of the 183 women in the two surgery groups, 140 had documented medical records regarding diagnosis. The most common preoperative symptoms and diagnoses in the available medical records were suspected or diagnosed uterine fibroids (75.7%), suspected or diagnosed menorrhagia (58.6%) and chronic pelvic pain (25.7%). Suspected or diagnosed fibroids accompanied the presentation of menorrhagia (85.4%) and chronic pelvic pain (83.3%) in this sample.
Participants were followed for up to 11 years after study entry, with observations from up to 9 years before and after FMP or surgery and a mean (SD) number of exams per woman being 10.78 (.90), with 4.71 (2.33) on average after FMP or surgery. On average women reported HT use after FMP or surgery at 0.72 (1.58), 1.22 (1.75), and 3.42 (2.97) visits for natural menopause, hysterectomy with ovarian conservation, and hysterectomy with bilateral oophorectomy groups, respectively, p < .001. The proportion of visits on HT throughout the study before and after FMP or surgery for these groups were on average .14 (.34), .22 (.29), and .57 (.36), p < .001.
Women who subsequently reported hysterectomy with or without oophorectomy were more likely to be African American and younger. (Table 1). tPA-ag levels were elevated at study entry among women who later had hysterectomy with bilateral oophorectomy, whereas BMI, Factor VIIc levels, and use of lipid-lowering medications were higher at study entry among women who later had hysterectomy with ovarian conservation, as compared with women with naturally occurring menopause. No other risk factors differed by FMP/surgery status.
Table 1.
Characteristics of women in analytic sample
| Total (n=1952) |
Natural Menopause (n=1769 90.6%) n, col % |
Hysterectomy with ovarian conservation (n=77, 3.9%) n, col % |
Hysterectomy with bilateral oophorectomy (n=106, 5.4%) n, col % |
Group ANOVA‡ p-values |
||
|---|---|---|---|---|---|---|
| Race/ethnicity | <.001 | |||||
| White | 946 (48.5) | 870 (49.2) | 32 (41.6) | 44 (41.5) | ||
| African American | 615 (31.5) | 528 (29.8) | 40 (51.9)† | 47 (44.3)† | ||
| Chinese | 180 (9.2) | 172 (9.7) | 0 (0) | 8 (7.5) | ||
| Japanese | 211 (10.8) | 199 (11.2) | 5 (6.5) | 7 (6.6) | ||
|
Menopausal status at visit prior to FMP§/surgery |
<.001 | |||||
| Premenopausal | 415 (21.3) | 323 (18.3) | 44 (57.1)† | 48 (45.3) | ||
| Perimenopausal | 847 (43.4) | 840 (47.5) | 2 (2.6)† | 5 (4.7)† | ||
| Unknown | 309 (15.8) | 283 (16.0) | 9 (11.7) | 17 (16.0)† | ||
| Missing | 381 (19.5) | 323 (18.3) | 22 (28.6) | 36 (34.0) | ||
| Education | .25 | |||||
| ≤ High school | 400 (20.6) | 363 (20.6) | 13 (16.9) | 24 (22.6) | ||
| Some college | 634 (32.7) | 565 (32.1) | 26 (33.8) | 43 (40.6)† | ||
| ≥ College | 907 (46.7) | 830 (47.2) | 38 (49.4) | 39 (36.8) | ||
| Baseline self-rated health | .10 | |||||
| Excellent or very good | 426 (22.0) | 377 (21.5) | 24 (31.6)† | 25 (23.8) | ||
| Good/fair/poor | 1511 (78.0) | 1379 (78.5) | 52 (68.4) | 80 (76.2) | ||
|
Baseline antidepressant use |
.55 | |||||
| Yes | 172 (8.8) | 152 (8.6) | 9 (11.8) | 11 (10.4) | ||
| No | 1776 (91.2) | 1614 (91.4) | 67 (88.2) | 95 (89.6) | ||
|
Baseline lipid- lowering medication use |
.03 | |||||
| Yes | 20 (1.0) | 15 (.8) | 4 (5.3)† | 1 (.9) | ||
| No | 1927 (99.0) | 1750 (99.2) | 72 (94.7) | 105 (99.1) | ||
|
Baseline heart medication use |
.33 | |||||
| Yes | 53 (2.7) | 45 (2.5) | 3 (3.9) | 5 (4.7) | ||
| No | 1899 (97.3) | 1724 (97.5) | 74 (96.1) | 101 (95.3) | ||
|
Baseline current smoker |
.45 | |||||
| Yes | 311 (16.0) | 283 (16.1) | 15 (19.5) | 13 (12.6) | ||
| No | 1627 (84.0) | 1475 (83.9) | 62 (80.5) | 90 (87.4) | ||
|
Baseline past stroke/ myocardial infarction |
.70 | |||||
| Yes | 17 (0.9) | 16 (0.9) | 0 (0.0) | 1 (1.0) | ||
| No | 1886 (99.1) | 1709 (99.1) | 75 (100.0) | 102 (99.0) | ||
| Baseline | mean (SD║) | mean (SD) | mean (SD) | |||
|
Age at baseline (mean, SD) |
46.31 (2.61) | 46.41 (2.59) | 44.94 (2.55)† | 45.66 (2.62)† | <.001 | |
|
Age at FMP/surgery (mean, SD) |
51.44 (2.73) | 51.72 (2.53) | 48.00 (2.83)† | 49.25 (3.05)† | <.001 | |
|
Body mass index (kg/m2) |
27.95 (7.32) | 27.77 (7.28) | 30.12 (7.90)† | 29.47 (7.32) | <.01 | |
|
Physical activity score |
7.74 (1.77) | 7.75 (1.77) | 7.73 (2.00) | 7.58 (1.65) | .67 | |
| LDL-C¶(mg/dl) | 115.44 (31.38) | 115.19 (31.24) | 122.08 (31.01) | 114.79 (33.59) | .18 | |
| HDL-C#(mg/dl) | 57.28 (14.18) | 57.52 (14.25) | 55.22 (13.93) | 54.78 (13.02) | .07 | |
| Apo** B (mg/dl) | 110.33 (26.68) | 110.20 (29.57) | 115.87 (30.68) | 108.54 (30.73) | .22 | |
| ApoA1 (mg/dl) | 151.63 (24.18) | 151.96 (24.11) | 148.29 (25.06) | 148.55 (24.54) | .17 | |
|
Triglycerides (mg/dL) |
109.24 (78.36) | 109.03 (80.09) | 111.05 (65.35) | 111.41 (54.49) | .38* | |
| HOMA-IR†† | 2.94 (5.92) | 2.94 (6.14) | 3.01 (2.43) | 3.03 (3.71) | .11* | |
|
Systolic blood pressure (mmHg) |
117.82 (17.32) | 117.57 (17.25) | 118.16 (18.17) | 121.88 (17.56) | .06 | |
| tPA-ag‡‡(ng/ml) | 8.17 (7.05) | 7.95 (5.69) | 8.44 (4.50) | 11.82 (19.05)† | <.001 | |
| PAI-1§§(ng/ml) | 25.86 (39.20) | 25.80 (39.00) | 21.35 (26.17) | 30.37 (49.91) | .47 | |
|
Factor VIIc (mg/dL) |
127.81 (44.18) | 126.52 (32.59) | 155.70 (139.65)† | 125.13 (35.59) | <.01 | |
| CRP ║║ (mg/L) | 3.70 (6.17) | 3.62 (6.16) | 3.94 (4.13) | 4.89 (7.51) | .11 | |
P-value from logged analyses
Group differs significantly different (p<.05) from natural menopause in post-hoc analyses
ANOVA (analysis of variance)
Apo (apolipoprotein)
CRP(C-reactive Protein)
FMP (final menstrual period)
HDL-C (high density lipoprotein cholesterol)
HOMA-IR (Homeostasis Model of Assessment –Resistance)
LDL-C (low density lipoprotein cholesterol)
PAI-1 (Plasminogen activator inhibitor-1)
SD (standard deviation)
tPA-ag (Tissue plasminogen activator- antigen).
Lipid changes
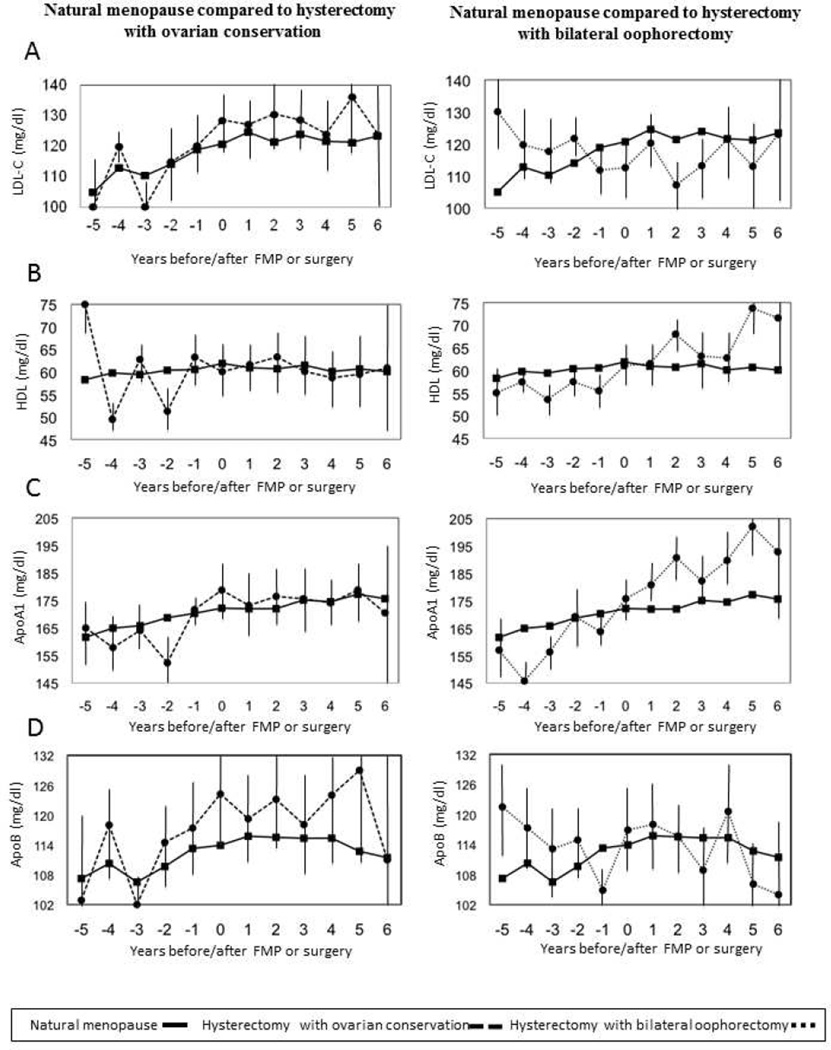
At the time of the index visit, women with hysterectomy with ovarian conservation had higher LDL-C and tended to have higher ApoB levels (which was significant when adjusted for BMI), whereas women with hysterectomy with bilateral oophorectomy had higher triglyceride levels, relative to the natural postmenopausal women (Table 2; Figure 1 shows the covariate-adjusted means (95% confidence intervals) on the basis of predicted values from linear regression models within each year.) LDL-C, triglycerides, and ApoA1 levels increased annually prior to and following FMP, whereas HDL-C decreased annually after FMP. ApoB increased prior to FMP and nonsignificantly declined after FMP. The change in these factors was similar before and after surgery compared to before and after FMP, with two exceptions. The increase in ApoA1 levels was larger, with a modest decline in triglyceride levels prior to surgery in women with hysterectomy with ovarian conservation, compared to those who became naturally postmenopausal. Further adjustments for BMI did not alter the results.
Table 2.
Regression coefficients (standard errors) and their p-values from fully adjusted models for lipid levels at index visit (visit after final menstrual period or surgery) and changes prior to and following index visit
| HDL* | LDL† | (log) Triglycerides | Apo‡A1 | ApoB | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient (SE)§ |
p-value | Coefficient (SE) |
p-value | Coefficient (SE) |
p-value | Coefficient (SE) |
p-value | Coefficient (SE) |
p-value | |
| Mean at index visit | ||||||||||
| Natural menopause (referent) |
59.39 (1.31) | — | 129.77 (3.01) | — | 2.05 (.02) | — | 170.99 (2.32) | — | 116.70 (2.64) | — |
| Hysterectomy with ovarian conservation |
58.06 (2.12) | .53 | 140.44 (5.01) | .03 | 2.07 (.03) | .47 | 174.22 (4.07) | .43 | 124.34 (4.19) | .07 |
| Hysterectomy with bilateral oophorectomy |
58.93 (1.97) | .82 | 126.33 (4.43) | .44 | 2.12 (.03) | .02 | 173.97 (3.66) | .42 | 117.20 (3.62) | .89 |
|
Mean annual rate of change from baseline to index visit |
||||||||||
| Natural menopause(referent) |
.09 (.10) | .35 | 3.68 (.26) | <.001 | .01 (.00) | <.001 | 2.07 (.24) | <.001 | .52 (.24) | .03 |
| Hysterectomy with ovarian conservation |
.82 (.57) | .20 | 3.95 (1.92) | .89 | −.01 (.01) | .04 | 5.18 (1.43) | .03 | −1.23 (1.99) | .38 |
| Hysterectomy with bilateral oophorectomy |
.25 (.54) | .76 | 2.64 (1.42) | .46 | −.01 (.01) | .85 | 2.83 (1.45) | .60 | .49 (1.18) | .98 |
|
Mean annual rate of change after index visit |
||||||||||
| Natural menopause (referent) |
−.82 (.15) | <.001 | 1.52 (.37) | <.001 | .01 (.00) | <.001 | 1.00 (.33) | <.001 | −.58 (.34) | .09 |
| Hysterectomy with ovarian conservation |
−.55 (.59) | .64 | 1.02 (1.78) | .78 | .02 (.01) | .40 | .06 (1.17) | .42 | −.62 (1.47) | .98 |
| Hysterectomy with bilateral oophorectomy |
−.50 (.62) | .61 | 3.44 (1.31) | .14 | .01 (.01) | .98 | 1.62 (1.45) | .67 | .98 (1.34) | .25 |
Models adjusted by site, race/ethnicity, educational attainment, age at FMP or surgery, menopausal status at visit prior to FMP or surgery, elapsed time between index visit and FMP or surgery (all time-invariant), and stroke or myocardial infarction, physical activity, antidepressant use (HDL, triglycerides), hormone therapy use, self-rated health, cholesterol medication use (HDL, LDL, triglycerides), and smoking status (time-varying). All covariates entered simultaneously.
P-values for natural menopause (referent) refer to significant difference from 0; p-values for hysterectomy with ovarian conservation and hysterectomy with bilateral oophorectomy refer to significant difference from natural menopause.
LDL-C (low density lipoprotein cholesterol)
HDL-C (high density lipoprotein cholesterol)
Apo (apolipoprotein)
SE (Standard Error)
Figure 1. Lipids annual means.
Figure shows the covariate-adjusted means (95% CI) at the time of surgery and before and after surgery compared to values at the time of final menstrual period (FMP) and before and after FMP based on predicted values from adjusted linear regression models within each year. Slopes before and after surgery compared to slopes before and after FMP were identical except for a steeper slope for ApoA1 prior to having hysterectomy with ovarian conservation, p = .03.
Other Cardiovascular Risk Factors
At the index visit, groups did not differ in HOMA-IR, SBP, tPA-ag, Factor VIIc, or CRP levels (Table 3). From study entry to FMP, women increased annually in SBP, tPA-ag, Factor VIIc, and declined annually in PAI-1 and CRP. These changes were similar to those experienced by women from baseline to surgery, except that tPA-ag decreased annually in women who had a hysterectomy with ovarian conservation and CRP levels increased annually more in the women who subsequently had a hysterectomy with bilateral oophorectomy (Table 3). After FMP, HOMA-IR increased annually and PAI-1 declined. After surgery the changes were similar to those experienced by the natural menopause group.
Table 3.
Regression coefficients (standard errors) and their p-values from fully adjusted models for other cardiovascular risk factor levels at index visit (visit after final menstrual period or surgery) and changes prior to and following index visit
| (log) HOMA-IR* | Systolic Blood Pressure |
TPA-ag‡ | PAI-1‡ | Factor VIIc | CRP§ | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient (SE)** |
p- value |
Coefficient (SE) |
p- value |
Coefficient (SE) |
p- value |
Coefficient (SE) |
p- value |
Coefficient (SE) |
p- value |
Coefficient (SE) |
p- value |
|
|
Mean at index visit |
||||||||||||
|
Natural Menopause (referent) |
.30 (.02) | — | 111.58 (1.23) |
— | 8.04 (.84) | — | 24.68 (3.14) | — | 129.77 (3.06) |
— | 2.97 (.54) | — |
|
Hysterectomy with ovarian conservation |
.32 (.04) | .58 | 112.56 (2.06) |
.63 | 7.68 (.78) | .64 | 21.40 (4.83) | .50 | 154.08 (21.56) |
.26 | 3.77 (.83) |
.33 |
|
Hysterectomy with bilateral oophorectomy |
.29 (.03) | .78 | 114.14 (1.66) |
.12 | 8.23 (1.09) | .86 | 26.15 (4.80) | .76 | 131.76 (5.19) |
.70 | 4.20 (.77) | .11 |
|
Mean annual rate of change from baseline to index visit |
||||||||||||
|
Natural menopause (referent) |
.00 (.00) | .06 | .40 (.12) | <.001 | .24 (.08) | <.01 | −1.94 (.57) | <.001 | 1.27 (.39) | <.01 | −.15 (.06) | .02 |
|
Hysterectomy with ovarian conservation |
.00(.02) | .88 | −.38 (1.19) | .51 | −.39 (.20) | <.01 | −1.73 (1.92) | .92 | 7.41 (6.40) | .34 | −.97 (1.33) | .53 |
|
Hysterectomy with bilateral oophorectomy |
.00 (.01) | .86 | 1.04 (.61) | .30 | .40 (.30) | .60 | −.27 (1.90) | .38 | −.80 (1.97) | .29 | .58 (.25) | <.01 |
|
Mean annual rate of change after index visit |
||||||||||||
|
Natural menopause (referent) |
.02 (.00) | <.001 | .01 (.10) | .92 | .27 (.22) | .23 | −2.54 (.53) | <.001 | −.33 (.59) | .58 | .14 (.08) | .07 |
|
Hysterectomy with ovarian conservation |
.01 (.01) | .42 | .02 (.34) | .98 | .15 (.27) | .67 | −1.67 (1.81) | .63 | −8.27 (9.31) | .40 | −.19 (.25) | .18 |
|
Hysterectomy with bilateral oophorectomy |
.02 (.01) | .88 | −.02 (.36) | .93 | −.18 (.36) | .21 | −3.21 (1.72) | .69 | −.50 (2.22) | .94 | .03 (.22) | .61 |
Models adjusted by site, race/ethnicity, educational attainment, age at FMP or surgery, menopausal status at visit prior to FMP or surgery, elapsed time between index visit and FMP or surgery (all time-invariant), and stroke or myocardial infarction, heart medication use (systolic blood pressure, TPA, PAI-1, factor VIIc), physical activity, antidepressant use (HOMA-IR, PAI-1, FactorVIIc, CRP), hormone therapy use, smoking status, and antihypertensive medication use (systolic blood pressure) (time-varying). All covariates entered simultaneously.
P-values for natural menopause (referent) refer to significant difference from 0; p-values for hysterectomy with ovarian conservation and hysterectomy with bilateral oophorectomy refer to significant difference from natural menopause.
CRP(C-reactive Protein)
HOMA-IR (Homeostasis Model of Assessment –Resistance)
PAI-1 (Plasminogen activator inhibitor-1)
tPA-ag (Tissue plasminogen activator- antigen)
SE (standard error)
Further adjustments for BMI did alter the results somewhat for tPA-ag and CRP: After FMP, tPA-ag increased annually, estimate = .74 (.35) p = .04, whereas it declined annually after either hysterectomy with ovarian conservation, estimate = −.14 (.43), p = .04, or after hysterectomy with bilateral oophorectomy, estimate = −. 13 (.47), p = .06. CRP levels following hysterectomy with ovarian conservation declined annually, estimate = −.24 (.17), p =.04, relative to after FMP, estimate = .11 (.08).
There were no significant effects of ethnicity and change in CV risk factors prior to and following FMP or surgery. In other words, the impact of hysterectomy with or without oophorectomy in relation to FMP did not differ between African Americans and Caucasians.
Discussion
The objective of the present paper was to compare the annual CVD risk factor changes that occur in mid-life women prior to and following natural menopause or hysterectomy with or without ovarian conservation. The influence of natural menopause and hysterectomy with or without ovarian conservation was similar for HDL-C, LDL-C, ApoB, HOMA-IR, SBP, PAI-1, and Factor VIIc over time. Several CVD risk factor changes did differ during the intervals prior to and following hysterectomy, compared to the changes prior to and following FMP, but not in a pattern suggesting increasing cardiovascular risk following hysterectomy. Prior to hysterectomy with ovarian conservation, triglycerides and tPA changes declined and ApoA1 increased, compared to the changes prior to natural menopause, suggesting a lower risk trajectory among these women pre-surgically. Prior to hysterectomy with bilateral oophorectomy, CRP increases were greater than prior to a natural menopause but no differences occurred after surgery, compared to after FMP. The absence of effects for the group that should be presumably be at the highest risk – women who had a hysterectomy with bilateral oophorectomy – may be due to the influence of HT use, although some evidence points to elevated CRP being associated with HT (23). Thus, we conclude that hysterectomy with or without bilateral oophorectomy does not introduce a substantial increase in cardiovascular risk factors among midlife women.
On the surface, our results differ from those of the Women’s Health Initiative. In that study, elderly women who had reported having a hysterectomy, regardless of oophorectomy status, had at baseline higher levels of CVD risk factors (8) and a higher proportion of hypertension, diabetes, high cholesterol, and obesity, compared to women who had natural menopause. There are likely to be a number of reasons for the differences. Our focus was on risk factor levels, adjusted for medications for hypertension, diabetes, and high cholesterol. In the Women’s Health Initiative, CVD risk factor data collection had typically occurred many years after surgery, whereas in the present study the risk factor, surgical status, and menstrual cycle information were collected annually and concurrently for 11 years. Our analyses were restricted to elective surgery and we excluded surgery due to cancer because of treatment and disease effects, whereas the Women’s Health Initiative did not exclude women with specific causes for the surgery. Finally, the frequency and reasons for surgical menopause are undoubtedly different for the two cohorts because of changes in recommendations regarding hysterectomy over the decades.
Limitations and Strengths
Our study findings are limited by a number of factors. First, the sample was composed of premenopausal women who were initially 42–52 years of age, all of whom had a uterus and at least one ovary at entry. Perhaps they were not young enough as bilateral oophorectomy may have a greater impact on CVD risk if the surgery was performed at younger ages (24), when the decine in ovarian hormones would have been more pronounced. Given that we could evaluate surgeries only occurring after study entry, restricting the range in age and time of surgery in the sample, we could not evaluate the influence of age at surgery on our results. Second, there were relatively few cases of hysterectomy and bilateral oophorectomy. Many women who have elective hysterectomy are entering the perimenopause and would not have been eligible initially for the cohort. Thus, the SWAN sample is not typical of the general population of mid-life women, many of whom had had a hysterectomy or oophorectomy by midlife. Given the large number of risk factors and comparisons tested, the few significant associations observed may be due to chance. Finally, the study could not address the impact of surgery on clinical events as the follow-up ended prior to the high risk period in women, i.e. above 65 years of age.
The study has a number of strengths. It is composed of a large, well characterized sample of mid-life women. Detailed data were collected annually with women having on average of over 10 assessments. The analytic approach compared annual changes in CVD risk before and after FMP or surgery and was robust to missing data. A large number of covariates, including use of medications known to influence risk factors and hormone therapy, were included. Thus, it is the only study that has carefully tracked prospective changes in CVD risk factors relative to FMP or surgical menopause.
Conclusions
Although women in mid-life experienced significant increases in CVD risk, the women who had surgical menopause in their 40’s and 50’s were not at any greater risk for increases in CVD risk factors compared to women who had a natural menopause. These results should provide reassurance to women and their clinicians that hysterectomy with or without ovarian conservation in mid-life is not likely to substantially accelerate women’s CVD risk.
Acknowledgments
Financial Support: The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants NR004061; AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
We thank the study staff at each site and all the women who participated in SWAN.
Abbreviations List
- Apo
apolipoprotein
- BMI
body mass index
- CRP-hs
C-reactive protein-high sensitivity
- FMP
final menstrual period
- HDL-C
high-density lipoprotein cholesterol
- LDL-C
low density lipoprotein cholesterol
- PAI-1
plasminogen activator inhibitor-1
- SBP
systolic blood pressure
- SWAN
Study of Women’s Health across the Nation
- tPA-ag
tissue plasminogen activator- antigen
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Relationship with Industry: None
Clinical Centers: University of Michigan, Ann Arbor – Siobán Harlow, PI 2011 – present, MaryFran Sowers, PI 1994–2011; Massachusetts General Hospital, Boston, MA – Joel Finkelstein, PI 1999 – present; Robert Neer, PI 1994 – 1999; Rush University, Rush University Medical Center, Chicago, IL – Howard Kravitz, PI 2009 – present; Lynda Powell, PI 1994 – 2009; University of California, Davis/Kaiser – Ellen Gold, PI; University of California, Los Angeles – Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY – Carol Derby, PI 2011 – present, Rachel Wildman, PI 2010 – 2011; Nanette Santoro, PI 2004 – 2010; University of Medicine and Dentistry – New Jersey Medical School, Newark – Gerson Weiss, PI 1994 – 2004; and the University of Pittsburgh, Pittsburgh, PA – Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD – Winifred Rossi 2012 - present; Sherry Sherman 1994 – 2012; Marcia Ory 1994 – 2001; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory: University of Michigan, Ann Arbor – Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: University of Pittsburgh, Pittsburgh, PA – Maria Mori Brooks, PI 2012 - present; Kim Sutton-Tyrrell, PI 2001 – 2012; New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995 – 2001.
Steering Committee: Susan Johnson, Current Chair
Chris Gallagher, Former Chair
Reference List
- 1.Keshavarz H, Hillis SD, Kieke BA, Marchbanks PA. Hysterectomy surveillance. United States 1994–1999. MMWR CDC Surveill Summ. 2002;51:1–8. [PubMed] [Google Scholar]
- 2.Whiteman MK, Hillis SD, Jamieson DJ, et al. Inpatient hysterectomy surveillance in the United States, 2000–2004. Am J Obstet Gynecol. 2008;198:34–37. doi: 10.1016/j.ajog.2007.05.039. [DOI] [PubMed] [Google Scholar]
- 3.El-Hemaidi I, Gharaibeh A, Shehata H. Menorrhagia and bleeding disorders. Curr Opin Obstet Gynecol. 2007;19:513–520. doi: 10.1097/GCO.0b013e3282f1ddbe. [DOI] [PubMed] [Google Scholar]
- 4.Colditz GA, Willett WC, Stampfer MJ, Rosner B, Speizer FE, Hennekens CH. Menopause and the risk of coronary heart disease in women. N Engl J Med. 1987;316:1105–1110. doi: 10.1056/NEJM198704303161801. [DOI] [PubMed] [Google Scholar]
- 5.Parker WH, Broder MS, Chang E, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the nurses' health study. Obstet Gynecol. 2009;113:1027–1037. doi: 10.1097/AOG.0b013e3181a11c64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ingelsson E, Lundholm C, Johansson AL, Altman D. Hysterectomy and risk of cardiovascular disease: a population-based cohort study. Eur Heart J. 2011;32:745–750. doi: 10.1093/eurheartj/ehq477. [DOI] [PubMed] [Google Scholar]
- 7.Jacoby VL, Grady D, Wactawski-Wende J, et al. Oophorectomy vs ovarian conservation with hysterectomy: cardiovascular disease, hip fracture, and cancer in the Women's Health Initiative Observational Study. Arch Intern Med. 2011;171:760–768. doi: 10.1001/archinternmed.2011.121. [DOI] [PubMed] [Google Scholar]
- 8.Howard BV, Kuller L, Langer R, et al. Risk of cardiovascular disease by hysterectomy status, with and without oophorectomy: the Women's Health Initiative Observational Study. Circulation. 2005;111:1462–1470. doi: 10.1161/01.CIR.0000159344.21672.FD. [DOI] [PubMed] [Google Scholar]
- 9.Laughlin SK. New directions in the epidemiology of uterine fibroids. Semin Reprod Med. 2010;28:204–217. doi: 10.1055/s-0030-1251477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freeman EW, Sammel MD, Grisso JA, Battistini M, Garcia-Espagna B, Hollander L. Hot flashes in the late reproductive years: risk factors for Africa American and Caucasian women. J Womens Health Gend Based Med. 2001;10:67–76. doi: 10.1089/152460901750067133. [DOI] [PubMed] [Google Scholar]
- 11.Merrill RM. Hysterectomy surveillance in the United States, 1997 through 2005. Med Sci Monit. 2008;14:CR24–CR31. [PubMed] [Google Scholar]
- 12.Gibson CJ, Bromberger JT, Weiss GE, Thurston RC, Sowers M, Matthews KA. Negative attitudes and affect do not predict elective hysterectomy: a prospective analysis from the Study of Women's Health Across the Nation. Menopause. 2011;18:499–507. doi: 10.1097/gme.0b013e3181f9fa35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matthews KA, Crawford SL, Chae CU, et al. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition? J Am Coll Cardiol. 2009;54:2366–2373. doi: 10.1016/j.jacc.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibson CJ, Thurston RC, El Khoudary SR, Sutton-Tyrrell K, Matthews KA. Body Mass Index Following Natural Menopause and Hysterectomy with and without Bilateral Oophorectomy. Int J Obes. 2012 Sep 25; doi: 10.1038/ijo.2012.164. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sowers M, Crawford SL, Sternfeld B, Morganstein D, Gold EB, Greendale GA, Evans D, Neer R, Matthews KA, Sherman S, Lo A, Weiss G, Kelsey J. SWAN: A multicenter, multiethnic, community-based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey J, Marcus R, editors. Menopause: Biology and Pathobiology. San Diego, CA: Academic Press; 2000. pp. 175–188. [Google Scholar]
- 16.Myers GL, Cooper GR, Winn CL, Smith SJ. The Centers for Disease Control-National Heart, Lung and Blood Institute Lipid Standardization Program. An approach to accurate and precise lipid measurements. Clin Lab Med. 1989;9:105–135. [PubMed] [Google Scholar]
- 17.Steiner P, Freidel J, Bremner W, Stein E. Standardization of micromethods for plasma cholesterol, triglyceride and HDL-cholesterol with the lipids clinics' methodolgy. J Clin Chem Clin Biochem. 1981;19:850. [Google Scholar]
- 18.Warnick GR, Albers JJ. A comprehensive evaluation of the heparin-manganese precipitation procedure for estimating high density lipoprotein cholesterol. J Lipid Res. 1978;19:65–76. [PubMed] [Google Scholar]
- 19.Sternfeld B, Ainsworth BE, Quesenberry CP. Physical activity patterns in a diverse population of women. Prev Med. 1999;28:313–323. doi: 10.1006/pmed.1998.0470. [DOI] [PubMed] [Google Scholar]
- 20.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 21.Ware J. Manual and Interpretation Guide. Boston, MA: The Health Institute, New England Medical Center; 1993. SF-36 211 Health Survey. [Google Scholar]
- 22.Bryk A, Raudenbush S. Hierarchical Linear Models: Applications and Data Analysis Methods. 2nd edition. London: Sage Publications, Inc; 2002. [Google Scholar]
- 23.Lakoski SG, Herrington DM. Effects of hormone therapy on C-reactive protein and IL-6 in postmenopausal women: a review article. Climacteric. 2005 Dec;8(4):317–326. doi: 10.1080/13697130500345109. [DOI] [PubMed] [Google Scholar]
- 24.Atsma F, Bartelink ML, Grobbee DE, van der Schouw YT. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: a meta-analysis. Menopause. 2006;13:265–279. doi: 10.1097/01.gme.0000218683.97338.ea. [DOI] [PubMed] [Google Scholar]