Abstract
Various PEG-Vitamin E conjugates including D-alpha-tocopheryl polyethylene glycol succinate 1000 (TPGS) have been extensively studied as a nonionic surfactant in various drug delivery systems. However, limited information is available about the structure-activity relationship of PEG-Vitamin E conjugates as a micellar formulation for paclitaxel (PTX). In this study, four PEG-Vitamin E conjugates were developed that vary in the molecular weight of PEG (PEG2K vs PEG5K) and the molar ratio of PEG/Vitamin E (1/1 vs 1/2) in the conjugates. These conjugates were systematically characterized with respect to CMC, PTX loading efficiency, stability, and their efficiency in delivery of PTX to tumor cells in vitro and in vivo. Our data show that PEG5K-conjugates have lower CMC values and are more effective in PTX loading with respect to both loading capacity and stability. The conjugates with two Vitamin E molecules also worked better than the conjugates with one molecule of Vitamin E, particularly for PEG2K-system. Furthermore, all of the PEG-Vitamin E conjugates can inhibit P-gp function with their activity being comparable to that of TPGS. More importantly, PTX-loaded PEG5K-VE2 resulted in significantly improved tumor growth inhibitory effect in comparison to PTX formulated in PEG2K-VE or PEG2K-VE2, as well as Cremophor EL (Taxol) in a syngeneic mouse model of breast cancer (4T1.2). Our study suggests that PEG5K-Vitmin E2 may hold promise as an improved micellar formulation for in vivo delivery of anticancer agents such as PTX.
Keywords: Nanomicelles, Paclitaxel, TPGS, Controlled and sustained drug delivery, Cancer, Nanotechnology
INTRODUCTION
The poor clinical efficacy and the associated severe side effects of conventional chemotherapy in cancer treatment have stimulated the development of novel and effective drug delivery systems. Recently, increasing efforts have been placed on the development of nanotechnology-based drug delivery platforms. Polymeric micelles, liposomes, dendrimers and nanoparticles of biodegradable polymers have been extensively studied as delivery systems to improve cancer treatment.1 Among the many studied delivery systems, polymeric micelles have drawn considerable attention as a versatile nanotherapeutic platform, owing to ease of preparation, good biocompatibility, and relatively high efficiency in drug delivery.2,3 It is well known that polymeric micelles can improve the aqueous solubility of poorly water-soluble chemotherapeutic agents by packing them in the hydrophobic core of the micelles. Besides, the blood circulation times of drug-loaded micelles can be significantly prolonged due to the steric hindrance imposed by the presence of the long hydrophilic PEG shell.2,3 Furthermore, compared to other delivery systems, micelles are highly effective in passive tumor targeting through the leaky vasculature via enhanced permeability and retention effect (EPR) because of their extremely small sizes ranging from 10 to 100 nm, resulting in favorable biodistribution and improved therapeutic index.4,5 Nevertheless, most polymeric micellar formulations employ “inert” excipients that not only lack therapeutic activity, but also potentially impose safety concern.6
D-alpha-tocopheryl polyethylene glycol succinate 1000 (TPGS) is a hydrophilic derivative of natural Vitamin E, which is generated via coupling of polyethylene glycol (PEG) to Vitamin E succinate via an ester linkage.7 Over the last decade, TPGS has been intensively studied in various types of delivery systems: TPGS has been used as an effective emulsifier, solubilizer, additive, permeability enhancer as well as absorption enhancer.8,9 As an inhibitor of P-gp, TPGS has also been utilized as an excipient to overcome multidrug resistance (MDR) and improve the bioavailability of anticancer drugs.9–12 Examples of TPGS application in nanomedicine platform include TPGS-emulsified PLGA nanoparticles, nanoparticles of TPGS-based copolymers, and TPGS-based micelles, liposomes, and prodrugs.3,13–19 In addition, several new derivatives of improved performance have been reported including TPGS5K, TPGS2K, and PEG2K-Vitamin E2 conjugate.3,15,19, However, the optimal structure of PEG-Vitamin E conjugates as a micellar delivery system remains incompletely understood.
We have recently developed a PEG-derivatized embelin-based micellar system that is suitable for delivery of poorly water-soluble drugs such as PTX.20 Structurally, PEG-embelin conjugate is very similar to TPGS. Embelin has various biological activities including anti-inflammatory, anti-diabetic, and hepatoprotective effect.21–23 Embelin also has antitumor activity and synergizes with other anticancer agents through blocking the activity of X-linked inhibitor of apoptosis protein (XIAP).20,21,24–27 Thus, similar to TPGS, PEG-embelin also functions as a dual functional system for delivery of anticancer agents but with different mechanism of action.20 Optimization of PEG-embelin system has shown that a conjugate with two embelin molecules coupled to PEG is significantly more effective than the conjugate with a 1: 1 molar ratio of PEG and embelin.28 In addition, the embelin conjugates with PEG5K worked better than the PEG3.5K conjugates.28 This has prompted us to conduct similar study with TPGS micellar system. We have developed four PEG-Vitamin E conjugates that vary in the molecular weight of PEG (PEG2K vs PEG5K) and the molar ratio of PEG/Vitamin E (1/1 vs 1/2) in the conjugates. Our data show that PEG5K-conjugates have lower CMC values and are more effective in PTX loading with respect to both loading capacity and stability. The conjugates with two Vitamin E molecules also worked better than the conjugates with one molecule of Vitamin E, particularly for PEG2K-system. All of the four PEG-Vitamin E conjugates showed the P-gp inhibition activity with their efficiency being comparable to that of TPGS. More importantly, PTX-loaded PEG5K-VE2 resulted in significantly improved tumor growth inhibitory effect in comparison to PTX formulated in PEG2K-VE or PEG2K-VE2, as well as Cremophor EL (Taxol) in a syngeneic mouse model of breast cancer (4T1.2).
EXPERIMENTAL SECTION
Materials
Paclitaxel (98%) was purchased from AK Scientific Inc. (CA, USA). Dulbecco’s phosphate buffered saline (DPBS) was purchased from Lonza (MD, USA). Methoxy-PEG2,000-OH, Methoxy-PEG5,000-OH, dimethyl sulfoxide (DMSO), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), Triton X-100, Dulbecco’s Modified Eagle’s Medium (DMEM) and succinate anhydride were all purchased from Sigma-Aldrich (MO, USA). Fetal bovine serum (FBS) and penicillin-streptomycin solution were from Invitrogen (NY, USA). D-alpha-tocopheryl was purchased from Tokyo Chemical Industry (OR, USA). DCC was purchased from Alfa Aesar (MA, USA). DMAP was purchased from Calbiochem-Novabiochem Corporation (CA, USA). All solvents used in this study were HPLC grade.
Synthesis of PEG2K-VE, PEG2K-VE2, PEG5K-VE and PEG5K-VE2
PEG5K-VE2 was synthesized via solution phase condensation reactions from MeO-PEG-OH with a molecular weight of 5000 Da. (Boc)lysine(Boc)-OH (2 equ.) was coupled onto the terminal-OH of PEG using DCC (2 equ.) and DMAP (0.1 equ.) as coupling reagents in DCM overnight. Di-Boc lysyl-PEG5K ester was precipitated and washed three times with cold ethanol and ether, respectively. Then, Boc groups were removed via treatment with 50% trifluoroacetic acid in DCM, and the lysyl-PEG5K ester was precipitated and washed three times by cold ethanol and ether, respectively. White powder precipitate was dried under vacuum. Vitamin E succinate was coupled to the deprotected amino groups of lysine with the assistance of DCC (2 equ.) and DMAP (0.1 equ.), resulting in PEG5K-VE2. This compound was subsequently dialyzed against water and lyophilized to yield a white powder. PEG2K-VE2 was similarly synthesized as PEG5K-VE2. PEG2K-VE (TPGS2K) and PEG5K-VE (TPGS5K) were synthesized following the literature.3
Preparation and characterization of free or PTX-loaded micelles
PTX-solubilized micelles were prepared by the following method. PTX (10 mM in chloroform) was added to different PEG-Vitamin E conjugates (10 mM in chloroform), respectively, with various carrier/drug molar ratios. The organic solvent was first removed by steady nitrogen flow to form a thin dry film of drug/carrier mixture. The film was further dried under high vacuum for 2 h to remove any traces of remaining solvent. Drug-loaded micelles were formed by suspending the film in DPBS. The drug-free micelles were similarly prepared as described above. The mean diameter of four different micelles with or without loaded drug was assessed by dynamic light scattering (DLS). The morphology and size distribution of PEG2K-VE, PEG2K-VE2, PEG5K-VE and PEG5K-VE2 micelles were observed, respectively, using transmission electron microscopy (TEM) after negative staining. The concentration of PTX in PTX-loaded micelles was evaluated by HPLC as described previously.20 The drug loading capacity (DLC) and drug loading efficiency (DLE) were calculated according to the following formula:
DLC (%) = [weight of drug used/(weight of polymer + drug used)] ×100%
DLE (%) = (weight of loaded drug/weight of input drug) ×100%
Stability study of micelles
A series of PTX-loaded micelles with different carrier/PTX molar ratios were prepared as described above and the PTX concentration in all samples was kept at 1 mg/mL. The sizes of samples were measured at different time points following the sample preparation. To examine the effect of serum on the particle stability, the samples were mixed with serum (FBS) at a final serum concentration of 50%. Size changes were monitored by DLS and measurement was terminated when the change of size reached significant difference.
Determination of the critical micelle concentration (CMC)
The CMCs of four different micelles were determined by employing pyrene as a fluorescence probe.29 A drug-free micelle solution in DPBS (2.5 mg/mL) was prepared via solvent evaporation method. A series of 2-fold dilutions was then made for PEG2K-VE, PEG2K-VE2, PEG5K-VE and PEG5K-VE2 micelles, with concentrations ranging from 2×10−4 to 0.5mg/mL. At the same time, aliquots of 50 µL of 4.8×10−6 M pyrene in chloroform were added into separate vials. The chloroform was first removed by nitrogen flow to form a thin film. The film was further dried under high vacuum for 2 h to remove any traces of remaining solvent. Then, the pre-prepared micelle solutions (400 µL in DPBS) of varying concentrations were added to the pyrene film to obtain a final pyrene concentration of 6×10−7 M in each vial. The solutions were kept on a shaker at 37 °C for 24 h to reach equilibrium before fluorescence measurement. The fluorescence intensity of samples was measured at the excitation wavelength of 334 nm and emission wavelength of 390 nm by Synergy H1 Hybrid Multi-Mode Microplate Reader (Winooski, VT). The CMC is determined from the threshold concentration, where the sharp increase in pyrene fluorescence intensity is observed.
In vitro drug release study
An in vitro drug release study was carried out by dialysis using DPBS (PH = 7.4) containing 0.5% (w/v) Tween 80 as the release medium. Two mL of PTX-loaded micelles (PEG2K-VE, PEG2K-VE2, PEG5K-VE or PEG5K-VE2) (1 mg PTX/mL) were sealed in dialysis tubes (MWCO = 12 KDa, Spectrum Laboratories) which were then immersed in 200 mL release medium in a beaker covered with parafilm. The beakers were placed in an incubator shaker at 100 rpm and 37°C. The concentration of PTX remaining in the dialysis tubes at various time points was measured by HPLC with the detector set at 227 nm. Values were reported as the means from triplicate samples.
Cell culture
DU145 and PC-3 are two androgen-independent human prostate cancer cell lines. 4T1.2 is a mouse metastatic breast cancer cell line. MCF-7 and MDA-MB-231 are human breast cancer cell lines. NCI/ADR-RES is Adriamycin (ADR)-resistant cell line. All cell lines were cultured in DMEM containing 10% FBS and 1% penicillin-streptomycin in a humidified environment at 37 °C with 5% CO2.
Hemolytic effect of micelles
Fresh blood samples were collected through cardiac puncture from rats. Heparin was immediately added into 10 mL of blood to prevent coagulation. Red blood cells (RBCs) were separated from plasma by centrifugation at 1500 rpm for 10 min at 4 °C. RBCs were washed three times with 30 mL ice-cold DPBS. RBCs were then diluted to 2% w/v with ice-cold DPBS and utilized immediately for the hemolysis assay. One mL of diluted RBC suspension was treated with various concentrations (0.0001, 0.001, 0.01, 0.1 and 1.0 mg/mL) of PEG2K-VE, PEG2K-VE2, PEG5K-VE or PEG5K-VE2 micelles, and PEI, respectively, and then incubated at 37 °C in an incubator shaker for 4 h. The samples were centrifuged at 1500 rpm for 10 min at 4 °C, and 100 µL of supernatant from each sample was transferred into a 96-well plate. The release of hemoglobin was determined by the absorbance at 540 nm using a microplate reader. RBCs treated with Triton X-100 (2%) and DPBS were considered as the positive and negative controls, respectively. Hemoglobin release was calculated as (ODsample-ODnegative control)/(ODpositive control-ODnegative control) × 100%
In vitro cytotoxicity study
The cytotoxicity of PTX formulated in PEG2K-VE, PEG2K-VE2, PEG5K-VE or PEG5K-VE2 micelles was assessed with two cancer cell lines (4T1.2 and NCI/ADR-RES) and compared to Taxol formulation. Briefly, 4T1.2 (1000 cells/well) or NCI/ADR-RES (3000 cells/well) cells were seeded in 96-well plates followed by 24 h of incubation in DMEM with 10% FBS and 1% streptomycin-penicillin. Various dilutions of PTX-loaded PEG2K-VE, PEG2K-VE2, PEG5K-VE or PEG5K-VE2 micelles, and Taxol (at the equal concentrations of PTX) were added to cells. Cells were incubated for 72 h and cell viability was assessed by MTT assay as described previously.20 The cytotoxicity of PEG2K-VE, PEG2K-VE2, PEG5K-VE or PEG5K-VE2 micelles alone was similarly tested in 4T1.2 (1000 cells/well), NCI/ADR-RES (3000 cells/well), MCF-7 (5000 cells/well), MDA-MB-231 (2000 cells/well) and PC-3 (5000 cells/well) cells as described above.
P-gp ATPase assay
The modulation of P-gp ATPase activity by PEG-derivatized Vitamin E conjugates was conducted by using P-gp-Glo™ assay system (Promega, USA). This assay system provides the necessary reagents for performing luminescent P-gp ATPase assay. Compounds that interact with P-gp can be identified as stimulator or inhibitor of the ATPase activity. The P-gp-Glo™ assay detects the effects of compounds on recombinant human P-gp in a cell membrane fraction. Essentially, the assay relies on an ATP-dependent light-generating reaction of firefly luciferase. The effect of PEG-derivatized Vitamin E conjugates on P-gp ATPase activity was evaluated on a verapamil-stimulated ATPase activity. In this assay, sodium orthovanadate (Na3VO4) was employed as a selective inhibitor of P-gp. First, test samples containing verapamil (50µM) and PEG-derivatized Vitamin E conjugates (final concentrations at 10 and 100 µM, respectively) or Na3VO4 were added to 96-well plates and incubated with P-gp membrane for 5 min at 37 °C. Then, the reaction was initiated by the addition of MgATP followed by another 40 minutes’ incubation at 37°C. Afterwards, the samples were removed from 37°C incubator and then ATP detection reagent was added in order to develop the luminescence. Signals were measured 20 minutes later on a plate reading luminometer (Victor2 1420 multilabel counter). The changes of relative light unit (ΔRLU) were determined as follows: ΔRLU = (luminescence of Na3VO4-treated group) – (luminescence of the samples treated by the mixture of verapamil and PEG-derivatized Vitamin E conjugates).
Animals
Female BALB/c mice, 10–12 weeks were purchased from Charles River (Davis, CA). All animals were housed under pathogen-free conditions according to AAALAC guidelines. All animal-related experiments were performed in full compliance with institutional guidelines and approved by the Animal Use and Care Administrative Advisory Committee at the University of Pittsburgh.
In vivo therapeutic study
A syngeneic murine breast cancer model (4T1.2) was used to examine the therapeutic effect of PTX formulated in PEG2K-VE, PEG2K-VE2, PEG5K-VE or PEG5K-VE2 micelles, and Taxol. 2 × 105 4T1.2 cells in 200 µL PBS were inoculated s.c. at the right flank of female BALB/c mice. Treatments were initiated when tumors in the mice reached a tumor volume around 50 mm3 and this day was designated as day 1. On day 1, mice were randomly divided into six groups (n=5) and received i.v. administration of PTX formulated in PEG2K-VE, PEG2K-VE2, PEG5K-VE or PEG5K-VE2 micelles, as well as Taxol (10 mg PTX/kg), respectively on days 1, 3, 5, 9, and 12, while control mice received saline. Tumor sizes were measured with digital caliper on days 1, 3, 5, 9, 12, 15 and 18, and calculated according to the following formula: (L×W2)/2, where L is the longest and W is the shortest in tumor diameters (mm). To compare between groups, relative tumor volume (RTV) was calculated at each measurement time point (where RTV equals the tumor volume at a given time point divided by the tumor volume prior to first treatment). Mice were sacrificed when tumor reached 2000 mm3 or developed ulceration. To monitor the potential toxicity, the body weights of all mice from different groups were measured on days 1, 3, 5, 9, 12, 15 and 18.
Statistical analysis
In all statistical analysis, the significance level was set at a probability of P < 0.05. All results were reported as the mean ± standard deviation (SD) unless otherwise indicated. Statistical analysis was performed by Student’s t-test for two groups, and one-way ANOVA for multiple groups.
RESULTS
Synthesis of PEG2K-VE, PEG2K-VE2, PEG5K-VE or PEG5K-VE2 conjugates
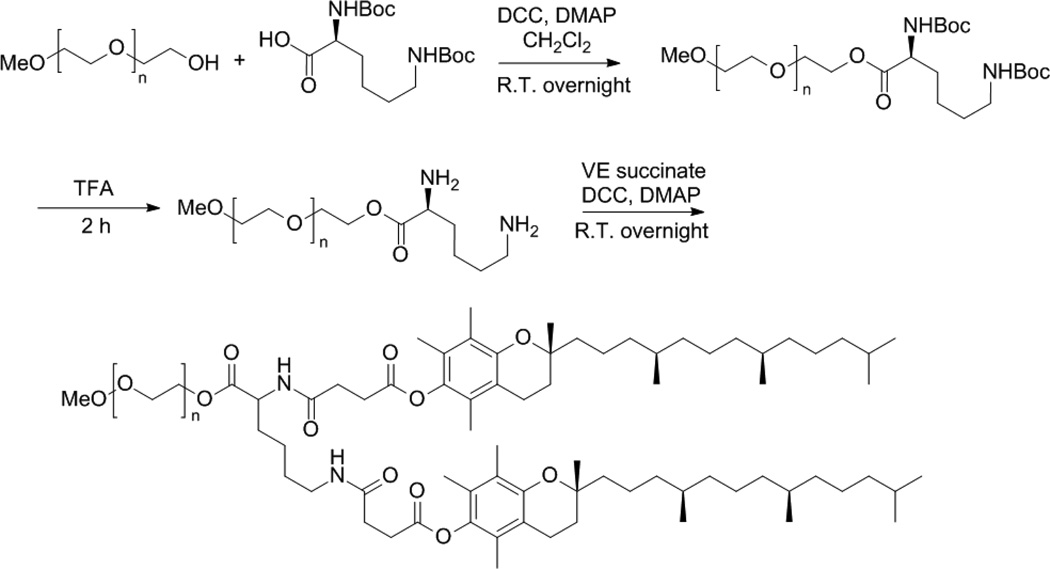
Lysine-linked di-tocopherol polyethylene glycol 5000 succinate was synthesized via solution phase reaction with two Vitamin E succinates attached to mPEG-5000 through the linker of lysine. The synthetic scheme is presented in Fig.1.
Fig. 1.
The synthesis scheme of PEG5K-VE2. First, PEG5K reacted with di-Boc-protected lysine to obtain PEG5K-conjugated di-Boc lysine. Then TFA was employed to remove the Boc groups in order to get free amine. Finally, free amine reacted with Vitamin E succinate to attain PEG5K-VE2.
Initially, (Boc)lysine(Boc)-OH was coupled onto the terminal −OH of PEG using DCC and DMAP as coupling reagents in DCM. Boc groups were removed by the treatment with 50% trifluoroacetic acid in DCM. Vitamin E succinate was coupled to the amino groups of lysine, yielding PEG5K-VE2. The structure of PEG5K-VE2 was confirmed by 1H NMR in CDCl3 (Fig.S1A).The intense peak at 3.66 ppm was assigned to the methane protons of the polyethylene glycol. The proton peaks below 3.0 ppm were ascribed to the section of Vitamin E succinate.
MALDI-TOF suggested that two Vitamin E succinates were successfully attached to mPEG5000 with the linker of lysine. (Fig. S2A). PEG2K-VE2 was synthesized following the same synthesis route of PEG5K-VE2. PEG2K-VE and PEG5K-VE were synthesized according to the literature.3 Their structures were confirmed by 1H NMR and MALDI-TOF as reported (Figs. S1 & S2). The purity for each conjugates was assessed via HPLC and shown in Fig. S3.
Size & size distribution of micelles
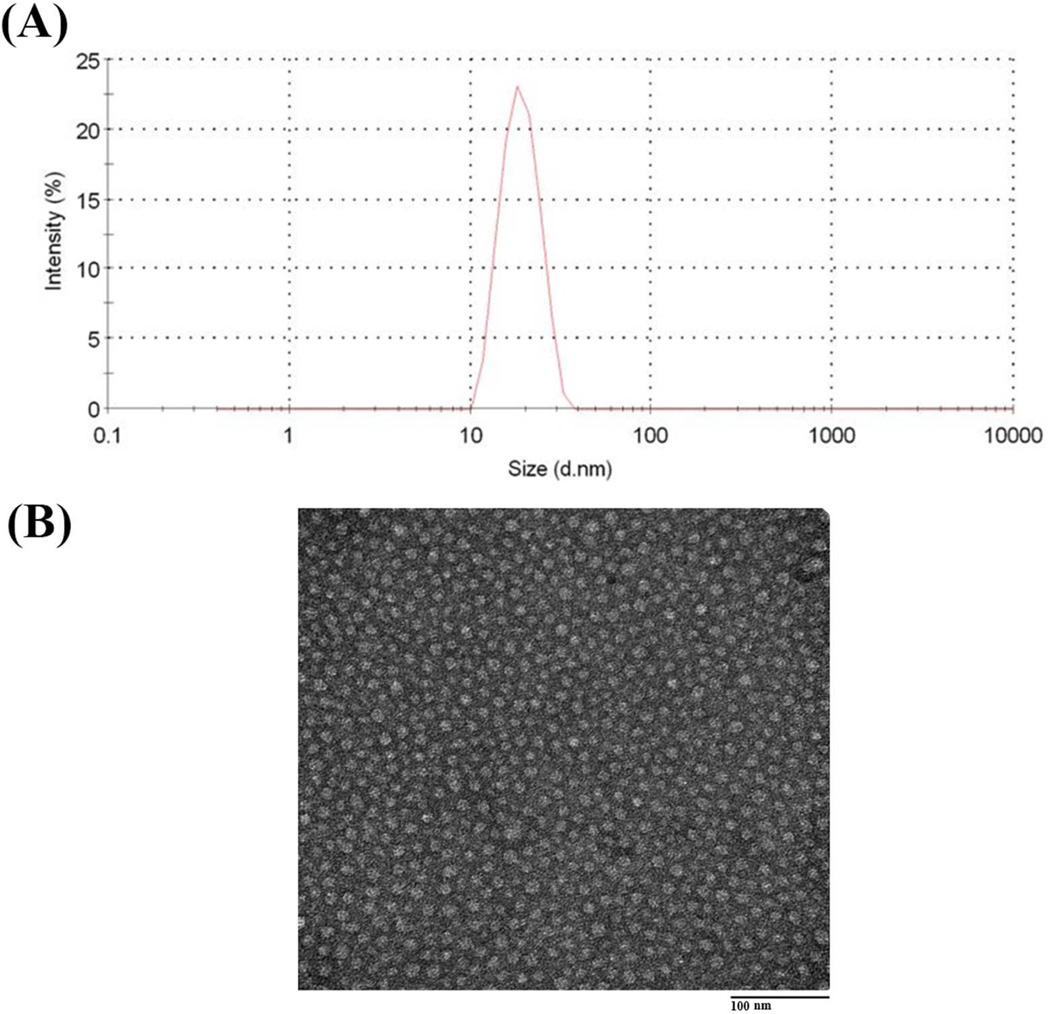
In aqueous solution, the four PEG-derivatized Vitamin E conjugates readily self-assemble to form micellar nanoparticles with the particle sizes of around 20 nm as determined by DLS analysis (Table.1).
Table 1.
Size of PEG-derivatized Vitamin E micelles
| Conjugates | size | PDI |
|---|---|---|
| PEG2K-VE | 21.5±0.68 | 0.08 |
| PEG2K-VE2 | 18.9±0.08 | 0.07 |
| PEG5K-VE | 19.7±0.09 | 0.08 |
| PEG5K-VE2 | 18.8±0.12 | 0.09 |
Fig. 2A shows a single peak for PEG5K-VE2 micelles in size distribution. Negative EM staining revealed spherical particles of uniform size (Fig.2B).
Fig. 2.
(A) The size distribution of free PEG5K-VE2 nanoparticles in DPBS measured by dynamic light scattering (DLS) and (B) Transmission electron microscopic (TEM) images of PEG5K-VE2 micelles. The spherical micelles with diameter around 20 nm were observed. The concentration of PEG5K-VE2 was kept at 20 mg/mL during the measurements.
The sizes of the micelles observed under TEM were quite consistent with those measured by DLS. Similar results were shown for the other three micelles (Figs. S4 & S5).
Critical micelle concentration (CMC)
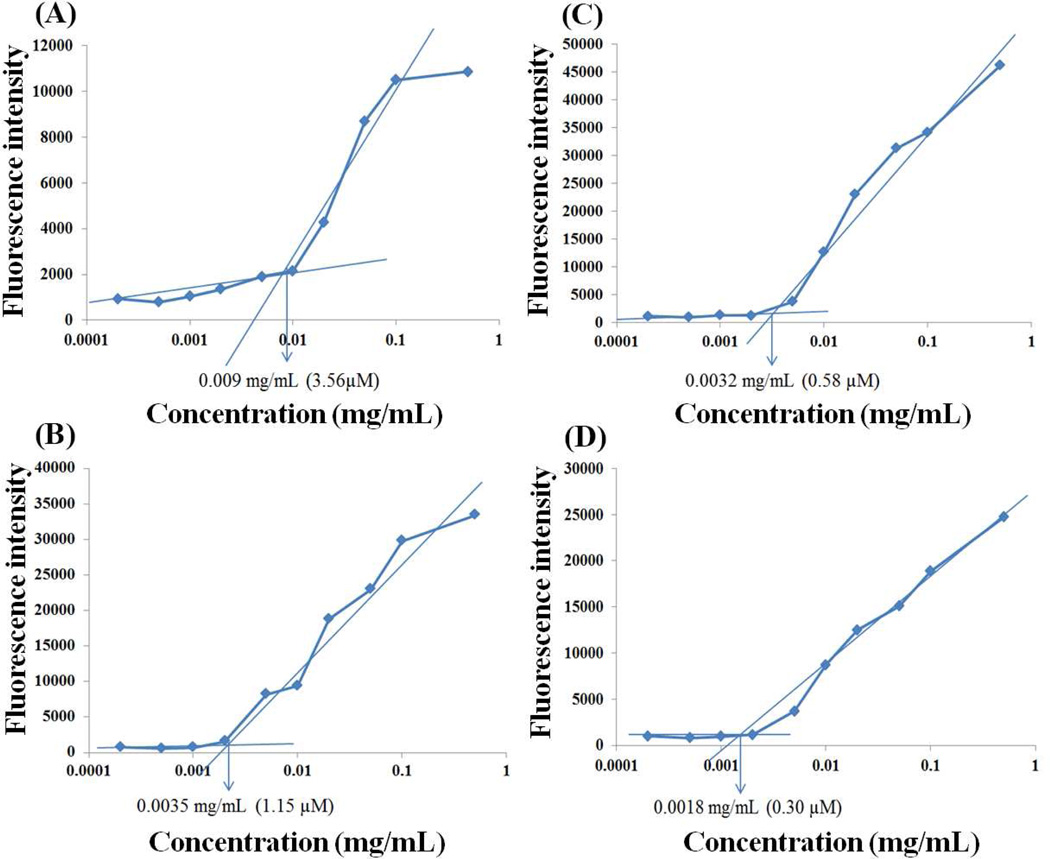
Fig. 3 shows the CMC measurements of PEG2K-VE, PEG2K-VE2, PEG5K-VE, and PEG5K-VE2 micelles using pyrene as a fluorescence probe.
Fig. 3.
Critical micelle concentration (CMC) measurements of PEG2K-VE (A), PEG2K-VE2 (B), PEG5K-VE (C) and PEG5K-VE2 (D) by using pyrene as a hydrophobic fluorescence probe. The fluorescence intensity of pyrene was collected at the excitation wavelength of 334 nm and the emission wavelength of 390 nm. The fluorescence intensity was plotted as a function of logarithmic concentration of micelles. [pyrene] =6 × 10−7 mol/L.
Upon incorporation into the micelles, the fluorescence intensity of pyrene increases substantially at the concentration of micelles above the CMC.30 Based on the partition of the pyrene, the CMC of micelles was obtained by plotting the fluorescence intensity versus logarithm concentration of the polymer. The CMCs of PEG-derivatized micelles were determined from the crossover point at the low concentration range. The CMCs of the PEG5K-VE and PEG5K-VE2 conjugates are 0.58 µM and 0.30 µM, respectively, which are lower than those of PEG2K-VE (3.56 µM) and PEG2K-VE2 (1.15 µM).
Drug loading efficiency (DLE)
DLE is one of the important parameters in drug delivery systems. The PTX loading efficiency of PEG2K-VE, PEG2K-VE2, PEG5K-VE, and PEG5K-VE2 micelles with different carrier to drug molar ratios was determined by HPLC (Table. 2). The sizes of micelles were also examined under corresponding conditions.
Table 2.
Physicochemical characterization of PTX-loaded miscelles
| carrier/PTX ratio |
PTX-loaded micellesa |
size (nm)b | PDIc | DLE (%)d | stabilitye in DPBS (hour) |
stabilitye in 50% FBS (hour) |
|---|---|---|---|---|---|---|
| 2.5:1 | PEG2K-VE/PTX | 34.4±1.22 | 0.34 | 42.9 | 0.1 | Milky |
| PEG2K-VE2/PTX | 26.1±2.42 | 0.23 | 64.8 | 0.2 | milky | |
| PEG5K-VE/PTX | 21.3±0.60 | 0.04 | 85.4 | 0.8 | 0.3 | |
| PEG5K-VE2/PTX | 20.1±0.72 | 0.05 | 86.4 | 1 | 0.4 | |
| 5:1 | PEG2K-VE/PTX | 224±0.36 | 0.14 | 66.6 | 0.8 | 0.5 |
| PEG2K-VE2/PTX | 23.5±0.35 | 0.15 | 69.8 | 1 | 0.7 | |
| PEG5K-VE/PTX | 20.3±0.59 | 0.05 | 86.4 | 2.3 | 1.5 | |
| PEG5K-VE2/PTX | 19.7±0.38 | 0.04 | 88.4 | 3 | 2.1 | |
| 7.5:1 | PEG2K-VE/PTX | 23.6±1.31 | 0.12 | 71.6 | 3 | 0.9 |
| PEG2K-VE2/PTX | 22.5±0.78 | 0.11 | 72.2 | 3.5 | 2.1 | |
| PEG5K-VE/PTX | 21.1±0.98 | 0.19 | 96.4 | 22 | 16.5 | |
| PEG5K-VE2/PTX | 20.9±0.56 | 0.16 | 98.2 | 24 | 19.3 | |
| 10:1 | PEG2K-VE/PTX | 21.9±l.34 | 0.10 | 86.4 | 4.2 | 2.8 |
| PEG2K-VE2/PTX | 23.6±0.84 | 0.16 | 89.7 | 55.5 | 30.8 | |
| PEG5K-VE/PTX | 19.8±1.23 | 0.09 | 96.9 | 60 | 39.5 | |
| PEG5K-VE2/PTX | 19.6±1.15 | 0.12 | 98.7 | 68 | 45.3 |
The PTX concentration were kept at 1 mg/mL.
Data represents the mean ± standard deviation (n±3) in DPBS.
Polydispersity index.
Drug Loading efficiency (%) = (weight of Loaded drug/weight of input drug) × 100° 0.
Data means there was no noticeable size change during the follow-up period.
PEG5K-VE and PEG5K-VE2 were comparable with respect to DLE at all carrier/drug ratios examined. Both effectively solubilized PTX in aqueous solution in a molar ratio as low as 0.5:1 with particle size remaining around 20 nm. However, these drug-loaded particles were only stable for less than 1 h. At a carrier/drug ratio of 7.5/1, they formed stable mixed micelles with PTX that were stable for about one day in DPBS. Increasing the carrier/drug ratio to 10:1 led to formation of particles that are stable over 65 h in DPBS. Essentially, all of the added PTX was incorporated into the micelles. In addition, the sizes of the particles remained the same following lyophilization and reconstitution with water (data not shown).
For PEG2K-VE and PEG2K-VE2 micelles, a minimal carrier/drug ratio of 2.5/1 (m/m) was required to solubilize the drug. PEG2K-VE2 was more effective than PEG2K-VE in solubilizing PTX with higher DLE at all carrier/drug ratios examined. At a carrier/drug ratio of 10/1, PTX-loaded PEG2K-VE2 micelles were significantly more stable than PTX formulated in PEG2K-VE micelles (55.5 vs 4.2 h). In addition to evaluating the stability of PTX-loaded micelles in DPBS, their stability in 50% FBS over time was also examined. All of the formulations tested were less stable in serum than in DPBS. Addition of serum to PEG5K-VE2/PTX (10/1, m/m) mixed micelles resulted in an increase of the particle size from 19.6 nm to 31.7 nm, which stayed stable for 45 h. Again, PEG5K-VE2/PTX shows the best stability in serum among the 4 mixed micelles tested. Overall, the four conjugates were ranked in the order of PEG5K-VE2 > PEG5K-VE > PEG2K-VE2 > PEG2K-VE with respect to their efficiency in forming stable mixed micelles with PTX in both DPBS and 50% FBS.
In vitro PTX release kinetics
A dialysis method was used to evaluate the release kinetics of PTX from PEG2K-VE, PEG2K-VE2, PEG5K-VE or PEG5K-VE2 micelles with DPBS (PH = 7.4) containing 0.5% w/v Tween 80 as the release medium. As shown in Fig. 4, PTX formulated in PEG5K-VE and PEG5K-VE2 micelles exhibited significantly better stability than PTX-loaded PEG2K-VE and PEG2K-VE2 micelles.
Fig. 4.
Cumulative PTX release profile from PTX-loaded micelles. DPBS (PH = 7.4) containing 0.5% (w/v) Tween 80 was used as the release medium to solubilize released PTX. At various time points, samples from different formulations were collected and measured by HPLC. PTX loading level was 1 mg/mL in micelles. Values are reported as the means ± SD for triplicate samples.
For the first 7 h, there was no significant difference among the 4 micellar systems, during which a burst release due to the relatively high drug concentrations at the very beginning may account for this result. However, significant differences were observed among the 4 formulations during the remaining experimental period. The size of PEG significantly affects the release kinetics: the two conjugates with PEG5K showed significantly slower release kinetics compared to the two conjugates with PEG2K. In addition, the conjugates with two molecules of Vitamin E gave better stability than the PEG-VE conjugates of 1: 1 molar ratio, particularly for PEG2K conjugates. Overall, the four conjugates were ranked in the order of PEG5K-VE2 > PEG5K-VE > PEG2K-VE2 > PEG2K-VE with respect to their stability in the release study.
Hemolytic effect of micelles
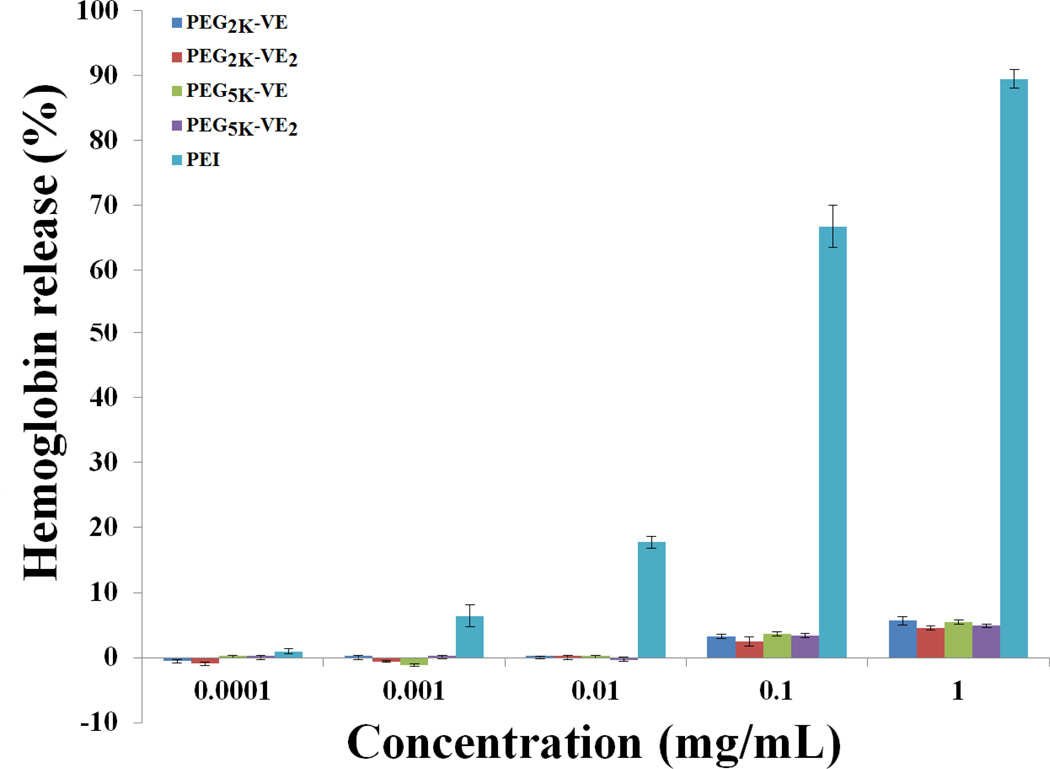
One concern for micellar systems is whether or not the surface activity of the surfactants affects cell membrane integrity. Therefore, free PEG2K-VE, PEG2K-VE2, PEG5K-VE and PEG5K-VE2 micelles were examined for the hemolytic activity and compared to polyethylenimine (PEI), a cationic polymer with potent cell surface activity. As shown in Fig. 5, treatment with PEI resulted in significant hemolysis in a dose-dependent manner. In contrast, only a very low level of hemolysis (~5%) was observed for all four blank micelles at the high doses (0.1 and 1 mg/mL) examined. The negligible hemolytic activity suggests that all of the 4 conjugates are mild surfactants that can be suitable for in vivo delivery of potent hydrophobic anticancer drugs.
Fig. 5.
In vitro hemolysis assay of PEG-derivatized Vitamin E micelles compared with PEL PEG-VE micelles and PEI of various concentrations were incubated with rat red blood cells (RBCs) for 4 h at 37 °C in an incubator shaker. The degree of RBCs lysis was measured spectrophotometrically (λ=540 nm) according to the release of hemoglobin. 2% Triton X-100 and DPBS were used as a positive and negative control, respectively. Values reported are the means ± SD for triplicate samples.
In vitro cytotoxicity of free and PTX-loaded micelles
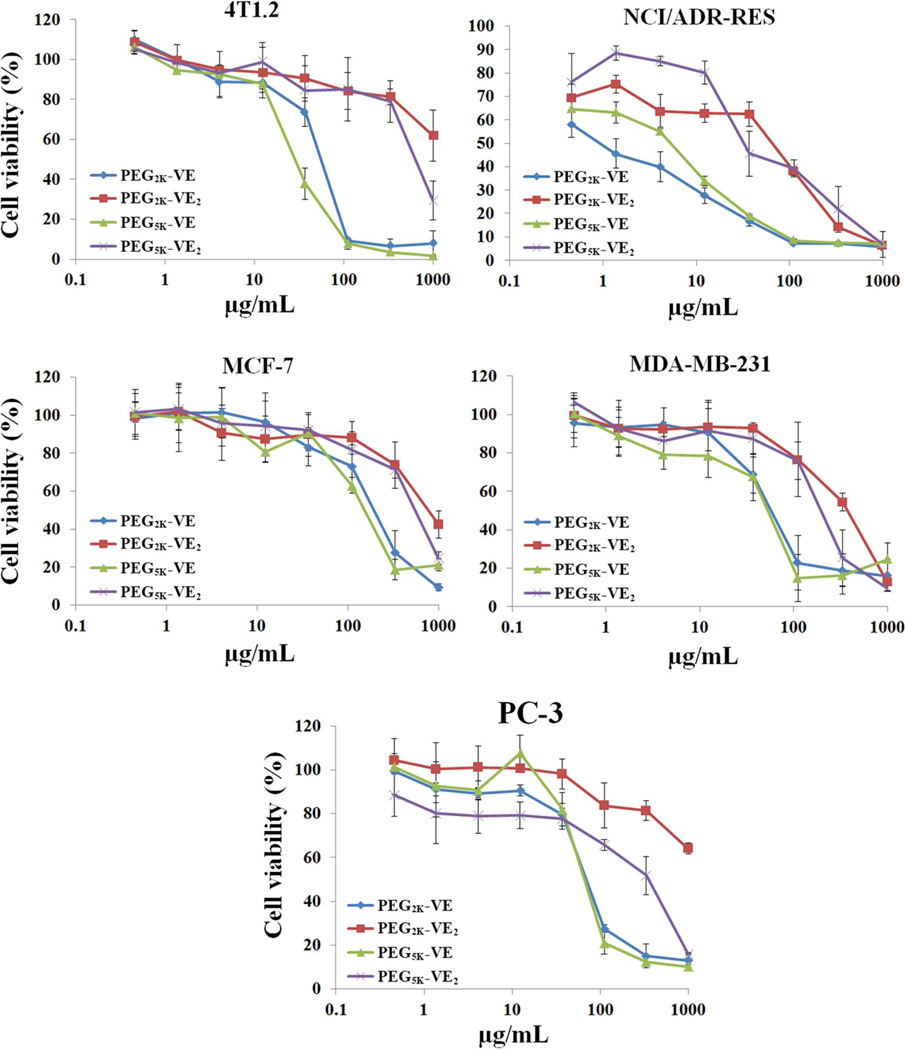
The cytotoxicity of carriers alone was examined in 4T1.2, NCI/ADR-RES, MCF-7, MDA-MB-231, and PC-3 cells, respectively. It was apparent that the single Vitamin E conjugates (PEG2K-VE and PEG5K-VE) showed significantly higher levels of cytotoxicity than those of double Vitamin E conjugates (PEG2K-VE2 and PEG5K-VE2) in all five cancer cell lines tested (Fig. 6).
Fig. 6.
Cell viability following treatment with free PEG2K-VE, PEG2K-VE2, PEG5K-VE, or PEG5K-VE2 micelles in a mouse breast cancer cell line, 4T1.2, a drug-resistant cell line, NCI/ADR-RES, two human breast cancer cell lines, MCF-7, and MDA-MB-231, and an androgen-independent human prostate cancer cell line. PC-3. Cells were incubated with indicated concentrations of micelles for 72 h and cytotoxicity was determined by MTT assay. Values reported are the means ± SD for triplicate samples
In MDA-MB-231 cells, the IC50 for PEG2K-VE2 and PEG5K-VE2 is 6 and 4.8 times higher than their single Vitamin E counterparts (Table. 3). Similar results were shown for the other four cancer cell lines (Table. 3). It is also apparent that NCI/ADR-RES cells were more sensitive than the other four cancer cell lines to all of the conjugates (Fig. 6 & Table. 3). Again, the single Vitamin E conjugates showed more potent cytotoxicity than the double Vitamin E conjugates in this drug-resistant cell line (Fig. 6 & Table. 3).
Table 3.
IC50 of free PEG2K-VE, PEG2K-VE2, PEG5K-VE and PEG5K-VE2 micelles in different cancer cell lines
| IC50(µg/mL) |
||||
|---|---|---|---|---|
| PEG2K-VE | PEG2K-VE2 | PEG5K-VE | PEG5K-VE2 | |
| 4T1.2 | 64.30 | N/A | 30.98 | 726.44 |
| NCI/ADR-RES | 1.04 | 74.83 | 6.05 | 33.83 |
| MCF-7 | 223.78 | 839.89 | 176.29 | 637.71 |
| MDA-MB-231 | 66.82 | 403.93 | 61.52 | 224.98 |
| PC-3 | 79.17 | N/A | 75.91 | 366.79 |
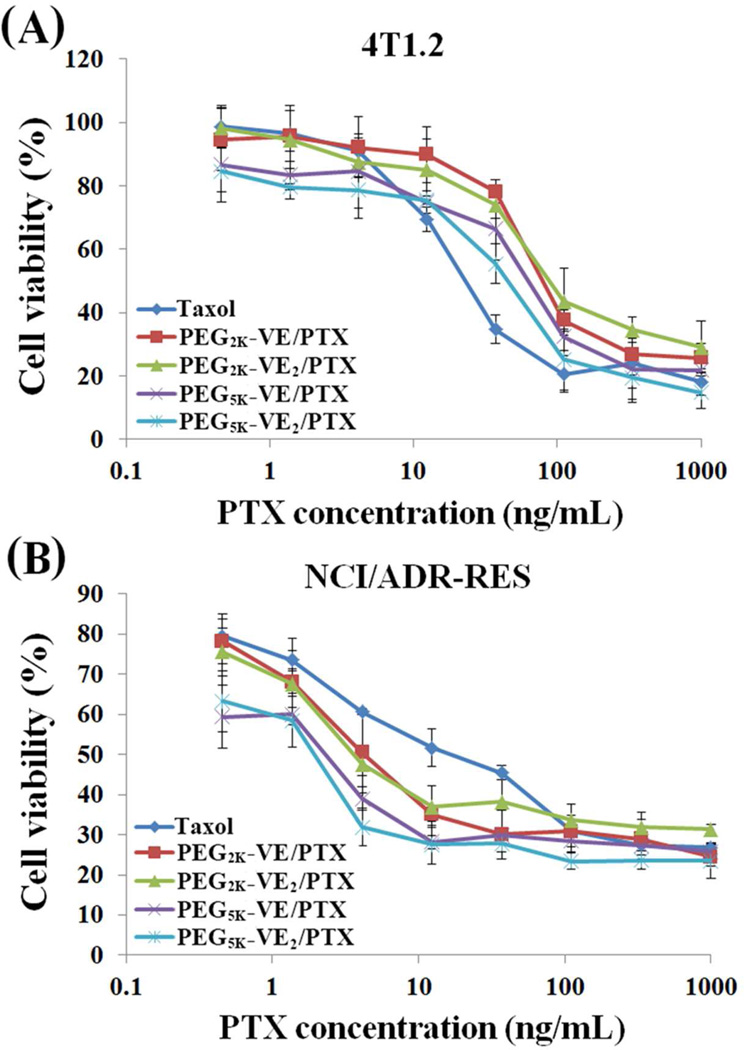
Fig. 7A shows the in vitro cytotoxicity of PTX formulated in PEG2K-VE, PEG2K-VE2, PEG5K-VE and PEG5K-VE2 micelles in comparison with Taxol in 4T1.2 cancer cells. All of the four PTX mixed micelles were less active than Taxol in antitumor activity. Interestingly, different from the study of carriers alone in which single Vitamin E conjugates were more active, PTX-loaded PEG5K-VE2 micelles were more potent than PTX formulated in the other three micelle formulations. Similar to the study of carriers alone, NCI/ADR-RES tumor cells are also more sensitive than 4T1.2 cancer cells to PTX formulated in either PEG-Vitamin E micelles or Cremophor/ethanol (Taxol) (Fig. 7B).
Fig. 7.
The cytotoxicity of PTX-loaded PEG2K-VE, PEG2K-VE2, PEG5K-VE, or PEG5K-VE2 micelles against a mouse breast cancer cell line, 4T1.2 (A) and a drug-resistant cell line, NCI/ADR-RES (B) in comparison to Taxol. Cells were treated with indicated concentrations of different PTX formulations for 72 h and cytotoxicity was then determined by MTT assay. Values reported are the means ± SD for triplicate samples.
Again, PTX-loaded PEG5K-VE2 micelles showed the highest level of in vitro cytotoxicity followed by PTX-loaded PEG5K-VE micelles. PTX-PEG2K-VE and PTX-PEG2K-VE2 are comparable in antitumor activity. However, all of the four PTX micellar formulations were more active than Taxol in NCI/ADR-RES tumor cells, which is quite different from the data in 4T1.2 cells. The IC50 of Taxol and several PTX-loaded micelles in the two cancer cell lines were summarized in Table. 4.
Table 4.
IC50 of PTX-loaded micelles in 4T1.2 and NCI/ADR-RES cancer cell lines
| IC50 (ng/mL) |
|||||
|---|---|---|---|---|---|
| Taxol | PEG2K-VE/PTX | PEG2K-VE2/PTX | PEG5k-VE/PTX | PEG5K-VE2/PTX | |
| 4T1.2 | 26.16 | 88.17 | 95.52 | 72.75 | 50.38 |
| NCI/ADR-RES | 18.92 | 4.40 | 3.78 | 2.67 | 2.24 |
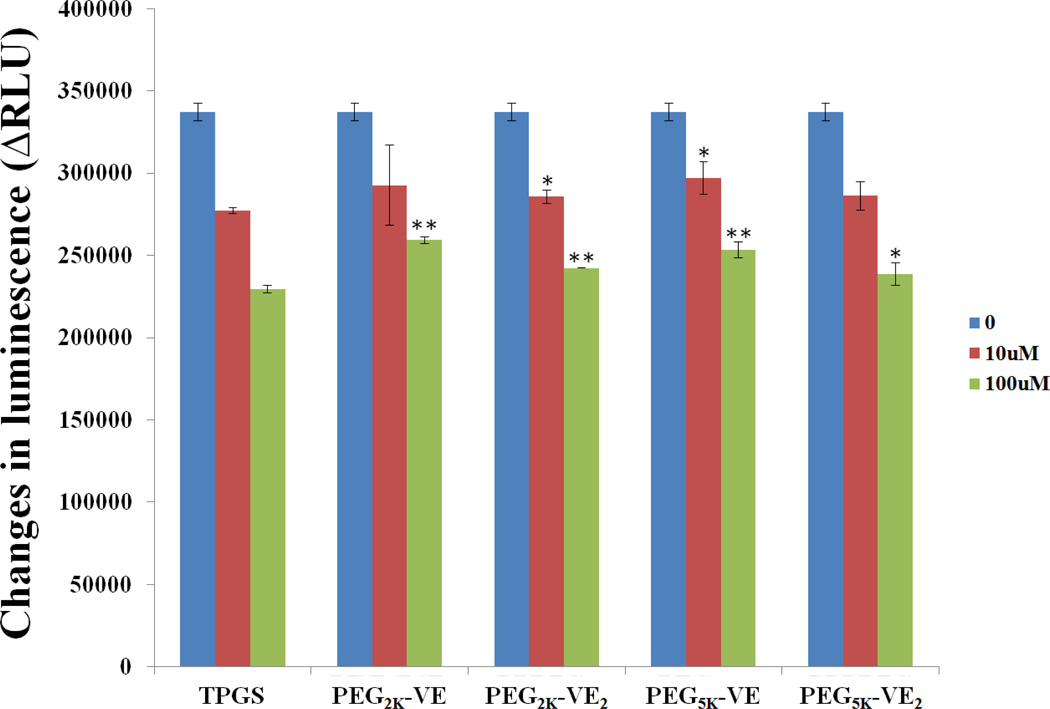
Inhibition of P-gp ATPase
Fig. 8 shows that P-gp ATPase activity was significantly inhibited by TPGS in a concentration-dependent manner. The P-gp ATPase activity was also significantly inhibited by the four PEG-Vitamin E conjugates although they were less active compared to TPGS, which was consistent with the published work in the literature31.
Fig. 8.
Inhibitory effect of TPGS, PEG2K-VE, PEG2K-VE2, PEG5K-VE or PEG5K-VE2 on verapamil-stimulated P-gp ATPase activity. Test samples containing verapamil (50µM) and PEG-derivatized Vitamin E conjugates (final concentrations at 10 and 100 µM, respectively) or Na3VO4 (a selective inhibitor of P-gp) were incubated with P-gp membrane for 5 min at 37 °C. The reaction was initiated by the addition of MgATP followed by another 40 minutes’ incubation at 37°C. ATP detection reagent was then added luminescence was examined. *p < 0.05 and **p < 0.001 (vs TPGS of equivalent concentration). Values are reported as the means ± SD for triplicate samples.
In vivo therapeutic study
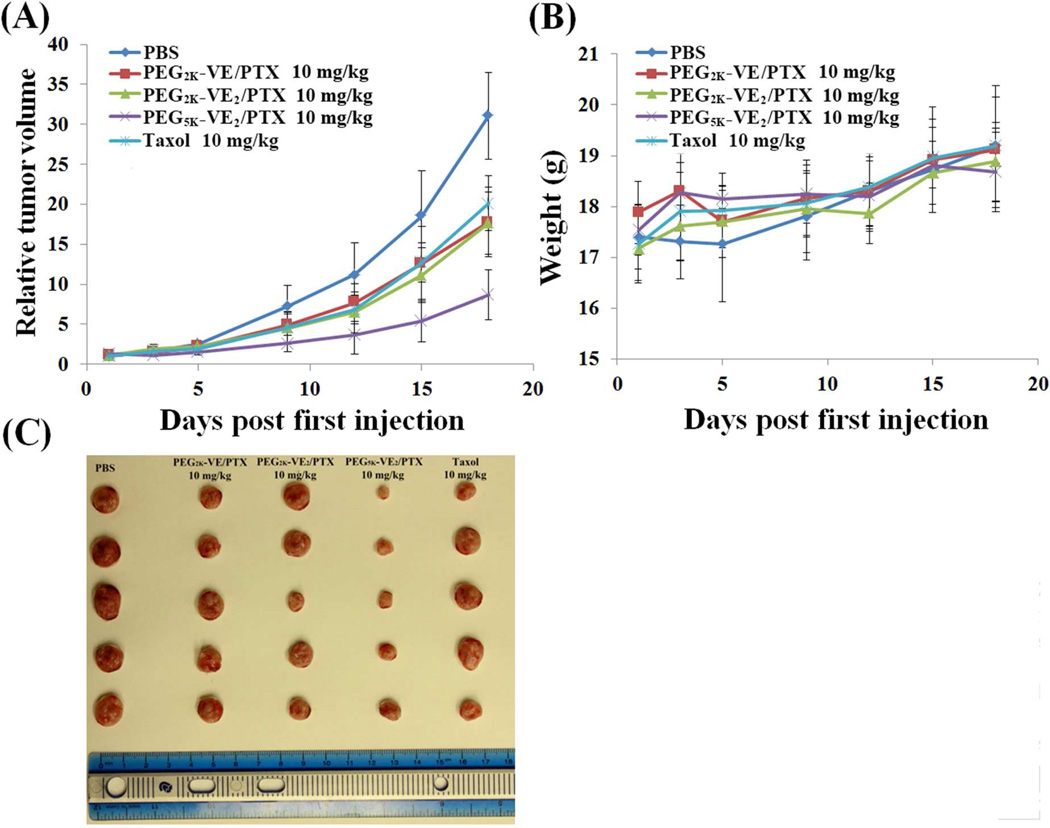
The in vivo therapeutic activity of PTX formulated in PEG2K-VE, PEG2K-VE2, and PEG5K-VE2 micelles was evaluated in a syngeneic murine breast cancer model (4T1.2), and compared to Taxol.
4T1.2 is a highly metastatic breast cancer cell line and was selected to rigorously assess the in vivo therapeutic efficacy of different PTX formulations. As shown in Fig. 9A, Taxol formulation showed moderate effect in inhibiting the tumor growth at a dose of 10 mg PTX/kg. Compared to Taxol treatment group, PTX formulated in PEG2K-VE or PEG2K-VE2 exhibited similar tumor growth inhibitory effect. In contrast, PTX formulated in PEG5K-VE2 micelles showed a significantly more pronounced antitumor activity at the same dosage. No significant changes in body weight were noticed in all treatment groups compared to PBS control group (Fig. 9B), suggesting that significant therapeutic effect can be achieved with minimal toxicity.
Fig. 9.
(A) Enhanced antitumor activity of PTX formulated in PEG5K-VE2 micelles. BABL/c mice were inoculated s.c. with 4T1.2 cells (2 × 105 cells/mouse). Five days later, mice received various treatments on days 1, 3, 5, 9, and 12, and tumor growth was monitored and plotted as relative tumor volume. P < 0.02 (PEG5K-VE2/PTX vs. Taxol, PEG2K-VE/PTX or PEG2K-VE2/PTX), N = 5. (B) Changes of body weight in mice receiving different treatments. (C) Images of tumors removed from the tumor-bearing mice at the completion of the study.
DISCUSSION
We have systematically compared the biophysical property and in vitro and in vivo efficiency of PTX delivery of four PEG-Vitamin E conjugates that differ in the size of PEG motif (PEG2K vs PEG5K) and the molar ratio of PEG/Vitamin E (1/1 vs 1/2) in the conjugates. Our data showed that PEG5K-conjugates were significantly more effective than PEG2K-conjugates in forming stable mixed micelles with PTX and in mediating delivery of PTX to tumor cells, particularly in vivo. In addition, conjugates with two Vitamin E molecules work better than the conjugates with one molecule of Vitamin E.
It is likely that various mechanisms are involved in the carrier/drug interaction for the Vitamin E-based micellar system. Vitamin E has a benzene ring and a long alkyl chain. In addition to hydrophobic interaction with PTX, the hydrogen bonding and the π-π stacking may also contribute to the overall carrier/PTX interaction. The close proximity of two Vitamin E molecules in PEG-VE2 conjugates is likely to facilitate the formation of a binding pocket that enhances the interaction between the carriers and PTX. This is supported by data from our recent work that inclusion of a drug-interactive motif at the interfacial region of surfactants significantly improves the carrier-drug interaction, leading to improvement in both drug-loading capacity and formulation stability32. Recently, Wang and colleagues reported a similar work in which they showed that PEG2K-Vitamin E2 conjugate was more effective than PEG2K-Vitamin E in mediating delivery of doxorubicin to tumors.15 In an independent study with two similar delivery systems based on PEG-embelin and PEG-farnesylthiosalicylic acid (FTS) conjugates, we also showed that conjugates with two embelin or FTS molecules were more effective than conjugates with one embelin or FTS molecule regardless whether PEG3.5K or PEG5K was used.20,27,34
As a hydrophilic motif of amphiphilic molecules, the size of PEG also critically affects the performance of the micelles. PEG provides steric hindrance, which is critical for ensuring long circulation property of the micelles. PEG decoration has also been shown to facilitate the penetration of nanoparticles through the mucus layer19,35 In this regard, PEGs of higher MW are expected to be more effective than those of lower MW. However, the size of PEG also affects the CMC which in turn significantly affects the performance of the micelles, particularly in vivo. Different micellar systems appear to be differentially affected by the size of PEG.3,20,28,33 In a systematic study on the SAR of PEG-cholic acid cluster-based micellar system, PEG2K was shown to be the optimal hydrophilic motif.33 Our data clearly showed that PEG5K-conjugates (with either one or two Vitamin E molecules) were more active than PEG2K-conjugates in forming stable mixed micelles with PTX. PEG5K-conjugates formed stable complexes with PTX at lower carrier/PTX molar ratios compared to PEG2K-conjugates. In addition, PTX formulated in PEG5K-micelles displayed much slower release kinetics. We have similarly demonstrated the advantages of PEG5K over PEG3.5K in PEG-embelin and PEG-FTS micellar systems.20,28,34 In the study by Hanes and colleagues, PLGA particles coated with TPGS5K were more effective than the particles decorated with TPGS1K in penetrating human cervicovaginal mucus19.
The four different PEG-Vitamin E conjugates showed varied levels of activity by themselves in four cancer cell lines. Overall, the single Vitamin E conjugates were more potent than the conjugates with two Vitamin E molecules in all cell lines tested. The more potent activity of single Vitamin E conjugates is unlikely due to the more active surface activity of the single chain conjugates as all of the four conjugates showed minimal hemolytic activity at much higher concentrations tested. It is possible that active Vitamin E is more readily released from the single Vitamin E conjugates than the ones with two Vitamin E molecules due to less steric hindrance to intracellular esterases. More studies are needed in the future to examine if the single Vitamin E conjugates indeed yield greater amounts of active free Vitamin E intracellularly.
Different from the cytotoxicity profiles of the conjugates alone, PTX formulated in PEG5K-VE2 micelles showed higher levels of cytotoxicity than PTX formulated in other three micellar systems in both 4T1.2 and NCI/ADR-RES tumor cell lines. This might be attributed to a more efficient intracellular delivery of PTX via PEG5K-VE2 micelles as PEG5K-VE2 formed the most stable mixed micelles with PTX among the four micellar systems tested. Despite the difference in the levels of cytotoxicity among the four types of micellar PTX, all of them were less active than Taxol formulation in 4T1.2 tumor cells. Interestingly we saw a reversal of the pattern in NCI/ADR-RES tumor cells: all of the four micellar PTX were more active than Taxol in this drug resistant cell line. The improved in vitro cytotoxicity of the PTX micellar formulations in this drug resistant cell line can be ascribed to the well-known inhibitory effect of P-gp efflux pump by Vitamin E derivatives and thus an improved bioavailability of PTX inside the tumor cells9–12. This hypothesis was supported by the P-gp ATPase activity assay in this work (Fig. 8). Our results were consistent with previous studies with various types of delivery system that involve the use of TPGS.9–12
In vivo therapy study clearly showed a significantly higher level of antitumor activity for PTX formulated in PEG5K-VE2 micelles compared to either Taxol or other two micellar formulations. This is likely due to the significantly improved loading capacity and stability for PEG5K-VE2 micelles, which shall lead to more effective delivery of PTX to tumor tissue in vivo. No significant difference was noticed between PEG2K-Vitamin E2 and PEG2K-Vitamin E in antitumor activity despite the demonstrated advantages of PEG2K-Vitamin E2 over PEG2K-Vitamin E in biophysical property. This might be due to the aggressive nature of 4T1.2 tumor model, which requires significant improvement of the formulation to achieve a significant gain in the therapeutic benefit.
CONCLUSION
In summary, we have shown that PEG5K-conjugates have lower CMC values and are more effective in PTX loading with respect to both loading capacity and stability. The conjugates with two Vitamin E molecules also worked better than the conjugates with one molecule of Vitamin E. All of the four PEG-Vitamin E were comparable to TPGS in inhibiting the function of P-gp. More importantly, PTX-loaded PEG5K-VE2 resulted in significantly improved tumor growth inhibitory effect in comparison to PTX formulated in PEG2K-VE or PEG2K-VE2, as well as Taxol in a syngeneic mouse model of breast cancer (4T1.2). More studies on the SAR of the Vitamin E-based micellar system and the mechanism of antitumor effect may lead to further improvement in this system for in vivo delivery of anticancer agents such as PTX.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported in part by NIH grants (R21CA128415 and R21CA155983) and a DOD grant (BC09603). We would like to thank Drs. Donna Stolz and Ming Sun for their help with negative EM study.
Footnotes
Supporting Information Available: HPLC trace, 1H NMR spectra and MALDI-TOF of PEG2K-VE, PEG2K-VE2, PEG5K-VE and PEG5K-VE2. Size distribution and TEM of PEG2K-VE, PEG2K-VE2 and PEG5K-VE. These materials are available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Torchilin VP. Micellar nanocarriers: pharmaceutical perspectives. Pharm. Res. 2007;24:1–16. doi: 10.1007/s11095-006-9132-0. [DOI] [PubMed] [Google Scholar]
- 2.Sutton D, Nasongkla N, Blanco E, Gao J. Functionalized micellar systems for cancer targeted drug delivery. Pharm. Res. 2007;24:1029–1046. doi: 10.1007/s11095-006-9223-y. [DOI] [PubMed] [Google Scholar]
- 3.Mi Y, Liu Y, Feng SS. Formulation of docetaxel by folic acid-conjugated D-α-tocopheryl polyethylene glycol succinate 2000 (Vitamin E TPGS2k) micelles for targeted and synergistic chemotherapy. Biomaterials. 2011;32:4058–4066. doi: 10.1016/j.biomaterials.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 4.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumouritropic accumulation of proteins and the antitumour agent smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 5.Gao Z, Lukyanov AN, Singhal A, Torchilin VP. Diacyllipid-polymer micelles as nanocarriers for poorly soluble anticancer drugs. Nano Lett. 2002;2:979–982. [Google Scholar]
- 6.Croy SR, Kwon GS. Polymeric micelles for drug delivery. Curr. Pharm. Des. 2006;12:4669–4684. doi: 10.2174/138161206779026245. [DOI] [PubMed] [Google Scholar]
- 7.Sokol RJ, Heubi JE, Butler-Simon N, McClung HJ, Lilly JR, Silverman A. Treatment of Vitamin E deficiency during chronic childhood cholestasis with oral d-alpha-tocopheryl polyethylene glycol-1000 succinate. Gastroenterology. 1987;93:975–985. doi: 10.1016/0016-5085(87)90559-2. [DOI] [PubMed] [Google Scholar]
- 8.Yu L, Bridgers A, Polli J, Vickers A, Long S, Roy A. Vitamin E-TPGS increases absorption flux of an HIV protease inhibitor by enhancing its solubility and permeability. Pharm. Res. 1999;16:1812–1817. doi: 10.1023/a:1018939006780. [DOI] [PubMed] [Google Scholar]
- 9.Dintaman JM, Silverman JA. Inhibition of P-glycoprotein by D-alpha-tocopheryl polyethylene glycol 1000 succinate (TPGS) Pharm. Res. 1999;16:1550–1556. doi: 10.1023/a:1015000503629. [DOI] [PubMed] [Google Scholar]
- 10.Varma MVS, Panchagnula R. Enhanced oral paclitaxel absorption with Vitamin E-TPGS: effect on solubility and permeability in vitro, in situ and in vivo . Eur. J. Pharm. Sci. 2005;25:445–453. doi: 10.1016/j.ejps.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Collnot EM, Baldes C, Schaefer UF, Edgar KJ, Wempe MF, Lehr CM. Vitamin E TPGS p-glycoprotein inhibition mechanism: influence on conformational flexibility, intracellular ATP levels, and role of time and site of access. Mol. Pharm. 2010;7:642–651. doi: 10.1021/mp900191s. [DOI] [PubMed] [Google Scholar]
- 12.Constantinides PP, Han JH, Davis SS. Advances in the use of tocols as drug delivery vehicles. Pharm. Res. 2006;23:243–255. doi: 10.1007/s11095-005-9262-9. [DOI] [PubMed] [Google Scholar]
- 13.Win KY, Feng SS. In vitro and in vivo studies on Vitamin E TPGS-emulsified poly(D,L-lactic-co-glycolic acid)nanoparticles for paclitaxel formulation. Biomaterials. 2006;27:2285–2291. doi: 10.1016/j.biomaterials.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 14.Ma Y, Zheng Y, Liu K, Tian G, Tian Y, Xu L, Yan F, Huang L, Mei L. Nanoparticles of poly(lactide-co-glycolide)-d-a-tocopheryl polyethylene glycol 1000 succinate random copolymer for cancer treatment. Nanoscale Res. Lett. 2010;6:1161–1169. doi: 10.1007/s11671-010-9620-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J, Sun J, Chen Q, Gao Y, Li L, Li H, Leng D, Wang Y, Sun Y, Jing Y, Wang S, He Z. Star-shape copolymer of lysine-linked di-tocopherol polyethylene glycol 2000 succinate for doxorubicin delivery with reversal of multidrug resistance. Biomaterials. 2012;28:6877–6888. doi: 10.1016/j.biomaterials.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 16.Muthu MS, Kulkarni SA, Xiong J, Feng SS. Vitamin E TPGS coated liposomes enhanced cellular uptake and cytotoxicity of docetaxel in brain cancer cells. Int. J. Pharm. 2011;421:332–340. doi: 10.1016/j.ijpharm.2011.09.045. [DOI] [PubMed] [Google Scholar]
- 17.Cao N, Feng SS. Doxorubicin conjugated to D-alpha-tocopheryl polyethylene glycol 1000 succinate (TPGS): conjugation chemistry, characterization, in vitro and in vivo evaluation. Biomaterials. 2008;29:3856–3865. doi: 10.1016/j.biomaterials.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 18.Anbharasi V, Cao N, Feng SS. Doxorubicin conjugated to D-alpha-tocopheryl polyethylene glycol succinate and folic acid as a prodrug for targeted chemotherapy. J. Biomed. Mater. Res. Part A. 2010;94:730–743. doi: 10.1002/jbm.a.32734. [DOI] [PubMed] [Google Scholar]
- 19.Mert O, Lai SK, Ensign L, Yang M, Wang YY, Wood J, Hanes J. A poly(ethylene glycol)-based surfactant for formulation of drug-loaded mucus penetrating particles. J. Control Release. 2012;157:455–460. doi: 10.1016/j.jconrel.2011.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang Y, Lu J, Gao X, Li J, Zhao W, Sun M, Stolz DB, Venkataramanan R, Rohan LC, Li S. PEG-derivatized embelin as a dual functional carrier for the delivery of paclitaxel. Bioconjug. Chem. 2012;23:1443–1451. doi: 10.1021/bc3000468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chitra M, Sukumar E, Suja V, Devi CS. Antitumor, anti-inflammatory and analgesic property of embelin, a plant product. Chemotherapy. 1994;40:109–113. doi: 10.1159/000239181. [DOI] [PubMed] [Google Scholar]
- 22.Bhandari U, Jain N, Pillai KK. Further studies on antioxidant potential and protection of pancreatic beta-cells by Embelia ribes in experimental diabetes. Exp. Diabetes Res. 2007:15803–15808. doi: 10.1155/2007/15803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh D, Singh R, Singh P, Gupta RS. Effect of embelin on lipid peroxidation and free radical scavenging activity against liver damage in rats. Basic Clin. Pharmacol. Toxicol. 2009;105:243–248. doi: 10.1111/j.1742-7843.2009.00429.x. [DOI] [PubMed] [Google Scholar]
- 24.Danquah M, Li F, Duke CB, Miller DD, Mahato RI. Micellar delivery of bicalutamide and embelin for treating prostate cancer. Pharm. Res. 2009;26:2081–2092. doi: 10.1007/s11095-009-9903-5. [DOI] [PubMed] [Google Scholar]
- 25.Sreepriya M, Bali G. Chemopreventive effects of embelin and curcumin against N-nitrosodiethylamine/phenobarbital-induced hepatocarcinogenesis in Wistar rats. Fitoterapia. 2005;76:549–555. doi: 10.1016/j.fitote.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 26.Dai Y, Qiao L, Chan KW, Yang M, Ye J, Ma J, Zou B, Gu Q, Wang J, Pang R, Lan HY, Wong BC. Peroxisome proliferator-activated receptor-gamma contributes to the inhibitory effects of Embelin on colon carcinogenesis. Cancer Res. 2009;69:4776–4783. doi: 10.1158/0008-5472.CAN-08-4754. [DOI] [PubMed] [Google Scholar]
- 27.Heo JK, Kim HJ, Kim SM, Park KR, Park SY, Kim SW, Nam D, Jang HJ, Lee SG, Ahn KS, Kim SH, Shim BS, Choi SH, Ahn KS. Embelin suppresses STAT3 signaling, proliferation, and survival of multiple myeloma via the protein tyrosine phosphatase PTEN. Cancer Lett. 2011;308:71–80. doi: 10.1016/j.canlet.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 28.Lu J, Huang Y, Zhao W, Marquez RT, Meng X, Li J, Gao X, Venkataramanan R, Wang Z, Li S. PEG-Derivatized Embelin as a Nanomicellar Carrier for Delivery of Paclitaxel to Breast and Prostate Cancers. Biomaterials. 2013;34:1591–1600. doi: 10.1016/j.biomaterials.2012.10.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.La SB, Okano T, Kataoka K. Preparation and characterization of the micelle-forming polymeric drug indomethacin incorporated poly(ethylene oxide)-poly(beta-benzyl L-aspartate) block copolymer micelles. J. Pharm. Sci. 1996;85:85–90. doi: 10.1021/js950204r. [DOI] [PubMed] [Google Scholar]
- 30.Kabanov AV, Nazarova IR, Astafieva IV, Batrakova EV, Alakhov VY, Yaroslavov AA, Kabanov VA. Micelle formation and solubilization of fluorescent probes in poly-(oxyethylene-b-oxypropylene-b-oxyethylene) solutions. Macromolecules. 1995;28:2303–2314. [Google Scholar]
- 31.Collnot EM, Baldes C, Wempe MF, Kappl R, Hüttermann J, Hyatt JA, Edgar KJ, Schaefer UF, Lehr CM. Mechanism of inhibition of P-glycoprotein mediated efflux by vit amin E TPGS: influence on ATPase activity and membrane fluidity. Mol. Pharm. 2007;4:465–474. doi: 10.1021/mp060121r. [DOI] [PubMed] [Google Scholar]
- 32.Gao X, Huang Y, Makhov AM, Epperly M, Lu J, Grab S, Zhang P, Rohan L, Xie XQ, Wipf P, Greenberger J, Li S. Nanoassembly of surfactants with interfacial drug-interactive motifs as tailor-designed drug carriers. Mol. Pharm. 2013;10:187–198. doi: 10.1021/mp300319m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luo J, Xiao K, Li Y, Lee JS, Shi L, Tan YH, Xing L, Holland Cheng R, Liu GY, Lam KS. Well-defined, size-tunable multi-functional micelles for efficient paclitaxel delivery for cancer treatment. Bioconjug. Chem. 2010;21:1216–1224. doi: 10.1021/bc1000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang X, Lu J, Huang Y, Zhao W, Chen Y, Li J, Gao X, Venkataramanan R, Sun M, Stolz DB, Zhang L, Li S. PEG-Farnesylthiosalicylate conjugate as a nanomicellar carrier for delivery of paclitaxel. Bioconjug. Chem. 2013;24:464–472. doi: 10.1021/bc300608h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lai SK, Wang YY, Hanes J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv. Drug Deliv. Rev. 2009;61:158–171. doi: 10.1016/j.addr.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.