Abstract
Study Objective:
To determine the impact of continuous positive airway pressure (CPAP) on weight change in persons with obstructive sleep apnea (OSA).
Design, Setting, and Participants:
The Apnea Positive Pressure Long-term Efficacy Study (APPLES) was a 6-month, randomized, double-blinded sham-controlled multicenter clinical trial conducted at 5 sites in the United States. Of 1,105 participants with an apnea hypopnea index ≥ 10 events/ hour initially randomized, 812 had body weight measured at baseline and after 6 months of study.
Intervention:
CPAP or Sham CPAP.
Measurements:
Body weight, height, hours of CPAP or Sham CPAP use, Epworth Sleepiness Scale score.
Results:
Participants randomized to CPAP gained 0.35 ± 5.01 kg, whereas those on Sham CPAP lost 0.70 ± 4.03 kg (mean ± SD, p = 0.001). Amount of weight gain with CPAP was related to hours of device adherence, with each hour per night of use predicting a 0.42 kg increase in weight. This association was not noted in the Sham CPAP group. CPAP participants who used their device ≥ 4 h per night on ≥ 70% of nights gained the most weight over 6 months in comparison to non-adherent CPAP participants (1.0 ± 5.3 vs. -0.3 ± 5.0 kg, p = 0.014).
Conclusions:
OSA patients using CPAP may gain a modest amount of weight with the greatest weight gain found in those most compliant with CPAP.
Commentary:
A commentary on this article appears in this issue on page 995.
Citation:
Quan SF; Budhiraja R; Clarke DP; Goodwin JL; Gottlieb DJ; Nichols DA; Simon RD; Smith TW; Walsh JK; Kushida CA. Impact of treatment with continuous positive airway pressure (CPAP) on weight in obstructive sleep apnea. J Clin Sleep Med 2013;9(10):989-993.
Keywords: Continuous positive airway pressure, weight, obstructive sleep apnea
Changes in weight are inextricably linked to obstructive sleep apnea (OSA).1 Weight gain is a risk for both the development and increased severity of OSA.2 Conversely, weight loss may result in remission or improvement in OSA.3 In one review, it has been estimated that 58% of OSA can be attributable to excess weight.4 There also is evidence that OSA itself contributes to weight gain,5–7 thus creating an adverse positive feedback loop whereby increasing weight leads to OSA, and OSA results in further weight gain. Treatment of OSA with continuous positive airway pressure (CPAP) should therefore prevent further weight gain or facilitate weight loss. However, available evidence is conflicting, with two studies demonstrating CPAP users losing weight8,9 while others observed either no change or increases in weight.11–15 However, these studies have been retrospective, small in size, lacked a parallel control group, or were not blinded. Thus, it is unclear whether treatment of OSA with CPAP results in any changes in weight.
The Apnea Positive Pressure Long-term Efficacy Study (APPLES) was a 6-month randomized controlled clinical trial to determine the impact of CPAP or Sham CPAP on a variety of neurocognitive outcomes in persons with OSA.16 As part of this study, weight measurements at the baseline and follow-up study visits were included in the protocol. Thus, APPLES is an ideal vehicle to test the hypothesis that CPAP treatment of OSA engenders weight loss.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Obese OSA patients commonly believe that treatment with CPAP will facilitate weight loss. However, empirical observations and uncontrolled studies suggest otherwise. Data from randomized controlled studies are lacking.
Study Impact: In this 6 month randomized controlled trial, treatment with CPAP resulted in weight gain, not weight loss. Therefore, in addition to treatment with CPAP, obese OSA patients should engage in other behaviors such as increased physical activity and reduction in caloric intake to lose weight.
METHODS
Participants
The recruitment procedures and inclusion and exclusion criteria for APPLES have been described extensively.16 Briefly, APPLES was a multisite study conducted at 5 Clinical Centers: Stanford University, Stanford, CA; University of Arizona, Tucson, AZ; Providence St. Mary Medical Center, Walla Walla, WA; St. Luke's Hospital, Chesterfield, MO; and Brigham and Women's Hospital, Boston, MA. The institutional review board (IRB) at each site approved the study protocol. Enrollment began in November 2003 and was completed in August 2008. All participants were adults with age ≥ 18 years and with an apnea hypopnea index (AHI) ≥ 10 events per hour. Excluded were individuals who had (1) prior OSA treatment with CPAP or surgery, (2) household members with current/past CPAP use, (3) a sleepiness-related automobile accident within the year prior to potential enrollment, (4) oxygen saturation < 75% for > 10% of the diagnostic polysomnogram (PSG) total sleep time; or (5) conditions or use of medications that could potentially affect neurocognitive function and/or alertness. For this analysis, only participants who had been randomized and had valid weight measurements at baseline and at the 6-month time point are included.
Study Design
The APPLES protocol has been described in detail elsewhere.16 In brief, participants were recruited from sleep clinics and by public advertisement. If an initial interview determined that there were no exclusion criteria, consenting participants underwent diagnostic PSG as previously described.16 Those who had an AHI ≥ 10/h and were not excluded because of severe oxygen desaturation were randomized to either active or sham CPAP (REMStar Pro, Phillips Respironics, Murrysville, PA) for 6 months. For those randomized to CPAP, the therapeutic pressure was determined during a sleep laboratory titration following their initial PSG. Those randomized to Sham underwent a sham PSG titration in the sleep laboratory. Neurocognitive assessments, the primary focus of the study, were performed at baseline and 2 and 6 months afterwards. The Epworth Sleepiness Scale was a component of the neuro-cognitive assessments. At these visits, weight measurements also were obtained using calibrated balance beam or digital scales, with the participant in street clothes without shoes. The same scale type was used at each visit. Height was measured at baseline using a stadiometer. Adherence to treatment was assessed objectively using Encore Pro SmartCards (Philips Respironics, Inc., Murrysville, PA), which were returned twice monthly.
Statistical Analyses
Body mass index (BMI) was computed as weight (kg)/height (m)2. Participants' race/ethnicity were classified as self-reported white or non-white. Assessment of adherence to CPAP or Sham used data obtained during the 1 month prior to the 6-month visit and was expressed as the mean hours of nightly use. Classification of participants as adherent or non-adherent was based on whether they used their assigned treatment > 4 h on ≥ 70% of nights. For continuous variables, unadjusted comparisons between CPAP and Sham were made using Students' t-test. Differences in proportions were assessed using the χ2 test. Repeated measures analysis of variance and covariance and linear regression were used to determine the impact of treatment group as well as other possible factors on weight change over the 6-month timeframe of the study. Data are expressed as mean ± standard deviation (SD) or percentages. Analyses were performed using IBM SPSS Statistics Version 20 (Chicago, IL).
RESULTS
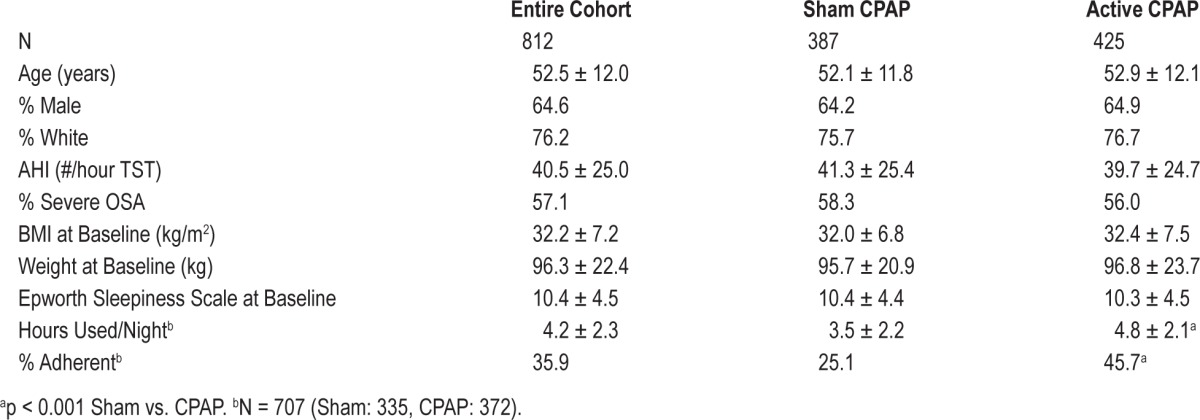
There were 1,105 participants initially randomized, but 7 were later excluded because they were later found to be ineligible or inadvertently received both study arms. Another 286 were excluded for missing measurements of body weight at 6 months. Of the remaining 812 participants, 387 had been randomized to Sham and 425 to active CPAP. There were no differences in baseline weight, height, gender, race, AHI, or ESS between participants with and without 6-month weight measurements. However, participants who had 6-month weight data were slightly older (52.5 ± 12.2 vs. 48.9 ± 12.4 years, p < 0.001). As shown in Table 1, participants generally were middle-aged, white, male, and obese. They also had moderate to severe OSA, were mildly sleepy, and had suboptimal adherence to their assigned treatment. There were no differences between the 2 groups with respect to age, gender, race, OSA severity, weight, BMI, or Epworth Sleepiness Scale score. However, participants in the Sham group were generally less adherent to their assigned treatment.
Table 1.
Characteristics of APPLES participants with 6-month weight data
The changes in weight from baseline to the 6-month time point for the Sham and CPAP groups are shown in Figure 1. Participants in the Sham group had a slight decrease in their weight, whereas those in the CPAP group had a slight increase. This difference in weight change was statistically significant (Sham = -0.70 ± 4.03 vs CPAP = 0.35 ± 5.01 kg, p = 0.001). There was no effect modification related to age, gender, race, baseline BMI, OSA severity, or ESS score, and these factors were not included in subsequent analytic models. However, change in weight was associated with adherence in the CPAP group, with each hour per night of CPAP use associated with a mean 0.42 kg increase in weight over 6 months (p = 0.001). In contrast, there was no association between weight change and adherence in the Sham group.
Figure 1. Weight change over 6 months in CPAP and.
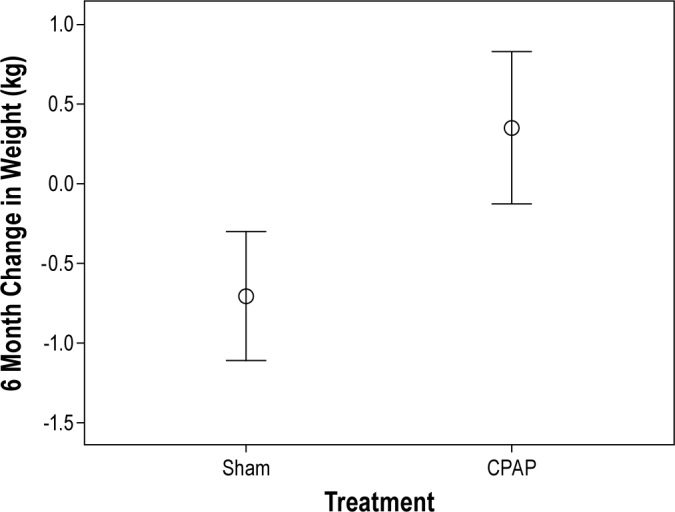
Mean weight change in CPAP = 0.35 ± 5.01 kg vs. Sham = -0.71 ± 4.03 kg, p = 0.001. N = 425 (CPAP) and 387 (Sham).
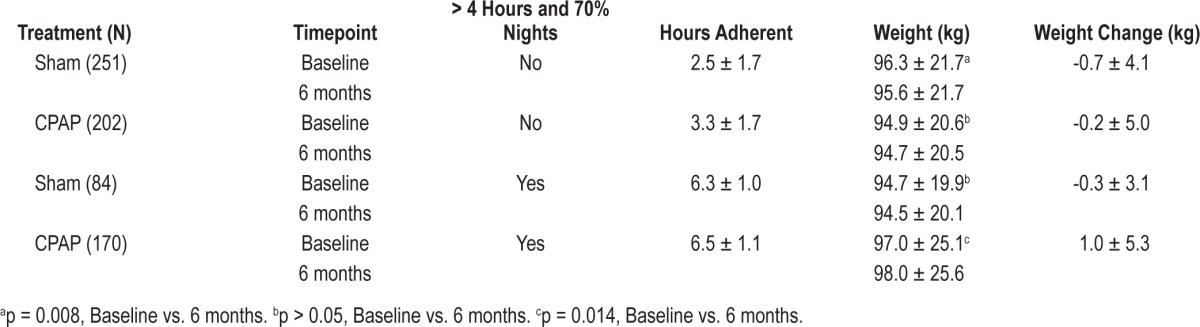
Table 2 shows mean baseline and 6-month weights stratified by treatment group and treatment adherence. There were 707 participants with analyzable adherence data. When classified by whether participants had 70% of nights with > 4 hours use, strikingly, adherent CPAP participants had a mean 1 kg increase in weight (97.0 ± 20.1 vs. 98.0 ± 25.6 kg, p = 0.014). In contrast, non-adherent Sham participants had a slight decrease in weight (96.3 ± 21.7 vs. 95.6 ± 21.7, p = 0.008).
Table 2.
Change in weight over 6 months stratified by CPAP adherence and treatment group
DISCUSSION
In this study we found that treatment of OSA with CPAP did not result in a reduction in weight, and actually was associated with weight gain. This observation was independent of age, gender, race, OSA severity, and sleepiness. Furthermore, those with the greatest adherence to CPAP appeared to have gained the most weight.
The primary finding in this study is that CPAP treatment of OSA does not result in weight loss over a 6-month time frame. Our findings are therefore consistent with those of Redenius et al., who failed to observe in a retrospective study any reduction in weight in 183 CPAP treated patients followed in a sleep center over 10 to 14 months.12 Similarly, several smaller studies also have failed to find a change in weight with CPAP treatment. In a randomized controlled trial of sibutramine in comparison to CPAP for weight loss, CPAP treatment for one year in 18 patients was not associated with a change in weight.11 Similarly, addition of CPAP did not result in greater weight loss when used as adjunctive therapy for a behavioral weight loss program in OSA patients.15 In two other randomized controlled studies of CPAP compared to sham CPAP, CPAP treatment for 12 weeks or 24 weeks also did not result in weight loss.13,14 However, the latter four studies may have been inadequately powered to detect a small change in weight. In a much larger, although non-blinded randomized study, CPAP treatment for a median of four years failed to result in weight loss.10 In contrast, Loube et al. observed that in 21 CPAP-treated OSA patients, those who self-reported being adherent were more likely to have greater than a 4.5 kg weight loss than those non-adherent. However, there was no significant weight change for the group overall.9 More recently, Sharma and colleagues in a randomized crossover design of 86 participants found that CPAP treatment for 3 months resulted in a mean weight loss of 0.37 kg compared to a 0.33 kg weight gain with sham CPAP.8 Our study of 812 participants is considerably larger than all but one of the aforementioned investigations and would be more likely to detect small changes in weight. Thus our results do not support the hypothesis that CPAP treatment of OSA facilitates weight loss.
We observed that aside from adherence to treatment assignment, no other factors modified the association between CPAP (or sham CPAP) and change in weight. In contrast, Redenius et al. found that women were more likely to gain weight after CPAP use.12 However, their data were derived retrospectively from a clinical population, whereas APPLES participants were largely recruited from the community. Thus, participant characteristics may account for this different observation.
In our study, participants treated with CPAP not only failed to lose weight, but actually appeared to gain a small amount of weight. Moreover, we found that those who were adherent to CPAP gained weight over six months of therapy in contrast to no significant weight increase observed in the sham or non-adherent CPAP participants. Furthermore, there was a dose response relationship between hours of adherence and amount of weight gained. These findings are consistent with Redenius et al., who found that in a subgroup analysis, those who used CPAP for more than 7 hours per night on 90% of nights had an increase in their BMI.12 Furthermore, in a small randomized controlled study, Garcia et al. observed a small amount of weight gain in 20 obese OSA subjects over the course of 6 months.13 Our study extends these latter findings by documenting weight gain with CPAP in a much larger cohort and showing that it is a function of adherence. Thus, our findings and those of previous studies suggest that CPAP is inducing a state of positive energy balance resulting in weight gain.
There are several mechanisms related to energy expenditure (EE) that could explain why persons treated with CPAP gain weight. Weight gain occurs when energy intake (EI) exceeds EE. Some but not all studies indicate that resting 24-hour as well as sleep EE in persons with OSA are higher than in those without OSA.17,18 Furthermore, EE may be higher in those with greater severity of OSA.19 Elevated EE during sleep in OSA patients may be related to greater work of breathing, similar to that observed in patients with chronic obstructive lung disease.20,21 Alternatively, there may be greater activation of the sympathetic nervous system as a result of more frequent arousals and intermittent hypoxia.22 Intervention with CPAP as well as upper airway surgery has been shown to reduce sleep EE, and in the absence of change in energy intake would favor weight gain.17,18,23 Alterations in level of physical activity and dietary habits with CPAP also could change the balance between EI and EE in OSA. Although patients with OSA often indicate that sleepiness and lack of energy are barriers to increased physical activity, a recent study observed that intervention with CPAP for three months improved sleepiness but did not alter activity levels.24 Inflammatory cytokines are elevated in OSA and decrease with use of CPAP.25,26 They may promote anorexia,27 and thus CPAP might reduce this effect leading to weight gain. However, we have previously demonstrated in APPLES that OSA participants eat high-caloric atherogenic diets.28 Preliminary data from a four-month follow-up of these participants indicate that their dietary habits do not change after intervention with CPAP.29 Thus, available evidence suggests that CPAP reduces sleep EE, but there is no corresponding increase in daytime EE or decrease in EI. This results in an energy balance that favors weight gain.
Fluid retention related to elevations in intrathoracic pressure from CPAP could explain some of the weight gain. In both clinical and experimental studies, positive airway pressure results in fluid retention related to a reduction in venous return and decreased secretion of antidiuretic hormone and atrial natriuretic peptide.30,31 However, any contribution from fluid retention is likely to be minor because CPAP is not used during wakefulness in OSA.
Our study is not without limitations. First, overall adherence to both treatment arms was suboptimal. However, the levels of adherence in this study are similar to those in other long-term randomized controlled trials.13,14,32 Furthermore, given our current findings, greater adherence would likely have made the differences in weight between CPAP and Sham even more striking. Second, in comparison to the CPAP group, more participants in the Sham group failed to complete the study. Differential dropout of Sham participants who were more likely to gain weight might explain the difference between the two groups, although there is no evidence that this occurred. Third, because the primary focus of this study was on neurocognitive outcomes, we did not collect data such as direct energy expenditure measurements, which might validate proposed mechanisms underlying the increase in weight observed in CPAP participants. Lastly, most of our participants were recruited from the community and not a sleep clinic. Thus, our findings may not be representative of the patient population seen in a clinical setting. Despite these limitations, our study had a number of strengths, including the large number of participants, a long 6-month follow-up period, use of sham CPAP as a control, and objective documentation of adherence to therapy.
In conclusion, treatment of OSA with CPAP may result in modest weight gain. Thus, if overweight, OSA patients should be encouraged to lose weight by other modalities and not rely on any direct physiologic effect of CPAP.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Quan is the Editor-in-Chief of the Journal of Clinical Sleep Medicine. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
APPLES was funded by contract 5UO1-HL-068060 from the National Heart, Lung and Blood Institute. The APPLES pilot studies were supported by grants from the American Academy of Sleep Medicine and the Sleep Medicine Education and Research Foundation to Stanford University and by the National Institute of Neurological Disorders and Stroke (N44-NS-002394) to SAM Technology. In addition, APPLES investigators gratefully recognize the vital input and support of Dr. Sylvan Green who died before the results of this trial were analyzed, but was instrumental in its design and conduct.
Administrative Core: Clete A. Kushida, MD, PhD; Deborah A. Nichols, MS; Eileen B. Leary, BA, RPSGT; Pamela R. Hyde, MA; Tyson H. Holmes, PhD; Daniel A. Bloch, PhD; William C. Dement, MD, PhD
Data Coordinating Center: Daniel A. Bloch, PhD; Tyson H. Holmes, PhD; Deborah A. Nichols, MS; Rik Jadrnicek, Microflow, Ric Miller, Microflow Usman Aijaz, MS; Aamir Farooq, PhD; Darryl Thomander, PhD; Chia-Yu Cardell, RPSGT; Emily Kees, Michael E. Sorel, MPH; Oscar Carrillo, RPSGT; Tami Crabtree, MS; Booil Jo, PhD; Ray Balise, PhD; Tracy Kuo, PhD
Clinical Coordinating Center: Clete A. Kushida, MD, PhD, William C. Dement, MD, PhD, Pamela R. Hyde, MA, Rhonda M. Wong, BA, Pete Silva, Max Hirshkowitz, PhD, Alan Gevins, DSc, Gary Kay, PhD, Linda K. McEvoy, PhD, Cynthia S. Chan, BS, Sylvan Green, MD
Clinical Centers:
Stanford University: Christian Guilleminault, MD; Eileen B. Leary, BA, RPSGT; David Claman, MD; Stephen Brooks, MD; Julianne Blythe, PA-C, RPSGT; Jennifer Blair, BA; Pam Simi, Ronelle Broussard, BA; Emily Greenberg, MPH; Bethany Franklin, MS; Amirah Khouzam, MA; Sanjana Behari Black, BS, RPSGT; Viola Arias, RPSGT; Romelyn Delos Santos, BS; Tara Tanaka, PhD
University of Arizona: Stuart F. Quan, MD; James L. Goodwin, PhD; Wei Shen, MD; Phillip Eichling, MD; Rohit Budhiraja, MD; Charles Wynstra, MBA; Cathy Ward, Colleen Dunn, BS; Terry Smith, BS; Dane Holderman, Michael Robinson, BS; Osmara Molina, BS; Aaron Ostrovsky, Jesus Wences, Sean Priefert, Julia Rogers, BS; Megan Ruiter, BS; Leslie Crosby, BS, RN
St. Mary Medical Center: Richard D. Simon Jr., MD; Kevin Hurlburt, RPSGT; Michael Bernstein, MD; Timothy Davidson, MD; Jeannine Orock-Takele, RPSGT; Shelly Rubin, MA; Phillip Smith, RPSGT; Erica Roth, RPSGT; Julie Flaa, RPSGT; Jennifer Blair, BA; Jennifer Schwartz, BA; Anna Simon, BA; Amber Randall, BA
St. Luke's Hospital: James K. Walsh, PhD, Paula K. Schweitzer, PhD, Anup Katyal, MD, Rhody Eisenstein, MD, Stephen Feren, MD, Nancy Cline, Dena Robertson, RN, Sheri Compton, RN, Susan Greene, Kara Griffin, MS, Janine Hall, PhD
Brigham and Women's Hospital: Daniel J. Gottlieb, MD, MPH, David P. White, MD, Denise Clarke, BSc, RPSGT, Kevin Moore, BA, Grace Brown, BA, Paige Hardy, MS, Kerry Eudy, PhD, Lawrence Epstein, MD, Sanjay Patel, MD
The authors also acknowledge Sleep HealthCenters for the use of their clinical facilities to conduct this research.
Consultant Teams:
Methodology Team: Daniel A. Bloch, PhD, Sylvan Green, MD, Tyson H. Holmes, PhD, Maurice M. Ohayon, MD, DSc, David White, MD, Terry Young, PhD
Sleep-Disordered Breathing Protocol Team: Christian Guilleminault, MD, Stuart Quan, MD, David White, MD
EEG/Neurocognitive Function Team: Jed Black, MD, Alan Gevins, DSc, Max Hirshkowitz, PhD, Gary Kay, PhD, Tracy Kuo, PhD
Mood and Sleepiness Assessment Team: Ruth Benca, MD, PhD, William C. Dement, MD, PhD, Karl Doghramji, MD, Tracy Kuo, PhD, James K. Walsh, PhD
Quality of Life Assessment Team: W. Ward Flemons, MD, Robert M. Kaplan, PhD APPLES Secondary Analysis-Neurocognitive (ASA-NC) Team: Dean Beebe, PhD, Robert Heaton, PhD, Joel Kramer, PsyD, Ronald Lazar, PhD, David Loewenstein, PhD, Frederick Schmitt, PhD
National Heart, Lung, and Blood Institute (NHLBI)
Michael J. Twery, PhD, Gail G. Weinmann, MD, Colin O. Wu, PhD Data and Safety Monitoring Board (DSMB)
Seven year term: Richard J. Martin, MD (Chair), David F. Dinges, PhD, Charles F. Emery, PhD, Susan M. Harding MD, John M. Lachin, ScD, Phyllis C. Zee, MD, PhD
Other term: Xihong Lin, PhD (2 years), Thomas H. Murray, PhD (1 year)
REFERENCES
- 1.Ong CW, O'Driscoll DM, Truby H, Naughton MT, Hamilton GS. The reciprocal interaction between obesity and obstructive sleep apnoea. Sleep Med Rev. 2013;17:123–31. doi: 10.1016/j.smrv.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284:3015–21. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 3.Thomasouli MA, Brady EM, Davies MJ, et al. The impact of diet and lifestyle management strategies for obstructive sleep apnoea in adults: a systematic review and meta-analysis of randomised controlled trials. Sleep Breath. 2013 Jan 30; doi: 10.1007/s11325-013-0806-7. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol. 2005;99:1592–9. doi: 10.1152/japplphysiol.00587.2005. [DOI] [PubMed] [Google Scholar]
- 5.Brown MA, Goodwin JL, Silva GE, et al. The impact of sleep-disordered breathing on body mass index (BMI): The Sleep Heart Health Study (SHHS) Southwest J Pulm Crit Care. 2011;3:159–68. [PMC free article] [PubMed] [Google Scholar]
- 6.Phillips BG, Hisel TM, Kato M, et al. Recent weight gain in patients with newly diagnosed obstructive sleep apnea. J Hypertens. 1999;17:1297–300. doi: 10.1097/00004872-199917090-00009. [DOI] [PubMed] [Google Scholar]
- 7.Traviss KA, Barr SI, Fleming JA, Ryan CF. Lifestyle-related weight gain in obese men with newly diagnosed obstructive sleep apnea. J Am Diet Assoc. 2002;102:703–6. doi: 10.1016/s0002-8223(02)90160-4. [DOI] [PubMed] [Google Scholar]
- 8.Sharma SK, Agrawal S, Damodaran D, et al. CPAP for the metabolic syndrome in patients with obstructive sleep apnea. N Engl J Med. 2011;365:2277–86. doi: 10.1056/NEJMoa1103944. [DOI] [PubMed] [Google Scholar]
- 9.Loube DI, Loube AA, Erman MK. Continuous positive airway pressure treatment results in weight loss in obese and overweight patients with obstructive sleep apnea. J Am Diet Assoc. 1997;97:896–7. doi: 10.1016/s0002-8223(97)00220-4. [DOI] [PubMed] [Google Scholar]
- 10.Barbe F, Duran-Cantolla J, Sanchez-de-la-Torre M, et al. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;307:2161–8. doi: 10.1001/jama.2012.4366. [DOI] [PubMed] [Google Scholar]
- 11.Ferland A, Poirier P, Series F. Sibutramine versus continuous positive airway pressure in obese obstructive sleep apnoea patients. Eur Respir J. 2009;34:694–701. doi: 10.1183/09031936.00167308. [DOI] [PubMed] [Google Scholar]
- 12.Redenius R, Murphy C, O'Neill E, Al-Hamwi M, Zallek SN. Does CPAP lead to change in BMI? J Clin Sleep Med. 2008;4:205–9. [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia JM, Sharafkhaneh H, Hirshkowitz M, Elkhatib R, Sharafkhaneh A. Weight and metabolic effects of CPAP in obstructive sleep apnea patients with obesity. Respir Res. 2011;12:80. doi: 10.1186/1465-9921-12-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoyos CM, Killick R, Yee BJ, Phillips CL, Grunstein RR, Liu PY. Cardiometabolic changes after continuous positive airway pressure for obstructive sleep apnoea: a randomised sham-controlled study. Thorax. 2012;67:1081–9. doi: 10.1136/thoraxjnl-2011-201420. [DOI] [PubMed] [Google Scholar]
- 15.Kajaste S, Brander PE, Telakivi T, Partinen M, Mustajoki P. A cognitive-behavioral weight reduction program in the treatment of obstructive sleep apnea syndrome with or without initial nasal CPAP: a randomized study. Sleep Med. 2004;5:125–31. doi: 10.1016/j.sleep.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Kushida CA, Nichols DA, Quan SF, et al. The Apnea Positive Pressure Long-term Efficacy Study (APPLES): rationale, design, methods, and procedures. J Clin Sleep Med. 2006;2:288–300. [PubMed] [Google Scholar]
- 17.Lin CC, Chang KC, Lee KS. Effects of treatment by laser-assisted uvuloplasty on sleep energy expenditure in obstructive sleep apnea patients. Metabolism. 2002;51:622–7. doi: 10.1053/meta.2002.31969. [DOI] [PubMed] [Google Scholar]
- 18.Stenlof K, Grunstein R, Hedner J, Sjostrom L. Energy expenditure in obstructive sleep apnea: effects of treatment with continuous positive airway pressure. Am J Physiol. 1996;271:E1036–43. doi: 10.1152/ajpendo.1996.271.6.E1036. [DOI] [PubMed] [Google Scholar]
- 19.Kezirian EJ, Kirisoglu CE, Riley RW, Chang E, Guilleminault C, Powell NB. Resting energy expenditure in adults with sleep disordered breathing. Arch Otolaryngol Head Neck Surg. 2008;134:1270–5. doi: 10.1001/archotol.134.12.1270. [DOI] [PubMed] [Google Scholar]
- 20.Crisafulli E, Beneventi C, Bortolotti V, et al. Energy expenditure at rest and during walking in patients with chronic respiratory failure: a prospective two-phase case-control study. PLoS One. 2011;6:e23770. doi: 10.1371/journal.pone.0023770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loring SH, Garcia-Jacques M, Malhotra A. Pulmonary characteristics in COPD and mechanisms of increased work of breathing. J Appl Physiol. 2009;107:309–14. doi: 10.1152/japplphysiol.00008.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonnet MH, Berry RB, Arand DL. Metabolism during normal, fragmented, and recovery sleep. J Appl Physiol. 1991;71:1112–8. doi: 10.1152/jappl.1991.71.3.1112. [DOI] [PubMed] [Google Scholar]
- 23.Lin CC, Lee KS, Wang YP, et al. Effect of uvulopalatopharyngoplasty on work of breathing during wakefulness in obstructive sleep apnea syndrome. Ann Otol Rhinol Laryngol. 2007;116:271–7. doi: 10.1177/000348940711600409. [DOI] [PubMed] [Google Scholar]
- 24.West SD, Kohler M, Nicoll DJ, Stradling JR. The effect of continuous positive airway pressure treatment on physical activity in patients with obstructive sleep apnoea: A randomised controlled trial. Sleep Med. 2009;10:1056–8. doi: 10.1016/j.sleep.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Testelmans D, Tamisier R, Barone-Rochette G, et al. Profile of circulating cytokines: Impact of OSA, obesity and acute cardiovascular events. Cytokine. 2013;62:210–6. doi: 10.1016/j.cyto.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 26.Ryan S, Taylor CT, McNicholas WT. Systemic inflammation: a key factor in the pathogenesis of cardiovascular complications in obstructive sleep apnoea syndrome? Thorax. 2009;64:631–6. doi: 10.1136/thx.2008.105577. [DOI] [PubMed] [Google Scholar]
- 27.Wong S, Pinkney J. Role of cytokines in regulating feeding behaviour. Curr Drug Targets. 2004;5:251–63. doi: 10.2174/1389450043490532. [DOI] [PubMed] [Google Scholar]
- 28.Vasquez MM, Goodwin JL, Drescher AA, Smith TW, Quan SF. Associations of dietary intake and physical activity with sleep disordered breathing in the Apnea Positive Pressure Long-Term Efficacy Study (APPLES) J Clin Sleep Med. 2008;4:411–8. [PMC free article] [PubMed] [Google Scholar]
- 29.Quan SF, Goodwin JL, Drescher AA, Baldwin CM, Simon RD, Smith TW. Impact of CPAP on activity patterns and diet in patients with obstructive sleep apnea (OSA) Sleep. 2013;36(Suppl):A141. doi: 10.5664/jcsm.3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hemmer M, Viquerat CE, Suter PM, Vallotton MB. Urinary antidiuretic hormone excretion during mechanical ventilation and weaning in man. Anesthesiology. 1980;52:395–400. doi: 10.1097/00000542-198005000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Frass M, Popovic R, Hartter E, Auinger C, Woloszczuk W, Leithner C. Atrial natriuretic peptide decrease during spontaneous breathing with continuous positive airway pressure in volume-expanded healthy volunteers. Crit Care Med. 1988;16:831–5. doi: 10.1097/00003246-198809000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Ip S, D'Ambrosio C, Patel K, et al. Auto-titrating versus fixed continuous positive airway pressure for the treatment of obstructive sleep apnea: a systematic review with meta-analyses. Syst Rev. 2012;1:20. doi: 10.1186/2046-4053-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]


