Abstract
Background
Affinity and clonality of allergen-specific IgE antibodies are important determinants for the magnitude of IgE-mediated allergic inflammation.
Objective
We sought to analyze the contribution of heavy and light chains of human allergen-specific IgE antibodies for allergen specificity and to test whether promiscuous pairing of heavy and light chains with different allergen specificity allows binding and might affect affinity.
Methods
Ten IgE Fabs specific for 3 non–cross-reactive major timothy grass pollen allergens (Phl p 1, Phl p 2, and Phl p 5) obtained by means of combinatorial cloning from patients with grass pollen allergy were used to construct stable recombinant single chain variable fragments (ScFvs) representing the original Fabs and shuffled ScFvs in which heavy chains were recombined with light chains from IgE Fabs with specificity for other allergens by using the pCANTAB 5 E expression system. Possible ancestor genes for the heavy chain and light chain variable region–encoding genes were determined by using sequence comparison with the ImMunoGeneTics database, and their chromosomal locations were determined. Recombinant ScFvs were tested for allergen specificity and epitope recognition by means of direct and sandwich ELISA, and affinity by using surface plasmon resonance experiments.
Results
The shuffling experiments demonstrate that promiscuous pairing of heavy and light chains is possible and maintains allergen specificity, which is mainly determined by the heavy chains. ScFvs consisting of different heavy and light chains exhibited different affinities and even epitope specificity for the corresponding allergen.
Conclusion
Our results indicate that allergen specificity of allergen-specific IgE is mainly determined by the heavy chains. Different heavy and light chain pairings in allergen-specific IgE antibodies affect affinity and epitope specificity and thus might influence clinical reactivity to allergens.
Keywords: Allergy, allergen, IgE antibodies, specificity, affinity
IgE is the key immunoglobulin mediating allergic inflammation.1 When allergens cross-link mast cell– or basophil-bound IgE, the release of inflammatory mediators, cytokines, and proteases is induced.2 Furthermore, IgE facilitates the presentation of allergens to T cells and thus might contribute to T cell–mediated chronic inflammation.3
Although the levels of IgE antibodies in allergic patients are higher than in nonallergic persons, they are much lower than the levels of IgG. Furthermore, the amounts of allergen to which allergic patients are usually exposed are extremely low.1 Nevertheless, small amounts of IgE-allergen immune complexes are able to induce strong inflammatory reactions, which might be explained by the high affinity of IgE to FcεRI on mast cells and basophils and also by high-affinity recognition of allergens by IgE.1,4,5 In fact, the 3-dimensional structures of 2 IgE-allergen complexes have recently been solved by x-ray crystallography, and affinity measurements done with surface plasmon resonance have revealed the high affinity binding of allergen-specific human IgE to conformational epitopes on the corresponding allergens.4,5
Several studies suggest that the mode of interaction between IgE and allergens has profound effects on clinical symptoms in allergic patients. It has been shown that the magnitude of basophil degranulation increases with the number of IgE epitopes on a given allergen and thus with the clonality of the IgE response, as well as with the levels of allergen-specific IgE and the affinities of IgE antibodies for the allergen.6,7 Despite the importance of the IgE-allergen interaction in allergy, only limited information regarding the structure of allergen-specific human IgE antibodies and their mode of interaction with the allergens is available.
The analysis of the primary structure of allergen-specific IgE from allergic patients has mainly become possible through combinatorial cloning because thus far no technology is available to establish a representative repertoire of IgE-producing cell lines from allergic patients.4,5,7-16 The analysis of allergic patients’ allergen-specific IgE Fabs or single chain variable fragments (ScFvs) shows that these molecules bind with high specificity and affinity to the corresponding allergens and show a varying degree of somatic mutations, and there seems to be quite a broad use of the individual heavy chain variable regions (VHs).4,5,7-17 Analyzing allergen-specific IgE Fabs, it has been noted that certain IgE heavy chains can recombine with different light chains and retain the specificity for the allergen.9,10,16 On the other hand, human IgE-derived allergen-specific Fabs or ScFvs have been described in which different heavy chains combined with the same light chain.7,8 However, it has not yet been studied whether and how shuffling of light chains between IgE Fabs with specificity for different allergens affects binding, affinity, and epitope specificity. Therefore in this study we analyzed the contribution of heavy and light chains for determining the specificity of allergen recognition by recombining IgE heavy chains from grass pollen allergen IgE Fabs with different light chains. Because it has been shown that both affinity and clonality of IgE recognition of allergens determines the intensity of effector cell degranulation6,7 and thus might have clinical importance, we investigated whether light chain shuffling can affect affinity and epitope specificity. For this purpose, we engineered stable ScFvs representing the Fab as isolated from the combinatorial library and shuffled versions in which light chains with specificities for other allergens were inserted. The recombinant ScFvs were then tested for allergen specificity and affinity by using ELISA and surface plasmon resonance measurements.
METHODS
Recombinant allergens
The recombinant grass pollen allergens rPhl p 1, rPhl p 2, rPhl p 5, rPhl p 6, and rPhl p 7 (timothy grass) and the recombinant major birch pollen allergen rBet v 1 were purchased from Biomay (Vienna, Austria), and rPhl p 12 and rPhl p 13 (timothy grass) were expressed and purified as previously described.18,19
Pollen from timothy grass (Phleum pratense), Kentucky bluegrass (Poa pratensis), and ryegrass (Lolium perenne) were purchased from Allergon (Välinge, Sweden). Aqueous pollen extracts were prepared from timothy grass pollen, as previously described.20 SDS extracts were prepared from all pollen, as previously described.21
Characterization of grass pollen allergen–specific IgE Fabs
IgE Fabs specific for the major timothy grass pollen allergens Phl p 1, Phl p 2, and Phl p 5 were isolated from a combinatorial pComb 3H–phagemid library constructed from PBMCs of a patient with grass pollen allergy.16 In total, 4 IgE Fab clones specific for Phl p 5 (clones 5, 14, 28, and 31), 3 clones specific for Phl p 2 (clones 94, 60, and 100), and 3 clones specific for Phl p 1 (clones 25, 43, and 10) were used as starting material for the engineering of defined ScFvs.11,14,16
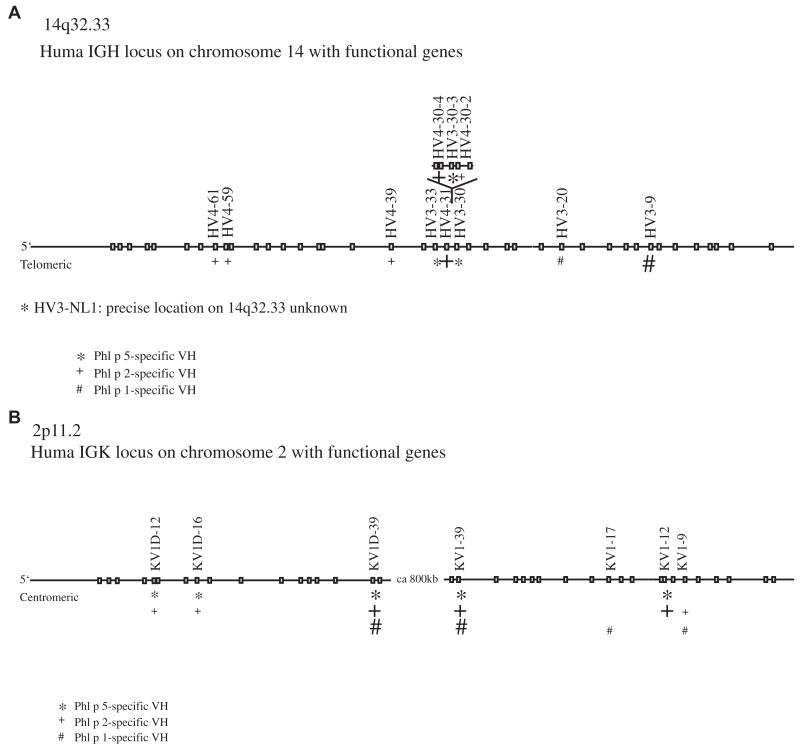
DNA sequences coding for the VHs and light chain variable regions (VLs) were aligned with human germline gene sequences (allele*01) from the ImMunoGeneTics database by using V-Quest software.22 For each of the analyzed heavy and light chain sequences, the corresponding germline V, D, and J genes with the highest sequence identities and those with sequence identities of no less than 5% to the latter (highlighted in bold in Fig E1 in this article’s Online Repository at www.jacionline.org) were mapped on chromosome locus 14q32.33 (heavy chains)23 or on chromosome locus 2p11.2 (light chains)24 (Fig 1). The light chain DNA sequences were translated (ExPASy, Swiss Institute of Bioinformatics, Geneva, Switzerland), and the deduced amino acid sequences were aligned (Fig 2) to compare the individual chains. Sequence identities were calculated by using the multiple sequence alignment program Clustal W (see Table E1 in this article’s Online Repository at www.jacionline.org).25
FIG 1.
Localization of the possible functional ancestor genes of the allergen-specific ScFvs in the human genome. A, Representation of the human IGH locus (14q32.33). B, Representation of the human IGK locus (2p11.2). Functional genes are boxed, and possible germline ancestors for the isolated allergen-specific ScFvs are indicated on top. Symbols (*, Phl p 5-specific; #, Phl p 1-specific; and +, Phl p 2-specific) represent the germline genes with high sequence identity to the allergen-specific variable regions (see Fig E1). The closest germline genes are indicated with large symbols. Gene IGHV3-NL1 on 14q32.33 could not be located with the ImMunoGeneTics/GENE-DB software.
FIG 2.
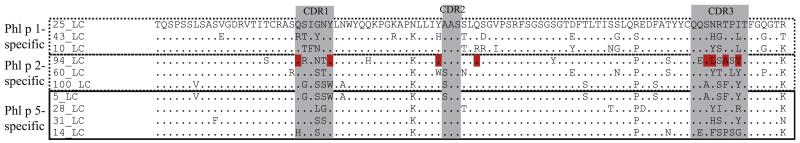
Amino acid sequence alignment of VL regions used in the allergen-specific IgE Fabs with the Phl p 1–specific region 25VL. Identical amino acids are indicated by dots, and complementarity-determining regions (CDR) are highlighted in gray. Amino acids involved in the binding of the light chain of the Phl p 2–2specific Fab with Phl p 24 are shown in red.
Conversion of allergen-specific IgE Fabs into ScFvs and light chain–shuffled ScFvs
By using the pComb 3H–phagemid DNA from clones 5 (Phl p 5), 94 (Phl p 2), and 25 (Phl p 1) as template, the heavy chain cDNAs were amplified by means of PCR with 20 pmol of an equimolar mix of IgHV and IgHJ family–specific ScFv primers.26 In parallel, cDNAs coding for 10 light chains of the original IgE Fabs (Phl p 5: clones 5, 14, 28, and 31; Phl p 2: clones 94, 60, and 100; and Phl p 1: clones 25, 43, and 10) were PCR amplified by using IGKV and IGKJ family–specific primers.26 PCR products were purified on a 1% agarose gel.
Fifty nanograms of DNA coding for VH and VL fragments was assembled to obtain ScFv cDNAs. The combination of heavy and light chain fragment–encoding cDNAs of the original Fab clones (clones 5, 94, and 25) resulted in corresponding ScFv cDNAs, giving rise to ScFvs specific for Phl p 5 (5VH/5VL), Phl p 2 (94VH/94VL), and Phl p 1 (25VH/25VL). In parallel, cDNAs coding for the VH fragments were recombined only with light chains from IgE Fabs with other specificities to obtain shuffled ScFvs, which were designated according to the clones from which the heavy chain and light chain cDNAs were obtained (heavy chain/light chain: 5VH/94VL, 5VH/60VL, 5VH/100VL, 5VH/25VL, 5VH/43VL, 5VH/10VL, 94VH/5VL, 94VH/31VL, 94VH/14VL, 94VH/28VL, 94VH/25VL, 94VH/43VL, 94VH/10VL, 25VH/5VL, 25VH/31VL, 25VH/14VL, 25VH/28VL, 25VH/94VL, 25VH/60VL, and 25VH/100VL).
The PCR assembly was performed by introducing a linker DNA coding for a 15-amino-acid linker (Gly4Ser)326. The production of the human linker DNA is described in the Methods section in this article’s Online Repository at www.jacionline.org. Assembly PCR was run for 7 cycles (Mouse ScFv module; Amersham Biosciences, LittleChalfont, UnitedKingdom),and the assembledsequence was further amplified by adding an equimolar mix of 20 pmol IGHV and IGKJ outer primers containing restriction sites for SfiI (IGHV) and NotI (IGKJ).26 Products were purified on a 1% agarose gel and eluted as previously described.
Purified ScFv cDNA was digested with SfiI and NotI (New England Biolabs, Ipswich, Mass) purified on a 1% agarose gel and further ligated into the phagemid vector pCANTAB 5 E (Amersham Biosciences). Recombinant phagemid DNA was transformed into the Escherichia coli strain HB2151 by means of electroporation for the production of soluble ScFv fragments (Expression module/Recombinant phage antibody system, Amersham Biosciences). E coli cells were grown on SOBAG plates (Expression module, Amersham Biosciences) at 30°C overnight.
ScFv-expressing clones were identified by means of sequencing (Eurofins MWG Operon, Ebersberg, Germany) with the ScFv-specific primers pCAN-TAB 5 E_fwd (5′ CCA TGA TTA CGC CAA GCT TTG GAG CC 3′) and pCANTAB 5 E_rev (5′ GTA AAT GAA TTT TCT GTA TGA GG 3′) and by testing supernatants for reactivityto the allergens. For this purpose, transformed bacteria were induced with 1 mmol/L isopropyl β-D-1-thiogalactopyranoside to express soluble ScFv and tested for allergen specificity by using ELISA.
Determination of binding specificities by using ELISA
Bacterial lysates containing soluble ScFvs were analyzed for specific binding to recombinant timothy grass pollen allergens (Phl p 1, Phl p 2, Phl p 5, Phl p 6, Phl p 7, Phl p 12, and Phl p 13), to the major birch pollen allergen Bet v 1, and to HSA as controls. Five micrograms per milliliter of the proteins in PBS were coated to ELISA plates (Nunc, Roskilde, Denmark) and incubated with the ScFv-containing lysates diluted 1:2 in blocking buffer (PBS/3% wt/vol BSA) or, for control purposes, with blocking buffer alone. For calibration, lysates from the 5VH/5VL clone were added to each plate. Bound ScFv fragments were detected with the monoclonal mouse anti–E tag antibody (Amersham Biosciences) and traced with a horseradish peroxidase–labeled sheep anti-mouse antiserum (Amersham Biosciences). The color reaction was read at 405 nm.27 All samples were analyzed in duplicates, and the means were calculated and displayed as mean OD values after subtraction of the baseline signal (ie, signal obtained with the unrelated allergen Bet v 1).
Experiments testing the cross-reactivity of the Phl p 5–specific ScFvs to nitrocellulose-blotted natural allergens from different grass species are described in the Methods section in the article’s Online Repository.
Determination of kinetics and binding affinities of ScFvs
All measurements were performed on a BIACORE 2000 instrument (GE Healthcare Biacore AB, Uppsala, Sweden) at 25°C. A 1:1 mixture of 1-ethyl-3-(3-dimethylaminopropyl carbodiimide) hydrochloride and N-hydroxysuccinimidewas injected at a flow rate of 5 μL/min for 7 minutes to activate the surface of a CM5 sensor chip flow cell (GE Healthcare Biacore AB). To serve as capturing antibody, 60 μg/mL of the monoclonal anti–E tag antibody (Amersham Biosciences) diluted in 10 mmol/L sodium acetate (pH 5) was injected at a flow rate of 10 μL/min until the maximal immobilization level of approximately 10,000 resonance units (RU) was reached. The flow cell was then deactivated by means of injection of 1 mol/L ethanolamine-HCl (pH 8.5; 5 μL/min) for 7 minutes. The reference cell was loaded with an isotype control antibody in an analogous way. Lysates containing ScFvs diluted in HBS-EP (0.01 mol/L HEPES, 0.15 mol/L NaCl, 3 mmol/L EDTA, and 0.005% vol/vol surfactant P20, pH 7.4) were injected at 5 μL/min for different periods of time, followed by a stabilization phase of 30 minutes to capture 100 RU of ScFv for a calculated maximum response of 100 RU.
For the determination of kinetics and binding affinities, multicycle kinetics were performed. For this purpose, the tested allergen was diluted in HBS-EP in 2-fold increasing concentrations (0.125-128 nmol/L) and injected in random order at 30 μL/min for 5 minutes. Dissociation was determined by means of injection of HBS-EP buffer at 30 μL/min for 30 minutes. For control purposes, 2 runs were performed with the same allergen dilutions. In addition, 2 cycles were run with HBS-EP alone to subtract background signals. Regeneration of the sensor chip was performed by using injection of 10 mmol/L glycine-HCl, pH 2.5, at 30 μL/min for 30 seconds. BIAEvaluation 3.2 (Biacore AB) software was used to calculate kinetics and affinity constants, and fittings were performed with a 1:1 (Langmuir) binding model.
For ScFvs showing weak binding in the ELISA (ie, 25VH/14VL and 25VH/28VL), Biacore experiments were performed to verify binding reactions. After capturing of 100 RU of ScFv, 256 nmol/L of the respective allergens was injected at a flow rate of 30 μL/min for 5 minutes. For double referencing, running buffer (HBS-EP) was injected under the same conditions.
Sandwich ELISA for detecting alterations in epitope recognition of shuffled ScFvs
ELISA plates (Nunc; Thermo Scientific, Uppsala, Sweden) were coated with either a human monoclonal Phl p 5– or a Phl p 2–specific IgG1 antibody (1 μg/mL),14,28 which contained the very same variable regions as the originally isolated ScFvs 5VH/5VL and 94VH/94VL, respectively. Coated plates containing the Phl p 5–specific IgG1 were incubated with rPhl p 5 (1 μg/mL) or a natural timothy grass pollen extract (40 μg/mL) containing nPhl p 5 diluted in blocking buffer. Plates containing Phl p 2–specific IgG1 were incubated with rPhl p 2 (1 μg/mL). Captured allergens were then exposed to E coli lysates containing Phl p 5– or Phl p 2–specific ScFvs, which had been diluted 1:2 in blocking buffer. For control purposes, rabbit anti–Phl p 529 or anti–Phl p 214 antibodies were used. Bound ScFv fragments were detected as described for the ELISA determining binding specificities. Bound rabbit antibodies were detected with a horseradish peroxidase–labeled donkey anti-rabbit antiserum (Amersham Biosciences).30 All determinations were performed in duplicates, and the results represent mean values with a mean coefficient of variation of less than 10%.
RESULTS
Sequence analysis of human IgE Fabs specific for 3 non–cross-reactive grass pollen allergens
VH and κ VL cDNAs from the 10 IgE Fabs specific for 3 non–cross-reactive allergens obtained from the combinatorial library16 were compared with human germline genes from the ImMunoGeneTics database.
Sequence analysis and comparison with the germline genes revealed that the 4 Phl p 5–specific IgE Fabs used the very same heavy chain (5VH) that had recombined with 4 different light chains (5VL, 14VL, 28VL, and 31VL; see Fig E1, A). The heavy chains of the 3 Phl p 2–specific IgE Fabs (94VH, 60VH, and 100VH) seemed to use the same IGHV genes (see Fig E1, A). Interestingly, 2 (94VH and 60VH) seemed to have recombined with similar light chains (94VL and 60VL), which were also used by Phl p 5–specific IgE Fabs (14, 28, and 31), and 1 (100VH) used a light chain identical to that of one of the 4 Phl p 5–specific IgE Fabs (5VL; see Fig E1 and Table E1). All 3 Phl p 1–specific IgE Fabs were derived from the same IGHV gene and also contained light chains based on IGKV genes, which appeared similar to those used by 3 of the Phl p 5–specific IgE Fabs (ie, 14, 28, and 31; see Fig E1). However, the alignment of the light chains in Fig 2 showed that their similarity was mainly due to the use of the same V genes (see Fig E1, B), whereas most of them showed considerable diversity in the amino acid composition within complementarity-determining region (CDR) 1 and CDR3 (Fig 2).
Possible ancestor genes for Phl p 5–, Phl p 2–, and Phl p 1–specific IgE VHs are distributed along the heavy chain locus on chromosome 14 (14q32.33; Fig 1, A) and arise from different VH genes belonging to the IGHV3 and IGHV4 families (Fig 1, A, and see Fig E1, A). In Fig E1 the closest related IGHV germline gene and those germline genes with a difference in sequence of less than 5% are colored. Within this range, 4 and 2 of the 23 functional IGHV3 genes might be considered as possible ancestors for Phl p 5–specific (IGHV3-30-3, IGHV3-30, IGHV3-33, and IGHV3-NL1) and Phl p 1–specific (IGHV3-9 and IGHV3-20) variable regions, respectively. Considering the same range of sequence identity, at least 5 of the 11 functional genes might be considered as possible progenitors (IGHV4-31, IGHV4-30-4, IGHV4-30-2, IGHV4-61, and IGHV4-59) for the Phl p 2–specific VH genes.
The sequence comparison for CDR3 with the possible corresponding IGHD and IGHJ genes was not possible due to their junctional diversity. Two of the Phl p 1–specific IgE Fabs (ie, 43 and 10) used similar IGHD genes, whereas the third Phl p 1–specific IgE Fab and the Phl p 2–specific IgE Fabs seemed to use different IGHD genes. The IGHJ genes in the IgE Fabs were similar (mainly IGHJ4*01), whereas the light chains used different IGKJ genes and thus might result from different somatic recombinations (Fig E1).
Interestingly, the light chains of the IgE Fabs specific for different unrelated allergens (ie, Phl p 5, Phl p 2, and Phl p 1) seemed to evolve from one germline gene pool (see Fig E1, B) located on the κ light chain locus on chromosome 2 (2p11.2; Fig 1, B). The possible germline genes of the light chains all belonged to the IGKV1 family (IGKV1-12, IGKV1D-12, IGKV1-39, IGKV1D-39, IGKV1D-16, IGKV1-17, and IGKV1-9; Fig 1, B, and see Fig E1, B) involving 7 of 20 functional genes.
Conversion of allergen-specific IgE Fab fragments into ScFv fragments with identical binding specificity and shuffling of heavy and light chains
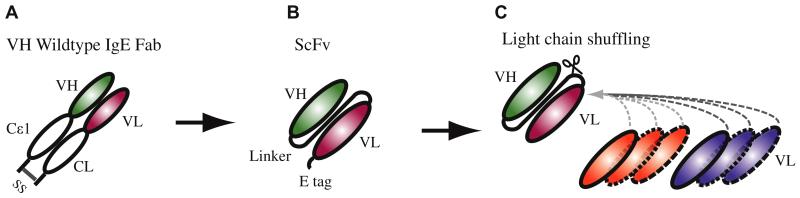
Expression of recombinant Fab fragments using the pComb 3H system might yield Fab preparations that consist of difficult to define mixtures of correctly formed Fabs and also incorrectly formed homodimers consisting of only heavy or light chains.31 Recombinant IgE Fabs were converted into ScFvs to obtain defined and stable combinations of heavy and light chain fragments for binding experiments.31 On the basis of the sequences of the original IgE Fab fragments, stable ScFv fragments were generated in a first step (Fig 3). ScFvs containing the original combinations of heavy and light chains were termed “original,” whereas those in which heavy chains were newly combined with light chains taken from Fabs with other specificities were designated “light chain shuffled.”
FIG 3.
Schematic illustration of the light chain–shuffling approach. Allergen-specific IgE Fabs (A) were converted into the ScFv format (B). Subsequently, the heavy chains were recombined with the light chains from the original Fabs (C).
Table I provides an overview of the 3 original ScFvs (5VH/5VL, 94VH/94VL, and 25VH/25VL) and 18 light chain–shuffled ScFvs. The recombinant ScFvs contain an E tag at their C-terminus and therefore could be detected in E coli lysates by means of immunoblotting, as shown in Fig E2 in this article’s Online Repository at www.jacionline.org. Light chain–shuffled ScFvs were obtained for all combinations except 94VH/28VL, which carried a frameshift mutation, and 25VH/31VL, which contained a substitution mutation, resulting in a premature stop of translation, and thus could not be studied (see Fig E2 and data not shown).
TABLE I.
Characterization of ScFvs regarding expression and binding specificity
| Combined with: |
Phl p 5–specific 5VH |
Phl p 2–specific 94VH |
Phl p 1–specific 25VH |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Phl p 5–
specific |
Phl p 2– specific |
Phl p 1– specific |
Phl p 5– specific |
Ph1 p 2–
specific |
Phl p 1– specific |
Phl p 5– specific |
Phl p 2– specific |
Phl p 1–
specific |
|||||||||||||
| 5VL | 60VL | 94VL | 100VL | 10VL | 25VL | 43VL | 5VL | 31VL | 14VL | 94VL | 10VL | 25VL | 43VL | 5VL | 14VL | 28VL | 60VL | 94VL | 100VL | 25VL | |
| E tag detection | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ± | + | + | + | + | + |
| ELISA | + | + | + | + | + | + | + | + | − | − | + | − | + | − | − | ± | ± | + | + | − | + |
| Biacore | + | + | + | + | + | + | + | + | ND | ND | + | ND | + | ND | ND | − | − | + | + | ND | + |
|
| |||||||||||||||||||||
| Phl p 5–specific 5VH | Phl p 2–specific 94VH | Phl p 1–specific 25VH | |||||||||||||||||||
|
| |||||||||||||||||||||
| Binding to: | Phl p 5 | Phl p 2 | Phl p 1 | ||||||||||||||||||
ScFvs grouped by their heavy chains and combined light chains (top) were classified according to expression (E tag detection) and binding to the allergens (bottom) by ELISA and Biacore. Original clones are shown in italics.
+ , Positive; ± , weak; − , negative; ND, not done.
Light chain shuffling in ScFvs indicates that allergen specificity is mainly determined by the heavy chain
The majority of the shuffled clones (12/18) reacted with the allergen that was recognized by the original ScFv when tested by using ELISA (Table I).
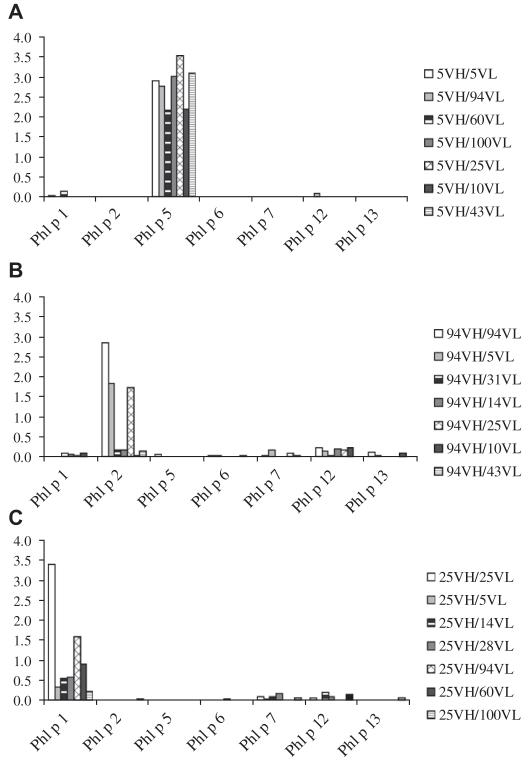
The Phl p 5–specific 5VH was successfully recombined with each of the 6 light chains and each of the light chain–shuffled ScFvs bound to Phl p 5 but not to other timothy grass pollen allergens (ie, Phl p 1, Phl p 2, Phl p 6, Phl p 7, Phl p 12, and Phl p 13; Fig 4, A, and Table I). The original and shuffled Phl p 5–specific ScFvs showed reactivity to nitrocellulose-blotted natural group 5 allergens from P pratense, P pratensis, and L perenne (see Fig E3 in this article’s Online Repository at www.jacionline.org).
FIG 4.
Reactivities of ScFvs to a panel of recombinant timothy grass pollen allergens (Phl p 1, Phl p 2, Phl p 5, Phl p 6, Phl p 7, Phl p 12, and Phl p 13) by means of ELISA. OD values (y-axes) corresponding to the binding intensities to the allergens (x-axes) are shown for ScFvs containing heavy chains from IgE Fabs specific for Phl p 5 (A, 5VH), Phl p 2 (B, 94VH), and Phl p 1 (C, 25VH; right margins).
Likewise, the Phl p 2–specific 94VH and the Phl p 1–specific 25VH recombined with the light chains, and many of the light-chain–shuffled ScFvs bound to the VH-specific allergen but not to the other tested grass pollen allergens (Fig 4, B and C, and Table I).
Surface plasmon resonance measurements confirm that light chain–shuffled ScFvs preserve the specificity of the original ScFvs and exhibit affinities in the nanomolar range
Kinetics (ie, association and dissociation rate constants) and binding affinities for the interaction between original and light chain–shuffled ScFvs and their respective allergens were measured by using surface plasmon resonance. For this purpose, ScFvs were immobilized to the Biacore chip by using the anti–E tag antibody for capturing, and 2-fold increasing concentrations of allergen were injected into the flow cell, allowing interaction with the captured ScFv. Six ScFvs (2 original and 4 light chain–shuffled ScFvs) with positive ELISA results could be analyzed in Biacore experiments because they could be successfully captured with the anti–E tag antibody (Table II).
TABLE II.
Summary of Biacore results for ScFvs with allergen specificity
| Phl p 5–specific 5VH |
Phl p 2–specific 94VH |
Phl p 1–specfic 25VH |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Combined with: |
Phl p 5–specific |
Phl p 2–specific |
Phl p 1–specific |
Phl p 5–specific |
Phl p 2– specific |
Phl p 1– specific |
Phl p 2–specific |
Phl p 1–specific |
|||||
| 5VL | 60VL | 94VL | 100VL | 10VL | 25VL | 43VL | 5VL | 94VL | 25VL | 60VL | 94VL | 25VL | |
| ka (1/Ms) | 2.23 × 105 | NA | NA | 1.84 × 105 | NA | 9.91 × 104 | 1.31 × 105 | 9.94 × 104 | NA | NA | NA | NA | 2.23 × 105 |
| kd (1/s) | 8.34 × 10−6 | NA | NA | 6.46 × 10−5 | NA | 2.57 × 10−4 | 5.23 × 10−4 | 6.66 × 10−4 | NA | NA | NA | NA | 1.23 × 10−4 |
| KD (M) | 3.74 × 10−11 | NA | NA | 3.5 × 10−10 | NA | 2.6 × 10−9 | 4.01 × 10−9 | 6.71 × 10−9 | NA | NA | NA | NA | 5.5 × 10−10 |
Association (ka) and dissociation (kd) rate constants, as well as dissociation constants (KD) calculated by fitting the analyzed data with a 1:1 binding model, are displayed for those ScFvs that could be analyzed by using Biacore.
NA, Not analyzable because of unstable capturing.
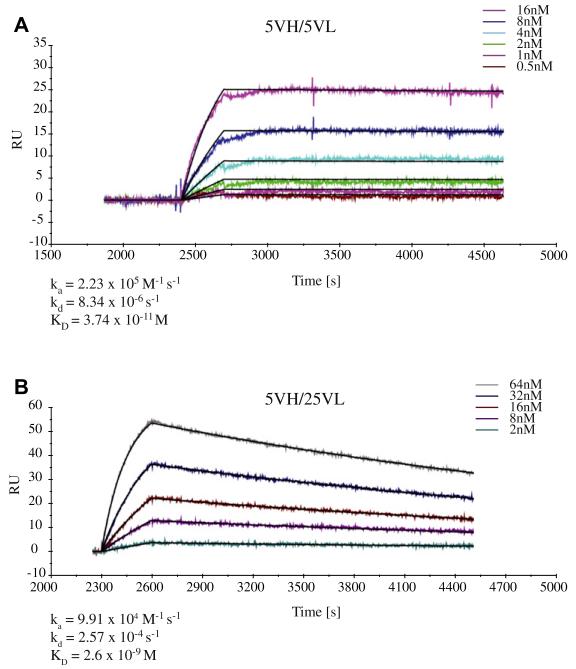
Among the ScFvs carrying the Phl p 5–specific 5VH, the original ScFv 5VH/5VL showed the highest affinity to Phl p 5 (dissociation constant = 3.74 × 10−11 mol/L; Fig 5, A, and Table II). The light chain–shuffled ScFv 5VH/25VL (Fig 5, B) still bound with high affinity to Phl p 5 (dissociation constant = 2.6 × 10−9 mol/L), but the affinity was approximately 100-fold reduced compared with that of the original ScFv (Table II). Likewise, the other 2 light chain–shuffled ScFvs (5VH/100VL and 5VH/43VL) showed lower affinities to Phl p 5 than the original ScFv (Table II).
FIG 5.
Sensor chip–based studies of the interaction between Phl p 5 and Phl p 5–specific ScFvs. ScFvs (A, 5VH/5VL; B, 5VH/25VL) were captured on the chip, and Phl p 5 was injected in 2-fold increasing concentrations from 0.5 to 16 nmol/L (Fig 5, A) or 2 to 64 nmol/L (Fig 5, B). Recorded (colored lines) and calculated (black lines) curves, which represent fittings of the data to a 1:1 binding model, were superimposed. Signal intensities (RU) are displayed (y-axes) versus time (in seconds; x-axes). Association (ka) and dissociation (kd) rate constants, as well as dissociation constants (KD) are indicated.
The original Phl p 1–specific ScFv 25VH/25VL showed an affinity comparable with that of the Phl p 5–specific shuffled ScFv (5VH/100VL, Table II). The shuffled Phl p 2–specific ScFv 94VH/5VL showed a lower affinity comparable with 2 of the Phl p 5–specific shuffled ScFvs (5VH/25VL and 5VH/43VL).
In this context we noted that certain amino acids, which, because of the resolved cocrystal structure, were known to be involved in the binding of the light chain of the Phl p 2–specific IgE Fab to Phl p 2,4 were different in the shuffled version, but binding was retained (Fig 2, printed in red). From the 2 contacts in the CDR1 of the light chain, one was conserved in all 3 ScFvs, which retained binding specificity (94VH/94VL, 94VH/5VL, and 94VH/25VL). In CDR2 only 1 residue made contact, which was conserved in each of the 3 binding ScFvs. Several contacts were observed for CDR3, but none of these amino acids were conserved in all 3 binding ScFvs. Despite the shuffling, each of the tested ScFvs showed binding affinities in the nanomolar range (Table II).
Light chain shuffling can change epitope specificity for the same allergen
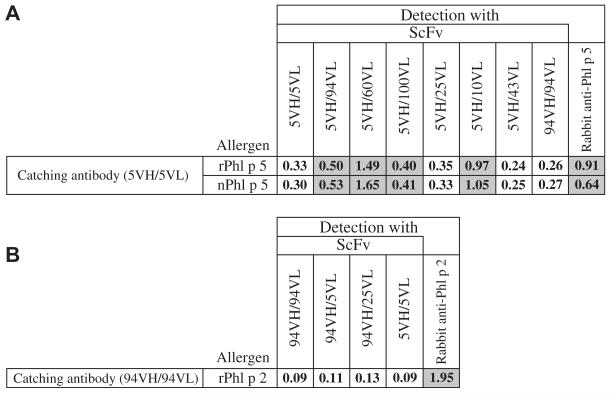
Sandwich ELISAs were performed to reveal possible changes in epitope specificity of shuffled ScFvs for the original allergens. Allergens were captured with antibodies containing the original VH/VL combination, and shuffled ScFvs were allowed to bind. Interestingly, 2 of 6 shuffled Phl p 5–specific ScFvs (5VH/60VL and 5VH/10VL) bound strongly to captured recombinant and natural Phl p 5 (Fig 6, A). Likewise, rabbit anti–Phl p 5 antibodies recognizing epitopes different from the catching antibody reacted with Phl p 5. Two other Phl p 5–specific ScFvs (5VH/94VL and 5VH/100VL) showed weak but distinct positive reactions to the recombinant and natural allergens, which were greater than the values obtained for a non–Phl p 5–specific ScFV (94VH/94VL) and the original ScFv (5VH/5VL; Fig 6, A). Neither of the 2 tested Phl p 2–specific shuffled ScFvs reacted with captured recombinant Phl p 2, whereas rabbit anti-Phl p 2 antibodies directed to other epitopes than those recognized by the capture antibody showed reactivity (Fig 6, B).
FIG 6.
Altered epitope recognition of certain ScFvs revealed by using sandwich ELISA. Plates coated with antibodies specific for Phl p 5 (5VH/5VL; A, left) or Phl p 2 (94VH/94VL; B, left) were incubated with allergens (Fig 6, A: rPhl p 5 and nPhl p 5; Fig 6, B: rPhl p 2) and exposed to different ScFvs or allergen-specific rabbit antibodies (positive controls), respectively. Mean OD values with less than 10% average variation are displayed, and binders are highlighted in gray.
We also studied whether shuffled ScFvs react with allergens or antigens different from the originally recognized allergen; however, shuffled ScFvs did not react with other antigens in extracts from grass pollen and unrelated allergens from olive and birch pollen (data not shown).
DISCUSSION
In this study the contribution of heavy and light chains to the binding specificity and affinity of ScFvs derived from human IgE antibodies specific to allergens was investigated. Using heavy and light chains from human IgE Fabs11,14,16 with specificity for 3 different immunologically unrelated major grass pollen allergens, Phl p 1, Phl p 2, and Phl p 5,32-34 as a starting material, we constructed and characterized stable recombinant ScFvs. Thus a defined experimental model system was obtained for testing the effects of variations in heavy and light chain pairing on allergen specificity and affinity. The question of whether light chain shuffling has an effect on allergen specificity, affinity, and epitope specificity has not been investigated until now. Thus far, it has not been possible to isolate and clone B cells producing allergen-specific IgE antibodies because the peripheral blood of allergic patients appears to contain only plasma cells secreting allergen-specific IgE.35,36 Therefore earlier studies only reported the isolation and characterization of allergen-specific human IgE Fabs by using combinatorial cloning.7-16 The only shuffling experiments carried out thus far were performed for antibodies of murine origin, which were specific for only 1 allergen.37
When the various ScFvs were tested for reactivity to 7 different recombinant grass pollen allergens, we found that the original ScFvs bound with high specificity only to the allergens recognized by the corresponding original IgE Fabs. Interestingly, several of the shuffled ScFvs in which the original light chain was replaced by a light chain derived from an IgE Fab with specificity for another unrelated allergen retained their specificity to the original allergen in a heavy chain–dependent manner. Furthermore, all Phl p 5–specific ScFvs were tested for their reactivity to natural allergens from cross-reactive grass species (ie, P pratense, P pratensis, and L perenne), which revealed that shuffled ScFvs bound to group 5 allergens with varying intensities. These results indicate that the heavy chain is important for determining specificity in human allergen-specific IgE antibodies. According to the sequence comparison of the cDNAs coding for the IgE VHs with the germline genes, it seemed that the analyzed heavy chains are derived from several distinct ancestor genes. Therefore allergen specificity might originate from several different genes, which indicates a certain redundancy in allergen recognition by IgE antibodies and might contribute to diversity.
Shuffled ScFvs containing different heavy and light chains showed different affinities to the corresponding allergens as measured by using surface plasmon resonance experiments. This finding suggests that in early B-cell development, in addition to junctional diversity, the choice of different light chains can contribute to the modulation of affinity in allergen recognition by allergen-specific IgE antibodies. Later in B-cell development, varying affinity maturation by somatic mutations may have additional effects on affinity.38
Interestingly, certain of the shuffled Phl p 5–specific ScFVs were shown to change their epitope specificity on recombinant and natural Phl p 5, indicating that light chain shuffling might increase clonality. Phl p 5 is a monomeric protein with very high allergenic activity causing effector cell degranulation already in very low concentrations.21,28 Therefore it might be speculated that light chain shuffling can increase allergenic activity.
It is quite likely that the findings made for allergen-specific IgE might be applicable also for allergen-specific IgG antibodies. However, a direct comparison is not possible because several studies indicate that allergen-specific IgE and IgG antibodies might recognize different allergens and epitopes in a given patient.39
The quaternary structure resulting from promiscuous heavy and light chain pairing in allergen-specific IgE antibodies might thus represent an important feature responsible for varying clinical sensitivities in allergic patients.
Supplementary Material
Key messages.
Allergen specificity in IgE-derived grass pollen allergen–specific ScFvs was dictated by the heavy chains.
Different pairings of heavy and light chains resulted in differences in affinity and epitope specificity of allergen recognition and thus might affect clinical sensitivity.
Acknowledgments
Supported by grant 813003 from the Austrian Research Promotion Agency, grant P23318-B11 (FWF) and special research program F46 of the Austria Science Fund (FWF), the Christian Doppler Research Association, and a research grant from Biomay, Vienna, Austria.
Abbreviations used
- CDR
Complementarity-determining region
- RU
Resonance units
- ScFv
Single chain variable fragment
- VH
Heavy chain variable region
- VL
Light chain variable region
Footnotes
Disclosure of potential conflict of interest: R. Valenta has received research support from the Austrian Science Fund, the Christian Doppler Research Association, Biomay, and Phadia–Thermo Fisher and has received consultancy fees from Biomay and Phadia–Thermo Fisher. The rest of the authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Geha RS, Jabara HH, Brodeur SR. The regulation of immunoglobulin E class-switch recombination. Nat Rev Immunol. 2003;3:721–32. doi: 10.1038/nri1181. [DOI] [PubMed] [Google Scholar]
- 2.Bischoff SC. Role of mast cells in allergic and non-allergic immune responses: comparison of human and murine data. Nat Rev Immunol. 2007;7:93–104. doi: 10.1038/nri2018. [DOI] [PubMed] [Google Scholar]
- 3.van Neerven RJ, Knol EF, Ejrnaes A, Wurtzen PA. IgE-mediated allergen presentation and blocking antibodies: regulation of T-cell activation in allergy. Int Arch Allergy Immunol. 2006;141:119–29. doi: 10.1159/000094714. [DOI] [PubMed] [Google Scholar]
- 4.Padavattan S, Flicker S, Schirmer T, Madritsch C, Randow S, Reese G, et al. High-affinity IgE recognition of a conformational epitope of the major respiratory allergen Phl p 2 as revealed by X-ray crystallography. J Immunol. 2009;182:2141–51. doi: 10.4049/jimmunol.0803018. [DOI] [PubMed] [Google Scholar]
- 5.Niemi M, Jylha S, Laukkanen ML, Soderlund H, Makinen-Kiljunen S, Kallio JM, et al. Molecular interactions between a recombinant IgE antibody and the betalactoglobulin allergen. Structure. 2007;15:1413–21. doi: 10.1016/j.str.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 6.Gieras A, Focke-Tejkl M, Ball T, Verdino P, Hartl A, Thalhamer J, et al. Molecular determinants of allergen-induced effector cell degranulation. J Allergy Clin Immunol. 2007;119:384–90. doi: 10.1016/j.jaci.2006.09.034. [DOI] [PubMed] [Google Scholar]
- 7.Christensen LH, Riise E, Bang L, Zhang C, Lund K. Isoallergen variations contribute to the overall complexity of effector cell degranulation: effect mediated through differentiated IgE affinity. J Immunol. 2010;184:4966–72. doi: 10.4049/jimmunol.0904038. [DOI] [PubMed] [Google Scholar]
- 8.Jylha S, Makinen-Kiljunen S, Haahtela T, Soderlund H, Takkinen K, Laukkanen ML. Selection of recombinant IgE antibodies binding the beta-lactoglobulin allergen in a conformation-dependent manner. J Immunol Methods. 2009;350:63–70. doi: 10.1016/j.jim.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Persson H, Sadegh MK, Greiff L, Ohlin M. Delineating the specificity of an IgE-encoding transcriptome. J Allergy Clin Immunol. 2007;120:1186–92. doi: 10.1016/j.jaci.2007.06.041. [DOI] [PubMed] [Google Scholar]
- 10.Andreasson U, Flicker S, Lindstedt M, Valenta R, Greiff L, Korsgren M, et al. The human IgE-encoding transcriptome to assess antibody repertoires and repertoire evolution. J Mol Biol. 2006;362:212–27. doi: 10.1016/j.jmb.2006.06.062. [DOI] [PubMed] [Google Scholar]
- 11.Flicker S, Steinberger P, Ball T, Krauth MT, Verdino P, Valent P, et al. Spatial clustering of the IgE epitopes on the major timothy grass pollen allergen Phl p 1: importance for allergenic activity. J Allergy Clin Immunol. 2006;117:1336–43. doi: 10.1016/j.jaci.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 12.Jakobsen CG, Bodtger U, Kristensen P, Poulsen LK, Roggen EL. Isolation of high-affinity human IgE and IgG antibodies recognising Bet v 1 and Humicola lanuginosa lipase from combinatorial phage libraries. Mol Immunol. 2004;41:941–53. doi: 10.1016/j.molimm.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 13.Laukkanen ML, Makinen-Kiljunen S, Isoherranen K, Haahtela T, Soderlund H, Takkinen K. Hevein-specific recombinant IgE antibodies from human single-chain antibody phage display libraries. J Immunol Methods. 2003;278:271–81. doi: 10.1016/s0022-1759(03)00070-x. [DOI] [PubMed] [Google Scholar]
- 14.Flicker S, Steinberger P, Norderhaug L, Sperr WR, Majlesi Y, Valent P, et al. Conversion of grass pollen allergen-specific human IgE into a protective IgG(1) antibody. Eur J Immunol. 2002;32:2156–62. doi: 10.1002/1521-4141(200208)32:8<2156::AID-IMMU2156>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 15.Edwards MR, Brouwer W, Choi CH, Ruhno J, Ward RL, Collins AM. Analysis of IgE antibodies from a patient with atopic dermatitis: biased V gene usage and evidence for polyreactive IgE heavy chain complementarity-determining region 3. J Immunol. 2002;168:6305–13. doi: 10.4049/jimmunol.168.12.6305. [DOI] [PubMed] [Google Scholar]
- 16.Steinberger P, Kraft D, Valenta R. Construction of a combinatorial IgE library from an allergic patient. Isolation and characterization of human IgE Fabs with specificity for the major timothy grass pollen allergen, Phl p 5. J Biol Chem. 1996;271:10967–72. doi: 10.1074/jbc.271.18.10967. [DOI] [PubMed] [Google Scholar]
- 17.Marth K, Novatchkova M, Focke-Tejkl M, Jenisch S, Jager S, Kabelitz D, et al. Tracing antigen signatures in the human IgE repertoire. Mol Immunol. 2010;47:2323–9. doi: 10.1016/j.molimm.2010.05.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valenta R, Ball T, Vrtala S, Duchene M, Kraft D, Scheiner O. cDNA cloning and expression of timothy grass (Phleum pratense) pollen profilin in Escherichia coli: comparison with birch pollen profilin. Biochem Biophys Res Commun. 1994;199:106–18. doi: 10.1006/bbrc.1994.1201. [DOI] [PubMed] [Google Scholar]
- 19.Swoboda I, Grote M, Verdino P, Keller W, Singh MB, De Weerd N, et al. Molecular characterization of polygalacturonases as grass pollen-specific marker allergens: expulsion from pollen via submicronic respirable particles. J Immunol. 2004;172:6490–500. doi: 10.4049/jimmunol.172.10.6490. [DOI] [PubMed] [Google Scholar]
- 20.Vrtala S, Grote M, Duchene M, van Ree R, Kraft D, Scheiner O, et al. Properties of tree and grass pollen allergens: reinvestigation of the linkage between solubility and allergenicity. Int Arch Allergy Immunol. 1993;102:160–9. doi: 10.1159/000236567. [DOI] [PubMed] [Google Scholar]
- 21.Madritsch C, Flicker S, Scheiblhofer S, Zafred D, Pavkov-Keller T, Thalhamer J, et al. Recombinant monoclonal human immunoglobulin E to investigate the allergenic activity of major grass pollen allergen Phl p 5. Clin Exp Allergy. 2011;41:270–80. doi: 10.1111/j.1365-2222.2010.03666.x. [DOI] [PubMed] [Google Scholar]
- 22.Brochet X, Lefranc MP, Giudicelli V. IMGT/V-QUEST: the highly customized and integrated system for IG and TR standardized V-J and V-D-J sequence analysis. Nucleic Acids Res. 2008;36:W503–8. doi: 10.1093/nar/gkn316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lefranc MP. Nomenclature of the human immunoglobulin heavy (IGH) genes. Exp Clin Immunogenet. 2001;18:100–16. doi: 10.1159/000049189. [DOI] [PubMed] [Google Scholar]
- 24.Lefranc MP. Nomenclature of the human immunoglobulin kappa (IGK) genes. Exp Clin Immunogenet. 2001;18:161–74. doi: 10.1159/000049195. [DOI] [PubMed] [Google Scholar]
- 25.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–8. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 26.Marks JD, Hoogenboom HR, Bonnert TP, McCafferty J, Griffiths AD, Winter G. By-passing immunization. Human antibodies from V-gene libraries displayed on phage. J Mol Biol. 1991;222:581–97. doi: 10.1016/0022-2836(91)90498-u. [DOI] [PubMed] [Google Scholar]
- 27.Gadermaier E, Flicker S, Aberer W, Egger C, Reider N, Focke M, et al. Analysis of the antibody responses induced by subcutaneous injection immunotherapy with birch and Fagales pollen extracts adsorbed onto aluminum hydroxide. Int Arch Allergy Immunol. 2010;151:17–27. doi: 10.1159/000232567. [DOI] [PubMed] [Google Scholar]
- 28.Flicker S, Vrtala S, Steinberger P, Vangelista L, Bufe A, Petersen A, et al. A human monoclonal IgE antibody defines a highly allergenic fragment of the major timothy grass pollen allergen, Phl p 5: molecular, immunological, and structural characterization of the epitope-containing domain. J Immunol. 2000;165:3849–59. doi: 10.4049/jimmunol.165.7.3849. [DOI] [PubMed] [Google Scholar]
- 29.Focke M, Marth K, Flicker S, Valenta R. Heterogeneity of commercial timothy grass pollen extracts. Clin Exp Allergy. 2008;38:1400–8. doi: 10.1111/j.1365-2222.2008.03031.x. [DOI] [PubMed] [Google Scholar]
- 30.Casset A, Mari A, Purohit A, Resch Y, Weghofer M, Ferrara R, et al. Varying allergen composition and content affects the in vivo allergenic activity of commercial Dermatophagoides pteronyssinus extracts. Int Arch Allergy Immunol. 2012;159:253–62. doi: 10.1159/000337654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barbas CF, III, Burton DR, Scott JK, Silverman GJ. Phage display. A laboratory manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor (NY): 2001. [Google Scholar]
- 32.Laffer S, Valenta R, Vrtala S, Susani M, van Ree R, Kraft D, et al. Complementary DNA cloning of the major allergen Phl p I from timothy grass (Phleum pratense); recombinant Phl p I inhibits IgE binding to group I allergens from eight different grass species. J Allergy Clin Immunol. 1994;94:689–98. doi: 10.1016/0091-6749(94)90176-7. [DOI] [PubMed] [Google Scholar]
- 33.Dolecek C, Vrtala S, Laffer S, Steinberger P, Kraft D, Scheiner O, et al. Molecular characterization of Phl p II, a major timothy grass (Phleum pratense) pollen allergen. FEBS Lett. 1993;335:299–304. doi: 10.1016/0014-5793(93)80406-k. [DOI] [PubMed] [Google Scholar]
- 34.Vrtala S, Sperr WR, Reimitzer I, van Ree R, Laffer S, Muller WD, et al. cDNA cloning of a major allergen from timothy grass (Phleum pratense) pollen; characterization of the recombinant Phl pV allergen. J Immunol. 1993;151:4773–81. [PubMed] [Google Scholar]
- 35.Horst A, Hunzelmann N, Arce S, Herber M, Manz RA, Radbruch A, et al. Detection and characterization of plasma cells in peripheral blood: correlation of IgE1 plasma cell frequency with IgE serum titre. Clin Exp Immunol. 2002;130:370–8. doi: 10.1046/j.1365-2249.2002.02025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eckl-Dorna J, Pree I, Reisinger J, Marth K, Chen K-W, Vrtala S, et al. The majority of allergen-specific IgE in the blood of allergic patients does not originate from blood-derived B cells or plasma cells. Clin Exp Allergy. 2012;42:1347–55. doi: 10.1111/j.1365-2222.2012.04030.x. [DOI] [PubMed] [Google Scholar]
- 37.Christensen LH, Holm J, Lund G, Riise E, Lund K. Several distinct properties of the IgE repertoire determine effector cell degranulation in response to allergen challenge. J Allergy Clin Immunol. 2008;122:298–304. doi: 10.1016/j.jaci.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 38.Collins AM, Sewell WA, Edwards MR. Immunoglobulin gene rearrangement, repertoire diversity, and the allergic response. Pharmacol Ther. 2003;100:157–70. doi: 10.1016/j.pharmthera.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 39.Valenta R, Ball T. Antibody recognition of recombinant allergens and allergen fragments: evidence for a predominantly direct class switch to IgE in allergic patients. In: Vercelli D, editor. IgE regulation: molecular mechanisms. 1997. pp. 225–36. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.