To the Editor:
Measurement of fraction of exhaled nitric oxide (FeNO) is an established tool for the assessment of airway inflammation in asthma. Despite increasing evidence of a poor relationship between the two (1), FeNO continues to be widely regarded as a surrogate marker of sputum eosinophilia (2–4). Furthermore, recent American Thoracic Society (ATS) guidelines have suggested clinically significant cut points for low and high FeNO levels that can be used to indicate the presence or absence of sputum eosinophilia (5). It is known that levels of FeNO may be elevated even in the absence of eosinophilic inflammation and are strongly associated with atopy (6, 7). Furthermore, adult studies have also reported some patients with low FeNO levels despite high sputum eosinophils (8). These discordant relationships make interpretation of FeNO levels, in the individual, more difficult. In view of these published guidelines, we retrospectively analyzed data from two previous studies (9, 10) to assess the validity of these cut points in our population and whether in an individual the relationship between sputum eosinophils and FeNO would be consistent over time and thus aid the interpretation of FeNO results by identifying those in whom FeNO consistently reflects the presence or absence of sputum eosinophilia. Some of these results have been previously reported in the form of an abstract (11).
We analyzed paired sputum eosinophil and FeNO levels in children with a range of asthma severity up to four times per child in a 1-year period. A total of 79 school-aged children were recruited. Fifty-one had severe asthma and were recruited for a previously reported randomized controlled trial comparing a management strategy aimed at controlling sputum eosinophils with a conventional symptom-based strategy (NCT00262340) (9). Twenty-eight had mild to moderate asthma. They were prospectively recruited for a previously described observational study of inflammatory phenotypes (10). Their demographics are shown in Table 1. A total of 197 sputum and FeNO pairs were obtained.
TABLE 1.
DEMOGRAPHICS OF THE SEVERE ASTHMA AND MILD TO MODERATE ASTHMA COHORTS
| Severe Asthma (N = 51) | Mild to Moderate Asthma (N = 28) | P Value | |
|---|---|---|---|
| Age, yr, mean (SD) |
12.9 (2.7) |
11.1 (2.2) |
0.003 |
| Sex, M/F |
25/26 |
13/15 |
0.825 |
| Baseline FEV1, % predicted, mean (SD) |
79.4 (13.8) |
76.9 (16.3) |
0.473 |
| Subjects with atopy, n (%) |
43 (84%) |
22 (79%) |
0.523 |
| Dose of ICS, FP equivalent, μg/d, median (IQR) |
500 (500–1,000) |
200 (100–344) |
<0.001 |
| Subjects prescribed ICS, n (%) |
51 (100%) |
23 (82%) |
0.002 |
| Subjects prescribed maintenance OCS, n (%) |
8 (16%) |
0 |
0.027 |
| Subjects prescribed LABA, n (%) |
51 (100%) |
18 (64%) |
<0.001 |
| Subjects prescribed LRTA, n (%) | 20 (39%) | 3 (11%) | 0.008 |
Definition of abbreviations: F = female; FP = fluticasone propionate; ICS = inhaled corticosteroids; IQR = interquartile range; LABA = long-acting β-agonist; LRTA = leukotriene receptor antagonist; M = male; OCS = oral corticosteroids; SD = standard deviation.
Significant differences are shown in italics.
FeNO was measured at a flow rate of 50 ml/s according to published European Respiratory Society/ATS guidelines (12). Spirometry, sputum induction, and processing were performed as previously described (13–15). Atopy was defined as one or more positive skin-prick tests (wheal ≥ 3 mm) or serum-specific IgE > 0.34 kU/L to a standard panel of six aeroallergens.
Concordance and discordance between sputum eosinophils and FeNO were defined using the FeNO cut points recommended by the recent ATS guidelines to define high and low FeNO levels (5). Concordance: high eosinophils and high FeNO (eos+FeNO+), eosinophils > 2.5%, FeNO ≥ 20 ppb; low eosinophils and low FeNO (eos−FeNO−), eosinophils ≤ 2.5%, FeNO ≤ 35 ppb. Discordance: high eosinophils and low FeNO (eos+FeNO−), eosinophils > 2.5%, FeNO < 20 ppb; low eosinophils and high FeNO (eos−FeNO+), eosinophils ≤ 2.5%, FeNO > 35 ppb. FeNO values between 20 and 35 ppb were always considered concordant whatever the eosinophil value.
The relationship between sputum eosinophils and FeNO was calculated using one pair per subject (the first available). There was a significant positive correlation between sputum eosinophils and FeNO (r = 0.49, P < 0.0001). Receiver operating characteristic curves were constructed to assess the utility of the ATS cut points for predicting sputum eosinophil counts of >2.5% and ≤2.5% (Figure E1 in the online supplement). FeNO levels of >35 ppb gave a sensitivity of 58%, specificity of 73%, positive predictive value of 67%, and negative predictive value of 65% for identifying a sputum eosinophil count of >2.5%. FeNO levels of <20 ppb gave a sensitivity of 51%, specificity of 76%, positive predictive value of 70%, and negative predictive value of 59% for identifying a sputum eosinophil count of ≤2.5%.
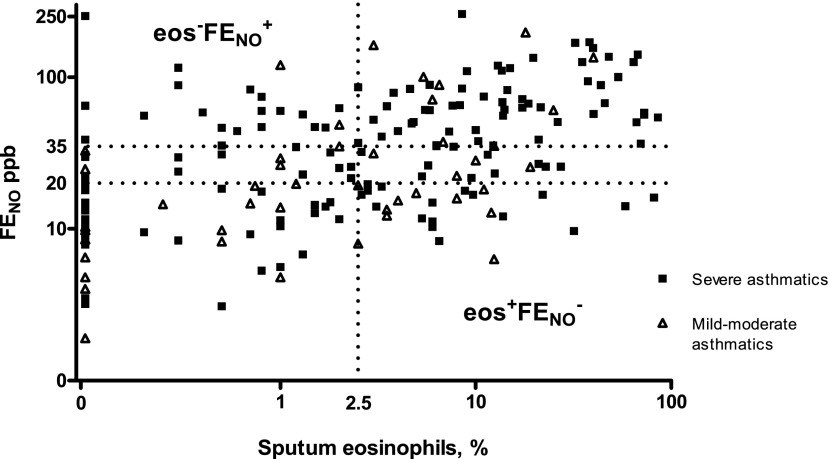
When all 179 samples were considered, 148 samples were concordant (eos+FeNO+ = 77; eos−FeNO− = 71) and 49 (25%) were discordant (eos+FeNO− = 25; eos−FeNO+ = 24) (Figure 1). Fifty-nine children produced two or more sputum samples. Of these, 31 children (53%) were consistently concordant, 24 (41%) had discordant eosinophil/FeNO levels in one sample but concordant levels on at least one other occasion (1 of whom showed discordance in both directions and concordance in a third sample), and only 4 (7%) demonstrated consistently discordant levels. Those who demonstrated the eos−FeNO+ relationship on at least one occasion were significantly more likely to have severe asthma (P = 0.027). There were no other differences between those who were consistently concordant and those who had discordant levels on at least one occasion in terms of age, sex, atopic status, or dose of inhaled corticosteroids (ICS) (Table E1).
Figure 1.
Relationship between sputum eosinophils and fraction of exhaled nitric oxide (FeNO) for all available samples. The dotted line denotes the 2.5% level for sputum eosinophils and the 20 and 35 ppb levels for FeNO. Levels of sputum eosinophils and FeNO have been log-transformed for clarity.
To our knowledge, this is the first study to assess over time the relationship between sputum eosinophils and FeNO. Almost half of all children did not have the expected relationship between sputum eosinophils and FeNO when more than one sputum eosinophil/FeNO pair per subject were included. Previous studies in children have reported high levels of FeNO in the absence of sputum eosinophilia; however, in our study, low levels of FeNO despite high levels of eosinophils were just as likely to occur. There is poor sensitivity and only moderate specificity at the ATS-suggested cut points for identifying the absence of sputum eosinophilia in children (20 ppb) and the presence of sputum eosinophilia (35 ppb). It was not possible in this study to identify those in whom FeNO would be a useful predictor of sputum eosinophilia.
A number of previous studies have reported an association between FeNO and atopy independent of asthma (6, 7), and high FeNO in the absence of eosinophils is well recognized (4, 16). This in part is due to the fact that FeNO is only indirectly related to airway eosinophilia and that NO production can be independent of eosinophilic inflammation (17). The presence of low levels of FeNO despite high eosinophil levels (eos+FeNO−) is much less well described and understood in childhood asthma and yet in our study was just as common as eos−FeNO+. This may be because of ICS causing FeNO suppression but only partial eosinophil suppression or because of stimulation of eosinophils by cytokines not involved in inducing iNOS. This study cannot answer these questions, but it does highlight that using FeNO to predict sputum eosinophilia is equally likely to overestimate and underestimate sputum eosinophilia.
The relationship between sputum eosinophils and FeNO was variable in almost half the children who produced more than one sputum sample. Children with severe asthma were more likely to have a variable relationship, but this in part was due to the fact that we collected more sputum samples per child in this group. It was not otherwise possible to identify a group in whom FeNO would consistently reflect eosinophilia. Each of these biomarkers measures different things and give us information that, although under some circumstances it is largely overlapping, is nonetheless independent of the other. This study cannot answer which is better, and further work is needed to establish the utility of FeNO, independent of sputum eosinophils, particularly as an indicator of steroid responsiveness in children already treated with ICS.
Acknowledgments
Acknowledgment
The authors thank all the children and their parents who participated in the study and the staff in the Paediatric Respiratory Department at the Royal Brompton Hospital who supported this study, in particular Drs. Ian Balfour-Lynn, Mark Rosenthal, and Claire Hogg.
Footnotes
Supported by a grant from the British Lung Foundation, UK, the NIHR Respiratory Disease Biomedical Research Unit at the Royal Brompton and Harefield NHS Foundation Trust, and Imperial College London.
Author Contributions: L.F. performed the study visits for the severe asthma cohort and data analyses for both cohorts and wrote the report. L.T. performed the study visits for the mild to moderate asthma cohorts and participated in writing the report. A.B. and N.W. were the principal investigators and participated in the study design and writing of the report. N.R. performed the sputum cell counts for both cohorts and participated in idea formation and writing of the report.
This letter has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Lemiere C, Ernst P, Olivenstein R, Yamauchi Y, Govindaraju K, Ludwig MS, Martin JG, Hamid Q. Airway inflammation assessed by invasive and noninvasive means in severe asthma: eosinophilic and noneosinophilic phenotypes. J Allergy Clin Immunol. 2006;118:1033–1039. doi: 10.1016/j.jaci.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Berry MA, Shaw DE, Green RH, Brightling CE, Wardlaw AJ, Pavord ID. The use of exhaled nitric oxide concentration to identify eosinophilic airway inflammation: an observational study in adults with asthma. Clin Exp Allergy. 2005;35:1175–1179. doi: 10.1111/j.1365-2222.2005.02314.x. [DOI] [PubMed] [Google Scholar]
- 3.Jatakanon A, Lim S, Kharitonov SA, Chung KF, Barnes PJ. Correlation between exhaled nitric oxide, sputum eosinophils, and methacholine responsiveness in patients with mild asthma. Thorax. 1998;53:91–95. doi: 10.1136/thx.53.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lex C, Ferreira F, Zacharasiewicz A, Nicholson AG, Haslam PL, Wilson NM, Hansel TT, Payne DN, Bush A. Airway eosinophilia in children with severe asthma: predictive values of noninvasive tests. Am J Respir Crit Care Med. 2006;174:1286–1291. doi: 10.1164/rccm.200603-352OC. [DOI] [PubMed] [Google Scholar]
- 5.Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, Olin AC, Plummer AL, Taylor DR. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184:602–615. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franklin PJ, Turner SW, Le Souef PN, Stick SM. Exhaled nitric oxide and asthma: complex interactions between atopy, airway responsiveness, and symptoms in a community population of children. Thorax. 2003;58:1048–1052. doi: 10.1136/thorax.58.12.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scott M, Raza A, Karmaus W, Mitchell F, Grundy J, Kurukulaaratchy RJ, Arshad SH, Roberts G. Influence of atopy and asthma on exhaled nitric oxide in an unselected birth cohort study. Thorax. 2010;65:258–262. doi: 10.1136/thx.2009.125443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nair P, Kjarsgaard M, Armstrong S, Efthimiadis A, O'Byrne PM, Hargreave FE. Nitric oxide in exhaled breath is poorly correlated to sputum eosinophils in patients with prednisone-dependent asthma. J Allergy Clin Immunol. 2010;126:404–406. doi: 10.1016/j.jaci.2010.05.032. [DOI] [PubMed] [Google Scholar]
- 9.Fleming L, Wilson N, Regamey N, Bush A. Use of sputum eosinophil counts to guide management in children with severe asthma. Thorax. 2011;67:193–198. doi: 10.1136/thx.2010.156836. [DOI] [PubMed] [Google Scholar]
- 10.Fleming L, Tsartsali L, Wilson N, Regamey N, Bush A. Sputum inflammatory phenotypes are not stable in children with asthma. Thorax. 2012;67:675–681. doi: 10.1136/thoraxjnl-2011-201064. [DOI] [PubMed] [Google Scholar]
- 11.Fleming L, Tsartsali L, Wilson N, Regamey N, Bossley C, Bush A. Discordance between sputum eosinophils and exhaled nitric oxide in children with asthma [abstract] Thorax. 2008;6:A32–A35. [Google Scholar]
- 12.American Thoracic Society; European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171:912–930. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 13.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 14.Lex C, Payne DN, Zacharasiewicz A, Li AM, Wilson NM, Hansel TT, Bush A. Sputum induction in children with difficult asthma: safety, feasibility, and inflammatory cell pattern. Pediatr Pulmonol. 2005;39:318–324. doi: 10.1002/ppul.20159. [DOI] [PubMed] [Google Scholar]
- 15.Pizzichini E, Pizzichini MM, Efthimiadis A, Evans S, Morris MM, Squillace D, Gleich GJ, Dolovich J, Hargreave FE. Indices of airway inflammation in induced sputum: reproducibility and validity of cell and fluid-phase measurements. Am J Respir Crit Care Med. 1996;154:308–317. doi: 10.1164/ajrccm.154.2.8756799. [DOI] [PubMed] [Google Scholar]
- 16.Zacharasiewicz A, Wilson N, Lex C, Erin EM, Li AM, Hansel T, Khan M, Bush A. Clinical use of noninvasive measurements of airway inflammation in steroid reduction in children. Am J Respir Crit Care Med. 2005;171:1077–1082. doi: 10.1164/rccm.200409-1242OC. [DOI] [PubMed] [Google Scholar]
- 17.Dweik RA, Comhair SA, Gaston B, Thunnissen FB, Farver C, Thomassen MJ, Kavuru M, Hammel J, Abu-Soud HM, Erzurum SC. NO chemical events in the human airway during the immediate and late antigen-induced asthmatic response. Proc Natl Acad Sci USA. 2001;98:2622–2627. doi: 10.1073/pnas.051629498. [DOI] [PMC free article] [PubMed] [Google Scholar]