Abstract
Background
Fatigue, cognitive deficits, and depression are frequently reported but often undertreated symptoms that can profoundly affect daily life in patients with primary brain tumors (PBTs). To evaluate the effects of the psychostimulant modafinil on fatigue, depression, health-related quality of life (HRQOL), and cognitive functioning in PBT patients, we performed a multicenter, double-blind placebo-controlled crossover trial.
Methods
Patients randomly received either 6 weeks of treatment with modafinil (up to 400 mg/day) or 6 weeks with placebo. After a 1-week washout period, the opposite treatment was provided. Assessments took place at baseline and immediately after the first and second condition. Patients completed self-report questionnaires on fatigue (Checklist Individual Strength [CIS]), depression (Center for Epidemiologic Studies Depression Scale [CES-D]), HRQOL (Short-Form Health Survey [SF-36]), and self-perceived cognitive functioning (Medical Outcomes Study [MOS]). They also underwent comprehensive neurocognitive testing.
Results
In total, 37 patients participated. Relative to baseline, patients reported lower fatigue severity (CIS) and better motivation (CIS) in both the modafinil (P = .010 and P = .021, respectively) and the placebo condition (P < .001 and P = .027, respectively). The same held for physical health (SF-36 Physical Component Summary score; P = .001 and P = .008, respectively), working memory (P = .040 and P = .043), and information processing capacity (P = .036 and P = .040). No improvement in depressive symptoms was found in either condition.
Conclusions
Modafinil did not exceed the effects of placebo with respect to symptom management. Patient accrual was slow, and relatively many patients dropped out during the trial, due mostly to side effects. Other, preferably nonpharmacologic intervention studies should be considered to improve symptom management of PBT patients.
Keywords: brain tumor, cognitive functioning, fatigue, HRQOL, modafinil, mood
Fatigue, cognitive deficits, and depression are frequently reported symptoms in patients with primary brain tumors (PBTs).1,2 Over 80% of PBT patients treated with cranial irradiation experience some degree of somnolence, defined as symptoms of drowsiness, lethargy, and fatigue.3,4 Unrelated to radiation treatment, 39% of long-term survivors of low-grade glioma report severe symptoms of fatigue.5 In addition to fatigue, cognitive impairments are experienced by 80% of PBT patients.6 The prevalence of depression in PBT patients ranges from 15% to 27%.7 All of these symptoms have a large impact on the everyday life of patients and may lead to a significant decrease in health-related quality of life (HRQOL).8 It is suggested that effective treatment of these symptoms may increase HRQOL,9 although an optimal strategy to reach this goal has not been defined yet.
Modafinil (2-benzhydrylsulfinylethanamide) is a wakefulness-promoting agent that targets fatigue, cognitive functioning, and mood. Although categorized as a psychostimulant, it differs from amphetamine in both physiological and behavioral aspects. It is highly selective for the central nervous system, has a lower abuse potential, and poses a lower risk for adverse effects on organ systems.10–14 The precise mechanism of action of modafinil is unknown, but it is theorized to act in a localized manner, utilizing hypocretin, histamine, epinephrine, γ-aminobutyric acid (GABA), and glutamate.10 It enhances catecholaminergic signaling and decreases GABA release, primarily at the level of the anterior hypothalamus and locus coeruleus.11,15 It has been shown to bind directly to dopamine and norepinephrine receptors.14 Forty percent to 65% of modafinil is readily absorbed, with only 10% of the drug being excreted in the urine in unchanged form.14,16 Modafinil appears to target the sleep-wake centers of the brain more specifically than other psychostimulants.11,12 With a half-life of 12–15 h,14,16 modafinil requires only a single daily dose for efficacy. Lower dosages (50–200 mg/day) are generally prescribed for fatigue and concentration problems, while higher dosages (up to 600 mg/day) are used for daytime sleepiness in narcolepsy.17 Although originally marketed for the latter,18,19 modafinil has recently been found to be of use in improving fatigue,20–27 mood,28–31 and overall HRQOL32 in several study populations. Moreover, there is evidence that it may even enhance cognitive functioning.14,33–36 In healthy adults, working memory, recognition memory, sustained attention, and cognitive control are improved after modafinil.14,36 In children and adolescents with attention deficit hyperactivity disorder (ADHD), improved attention and response inhibition have been reported, while in several adult psychiatric populations, modafinil appears to improve cognitive functions that depend on prefrontal structures.14 In patients with schizophrenia, especially working memory and problem-solving abilities improved.34
Despite these potentially beneficial effects, notably little is known about the effects of modafinil on symptom management in PBT patients. Compared with modafinil, methylphenidate is more similar to amphetamine in its pharmacologic profile. It inhibits dopamine and norepinephrine uptake and increases concentrations of these neurotransmitters in the brain.37 It targets primarily the prefrontal cortex.38 Immediate-release methylphenidate has a relatively short half-life, which necessitates 2 or 3 doses a day, while sustained-release methylphenidate requires only a single dose per day.39 In an open-label pilot study, 24 PBT patients were randomly assigned to 4 weeks of modafinil (200 mg q.d.), immediate-release methylphenidate (20 mg b.i.d.), or sustained-release methylphenidate (18 mg q.d.).39 Comparison of combined immediate- and sustained-release methylphenidate with modafinil showed the latter to have significant positive effects on information-processing speed and executive functioning requiring divided attention. Additionally, a general beneficial effect of both methylphenidate and modafinil on fatigue, mood, and HRQOL was found.39 Another pilot study, presented at the 2006 annual meeting of the American Society of Clinical Oncology, that had a double-blind dose-controlled randomization of 200 or 400 mg/day of modafinil for 3 weeks, a washout period of 1 week, and an open label extension of 8 weeks, also reported decreased fatigue and improvements in cognitive functioning and mood in PBT patients, although final results have not been published yet.40 Presently, we performed a multicenter, double-blind placebo-controlled crossover trial to evaluate the effects of modafinil on fatigue in PBT patients. As fatigue may interact with functional activities and HRQOL, and considering the encouraging study results described above, the effects of modafinil on cognition, mood, and overall HRQOL were also evaluated.
Materials and Methods
Participants
We identified patients who visited the outpatient departments of 3 tertiary referral centers for neuro-oncology (VU University Medical Center, Amsterdam; Academic Medical Center, Amsterdam; and Medical Center Haaglanden, the Hague) between January 2009 and December 2011. Participants were eligible if they (i) were ≥ 18 years old, (ii) had been diagnosed with a histologically confirmed glioma or meningioma (collectively called PBT throughout this report), and (iii) had no signs of tumor recurrence in the last 6 months. In addition, they were invited to participate only if they reported a heightened experience of fatigue (score >27 on the Checklist Individual Strength [CIS]41), as this was our primary outcome measure. Patients were excluded if (i) they had a history of psychiatric disease or symptoms, including depressive disorders; (ii) adverse interactions between modafinil and other prescribed medications were expected (eg, decreased effectiveness of oral contraceptives, levonorgestrel-releasing intrauterine systems and antidepressants, fluctuations in the effectiveness of antiepileptic drugs acting on similar enzymes); (iii) they were unable to communicate in Dutch. A priori sample size calculations based on statistical power (1-β) of 0.80, r = 0.30 and α = 0.05, yielded 64 patients to be included in the study. The study was approved by the institutional review boards of all participating centers and was registered at http://www.clinicaltrialsregister.eu/ with EudraCT number 2007-003102-10. All patients provided written informed consent.
Procedure
Eligible patients were introduced to the trial by their treating physician either in person or by mail. The researchers then contacted the patients by telephone to inquire if they were interested in participating. As fatigue was our primary outcome measure, interested patients were asked to fill out the CIS to assess the severity of their complaints and their suitability for participation. After obtaining informed consent, sociodemographic and clinical data were collected from patients' medical records. A pharmacy randomization system was used to assign participants to either the modafinil or the placebo condition, while patients, treating physicians, and researchers were blind to treatment allocation. Patients received 6 weeks of treatment with either modafinil or placebo starting with a 100-mg dose upon waking and at lunch (200 mg/day in total). After the first week, the dose was doubled to 400 mg/day. After treatment period 1, a washout period of 1 week was applied. Hereafter, the opposite treatment was provided during treatment period 2 (ie, those who first received modafinil now received placebo and vice versa). During the trial, patients were asked not to take benzodiazepines, as these might interfere with modafinil. Assessments took place at baseline (T1), immediately after treatment period 1 (after 6 wk; T2), and immediately after treatment period 2 (after 12 wk; T3). These assessments included self-report questionnaires and neuropsychological assessment, as well as physical and neurological examination carried out by a physician. If patients experienced adverse effects, they were allowed to decrease the medication to the lower dose (200 mg/day) or to stop participating in the trial after consulting the physician involved in the trial.
Patient-Reported Outcomes
Patients were asked to complete self-report questionnaires on measures of fatigue as the primary outcome measure and depression, HRQOL, and subjective cognitive functioning as secondary outcome measures.
Checklist Individual Strength (CIS).41
Fatigue was assessed with this multidimensional scale; each item was scored on a 7-point Likert scale. The CIS includes 4 aspects of fatigue (fatigue severity, concentration problems, reduced motivation, and reduced activity) and a total score. High scores indicate a high level of fatigue, a high level of concentration problems, low motivation, and a low activity level. Based on normative controls, a total score between 27 and 35 indicates a heightened experience of fatigue.
Center for Epidemiologic Studies Depression Scale (CES-D).42
This 20-item questionnaire was used to assess symptoms of depression. Participants were asked to indicate on a 4-point scale how often they felt that a statement was applicable to their situation during the past week. Scores range between 0 and 60, with higher scores indicating more feelings of depression. In the general population, respondents with a total score of ≥16 are considered depressed.
Medical Outcomes Study Short-Form Health Survey (SF-36).43
This HRQOL survey is composed of 36 items that are organized into 8 multi-item scales assessing (i) physical functioning, (ii) limitations in role functioning due to physical problems, (iii) limitations in role functioning due to emotional problems, (iv) pain, (v) vitality, (vi) social functioning, (vii) mental health, and (viii) general health perceptions. From these scales, 2 higher-order summary scores can be calculated: (i) the Physical Component Summary (PCS), measuring physical health and (ii) the Mental Component Summary (MCS), measuring mental health. In a normative sample from the general population, PCS and MCS scores have a mean of 50 with a standard deviation of 10.
MOS subjective cognitive functioning scale.44
This 6-item scale assesses everyday problems in cognitive functioning, including difficulty with reasoning and problem solving, slowed reaction time, and problems with concentration (range, 1–6).
Objective Cognitive Functioning
Using an extensive battery of neuropsychological tests, objective cognitive functioning was assessed. Tests included measures of verbal memory (Auditory Verbal Learning Test45), working memory (Memory Comparison Test46), attentional functioning (Stroop Color Word Test47), information processing (Letter Digit Substitution Test48), executive functioning (Concept Shifting Test,49 Categorical Word Fluency Test50), and psychomotor speed (Concept Shifting Test, Letter Digit Substitution Test).
Statistical Analysis
All statistical analyses were performed with SPSS software version 20. Standard scoring rules were applied to convert the data from the questionnaires. Mean imputation was used to handle missing values within completed questionnaires or neuropsychological assessments. To assess a change in cognitive functioning as accurately as possible, cognitive test scores were converted to z-scores using the means and SDs of the patients' scores at baseline. To achieve data reduction, 6 cognitive domains were formed (verbal memory, working memory, attentional functioning, information processing, executive functioning, and psychomotor speed; Table 3). Construction of these cognitive domains was based on a principal component analysis using Varimax rotation with Kaiser normalization performed on the z-scores of an extensive study among healthy subjects into the biological predictors of cognitive aging.51 To test whether the outcome measures were normally distributed, Kolmogorov–Smirnov tests were used. Since none of the outcome measures were normally distributed, Wilcoxon signed-rank tests were used to determine differences within patients in fatigue, depression, the PCS and MCS scales of the SF-36 (HRQOL), and subjective and objective cognitive functioning. Given the small sample size, no corrections for multiple statistical testing were applied. A P-value of ≤.05 was considered statistically significant; P ≤ .10 was considered a trend.
Table 3.
Overview of cognitive tests administered and results of comparisons between the baseline assessment and the modafinil and placebo conditions for cognitive functioning
| Baseline n = 36 M (SD) | Modafinil n = 25 M (SD) | Placebo n = 28 M (SD) | Baseline vs Modafinil z (P) | Baseline vs Placebo z (P) | Modafinil vs Placebo z (P) |
|---|---|---|---|---|---|
| Verbal memory | |||||
| Auditory Verbal Learning Test (AVLT)45 | |||||
| 0.00 (0.71) | 0.14 (0.86) | −0.12 (1.02) | −1.63 (.104) | −0.29 (.767) | −1.60 (.110) |
| Working memory | |||||
| Memory Comparison Test (MCT)46 | |||||
| 0.00 (0.86) | 0.24 (0.93) | 0.17 (0.90) | −2.06 (.040*) | −2.03 (.043*) | −1.26 (.209) |
| Attentional functioning | |||||
| Stroop Color Word Test47 | |||||
| 0.00 (1.04) | 0.15 (0.94) | 0.18 (0.75) | −1.28 (.201) | −2.44 (.015*) | −2.49 (.013*) |
| Information processing | |||||
| Letter Digit Substitution Test (LDST)48 | |||||
| 0.00 (0.94) | 0.36 (1.18) | 0.19 (1.12) | −2.01 (.036*) | −2.05 (.040*) | −0.24 (.808) |
| Executive functioning | |||||
| Concept Shifting Test (CST)49 | |||||
| Categorical Word Fluency Test (CWFT)50 | |||||
| 0.00 (0.80) | 0.19 (0.78) | 0.05 (0.94) | −1.17 (.242) | −1.25 (.210) | −0.03 (.977) |
| Psychomotor speed | |||||
| Concept Shifting Test40 | |||||
| Letter Digit Substitution Test48 | |||||
| 0.02 (0.61) | 0.26 (0.56) | 0.16 (0.53) | −1.60 (1.09) | −1.48 (.139) | −0.54 (.587) |
| Subjective cognitive functioning | |||||
| 31.79 (17.62) | 29.11 (16.04) | 27.49 (18.11) | −0.79 (.428) | −1.91 (.056) | −1.33 (.184) |
The cognitive domains included the following assessments: verbal memory: AVLT 1, AVLT recall, AVLT recognition, AVLT delta, AVLT total; working memory: MCT %, MCT 1, MCT 2, MCT 3, MCT4; attentional functioning: Stroop card 1, Stroop card 2, Stroop card 3; information processing: LDST reading, LDST writing; executive functioning: CST A, CST B, CST C, CWFT animals (60 s); psychomotor speed: CST 0, LDST delta.
*P < .05.
Results
Patient Characteristics
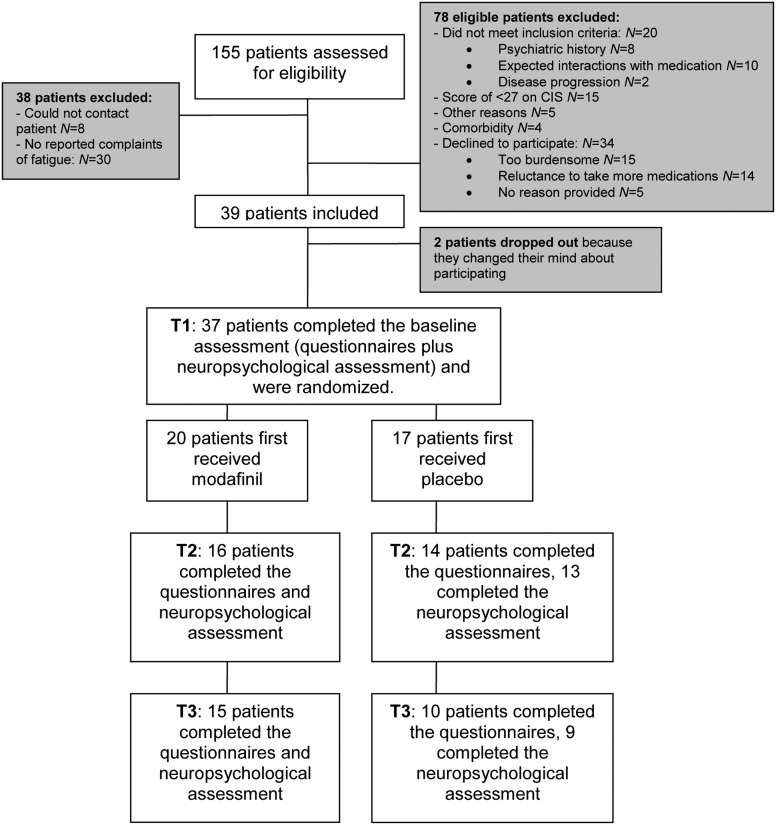
Figure 1 shows details of the participant flow. In total, 155 PBT patients were assessed for eligibility. A total of 39 (25.2%) patients met the inclusion criteria, agreed to participate, and subsequently signed the informed consent form. Thirty-seven patients were assigned randomly to a treatment condition, whereas 2 patients dropped out before randomization because they were no longer willing to participate. During the trial, overall 12 patients dropped out: 7 dropped out between T1 and T2, and 5 dropped out between T2 and T3. Reasons for not completing the trial were discontinuation of medication due to side effects (n = 7; patients reported a tingling sensation, nausea, vertigo, anxiety, depression, and feeling fidgety), missed follow-up (n = 4), and disease progression (n = 1). Table 1 presents sociodemographic and clinical characteristics of the study sample. The mean age of participants was 42.2 years, and more women than men participated (62.2% vs 37.8%). Most participants had a low-grade glioma (37.8%), and the mean time since diagnosis was 49.5 months.
Fig. 1.
Participant flow.
Table 1.
Demographic and clinical characteristics of the study sample
| Participants (n= 37) | |
|---|---|
| Age, y M (SD) | 48.16 (12.02) |
| Gender n (%) | |
| Male | 14 (37.8%) |
| Female | 23 (62.2%) |
| Educational level n (%) | |
| Low | 10 (27.0%) |
| Medium | 15 (40.5%) |
| High | 11 (29.7%) |
| Other | 1 (2.7%) |
| Marital status n (%) | |
| Single | 7 (18.9%) |
| Married or living together | 25 (67.6%) |
| Divorced | 2 (5.4%) |
| Widow(er) | 3 (8.1%) |
| Tumor grade n (%) | |
| Grade I | 15 (40.5%) |
| Grade II | 10 (27.0%) |
| Grade III | 7 (18.9%) |
| Grade IV | 5 (13.5%) |
| Tumor type n (%) | |
| Meningioma | 12 (32.4%) |
| Low-grade glioma | 14 (37.8%) |
| High-grade glioma | 11 (29.7%) |
| Tumor location n (%) | |
| Frontal | 13 (35.1%) |
| Temporal | 5 (13.5%) |
| Parietal | 6 (16.2%) |
| Occipital | 2 (5.4%) |
| Mixed | 6 (16.2%) |
| Other | 5 (13.5%) |
| Tumor lateralization n (%) | |
| Left | 15 (40.5%) |
| Right | 20 (54.1%) |
| Bilateral | 2 (5.4%) |
| Epilepsy n (%) | |
| Yes | 12 (32.4%) |
| No | 25 (67.6%) |
| Neurosurgical intervention n (%) | |
| Resection | 33 (89.2%) |
| Biopsy | 2 (5.4%) |
| None | 2 (5.4%) |
| Months since time of diagnosis M (range) | 49.46 (16–197) |
| <36 mo n (%) | 18 (48.6%) |
| >36 mo n (%) | 19 (51.4%) |
| Medication use prior to trial n (%) | |
| Antiepileptic drugs | 20 (54.1%) |
| Antihypertensive drugs | 8 (21.6%) |
| Cholesterol inhibitors | 4 (10.8%) |
| Anticoagulants | 3 (8.1%) |
| Analgesics (mild opioids) | 2 (5.4%) |
| Analgesics (non-opioids) | 1 (2.7%) |
| Antiallergic drugs | 2 (5.4%) |
| Bladder control drugs | 2 (5.4%) |
| Stomach protectors | 2 (5.4%) |
| Laxatives | 2 (5.4%) |
| Benzodiazepines | 2 (5.4%) |
| Anorexiants | 1 (2.7%) |
| Progestagens | 1 (2.7%) |
| Oral contraceptives | 1 (2.7%) |
| Change in medication use during trial n (%) | |
| Analgesics (non-opioids) | 4 (10.8%) |
| Antibiotics | 3 (8.1%) |
| Flu vaccine | 1 (2.7%) |
| Antidiabetics | 1 (2.7%) |
| Antiemetics | 1 (2.7%) |
| Radiotherapy (ever) n (%) | |
| Yes | 16 (43.2%)* |
| No | 21 (56.8%) |
| Chemotherapy (ever) n (%) | |
| Yes | 8 (21.6%)* |
| No | 29 (78.4%) |
| Progressive disease during intervention n (%) | |
| Yes | 2 (5.4%) |
| No | 35 (94.6%) |
*One participant had received radiotherapy not involving the CNS and chemotherapy for breast cancer, not for PBT. The patient did not have brain metastases.
Patient-Reported Outcomes
Fatigue
As can be seen in Table 2, scores on the CIS scales for fatigue severity and reduced motivation were significantly lower after treatment with both modafinil (P = .010 and P = .021, respectively) and placebo (P < .001 and P = .027, respectively) compared with the baseline assessment. The extent of decrease in fatigue as measured by these scales, however, did not significantly differ between the 2 conditions. Although not statistically significant, a trend can be seen for an improvement in reduced activity in the placebo condition (P = .093) compared with baseline. On the total CIS score, patients' symptoms were alleviated in both the modafinil and placebo conditions (P = .005 and P = .001, respectively), while scores between the experimental conditions did not differ.
Table 2.
Results of comparisons between the baseline assessment and the modafinil and placebo conditions for fatigue (CIS)
| Bas n = 36 M (SD) | Mod n = 26 M (SD) | Pla n = 29 M (SD) | Bas vs Mod z (P) | Bas vs Pla z (P) | Mod vs Pla z (P) |
|---|---|---|---|---|---|
| Concentration problems | |||||
| 20.75 (9.18) | 18.85 (7.90) | 19.91 (8.36) | −1.10 (.270) | −1.15 (.252) | −0.97 (.331) |
| Reduced motivation | |||||
| 17.00 (5.40) | 14.38 (5.72) | 14.86 (6.72) | −2.31 (.021*) | −2.22 (.027*) | −3.38 (.702) |
| Reduced activity | |||||
| 13.49 (4.87) | 11.58 (5.34) | 12.59 (5.17) | −1.37 (.170) | −1.68 (.093) | −0.43 (.671) |
| Fatigue severity | |||||
| 41.72 (9.22) | 34.92 (12.04) | 35.14 (10.86) | −2.56 (.010*) | −3.72 (<.001*) | −0.75 (.456) |
| Total score | |||||
| 93.80 (20.16) | 79.73 (26.45) | 82.48 (26.26) | −2.83 (.005*) | −3.35 (.001*) | −1.01 (.313) |
Abbreviations: Bas, baseline; Mod, modafinil; Pla, placebo.
*P < .05.
Depression
Scores on the CES-D did not differ significantly between treatment conditions. At baseline, reported scores were only slightly higher (M = 15.86, SD = 9.49) than in both the modafinil and placebo conditions (M = 14.16, SD = 9.06 and M = 13.97, SD = 9.44, respectively; P = n.s.). All mean scores were within the nondepressed range (ie, <16).
Health-related quality of life
Patients' physical health as measured by the PCS scale improved significantly after treatment with both modafinil (M = 46.05, SD = 7.96) and placebo (M = 44.71, SD = 9.13) compared with baseline (M = 40.60, SD = 7.58; P = .001 and P = .008, respectively). No other significant differences were observed for either physical or mental health scales.
Subjective cognitive complaints
A trend was observed where participants tended to report higher self-perceived cognitive functioning after treatment with placebo compared with baseline (P = .056; Table 3).
Objective Cognitive Functioning
Table 3 shows that patients improved after treatment with both modafinil and placebo compared with baseline for both the working memory domain (P = .040 and P = .043, respectively) and the information-processing domain (P = .036 and P = .040, respectively). Scores did not differ between the experimental conditions. For attentional functioning, scores improved significantly after the placebo treatment compared with baseline (P = .015) and after modafinil treatment (P = .013).
Discussion
Contrary to our hypotheses, we did not find beneficial effects of modafinil on fatigue, depression, overall HRQOL, or cognitive functioning in comparison with placebo. Patients reported a decrease in fatigue severity, an improvement in reduced motivation, and a better overall fatigue score in both the modafinil and the placebo condition compared with baseline, indicating a placebo effect. Counterintuitively, a trend was found for an improvement in the reduced activity scale after the placebo condition, but not after the modafinil condition. For depression, overall HRQOL, and cognitive functioning, we found no difference between the treatment conditions.
In different study samples of patients with various neurological conditions (Charcot-Marie-Tooth disease, fibromyalgia, amyotrophic lateral sclerosis, multiple sclerosis, schizophrenia, narcolepsy), beneficial effects of modafinil for symptoms of fatigue, HRQOL, and cognitive functioning have been shown.21–24,26,27,32,35 However, the majority of these studies were not placebo controlled, and study samples were often relatively small. Although the literature on modafinil for symptom management in PBT patients is scarce, 2 studies have been reported and both showed positive results. Gehring et al.39 reported beneficial effects of both modafinil and methylphenidate for patient-reported measures of fatigue, mood, and HRQOL as well as for objective neuropsychological testing. However, no differences between treatment arms over time were reported for fatigue, mood, and HRQOL, and findings with regard to cognitive functioning were inconsistent, indicating that nonspecific treatment effects may have played a role. In an unpublished pilot study, Kaleita et al.40 mention improvement in fatigue, mood, and cognitive functioning in PBT patients randomized to either a 200-mg or a 400-mg modafinil dose. However, since the results of Kaleita et al. remain unpublished, we cannot properly compare their methodology to ours. Importantly, in both studies, no placebo condition was used. As psychological mechanisms such as the presence of expectations prove to be powerful aspects of the experienced effects of medication use,52 it seems likely that the beneficial effects reported in these previous studies could at least, in part, be attributable to a placebo effect.
Despite this methodological advantage of the present study, there are also significant limitations. In spite of great efforts in recruiting patients, accrual was difficult, and ultimately, we did not reach the required sample size. As shown in Fig. 1, almost half of the eligible patients declined participation for several reasons, such as expecting participation to be too burdensome or declining to take more drugs than was strictly necessary. Furthermore, during the trial, a considerable number of patients (32%) dropped out for various reasons, although the majority of these (58.3%) decided to discontinue medication because of side effects. Interestingly, this also included patients in the placebo condition who should not have experienced any side effects. Although it is not uncommon to experience side effects with placebo use,53 the fact that these patients dropped out of the trial does suggest that possibly pharmaceutical trials for symptom management are less suitable for PBT patients. Another study limitation is the heterogeneity of the patient population. We included patients with meningiomas as well as gliomas, while these diseases are not equal in many respects (eg, nature of the disease, symptoms and treatment, prognosis). Although there is no indication that modafinil would be more effective in one subgroup of PBT patients than in another, it would have been preferable to study the effects of modafinil in a larger, more homogeneous group of patients.
Despite these limitations, we did find clear differences between the baseline assessment and outcome after both treatment conditions. This suggests that our lack of evidence for beneficial effects of modafinil for symptom management of PBT patients cannot be attributed to the small sample size. Rather, the participants in our sample did not experience better results from modafinil than from placebo. Given the apparent reluctance of a relatively large proportion of PBT patients to participate in pharmacologic trials for symptom management, as is shown in the present study as well as in the study by Gehring et al.,39 and given the high percentage of patients suffering from fatigue, cognitive deficits, and mood disorders, other intervention studies should be considered. Concerning fatigue, Armstrong and Gilbert54 provided an interesting overview of the guidelines of the National Comprehensive Cancer Network in relation to PBT patients. Although many nonpharmacologic interventions to treat fatigue (eg, activity enhancement, physically based therapy, psychosocial interventions, nutritional consultation, cognitive behavioral therapy for sleep hygiene) have been proven to be effective in the general cancer patient population, randomized controlled trials in PBT patients have not yet been performed. Concerning cognitive deficits, a cognitive function training program has been proven to be effective in PBT patients.55 For depressive symptoms, nonpharmacologic intervention studies in this patient population are also still scarce, although many therapeutic interventions already exist for different cancer patient populations.56 Because of their unique symptom pattern with neurological and cognitive sequelae, PBT patients are not easily comparable to other cancer patient groups. Therefore, the efficacy of the majority of interventions remains to be evaluated in this particular patient population. We recommend the development of nonpharmacologic interventions aimed specifically at symptom management in PBT patients, which may alleviate their symptom burden substantially and could improve their HRQOL significantly.
Acknowledgments
This study was supported by an unrestricted grant from Fonds NutsOhra. Special gratitude is owed to the study participants, to Fiore Giuseppin for her help in data analysis, to Eefje Sizoo and Femke Froklage for their availability as stand-in research physicians, and to Claudia Nijboer, Lies Braam, Alieke Weerdesteijn, Hanneke Zwinkels, Sanne Driessen, and Karen Hilverda for their help in recruiting patients.
Conflict of interest statement. None declared.
References
- 1.Taphoorn MJB, Klein M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol. 2004;3:159–168. doi: 10.1016/S1474-4422(04)00680-5. [DOI] [PubMed] [Google Scholar]
- 2.Weitzner MA. Psychosocial and neuropsychiatric aspects of patients with primary brain tumors. Cancer Invest. 1999;17:285–291. doi: 10.3109/07357909909040599. [DOI] [PubMed] [Google Scholar]
- 3.Powell C, Guerrero D, Sardell S, et al. Somnolence syndrome in patients receiving radical radiotherapy for primary brain tumours: a prospective study. Radiother Oncol. 2011;100:131–136. doi: 10.1016/j.radonc.2011.06.028. [DOI] [PubMed] [Google Scholar]
- 4.Faithful S, Brada M. Somnolence syndrome in adults following cranial irradiation for primary brain tumours. Clinical Oncol. 1998;10:250–254. doi: 10.1016/s0936-6555(98)80011-3. [DOI] [PubMed] [Google Scholar]
- 5.Struik K, Klein M, Heimans JJ, et al. Fatigue in low-grade glioma. J Neurooncol. 2008;92:73–78. doi: 10.1007/s11060-008-9738-7. [DOI] [PubMed] [Google Scholar]
- 6.Mukand JA, Blackinton DD, Crincoli MG, Lee JJ, Santos BB. Incidence of neurologic deficits and rehabilitation of patients with brain tumors. Am J Phys Med Rehabil. 2001;80:346–350. doi: 10.1097/00002060-200105000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Rooney AG, Carson A, Grant R. Depression in cerebral glioma patients: a systematic review of observational studies. J Nat Cancer Inst. 2011;103:61–76. doi: 10.1093/jnci/djq458. [DOI] [PubMed] [Google Scholar]
- 8.Pelletier G, Verhoef MJ, Khatri N, Hagen N. Quality of life in brain tumor patients: the relative contributions of depression, fatigue, emotional distress, and existential issues. J Neurooncol. 2002;57:41–49. doi: 10.1023/a:1015728825642. [DOI] [PubMed] [Google Scholar]
- 9.Shaw EG, Rosdhal R, D'Agostino RB, et al. Phase II study of donepezil in irradiated brain tumor patients: effect on cognitive function, mood, and quality of life. J Clin Oncol. 2006;24:1415–1420. doi: 10.1200/JCO.2005.03.3001. [DOI] [PubMed] [Google Scholar]
- 10.Ballon JS, Feifel D. A systematic review of modafinil: potential clinical uses and mechanisms of action. J Clin Psychiatry. 2006;67:554–566. doi: 10.4088/jcp.v67n0406. [DOI] [PubMed] [Google Scholar]
- 11.Lin JS, Hou Y, Jouvet M. Potential brain neuronal targets for amphetamine-, methylphenidate- and modafinil-induced wakefulness, as evidenced by c-fos immunocytochemistry in the cat. Proc Natl Acad Sci U S A. 1996;93:14128–14133. doi: 10.1073/pnas.93.24.14128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hou RH, Freeman C, Langley RW, Szabadi E, Bradshaw CM. Does modafinil activate the locus coeruleus in man? Comparison of modafinil and clonidine on arousal and autonomic functions in human volunteers. Psychopharmacology. 2005;181:537–549. doi: 10.1007/s00213-005-0013-8. [DOI] [PubMed] [Google Scholar]
- 13.Myrick H, Malcolm R, Taylor B, LaRowe S. Modafinil: preclinical, clinical, and post-marketing surveillance—a review of abuse liability issues. Ann Clin Psychiatry. 2004;16:101–109. doi: 10.1080/10401230490453743. [DOI] [PubMed] [Google Scholar]
- 14.Minzenberg MJ, Carter CS. Modafinil: a review of neurochemical actions and effects on cognition. Neuropsychopharmacology. 2008;33:1477–1502. doi: 10.1038/sj.npp.1301534. [DOI] [PubMed] [Google Scholar]
- 15.Lundorff LE, Jonsson BH, Sjogren P. Modafinil for attentional and psychomotor dysfunction in advanced cancer: a double-blind, randomised, cross-over trial. Palliat Med. 2009;23:731–738. doi: 10.1177/0269216309106872. [DOI] [PubMed] [Google Scholar]
- 16.Wong YN, King SP, Simcoe D, et al. Open-label, single-dose pharmacokinetic study of modafinil tablets: influence of age and gender in normal subjects. J Clin Pharmacol. 1999;39:281–288. [PubMed] [Google Scholar]
- 17.Harris JD. Fatigue in chronically ill patients. Curr Opin Support Palliat Care. 2008;2:180–186. doi: 10.1097/SPC.0b013e32830baed0. [DOI] [PubMed] [Google Scholar]
- 18.Besset A, Chetrit M, Carlander B, Billiard M. Use of modafinil in the treatment of narcolepsy: a long term follow-up study. Neurophysiol Clin. 1996;26:60–66. doi: 10.1016/0987-7053(96)81535-8. [DOI] [PubMed] [Google Scholar]
- 19.Boivin DB, Montplaisir J, Petit D, Lambert C, Lubin S. Effects of modafinil on symptomatology of human narcolepsy. Clin Neuropharmacol. 1993;16:46–53. doi: 10.1097/00002826-199302000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Jones DEJ, Newton JL. An open study of modafinil for the treatment of daytime somnolence and fatigue in primary biliary cirrhosis. Aliment Pharmacol Ther. 2007;25:471–476. doi: 10.1111/j.1365-2036.2006.03223.x. [DOI] [PubMed] [Google Scholar]
- 21.Carter GT, Han JJ, Mayadev A, Weiss MD. Modafinil reduces fatigue in Charcot-Marie-Tooth disease type 1a: a case series. Am J Hosp Palliat Care. 2006;23:412–416. doi: 10.1177/1049909106292169. [DOI] [PubMed] [Google Scholar]
- 22.Pachas WN. Modafinil for fatigue in fibromyalgia. J Clin Rheumatol. 2003;9:282–285. doi: 10.1097/01.RHU.0000081255.66597.df. [DOI] [PubMed] [Google Scholar]
- 23.Carter GT, Weiss MD, Lou JS, et al. Modafinil to treat fatigue in amyotrophic lateral sclerosis: an open label pilot study. Am J Hosp Palliat Care. 2005;22:55–59. doi: 10.1177/104990910502200112. [DOI] [PubMed] [Google Scholar]
- 24.Rabkin JG, Gordon PH, McElhiney M, Rabkin R, Chew S, Mitsumoto H. Modafinil treatment of fatigue in patients with ALS: a placebo-controlled study. Muscle Nerve. 2009;39:297–303. doi: 10.1002/mus.21245. [DOI] [PubMed] [Google Scholar]
- 25.Rabkin JG, McElhiney MC, Rabkin R, McGrath PJ. Modafinil treatment for fatigue in HIV/AIDS: a randomized placebo-controlled study. J Clin Psychiatry. 2010;71:707–715. doi: 10.4088/JCP.09m05171bro. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zifko UA, Rupp M, Schwarz S, Zipko HT, Maida EM. Modafinil in treatment of fatigue in multiple sclerosis: results of an open-label study. J Neurol. 2002;249:983–987. doi: 10.1007/s00415-002-0765-6. [DOI] [PubMed] [Google Scholar]
- 27.Niepel G, Bibani RH, Vilisaar J, et al. Association of a deficit of arousal with fatigue in multiple sclerosis: effect of modafinil. Neuropharmacology. 2012;64:380–388. doi: 10.1016/j.neuropharm.2012.06.036. [DOI] [PubMed] [Google Scholar]
- 28.Taneja I, Haman K, Shelton RC, Robertson D. A randomized, double-blind, crossover trial of modafinil on mood. J Clin Psychopharmacol. 2007;27:76–79. doi: 10.1097/jcp.0b013e31802eb7ea. [DOI] [PubMed] [Google Scholar]
- 29.Price CS, Taylor FB. A retrospective chart review of the effects of modafinil on depression as monotherapy and as adjunctive therapy. Depress Anxiety. 2005;21:149–153. doi: 10.1002/da.20075. [DOI] [PubMed] [Google Scholar]
- 30.Lundt L. Modafinil treatment in patients with seasonal affective disorder/winter depression: an open-label pilot study. J Affect Disord. 2004;81:173–178. doi: 10.1016/S0165-0327(03)00162-9. [DOI] [PubMed] [Google Scholar]
- 31.Joos L, Docx L, Schmaal L, Sabbe BGC, Dom G. Modafinil bij psychiatrische aandoeningen: de veelbelovende status opnieuw bekeken [Modafinil in psychiatric conditions: the promising status revisited] Tijdschrift voor Psychiatrie. 2010;52:763–773. [PubMed] [Google Scholar]
- 32.Beusterien KM, Rogers AE, Walsleben JA, et al. Health-related quality of life effects of modafinil for treatment of narcolepsy. Sleep. 1999;22:757–765. doi: 10.1093/sleep/22.6.757. [DOI] [PubMed] [Google Scholar]
- 33.McElhiney M, Rabkin J, Gorp van W, Rabkin R. Modafinil effects on cognitive function in HIV+ patients treated for fatigue: a placebo controlled study. J Clin Exp Neuropsychol. 2010;32:474–480. doi: 10.1080/13803390903201769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scoriels L, Jones PB, Sahakian BJ. Modafinil effects on cognition and emotion in schizophrenia and its neurochemical modulation in the brain. Neuropharmacology. 2012;64:168–184. doi: 10.1016/j.neuropharm.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 35.Hunter MD, Ganesan V, Wilkinson ID, Spence SA. Impact on modafinil on prefrontal executive function in schizophrenia. Am J Psychiatry. 2006;163:2184–2186. doi: 10.1176/appi.ajp.163.12.2184. [DOI] [PubMed] [Google Scholar]
- 36.Gill M, Haerich P, Westcott K, Godenick KL, Tucker JA. Cognitive performance following modafinil versus placebo in sleep-deprived emergency physicians: a double-blind randomized crossover study. Acad Emerg Med. 2008;13:158–165. doi: 10.1197/j.aem.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 37.Kuczenski R, Segal DS. Effects of methylphenidate on extracellular dopamine, serotonin and norepinephrine: comparison with amphetamine. J Neurochem. 1997;68:2032–2037. doi: 10.1046/j.1471-4159.1997.68052032.x. [DOI] [PubMed] [Google Scholar]
- 38.Berridge CW, Devilbiss DM. Psychostimulants as cognitive enhancers: the prefrontal cortex, catecholamines and attention deficit hyperactivity disorder. Biol Psychiatry. 2011;69:e101–e111. doi: 10.1016/j.biopsych.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gehring K, Patwardhan SY, Collins R, et al. A randomized trial on the efficacy of methylphenidate and modafinil for improving cognitive functioning and symptoms in patients with a primary brain tumor. J Neurooncol. 2012;107:165–174. doi: 10.1007/s11060-011-0723-1. [DOI] [PubMed] [Google Scholar]
- 40.Kaleita TA, Wellisch DK, Graham CA, et al. Pilot study of modafinil for treatment of neurobehavioral dysfunction and fatigue in adult patients with brain tumors [abstract] J Clin Oncol. 2006;24:1503. [Google Scholar]
- 41.Vercoulen JHMM, Albert M, Bleijenberg G. De Checklist Individual Strength (CIS) Gedragstherapie. 1999;32:131–136. [Google Scholar]
- 42.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psych Measure. 1977;1:385. [Google Scholar]
- 43.Aaronson NK, Muller M, Cohen PD, et al. Translation, validation, and norming of the Dutch language version of the SF-36 Health Survey in community and chronic disease populations. J Clin Epidemiol. 1998;51:1055–1068. doi: 10.1016/s0895-4356(98)00097-3. [DOI] [PubMed] [Google Scholar]
- 44.Stewart AL, Ware JE. Measuring Functioning and Well-Being: The Medical Outcome Study Approach. Durham: Duke University Press; 1992. NC. [Google Scholar]
- 45.Rey A. L'examen psychologique dans les cas d'encephalopathie traumatique. Arch Psychologie. 1941;28:21. [Google Scholar]
- 46.Elst van der W, Boxtel van MPJ, Breukelen van GJP, Jolles J. Assessment of information processing in working memory applied settings: the paper & pencil memory scanning test. Psych Med. 2007;37:1335–1344. doi: 10.1017/S0033291707000360. [DOI] [PubMed] [Google Scholar]
- 47.Stroop J. Studies of interference in serial verbal reactions. J Exper Psychol. 1935;18:643–662. [Google Scholar]
- 48.Elst van der W, Boxtel van MPJ, Breukelen van GJP, Jolles J. The Letter Digit Substitution Test: normative data for 1,858 healthy participants aged 24–81 from the Maastricht Aging Study (MAAS): influence of age, education, and sex. J Clin Exp Neuropsychol. 2006;28:998–1009. doi: 10.1080/13803390591004428. [DOI] [PubMed] [Google Scholar]
- 49.Elst van der W, Boxtel van MPJ, Breukelen van GJP, Jolles J. The concept shifting test: adult normative data. Psychol Assess. 2006;18:424–432. doi: 10.1037/1040-3590.18.4.424. [DOI] [PubMed] [Google Scholar]
- 50.Luteijn F, Ploeg FAF. GIT: Groninger Intelligentie Test. Lisse: Swets & Zeitlinger; 1983. [Google Scholar]
- 51.Bosma H, van Boxtel MP, Ponds RW, et al. Pesticide exposure and risk of mild cognitive dysfunction. Lancet. 2000;356:912–913. doi: 10.1016/s0140-6736(00)02685-4. [DOI] [PubMed] [Google Scholar]
- 52.Finniss DG, Kaptchuk TJ, Miller F, Benedetti F. Placebo effects: biological, clinical and ethical advances. Lancet. 2010;375:686–695. doi: 10.1016/S0140-6736(09)61706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barsky AJ, Saintfort R, Rogers MP, Borus JF. Nonspecific medication side effects and the nocebo phenomenon. JAMA. 2002;287:622–627. doi: 10.1001/jama.287.5.622. [DOI] [PubMed] [Google Scholar]
- 54.Armstrong TS, Gilbert MR. Practical strategies for management of fatigue and sleep disorders in people with brain tumors. Neuro Oncol. 2012;14:iv65–iv72. doi: 10.1093/neuonc/nos210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gehring K, Sitskoorn MM, Gundy CM, et al. Cognitive rehabilitation in patients with gliomas: a randomized, controlled trial. J Clin Oncol. 2009;27:3712–3722. doi: 10.1200/JCO.2008.20.5765. [DOI] [PubMed] [Google Scholar]
- 56.Faller H, Schuler M, Richard M, Heckl U, Weis J, Küffner R. Effects of psycho-oncologic interventions on emotional distress and quality of life in adult patients with cancer: systematic review and meta-analysis. J Clin Oncol. 2013;31:782–793. doi: 10.1200/JCO.2011.40.8922. [DOI] [PubMed] [Google Scholar]