Abstract
Liver transplantation is the only definitive treatment for end-stage cirrhosis and fulminant liver failure, but the lack of available donor livers is a major obstacle to liver transplantation. Recently, induced pluripotent stem cells (iPSCs) derived from the reprogramming of somatic fibroblasts, have been shown to resemble embryonic stem (ES) cells in that they have pluripotent properties and the potential to differentiate into all cell lineages in vitro, including hepatocytes. Thus, iPSCs could serve as a favorable cell source for a wide range of applications, including drug toxicity testing, cell transplantation, and patient-specific disease modeling. Here, we describe an efficient and rapid three-step protocol that is able to rapidly generate hepatocyte-like cells from human iPSCs. This occurs because the endodermal induction step allows for more efficient and definitive endoderm cell formation. We show that hepatocyte growth factor (HGF), which synergizes with activin A and Wnt3a, elevates the expression of the endodermal marker Foxa2 (forkhead box a2) by 39.3% compared to when HGF is absent (14.2%) during the endodermal induction step. In addition, iPSC-derived hepatocytes had a similar gene expression profile to mature hepatocytes. Importantly, the hepatocyte-like cells exhibited cytochrome P450 3A4 (CYP3A4) enzyme activity, secreted urea, uptake of low-density lipoprotein (LDL), and possessed the ability to store glycogen. Moreover, the hepatocyte-like cells rescued lethal fulminant hepatic failure in a nonobese diabetic severe combined immunodeficient mouse model. Conclusion: We have established a rapid and efficient differentiation protocol that is able to generate functional hepatocyte-like cells from human iPSCs. This may offer an alternative option for treatment of liver diseases.
Viral hepatitis or drugs often cause liver injury and cirrhosis. Liver transplantation is the only effective treatment for end-stage liver diseases1; however, serious side effects of chronic immunosuppression and lack of suitable donor livers are major obstacles to liver transplantation. Reprogramming of mouse and human somatic cells to become induced pluripotent stem cells (iPSCs) has recently been achieved by viral transduction using four transcription factors.2 Unlike human embryonic stem (ES) cells, human iPSCs provide an alternative approach that avoids the controversies associated with the use of human embryos to obtain pluripotent ES cells. Although their gene expression pattern is not identical to human ES cells,3 human iPSCs are pluripotent and able to differentiate into most, if not all, cell types of the body. Therefore, human iPSC-derived somatic cells, such as hepatocytes, would be able to serve as an alternative source for liver transplantation, as well as help with toxicity screening during drug discovery.
During embryonic development, epiblast cells receive sequential developmental cues and undergo epithelial-to-mesenchymal transition to generate mesoderm or definitive endoderm.4 Several studies have successfully generated hepatocyte-like cells from human ES cells5–11 and human iPSCs12–17 in vitro. Most of these studies have focused on how to develop an efficient differentiation protocol with which to generate functional hepatocyte-like cells. Under the culture conditions for the generation of the hepatocyte-like cells, human ES cells or human iPSCs are first differentiated into definitive endoderm,18 followed by generation of mature hepatocytes that express stage- and tissue-specific genes.
Hepatocyte growth factor (HGF) is essential for the development of liver. Previous studies demonstrated that HGF knockout mice fail to completely develop their liver architecture, with a loosened liver structure and dissociation of the parenchymal cells in the mouse model.19 HGF and its receptor c-MET also exert several important functions that are associated with cell proliferation, survival, motility, invasion, and morphogenesis.20 In addition to its pathophysiological functions, HGF has been shown to induce scattering of epithelial cells by up-regulating expression of Snail,21 which is a transcription repressor that directly targets E-cadherin.22 However, HGF-associated molecular mechanisms during embryonic development are still poorly understood.
In our previously published study, we successfully generated hepatocyte-like cells from mesenchymal stem cells in vitro by a novel two-step protocol involving HGF and oncostatin M.23 Here, we describe an efficient three-step differentiation protocol that significantly improves definitive endoderm formation during the endodermal induction step involving HGF and activin A signaling; subsequently, functional hepatocyte-like cells can be generated in vitro. These findings indicate that HGF is a critical mitogen during hepatic endoderm formation and participates in the epithelial-to-mesenchymal transition process during early definitive endoderm formation. Finally, we demonstrate that the carbon tetrachloride (CCl4)-induced lethal fulminant hepatic failure in nonobese diabetic severe combined immunodeficient (NOD-SCID) mice can be rescued by intrasplenic transplantation of iPSC-derived hepatocyte-like cells.
Materials and Methods
Cell and Cell Culture
Human ES cell line H9 (National Institutes of Health Code: GE09) and human iPSC line CFB4614 were maintained on mitomycin-C (Sigma-Aldrich, St Louis, MO) inactivated mouse embryonic fibroblast (MEF) feeder layer in ES cell medium (Dulbecco’s modified Eagle medium [DMEM]/F12 supplemented with 20% knockout serum replacement, 10 ng/mL basic fibroblast growth factor, 1 mM L-glutamine, 100 μM nonessential amino acids, 100 μM 2-mercaptoethanol, 50 U/mL penicillin, and 50 mg/mL streptomycin [Invitrogen, Carlsbad, CA]).24 Before differentiation, the cells were cultured on Matrigel-coated tissue culture dishes using MEF-conditioned medium.25
In Vitro Differentiation of Human iPSCs into Hepatocyte-Like Cells
The in vitro differentiation protocol was similar to our previously reported study and that of Hay et al.9 In brief, when human iPSCs had attained a confluence of 70%, the MEF-conditioned medium was replaced with Roswell Park Memorial Institute/B27 with 100 ng/mL activin A (PeproTech, London, UK), 50 ng/mL Wnt3a, and 10 ng/mL HGF (R&D Systems) for 3 days of endodermal induction. During the next step, the culture medium was replaced with hepatic commitment medium (knockout [KO]/DMEM containing 20% knockout serum replacement, 1 mM L-glutamine, 1% nonessential amino acids, 0.1 mM 2-mercaptoethanol, and 1% dimethyl sulfoxide). Finally, during the maturation step, the cells were culturing in Iscove’s modified Dulbecco’s medium (IMDM) supplemented with 20 ng/mL oncostatin M (Invitrogen), 0.5 μM dexamethasone, and 50 mg/mL ITS premix (BD Biosciences, San Jose, CA).
Histopathology, Immunohistochemical Staining, Immunofluorescence, Hepatic Gene Expression, and Functional Assays
Details of the materials and methods used are described in the Supporting Information.
Animal Model
Five- to 8-week-old NOD-SCID mice were purchased from National Laboratory Animal Center (Taipei, Taiwan). All the experimental procedures involving the use of animals were approved by the Animal Care Committee of the Taipei Veterans General Hospital. The lethality of CCl4 on NOD-SCID mice was tested by gavage. Hepatocyte-like cell transplants were performed at 24 hours after administration of CCl4 by intrasplenic injection, as previously reported.26
Microarray Gene Expression Analysis
RNA was isolated from human iPSCs and human iPSC-derived hepatocyte-like cells, using the RNeasy kit (Qiagen). Complementary DNA synthesis, fragmentation, hybridization, washing, staining, and scanning were performed at the National Research Progress for Genomic Medicine Microarray and Gene Expression Analysis Core Facility, National Yang-Ming University VYM Genome Research Center, Taiwan. To provide a visual impression of how the various sample groups are related, principal component analysis (PCA) was performed using the Partek Genomics Suite program (Partek Inc., St. Louis, MO). Array data of control iPSCs and differentiated hepatocyte-like cells were downloaded from the GEO database (accession number GSE14897).17
Results
Feeder-Free Human ES Cell Culture System and MEF-Conditioned Medium Maintain Human iPSCs in an Undifferentiated State
Human iPSC and ES cell colonies were plated onto a MEF feeder layer for several months with weekly passaging. Before hepatogenic differentiation, cells were passaged using Matrigel-coated feeder-free culture conditions. On growing from day −4 to day 0, human iPSC and ES cell colonies were able to reach 70% confluence, and the cells showed positive expression of human ES cell surface markers, including octamer-binding transcription factor 4 (Oct-4), also known as POU5F1, stage-specific embryonic antigen 4 (SSEA-4), or the tumor rejection antigens Tra 1–60 and Tra 1–81 (Fig. 1). These results demonstrate that the human iPSCs exhibit pluripotent properties before hepatogenic differentiation.
Fig. 1.
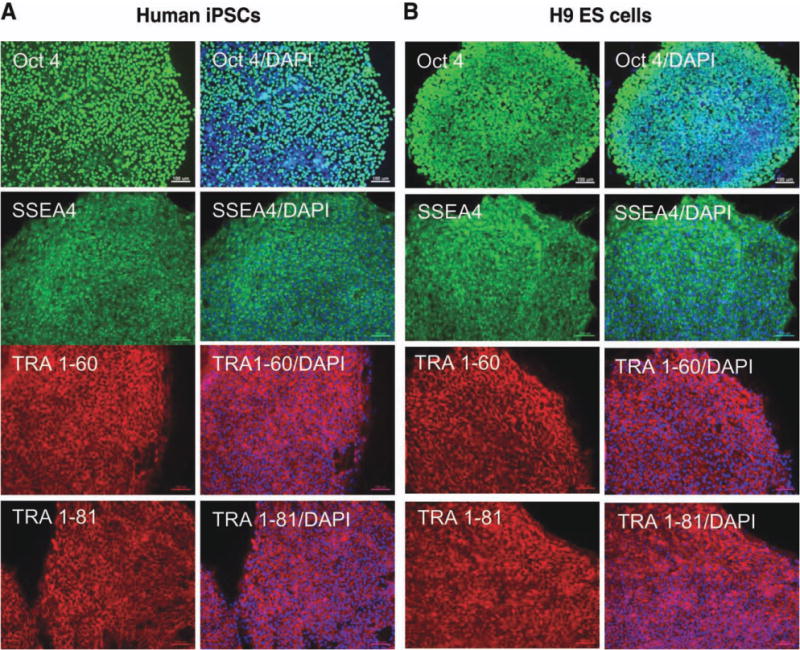
Characterization of human iPSCs cultured on Matrigel with MEF-conditioned medium. (A) Human iPSCs and (B) human ES cells show the expression of the stemness markers, Oct4, SSEA4, TRA-1-60, and TRA-1-81, when cultured on Matrigel-coated dishes with MEF-conditioned medium. Scale bars, 100 μm. (Original magnification, ×100). DAPI, 4′,6-diamidino-2-phenylindole.
Directed Hepatogenic Differentiation from Human iPSCs In Vitro
It is imperative to ensure the differentiation abilities of the human iPSCs prior to therapeutic application. Here, we developed a three-step protocol by modifying the culture condition described by Hay et al.,10 and Kuo et al.,26 in order to bring about the rapid generation of hepatocyte-like cells from human iPSCs.
In this protocol, which is described in the Materials and Methods section and Table 1, the human iPSCs were allowed to reach approximately 70% confluence in feeder cell-free culture system over 4 days, and this was followed by treatment with endodermal induction medium on day 0 (Fig. 2A, panel i) in the presence of activin A, Wnt3a, and HGF. This produced a human iPSC morphology with a spiky shape due to the loss of ES cell structure that occurred after dissociation from cell–cell contact (Fig. 2A, panel ii). Immunostaining revealed that most of the cells were positive for the definitive endoderm marker Sox17 (sex-determining region Y box 17; Fig. 2B), indicating that the human iPSCs efficiently differentiated into definitive endoderm during the endodermal induction step.
Table 1.
Hepatogenic Differentiation Condition
| Induction period | Medium Contents | |
|---|---|---|
| Preinduction | Day −4~Day 0 | CM (MEF-conditioned medium) |
| Endodermal induction | Day 1~Day 3 | RPMI/B27, activin A (100 ng/mL), Wnt 3a (50 ng/mL), HGF (10 ng/mL) |
| Hepatic lineage commitment | Day 4~Day 7 | KO/DMEM, L-glutamine (1 mM), nonessential amino acids (1%) 2-Mercaptoethanol (0.1 mM), dimethyl sulfoxide (1%) |
| Maturation step | Day 8~Day 12 | IMDM, oncostatin M (20 ng/mL), dexamethasone (0.5 μM), ITS premix (50 mg/mL) |
Fig. 2.
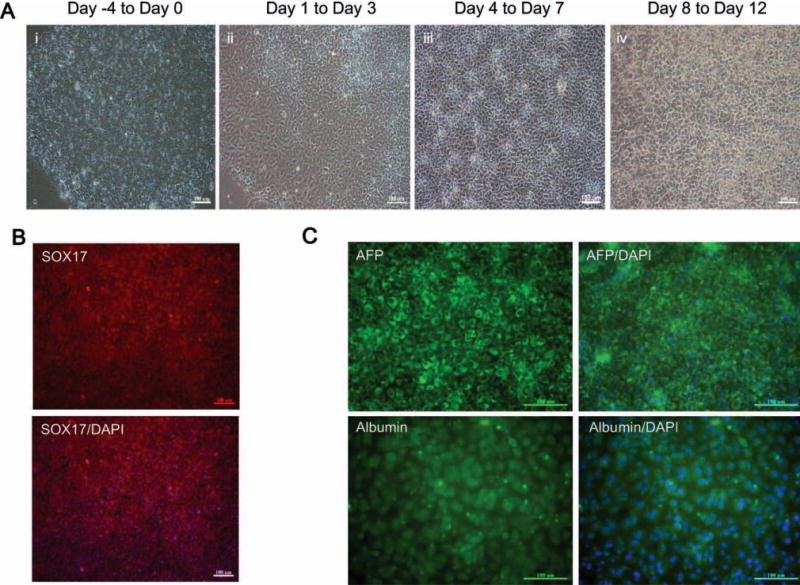
Generation of hepatocyte-like cells from human iPSCs. (A) Images showing the sequential morphological change from human iPSCs to hepatocyte-like cells. (Panel i) Morphology at the preinduction stage of human iPS cells; Panel ii shows the morphological changes that occur to give a spiky shape after culture in endodermal induction medium. (Panel iii) At day 7 after hepatic lineage commitment medium induction, the cell morphology has become polygonal in shape. (Panel iv) The morphology of the mature hepatocyte-like cells. (B) Immunocytochemisty showing that the expression of the definitive endoderm marker SOX17 at day 4 and (C) that the cells are positive for AFP and albumin at day 12 after the start of the differentiation procedure. Scale bars, 100 μm. (Original magnification, ×100). DAPI, 4′,6-diamidino-2-phenylindole.
Following the endodermal induction step, cells were treated with the hepatic commitment medium for 3 days; this changed the cell morphology from a spiky shape to a polygonal shape that had tight cell–cell contact (Fig. 2A, panel iii). Finally, the medium was changed to maturation medium, which resulted in the human iPSC morphology changing into a cuboidal shape (Fig. 2A, panel iv). Immunostaining of these cells confirmed that these hepatocyte-like cells were positive for alpha-fetoprotein (AFP) and albumin (ALB) (Fig. 2C).
Highly Efficient Endoderm Formation Requires Hepatocyte Growth Factor
HGF has multiple effects on target cells in culture and has been demonstrated to be involved in liver development.19 In our endodermal induction step, we were interested in how HGF acted synergistically with activin A and Wnt3a to accelerate definitive endoderm formation. To confirm this process, human iPSCs were induced in endodermal induction medium with or without HGF for 3 days. Consistent with definitive endoderm marker Sox17 expression, we observed that forkhead box a2 (Foxa2), which is another endodermal marker, could be detected after the endodermal induction step (Fig. 3A). Moreover, differentiation into Foxa2+ cells was detected in 39.35% ± 0.98% of iPSCs treated with HGF, compared to 14.18% ± 0.54% of iPSCs that did not have HGF treatment during the endodermal induction step (Fig. 3B).
Fig. 3.
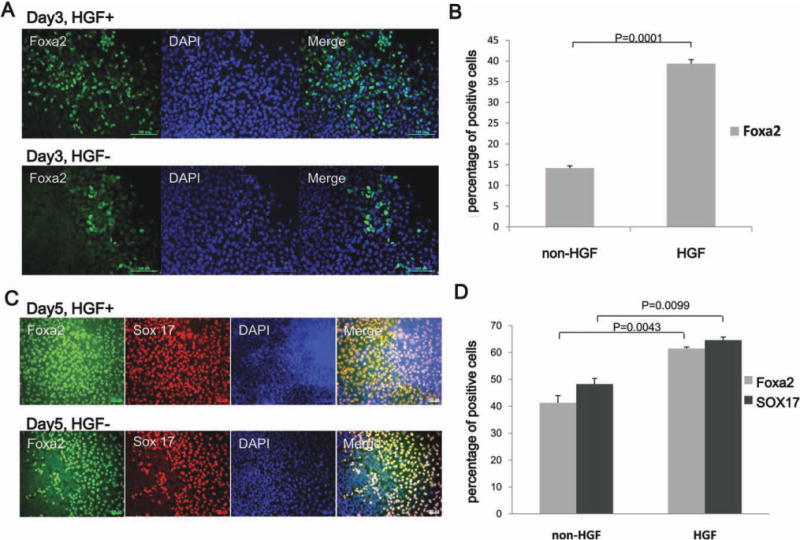
HGF plays an important role in endoderm formation during the hepatogenic differentiation of human iPSCs. (A) Immunocytochemical analysis of cells treated with endodermal induction medium with HGF or without HGF shows efficient production of the endoderm marker Foxa2 only in medium with HGF (C) Immunocytochemistry showing the expression of the colocalized definitive endoderm markers SOX17 and Foxa2 at day 5; the upper panel shows endodermal induction medium with HGF and the lower panel shows endodermal induction medium without HGF (Original magnification, ×100). (B,D) Quantitative analysis confirmed that there was a significant change between the cells grown in HGF+ and HGF− medium. Scale bars, 100 μm. (t test, n = 3). DAPI, 4′,6-diamidino-2-phenylindole.
To further investigate whether HGF treatment results in increased formation of hepatic lineage cells, we examined the expression of Sox17 and Foxa2 expression at day 5. The results showed that Sox17 and Foxa2 coexisted during the hepatic commitment step (Fig. 3C). Coincident with the endodermal induction step, there was only 41% Foxa2+ and 48% Sox17+ cells in the group without HGF, but this increased to 61% Foxa2+ and 64% Sox17+ cells in the HGF-treated group (Fig. 3C,D). These results suggest that HGF plays an important role in early hepatic lineage formation.
Gene Expression Profiles of the iPSC-Derived Hepatocyte-Like Cells
To determine whether iPSC-derived hepatocytes in our differentiation system displayed mature characteristics of a hepatic lineage, we examined the gene expression patterns of various early hepatic marker genes, namely hepatocyte nuclear factor 4 (HNF-4), albumin, cytokeratin 18 (CK-18), glucose 6-phosphate (G-6P), cytochrome P450 3A4 (CYP3A4), and cytochrome P450 7A1 (CYP7A1) by reverse transcription polymerase chain reaction (RT-PCR) (Fig. 4A). As seen, all of these genes were expressed in iPSC-derived hepatocyte cells. To determine the quantitative expression levels of the hepatic markers in iPSCs before and after induction, we examined the gene expression patterns by quantitative PCR and normalized the results against primary human hepatocytes. The results reveal that the expression levels of the hepatic genes AFP, TDO2, and transthyretin (TTR) were significantly higher in the iPSC-derived hepatocyte cells than in the primary human hepatocytes. Furthermore, if we compared iPSCs with iPSC-derived hepatocyte cells, it was found that ALB, cytokeratin 18 (CK-18), HNF-4A, tyrosine aminotransferase (TAT), and low-density lipoprotein receptor (LDLR) are more highly expressed in the iPSC-derived hepatocyte cells (Fig. 4B).
Fig. 4.
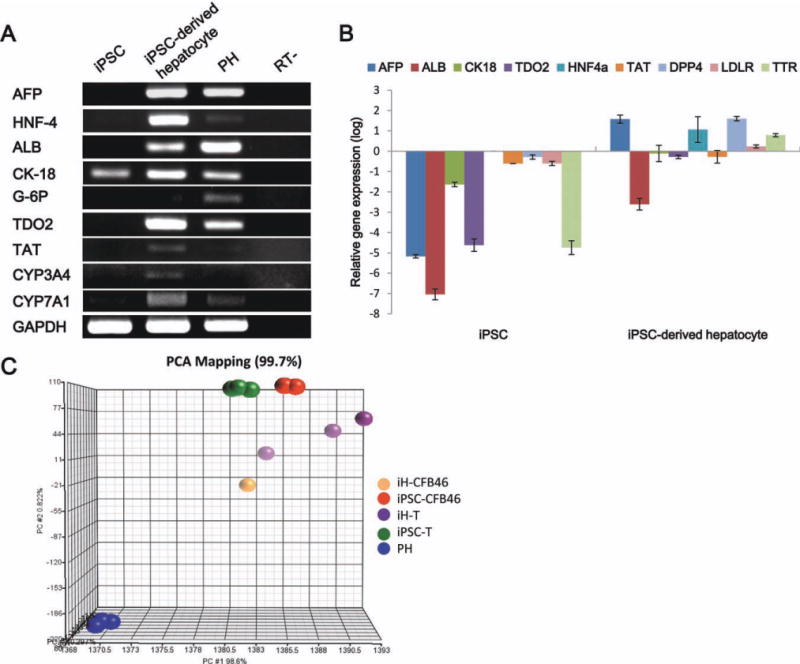
Gene expression patterns during differentiation from human iPSCs to hepatocyte-like cells. (A) Reverse transcription polymerase chain reaction (RT-PCR) gene expression of human iPSCs and iPSC-derived hepatocyte cells for the hepatocyte markers alpha-fetoprotein (AFP), hepatocyte nuclear factor-4 (HNF-4), albumin (ALB), cytokeratin 18 (CK-18), glucose-6-phosphatase (G-6P), tryptophan 2,3-dioxygenase (TDO2), tyrosine aminotransferase (TAT), cytochrome P450 3A4 (CYP3A4), and cytochrome P450 7A1 (CYP7A1). Gene expressions were normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH). (B) Quantitative PCR analysis of the hepatic markers AFP, ALB, CK-18, HNF4a, transthyretin (TTR), TAT, TDO2, dipeptidyl peptidase 4 (DPP4), and low-density lipoprotein receptor (LDLR). Gene expression levels were normalized against primary human hepatocytes. (C) The multidimensional scaling plot shows the discrimination ability of the molecular signatures of the cell groups. Each spot represents a single array sample. Each cell group exhibited a significant and distinct global gene expression profile (n = 3). iH-CFB46, iPSC-derived hepatocyte cells in our group; iPSC-CFB46, human iPS cells from the Kuo group14; iH-T, iPSC-derived hepatocyte cells from the Si-Tayeb group; iPSC-T, human iPS cells from the Si-Tayeb group17; PH, primary human hepatocyte cells.
Gene expression microarray analysis of the differentiated cells (orange spots, iH-CFB46, Fig. 4C) compared to the iPSC-derived hepatocyte cells of the Si-Tayeb group (purple spots, iH, Fig. 4C) showed that the iPSC-derived hepatocyte cells were different from the original iPSCs (green and red spots iPSC and CFB46, respectively, Fig. 4C) and were closer to primary hepatocyte cells (blue spots, PH, Fig. 4C), with our differentiated cells being closer to primary hepatocytes.
Functional Characterization of the Human iPSC-Derived Hepatocyte-Like Cells
To assess the functional status of the human iPSC-derived hepatocyte-like cells, we determined their metabolic capacity. The cytochrome P450 enzyme isoform, cytochrome P450 3A4, is one of the most important enzymes involved in the metabolism of xenobiotics in the liver. Our results demonstrated that the differentiated cells exhibited CYP3A4 activity similar to that found in primary human hepatocytes and that the expression level of the enzyme was remarkably higher than human iPSCs (Fig. 5A). Secretion of urea by the differentiated cells was also analyzed. Urea production was detectable on day 12 (Fig. 5B). In addition, iPSC-derived hepatocytes were competent for LDL uptake (Fig. 5C).
Fig. 5.
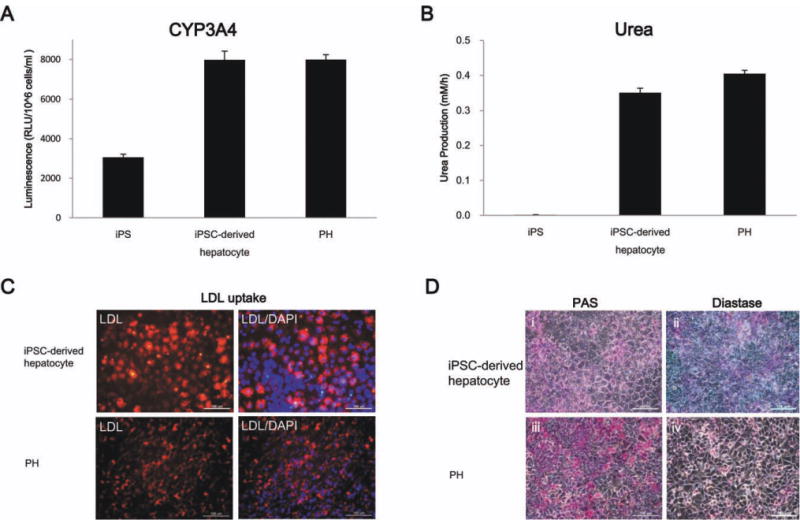
Functional analysis of the hepatocyte-like cells derived from iPSCs. (A) After 12 days induction, the human iPSC-derived hepatocytes exhibited cytochrome P450 isozyme activity similar to primary human hepatocytes (n = 3) and (B) they also secreted urea. (C) Immunofluorescence staining for LDL uptake in iPSC-derived hepatocytes. Glycogen storage was examined by PAS staining (D, panel i), which begins to show glycogen storage at differentiation day 12. (Panel ii) Glycogen stored in hepatic cells can be digested by diastase treatment and is then present as negative PAS staining. (Panels iii and iv) The primary human hepatocytes were used as a positive control. (Original magnification, ×100).
To further characterize the glycogen storage function of iPSC-derived hepatocyte-like cells, the presence of stored glycogen was determined by periodic acid-Schiff (PAS) staining. Glycogen was stained magenta and could be seen in the differentiated cells (day 12; Fig. 5D, panel i). Diastase digestion was subsequently performed, which confirmed that positive staining was due to the presence of glycogen (Fig. 5D, panel ii). Primary human hepatocytes were used as a positive control (Fig. 5D, panels iii and iv).
iPSC-Derived Hepatocyte-Like Cells Can Rescue Lethal Fulminant Hepatic Failure
To assess the therapeutic potential of iPSC-derived hepatocytes, a model of lethal fulminant hepatic failure caused by CCl4 in NOD-SCID mice was used. A dose of 0.35 mL/kg body weight was optimal and resulted in lethality in all animals in 2 weeks after administration of CCl4. ransplantation of 4.0 × 107 iPSCs per kilogram body weight failed to rescue recipient animals from fulminant hepatic failure (0 of 7 mice survived). However, in mice that received iPSC-derived hepatocyte cells, 71% of the animals were rescued from the transplantation of 4.0 × 107 iPSC-derived hepatocytes per kilogram body weight (5 of 7 mice survived) (Fig. 6A). Histopathologic analysis showed the presence of submassive hepatic necrosis liver in mice (Fig. 6B, panel viii), whereas the hepatic necrosis was rescued by transplantation of iPSC-derived hepatocytes, but not by iPSCs. Biochemical assays showed a dynamic change in the serum levels of hepatic marker proteins such as serum glutamyl oxaloacetic aminotransferase, glutamyl pyruvic aminotransferase, albumin, total bilirubin, and lactate dehydrogenase, confirming the infliction of acute liver failure by CCl4 (Table 2).
Fig. 6.
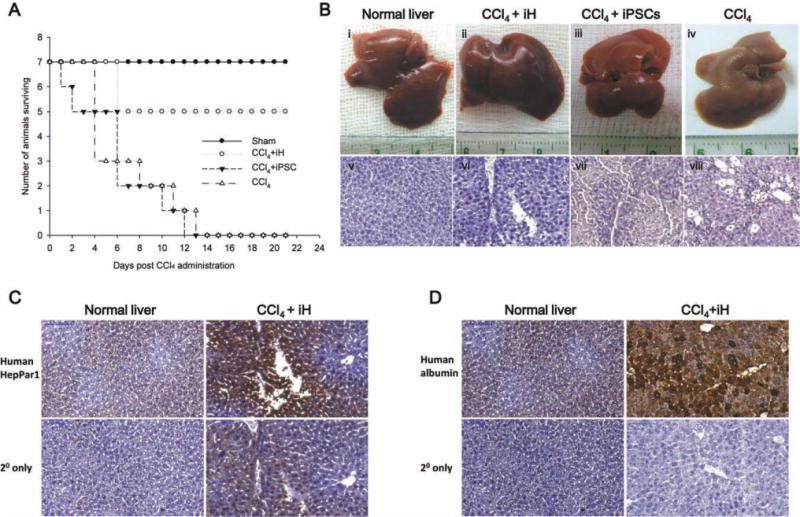
iPSC-Derived hepatocyte-like cells can rescue CCl4-induced lethal fulminant hepatic failure. (A) Survival curves of NOD-SCID mice (n = 7 in each group) that received intrasplenic cell transplantation with 4.0 × 107 iPSC-derived hepatocytes per kilogram body weight. (B) Appearance and histopathology of liver showed the organs with or without received cell transplantation (iPSC-derived hepatocytes or iPSCs) after administration of CCl4 (Panels i and v = normal liver; Panels ii and vi = iPSC-derived hepatocytes transplanted after administration of CCl4; Panels iii and vii = iPSCs transplanted after administration of CCl4; Panels iv and viii = intrasplenic saline injection after administration of CCl4). (C,D) Detection of human hepatocytes with anti-human HepPar1 and human albumin antibodies with immunohistochemistry. (Original magnification, ×200).
Table 2.
Liver Function Tests of NOD-SCID Mice That Were Administered 0.35 mL/kg CCl4 and Intrasplenic Transplantation of iPSC-Derived Hepatocyte Cells
| Serum Glutamyl Oxaloacetic Aminotransferase (IU/L) | Serum Glutamyl Pyruvic Aminotransferase (IU/L) | Albumin (g/dL) | Total Bilirubin (mg/dL) | Lactate Dehydrogenase (IU/L) | |
|---|---|---|---|---|---|
| Normal NOD-SCID | 60 ± 6.6 | 34 ± 5.8 | 2.5 ± 0.11 | 0.4 ± 0.08 | 336 ± 14.5 |
| Post-CCl4 day 1 | |||||
| Placebo | > 1000 | > 1000 | 3.4 ± 0.22 | 1.7 ± 0.23 | > 4000 |
| iH-CT | > 1000 | > 1000 | 3.4 ± 0.16 | 1.7 ± 0.11 | > 4000 |
| Post-CCl4 day 3 | |||||
| Placebo | 513 ± 29.6 | 536 ± 93.2 | 3.2 ± 0.17 | 1.6 ± 0.10 | 3738 ± 123.8 |
| iH-CT | 191 ± 57.2 | 128 ± 24.0 | 2.7 ± 0.19 | 0.6 ± 0.43 | 427 ± 31.8 |
| Post-CCl4 day 7 | |||||
| Placebo | 681 ± 31.6 | 557 ± 54.3 | 3.2 ± 0.08 | 1.7 ± 0.17 | 3754 ± 89.5 |
| iH-CT | 71 ± 12.9 | 39 ± 10.2 | 2.6 ± 0.12 | 0.4 ± 0.08 | 467 ± 41.3 |
| Post-CCl4 day 14 | |||||
| Placebo | ND | ND | ND | ND | ND |
| iH-CT | 70 ± 8.9 | 45 ± 8.2 | 2.6 ± 0.04 | 0.4 ± 0.13 | 407 ± 20.2 |
| Post-CCl4 day 21 | |||||
| Placebo | ND | ND | ND | ND | ND |
| iH-CT | 70 ± 4.6 | 44 ± 6.3 | 2.6 ± 0.09 | 0.5 ± 0.06 | 451 ± 37.0 |
| Post-CCl4 day 28 | |||||
| Placebo | ND | ND | ND | ND | ND |
| iH-CT | 76 ± 4.2 | 43 ± 7.6 | 2.6 ± 0.12 | 0.4 ± 0.09 | 435 ± 49.0 |
Abbreviations: iH-CT, iPSC-derived hepatocyte cell transplantation; ND, not done.
iPSC-Derived Hepatocyte-Like Cells Engraft in NOD-SCID Mice
To investigate whether the transplanted cells were engrafted in liver parenchyma of the recipients, two human hepatocyte-specific markers, HepPar128 and albumin, were used to detect human liver cells in mouse liver. Recipient mice that were rescued by intrasplenic transplantation of iPSC-derived hepatocytes were sacrificed on day 28 after transplantation. The immunohistochemical staining showed the presence of human HepPar1 and albumin in the liver parenchyma of recipient animals. These data indicate that the human iPSC-derived hepatocytes had been engrafted in recipient liver parenchyma (Fig. 6C,D).
Discussion
In this study, we developed a novel three-step protocol that efficiently generated hepatocyte-like cells from human iPSCs in vitro. During our differentiation protocol, human iPSCs are exposed to a high level of activin, Nodal, and Wnt signaling in a manner that is designed to mimic events during embryonic development in order to allow definitive endoderm formation.29, 30 This is followed by a hepatic lineage commitment and a maturation step. The results show that we successfully generated iPSC-derived hepatocyte cells that not only express hepatic markers but also have urea production and carry out glycogen storage. Although other methods for the hepatogenic differentiation of human iPSCs have been described, few have shown a close relationship between the iPSC-derived hepatocyte cells and primary human hepatocytes, using microarray gene expression profiling. A comparison of the gene expression profile with that of a previous study17 showed that our differentiated cells had a similar gene profile to the earlier study and that their profile is closely related to primary human hepatocytes.
During the endodermal induction step, morphology of the iPSCs changed from clustered to scattered with no cell–cell contact. Compared with cells cultured in media without HGF, we found that the presence of HGF may have a synergistic effect with activin A and Wnt3a and is able to efficiently drive iPSCs toward a definitive commitment to endoderm formation. Although several studies have demonstrated that HGF exerts several functions during angiogenesis and tumor progression, the role of HGF in embryonic development remains poorly understood. It has been previously reported that HGF induces a scattering of epithelial cells by up-regulating the expression of Snail, which is a transcription factor that controls the epithelial-to-mesenchymal transition. According to our findings, HGF induces a rapid increase in the expression of the definitive endoderm markers, Sox17 and Foxa2. The cell morphology of the iPSC also quickly changes into a spiky shape. Furthermore, the transcription factor Snail, which is a strong repressor of transcription of the E-cadherin gene, is up-regulated by the endodermal induction medium containing HGF, but not by medium without HGF (data not shown). Therefore, further analysis of the molecular mechanism related to HGF activities during early embryonic development is important to controlling hepatic lineage formation.
Using our protocol, it is possible to bring about the rapid and efficient generation of mature cells that exhibited characteristics of hepatocytes. The cytochrome P450 enzymes are critical enzymes associated with drug metabolism and the general metabolism of the human liver. The iPSC-derived hepatocyte cells expressed detectable enzyme activity for CYP3A4, which is the most important of the cytochrome P450s. This suggests strongly that these differentiated cells have the potential to be applied during in vitro model drug screening.
The in vitro differentiation system reported here that allows the differentiation of hepatocyte-like cells has numerous advantages. First, it should be possible to use these cells to treat diseases. This is because the method creates hepatocyte-like cells from human iPSCs, and these iPSCs can be reprogrammed from patient somatic cells. Second, the process is very rapid and highly efficient. Using our system, the differentiation of human iPSCs into functional hepatocyte-like cells requires only 12 days. This will facilitate the development of therapeutic protocols.
In conclusion, we have shown that human iPSCs can be directed to differentiate into hepatocyte-like cells in a rapid and efficient manner, through use of a three-step protocol. According to the gene expression pattern and functional analysis of the iPSC-derived hepatocyte-like cells, we believe that this study has advanced the hepatogenic differentiation field. Furthermore, using the differentiated cells as a source of hepatocytes should help the development of alternative methods that may supersede liver transplantation when patients have liver failure. The process also offers the possibility of using the hepatocyte-like cells for toxicity screening during drug discovery.
Supplementary Material
Acknowledgments
This work was supported in part by the University System of Taiwan–University of California San Diego International Center of Excellence in Advanced Bioengineering sponsored by the Taiwan National Science Council I-RiCE Program under grant NSC-99-2911-I-009-101. The authors also acknowledge financial support from the Taipei Veterans General Hospital (VGH100E1-010, VGH100C-056, VN100-05, and VGH100D-003-2), the National Science Council, Taiwan (NSC100-2120-M-010-001, NSC100-2314-B-010-030-MY3, NSC100-2321-B-010-019, NSC99-3111-B-010-002, NSC98-2314-B-010-001-MY3, NSC 99-2911-I-010-501, and NSC 99-3114-B-002-005). This work was technically assisted, in part, by the Division of Experimental Surgery of the Department of Surgery, Taipei Veterans General Hospital. This study was also supported by a grant from the Ministry of Education, Aim for the Top University Plan.
Abbreviations
- AFP
alpha-fetoprotein
- CK-18
cytokeratin 18
- CYP3A4
cytochrome P450 3A4
- CYP7A1
cytochrome P450 7A1
- ES cell
embryonic stem cell
- Foxa2
forkhead box a2
- G-6P
glucose 6-phosphate
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- HGF
hepatocyte growth factor
- HNF-4
hepatocyte nuclear factor 4
- iPSC
induced pluripotent stem cell
- LDL
low-density lipoprotein
- LDLR
low-density lipoprotein receptor
- MEF
mouse embryonic fibroblast
- NOD-SCID
nonobese diabetic severe combined immunodeficient
- Oct-4
octamer-binding transcription factor 4
- PAS
periodic acid-Schiff
- SSEA-4
stage-specific embryonic antigen 4
- SOX17
sex-determining region Y box 17
- TAT
tyrosine aminotransferase
- TDO2
tryptophan 2,3-dioxygenase
- Tra 1-60
tumor rejection antigens 1-60
- Tra 1-81
tumor rejection antigens 1-81
- TTR
transthyretin
- Wnt3a
wingless-type MMTV integration site family, member 3A
Footnotes
Potential conflict of interest: Nothing to report.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Lee WM. Acute liver failure. N Engl J Med. 1993;329:1862–1872. doi: 10.1056/NEJM199312163292508. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 3.Chin MH, Mason MJ, Xie W, Volinia S, Singer M, Peterson C, et al. Induced pluripotent stem cells and embryonic stem cells are distinguished by gene expression signatures. Cell Stem Cell. 2009;5:111–123. doi: 10.1016/j.stem.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shook D, Keller R. Mechanisms, mechanics and function of epithelial-mesenchymal transitions in early development. Mech Dev. 2003;120:1351–1383. doi: 10.1016/j.mod.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Rambhatla L, Chiu CP, Kundu P, Peng Y, Carpenter MK. Generation of hepatocyte-like cells from human embryonic stem cells. Cell Transplant. 2003;12:1–11. doi: 10.3727/000000003783985179. [DOI] [PubMed] [Google Scholar]
- 6.Lavon N, Yanuka O, Benvenisty N. Differentiation and isolation of hepatic-like cells from human embryonic stem cells. Differentiation. 2004;72:230–238. doi: 10.1111/j.1432-0436.2004.07205002.x. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz RE, Linehan JL, Painschab MS, Hu WS, Verfaillie CM, Kaufman DS. Defined conditions for development of functional hepatic cells from human embryonic stem cells. Stem Cells Dev. 2005;14:643–655. doi: 10.1089/scd.2005.14.643. [DOI] [PubMed] [Google Scholar]
- 8.Cai J, Zhao Y, Liu Y, Ye F, Song Z, Qin H, et al. Directed differentiation of human embryonic stem cells into functional hepatic cells. HEPATOLOGY. 2007;45:1229–1239. doi: 10.1002/hep.21582. [DOI] [PubMed] [Google Scholar]
- 9.Hay DC, Zhao D, Ross A, Mandalam R, Lebkowski J, Cui W. Direct differentiation of human embryonic stem cells to hepatocyte-like cells exhibiting functional activities. Cloning Stem Cells. 2007;9:51–62. doi: 10.1089/clo.2006.0045. [DOI] [PubMed] [Google Scholar]
- 10.Hay DC, Zhao D, Fletcher J, Hewitt ZA, McLean D, Urruticoechea-Uriguen A, et al. Efficient differentiation of hepatocytes from human embryonic stem cells exhibiting markers recapitulating liver development in vivo. Stem Cells. 2008;26:894–902. doi: 10.1634/stemcells.2007-0718. [DOI] [PubMed] [Google Scholar]
- 11.Touboul T, Hannan NR, Corbineau S, Martinez A, Martinet C, Branchereau S, et al. Generation of functional hepatocytes from human embryonic stem cells under chemically defined conditions that recapitulate liver development. HEPATOLOGY. 2010;51:1754–1765. doi: 10.1002/hep.23506. [DOI] [PubMed] [Google Scholar]
- 12.Song Z, Cai J, Liu Y, Zhao D, Yong J, Duo S, et al. Efficient generation of hepatocyte-like cells from human induced pluripotent stem cells. Cell Res. 2009;19:1233–1242. doi: 10.1038/cr.2009.107. [DOI] [PubMed] [Google Scholar]
- 13.Sullivan GJ, Hay DC, Park IH, Fletcher J, Hannoun Z, Payne CM, et al. Generation of functional human hepatic endoderm from human induced pluripotent stem cells. HEPATOLOGY. 2010;51:329–335. doi: 10.1002/hep.23335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang HP, Yu CY, Chen HF, Chen PH, Chuang CY, Lin SJ, et al. Factors from human embryonic stem cell-derived fibroblast-like cells promote topology-dependent hepatic differentiation in primate embryonic and induced pluripotent stem cells. J Biol Chem. 2010;285:33510–33519. doi: 10.1074/jbc.M110.122093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghodsizadeh A, Taei A, Totonchi M, Seifinejad A, Gourabi H, Pournasr B, et al. Generation of liver disease-specific induced pluripotent stem cells along with efficient differentiation to functional hepatocyte-like cells. Stem Cell Rev. 2010;6:622–632. doi: 10.1007/s12015-010-9189-3. [DOI] [PubMed] [Google Scholar]
- 16.Rashid ST, Corbineau S, Hannan N, Marciniak SJ, Miranda E, Alexander G, et al. Modeling inherited metabolic disorders of the liver using human induced pluripotent stem cells. J Clin Invest. 2010;120:3127–3136. doi: 10.1172/JCI43122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Si-Tayeb K, Noto FK, Nagaoka M, Li J, Battle MA, Duris C, et al. Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. HEPATOLOGY. 2010;51:297–305. doi: 10.1002/hep.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D’Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 2005;23:1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt C, Bladt F, Goedecke S, Brinkmann V, Zschiesche W, Sharpe M, et al. Scatter factor/hepatocyte growth factor is essential for liver development. Nature. 1995;373:699–702. doi: 10.1038/373699a0. [DOI] [PubMed] [Google Scholar]
- 20.Liu X, Newton RC, Scherle PA. Developing c-MET pathway inhibitors for cancer therapy: progress and challenges. Trends Mol Med. 2010;16:37–45. doi: 10.1016/j.molmed.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Grotegut S, von Schweinitz D, Christofori G, Lehembre F. Hepatocyte growth factor induces cell scattering through MAPK/Egr-1-mediated upregulation of Snail. EMBO J. 2006;25:3534–3545. doi: 10.1038/sj.emboj.7601213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cano A, Pérez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 23.Lee KD, Kuo TK, Whang-Peng J, Chung YF, Lin CT, Chou SH, et al. In vitro hepatic differentiation of human mesenchymal stem cells. HEPATOLOGY. 2004;40:1275–1284. doi: 10.1002/hep.20469. [DOI] [PubMed] [Google Scholar]
- 24.Park IH, Lerou PH, Zhao R, Huo HG, Daley GQ. Generation of human-induced pluripotent stem cells. Nat Protoc. 2008;3:1180–1186. doi: 10.1038/nprot.2008.92. [DOI] [PubMed] [Google Scholar]
- 25.Braam SR, Denning C, Matsa E, Young LE, Passier R, Mummery CL. Feeder-free culture of human embryonic stem cells in conditioned medium for efficient genetic modification. Nat Protoc. 2008;3:1435–1443. doi: 10.1038/nprot.2008.140. [DOI] [PubMed] [Google Scholar]
- 26.Kuo TK, Hung SP, Chuang CH, Chen CT, Shih YR, Fang SC, et al. Stem cell therapy for liver disease: parameters governing the success of using bone marrow mesenchymal stem cells. Gastroenterology. 2008;134:2111–2121. doi: 10.1053/j.gastro.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu C, Inokuma MS, Denham J, Golds K, Kundu P, Gold JD, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- 28.Newsome PN, Johannessen I, Boyle S, Dalakas E, McAulay KA, Samuel K, et al. Human cord blood-derived cells can differentiate into hepatocytes in the mouse liver with no evidence of cellular fusion. Gastroenterology. 2003;124:1891–1900. doi: 10.1016/s0016-5085(03)00401-3. [DOI] [PubMed] [Google Scholar]
- 29.Hay DC, Fletcher J, Payne C, Terrace JD, Gallagher RC, Snoeys J, et al. Highly efficient differentiation of hESCs to functional hepatic endoderm requires ActivinA and Wnt3a signaling. Proc Natl Acad Sci USA. 2008;105:12301–12306. doi: 10.1073/pnas.0806522105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vincent SD, Dunn NR, Hayashi S, Norris DP, Robertson EJ. Cell fate decisions within the mouse organizer are governed by graded Nodal signals. Genes Dev. 2003;17:1646–1662. doi: 10.1101/gad.1100503. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


