Alcoholic liver disease (ALD) is a major cause of liver-related morbidity and mortality worldwide.1 Although the most effective therapy for patients with ALD is alcohol cessation, targeted therapies are needed in patients with progressive disease and active drinking, and for those with life-threatening conditions such as alcoholic hepatitis. The development of such therapies is hampered by poor knowledge of the main disease drivers. During the last two decades, the cellular and molecular mechanisms of the hepatic wound-healing response to repeated exposure to alcohol have been partially delineated. ALD is characterised by steatosis, inflammatory infiltrate mainly composed of polymorphonuclear cells, hepatocellular injury and progressive fibrosis. The cellular and molecular basis includes paracrine stimulation by damaged hepatocytes, the innate immune system, oxidative stress and the resulting activation of non-parenchymal cells. Several inflammatory and fibrogenic mediators are released at the areas of tissue repair and perpetuate inflammation, leading to progressive fibrosis and organ failure. Among these mediators, a growing body of evidence indicates that endogenous cannabinoids have an important role in hepatic wound healing and represent promising druggable targets for therapy.2
The endocannabinoid system is composed of endocannabinoids (anandamide (AEA), 2-arachidonoyl glycerol (2-AG)), cannabinoid receptors (CB) (CB1 and CB2), and the enzymes involved in endocannabinoid biosynthesis and degradation.2 The degradation enzymes include fatty acid amide hydrolase and monoglyceride lipase for degradation of AEA and 2-AG, respectively. CB1 receptors are expressed at high levels in the brain, playing many important psychoactive functions. Recent studies revealed that peripheral tissues also expressed low levels of CB1 receptors with functionally relevant concentrations. For example, in the liver, low levels of CB1 receptors are detected in hepatocytes, cholangiocytes, hepatic stellate cells, which play important roles in controlling haemodynamics in cirrhosis, liver fibrogenesis, and lipogenesis in the liver.2 CB2 receptors are mainly expressed in immune cells, including liver Kupffer cells, but they are also detected in non-immune cells, such as hepatic stellate cells, myofibroblasts, hepatocytes, cholangiocytes, regulating liver fibrosis, steatosis and regeneration. Several recent reports suggest that the endocannabinoid system also participates in the pathogenesis of ALD via the activation of CB1 and CB2 receptors. It has been reported that activation of CB2 receptors protects against ALD by regulating M1/M2 balance in Kupffer cells,3 whereas, paracrine activation of hepatic CB1 receptors by stellate cell-derived 2-AG plays an important role in the development of alcoholic fatty liver via the stimulation of lipogenesis and suppression of fatty acid oxidation in hepatocytes.4 However, the effects of CB1 activation on alcoholic liver injury and inflammation are still not fully understood.
Kim DK and colleagues revealed an important mechanism by which CB1 receptors contribute to the pathogenesis of ALD.5 It has been known for many years that alcohol consumption upregulates expression of CYP2E1 in the liver, and that such upregulation plays a critical role in promoting ALD development via the stimulation of oxidative stress in the liver. Many mechanisms underlying hepatic CYP2E1 upregulation by ethanol have been identified. The findings provided by Kim DK et al that ethanol induction of CYP2E1 was abolished in CB1-deficient mice clearly imply that activation of CB1 is another mechanism underlying the upregulation of hepatic CYP2E1 in ethanol-fed mice. Further studies identified that the oestrogen-related receptor γ (ERR-γ) is an important downstream mediator of CB1 receptors contributing to ethanol-induced CYP2E1 expression, reactive oxygen species (ROS), and alcoholic liver injury. ERR-γ is a nuclear receptor and acts as a constitutive activator of transcription. No endogenous-activating ligand has been identified for this orphan receptor, but several inverse agonists have been found, including diethylstilbestrol, 4-hydroxytamoxifen (4-OHT) and its analogue GSK5182. Treatment with GSK5182 blocks CB1-mediated induction of CYP2E1 and subsequently ameliorates alcoholic liver damage.6 This suggests ERRγ is a critical link between CB1 receptors and CYP2E1 induction during alcoholic liver injury, which is an additional mechanism underlying the contribution of the endocannabinoid system to the pathogenesis of ALD besides to many previously identified mechanisms (figure 1).
Figure 1.
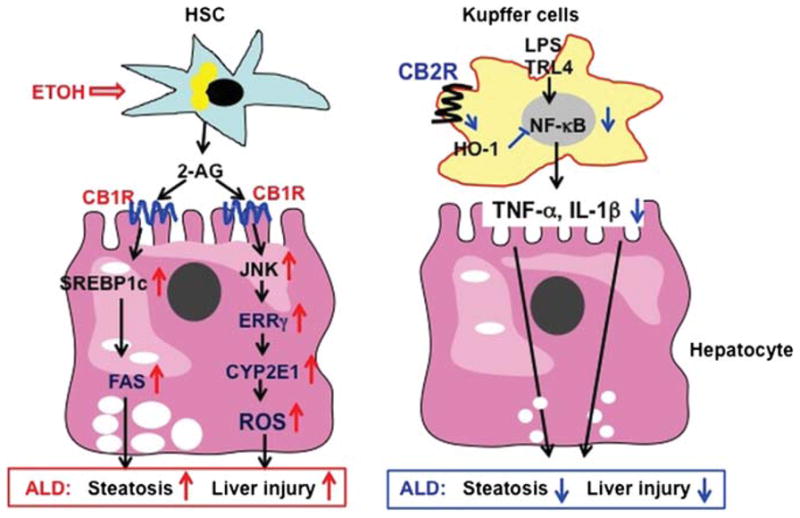
Opposing roles of CB1 and CB2 receptors in the pathogenesis of alcoholic liver disease (ALD). Chronic alcohol consumption stimulates hepatic stellate cell to release 2-arachidonoyl glycerol, which binds CB1 receptors on neighbouring hepatocytes, followed by activating SREBP-1c and CYP2E1, thereby exacerbating ALD. Chronic alcohol consumption may also activate CB2 receptors on Kupffer cells, followed by inhibiting the proinflammatory responses and ameliorating ALD.
The study by Kim DK et al has important pathophysiological implications. First, it suggests that activation of CB1 receptors is not only a fibrogenic pathway, but also represents an early step involved in the upregulation of the cell machinery that releases ROS during alcohol-induced liver damage. This finding, together with studies showing that endogenous cannabinoids regulate steatosis and cell damage in fatty liver disease,6 suggest that CB1 receptors are involved in several key pathological events that occur during ALD (ie, steatosis, inflammation and fibrogenesis). Targeting CB1 receptors may represent an appealing strategy to modulate both early and advanced ALD. However, the use of CB1 antagonists reaches the central nervous system in patients with liver diseases and is associated with many adverse effects, such as depression. Therefore, targeting peripheral rather than global CB1 receptors seems a more rational strategy to treat chronic liver diseases.7 Further preclinical studies using new peripheral CB1 antagonists in different models of ALD are required to confirm this hypothesis. Second, linking CB1 and CYP2E1 expression in the liver is a novel finding with important potential implications. CYP2E1 plays a role in the pathogenesis of ALD, and has also been implicated in oxidative stress in non-alcoholic fatty liver disease and chronic hepatitis C infection, and in the susceptibility to develop drug-induced liver injury.8 Moreover, CYP2E1 and oxidative stress have been implicated in the pathogenesis of hepatocellular carcinoma. Whether CB1-mediated CYP2E1 expression is also implicated in the pathogeneses of other chronic liver diseases deserves further investigation. Finally, the study reveals that a role for ERR-γ is an important downstream mediator of CB1 effects in the liver. This intriguing finding reinforces the role of nuclear receptor as key regulators of gene expression in fatty liver diseases.9 Although this study provides further insight into the important role of ERR-γ in experimental ALD, there are many unanswered questions that are needed to be addressed by future studies. First, do ERR-γ and other nuclear factors regulate other pathogenic effects of CB1 in the liver? Second, what is the role of ERR-γ in regulating gene expression during liver fibrogenesis? Finally, what are the signalling pathways linking ERR-γ and CB1? Whether the expression and/or activation of ERR-γ regulate the hepatic wound healing response to injury is largely unknown, and deserves further investigation. The study by Kim DK et al should help foster further interest in the role of nuclear receptors in chronic liver diseases.
Acknowledgments
Funding: None.
Footnotes
Contributors: Both authors contributed equally to revise the original paper by Kim et al, to write the commentary and approved the final version of our manuscript.
Competing interests: None.
Provenance and peer review: Commissioned; externally peer reviewed.
References
- 1.Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572–85. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tam J, Liu J, Mukhopadhyay B, et al. Endocannabinoids in liver disease. Hepatology. 2011;53:346–55. doi: 10.1002/hep.24077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Louvet A, Teixeira-Clerc F, Chobert MN, et al. Cannabinoid CB2 receptors protect against alcoholic liver disease by regulating Kupffer cell polarization in mice. Hepatology. 2011;54:1217–6. doi: 10.1002/hep.24524. [DOI] [PubMed] [Google Scholar]
- 4.Jeong WI, Osei-Hyiaman D, Park O, et al. Paracrine activation of hepatic CB1 receptors by stellate cell-derived endocannabinoids mediates alcoholic fatty liver. Cell Metab. 2008;7:227–35. doi: 10.1016/j.cmet.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 5.Kim DK, Jang H, Park J, et al. Estrogen-related receptor gamma controls hepatic CB1 receptor-mediated CYP2E1 expression and oxidative liver injury by alcohol. Gut. 2013;62:1044–54. doi: 10.1136/gutjnl-2012-303347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osei-Hyiaman D, Liu J, Zhou L, et al. Hepatic CB1 receptor is required for development of diet-induced steatosis, dyslipidemia, and insulin and leptin resistance in mice. J Clin Invest. 2008;118:3160–9. doi: 10.1172/JCI34827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kunos G, Osei-Hyiaman D, Bátkai S, et al. Should peripheral CB(1) cannabinoid receptors be selectively targeted for therapeutic gain? Trends Pharmacol Sci. 2009;30:1–7. doi: 10.1016/j.tips.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leung TM, Nieto N. CYP2E1 and oxidant stress in alcoholic and non-alcoholic fatty liver disease. J Hepatol. doi: 10.1016/j.jhep.2012.08.018. Published Online First: 28 Aug 2012. [DOI] [PubMed] [Google Scholar]
- 9.Vluggens A, Reddy JK. Nuclear receptors and transcription factors in the development of fatty liver disease. Curr Drug Metab. 2012 doi: 10.2174/138920012803762710. (in press) [DOI] [PubMed] [Google Scholar]


