Abstract
A new tracer, N-5-[18F]fluoropentylmaleimide ([18F]FPenM), for site-specific labeling of free thiol group in proteins and peptides was developed. The tracer was synthesized in three steps (18F displacement of the aliphatic tosylate, di-Boc removal by TFA to expose free amine and incorporation of the free amine into a maleimide). The radiosynthesis was completed in 110 min with 11–17% radiochemical yield (uncorrected), and specific activity of 20–49 GBq/µmol. [18F]FPenM showed comparable labeling efficiency with N-[2-(4-[18F]fluorobenzamido)ethyl]maleimide ([18F]FBEM). Its application was demonstrated by conjugation with glucagon-like peptide type 1 (GLP-1) analogue [cys40]-exendin-4. The cell uptake, binding affinity, imaging properties, biodistribution and metabolic stability of the radiolabeled [18F]FPenM-[cys40]-exendin-4 were studied using INS-1 tumor cells and INS-1 xenograft model. Positron emission tomography (PET) results showed that the new thiol-specific tracer, [18F]FPenM-[cys40]-exendin-4, had high tumor uptake (20.32 ± 4.36 %ID/g at 60 min post-injection) and rapid liver and kidney clearance, which was comparable to the imaging results with [18F]FBEM-[cys40]-exendin-4 reported by our group.
Introduction
Receptor targeting with radiolabeled peptides is an important and successful method for the diagnosis and treatment of neuroendocrine tumors1. The most prominent clinically applied peptides for in vivo imaging of human tumors are the somatostatin analogues2. The overexpression of many peptide receptors in cancers, compared to their relatively low density in normal organs, provides the rationale for in vivo molecular imaging and targeted radionuclide therapy. Among the available positron emitters for peptide labeling, fluorine-18 (18F) is most used due to its nuclear decay properties and chemical properties. The nuclide decays with a relatively low positron emission energy of 0.64 MeV, which provides a short tissue range (0.46 mm) and thus the potential for high image resolution. Its half-life (109.8 min) is long enough to allow complex, multistep radiosynthesis and allows image acquisitions for two or more hours post-injection. Labeling of organic molecules with 18F is amply described in the literature3–6.
Only a few examples for direct labeling of peptides or other macromolecules have been reported7–11, due to the harsh reaction conditions typically employed for 18F incorporation. To circumvent this drawback, a prosthetic group carrying the radioisotope is prepared and subsequently conjugated to a reactive functionality of macromolecule under mild conditions. N-succinimidyl-4-[18F]fluorobenzoate ([18F]SFB) is the most widely used 18F-prosthetic group for labeling peptides and proteins by conjugation of amine groups under mildly basic conditions (pH 8) and temperatures less than 40 °C12–17. Sometimes peptide labeling with [18F]SFB gives poor yield due to the steric hindrance of amino groups or unanticipated chemoselectivity and regioselectivity due to abundance of free amino groups in protein18, 19. Furthermore, a stoichiometric excess of valuable protein or peptide is often required to achieve sufficient radiochemical yields.
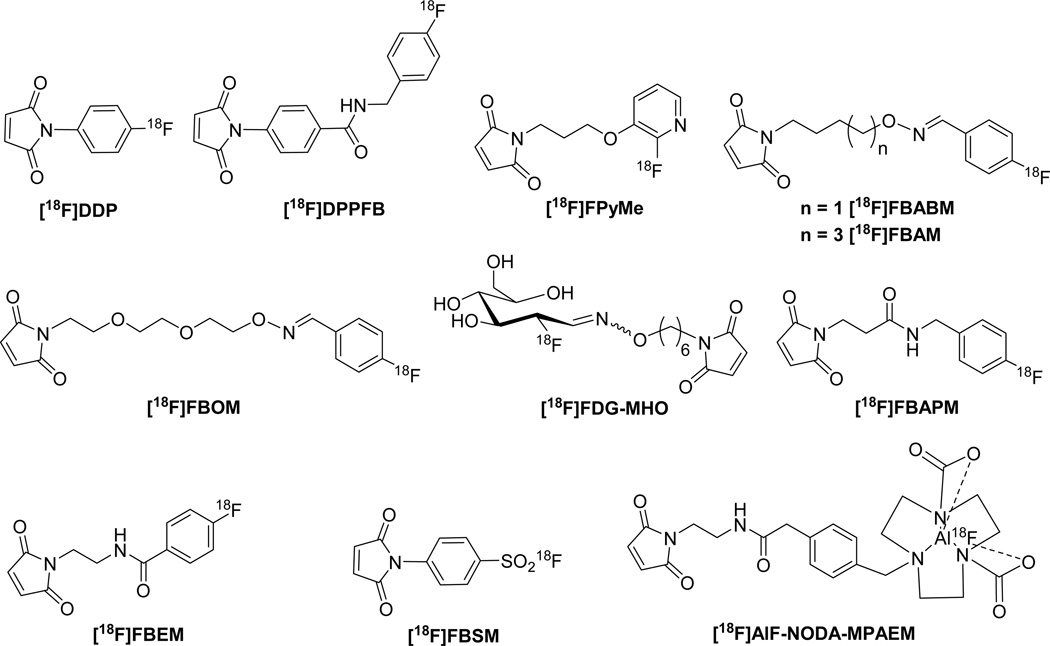
Alternative 18F-prosthetic groups for peptide labeling are maleimide-containing compounds that provide site-specific labeling of free thiol groups. Peptides and proteins can be engineered to incorporate or expose one cysteine residue to provide a site for specific labeling20, 21. There are several reports using maleimides as prosthetic groups for 18F PET study (Figure 1). The recently used 18F-prosthetic groups for thiol functions include [18F]FPPD and [18F]DDPFB developed by Shiue et al.22; aminooxy-aldehyde condensation series such as [18F]FBABM23, [18F]FBAM24, and [18F]FBOM25, which contained an aldehyde-reactive aminooxy group and a thiol-reactive maleimide group via readily available 4-[18F]fluorobenzaldehyde. [18F]FDG-MHO, another aminooxy-aldehyde condensation agent for thiol-specific labeling based on [18F]FDG was developed by Wuest et al.26. Dollé’s group reported the preparation of a heteroaromatic [18F]maleimide, 1-[3-(2-[18F]fluoropyridine-3-yloxy)propyl]pyrrole-2,5-dione ([18F]FPyME) in three steps with 17–20% uncorrected radiochemical yield27. N-[2-(4-[18F]fluorobenzamido)ethyl]maleimide ([18F]FBEM) is among the most often used prosthetic groups for site-specific radiolabeling of the free sulfhydryl groups of peptides and proteins. Two groups reported its synthetic methodology, either from previously reported [18F]SFB precursor28 or through more efficient direct condensation of 4-[18F]fluorobenzoic acid with N-(2-aminoethyl)maleimide29 with high specific activity. The other [18F]FBEM analogue, 4-[18F]fluorobenzylamidopropionyl maleimide ([18F]FBAPM) was synthesized through four radiolabeling steps with 55% decay corrected yield in 70 min and specific activity of 50–70 GBq/µmol. The route features highly efficient reduction of nitrile group using borohydride/transition metal catalyst method30.
Figure 1.
18F-labeled thiol site-specific prosthetic groups.
Recently, several investigators reported novel bifunctional prosthetic groups containing a maleimide group and a moiety for incorporation of [18F]fluoride in a single step. Inkster et al. reported sulfonyl fluoride-based prosthetic compounds as potential 18F labeling agents under aqueous conditions. One of the prosthetic groups, 4-N-maleimidobenzenesulfonyl [18F]fluoride ([18F]FBSM) was prepared in moderate yield, but the solvent and base selection during the preparation have a profound influence on the yield, and the exemplified 18F-peptide showed evidence of proteolytic defluorination and modification during incubation with mouse serum31. McBride et al., in an extension of their highly successful chelation of [18F]aluminum fluoride, developed [18F]AlF-NODA-MPAEM. This prosthetic group was conjugated to antibody Fab' fragment at room temperature in high yield without much loss of immunoreactivity. The method is useful for heat labile compounds32 (Figure 1).
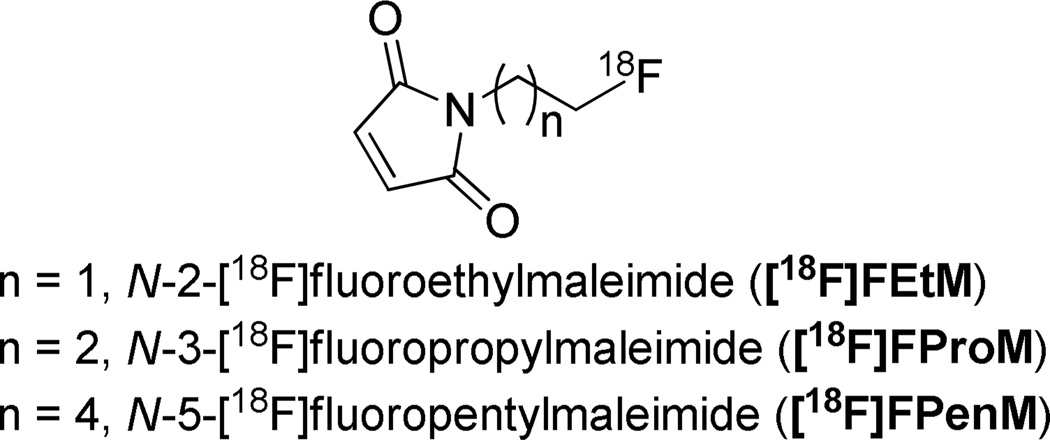
With the exceptions of [18F]FDG-MHO, [18F]AlF-NODA-MPAEM and [18F]FBSM, the above described radiofluorinations have been limited to nucleophilic aromatic substitutions. Activated aromatic rings bearing both a good leaving group and a strong electron-withdrawing component, especially located para to the leaving group are required during radiolabeling. However, heteroaromatic and aliphatic nucleophilic substitutions only require a good leaving group and the reactions are usually performed with dried no-carrier-added [18F]fluoride under basic conditions27, 33. Here we developed a new aliphatic maleimide-based prosthetic group based on aliphaticnucleophilic substitution. It was used for labeling GLP-1 receptor targeted peptide [cys40]-exendin-4 as an example (Figure 2).
Figure 2.
N-[18F]fluoroalkylmaleimide prosthetic groups.
Our group developed two new exendin-4 analogues by incorporation of a cysteine residue to either the C- or N-terminus of exendin-4, then site-specifically labeled with maleimide-containing prosthetic group [18F]FBEM34 or [18F]AlF-NOTA-MAL35. Results showed that both [18F]FBEM-[cys40]-exendin-4 and [18F]AlF-NOTA-MAL-[cys40]-exendin-4 have high tumor uptake but exhibit significantly different kidney uptake and clearance. In order to develop a more rapid radiochemical synthesis of a maleimide-based prosthetic group and to better understand the effect of the prosthetic group on the imaging profile of peptides, we describe here a new thiol-specific prosthetic agent, N-5-[18F]fluoropentylmaleimide ([18F]FPenM) based on aliphatic nucleophilic substitution and its conjugation with [cys40]-exendin-4. This new bifunctional agent has the lowest molecular weight among the maleimide-based tracers reported and, thus, would be expected to have a minimal effect on binding affinity compared to the parent exendin-4. The cell uptake, biodistribution, and imaging properties of [18F]FPenM-[cys40]-exendin-4 are described.
Experimental Section
Reagents and instrumentation
Unless otherwise stated, all chemicals were obtained from commercial sources and used without further purification. Analytical thin layer chromatography (TLC) was performed on Merck precoated silica gel 60 F254 plates with visualization by ultraviolet (UV) irradiation at λ = 254 nm or staining with KMnO4. Purifications were performed by silica gel chromatography. The water used in the experiments with [cys40]-exendin 4 (FW 4289.69) was deionized from Milli-Q Integral Water Purification System. [cys40]-exendin-4 was prepared by solid-phase peptide synthesis (CS Bio, Menlo Park, CA, USA). The [cys40]-exendin-4 sequence is His-Gly-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Leu-Ser-Lys-Gly-Met-Glu-Glu-Glu-Ala-Val-Arg-Leu-Phe-Ile-Glu-Trp-Leu-Lys-Asn-Gly-Gly-Pro-Ser-Ser-Gly-Ala-Pro-Pro-Pro-Ser-Cys-NH2.
Mass Spectrometry
LC/MS analysis was conducted on a Waters LC-MS system (Waters, Milford, MA) that included an Acquity UPLC unit coupled to the Waters Q-Tof Premier high resolution mass spectrometer. An Acquity BEH Shield RP18 column (150 × 2.1 mm) was employed for chromatography. Elution was achieved with isocratic 2 mM ammonium formate, 0.1% formic acid, and 5% CH3CN at 0.35 mL/min, The entire column elute was introduced into the Q-Tof mass spectrometer. Ion detection was achieved in ESI mode using a source capillary voltage of 3.5 kV, source temperature of 110 °C, desolvation temperature of 200 °C, cone gas flow of 50 L/Hr (N2), and desolvation gas flow of 700 L/Hr (N2).
Chemistry
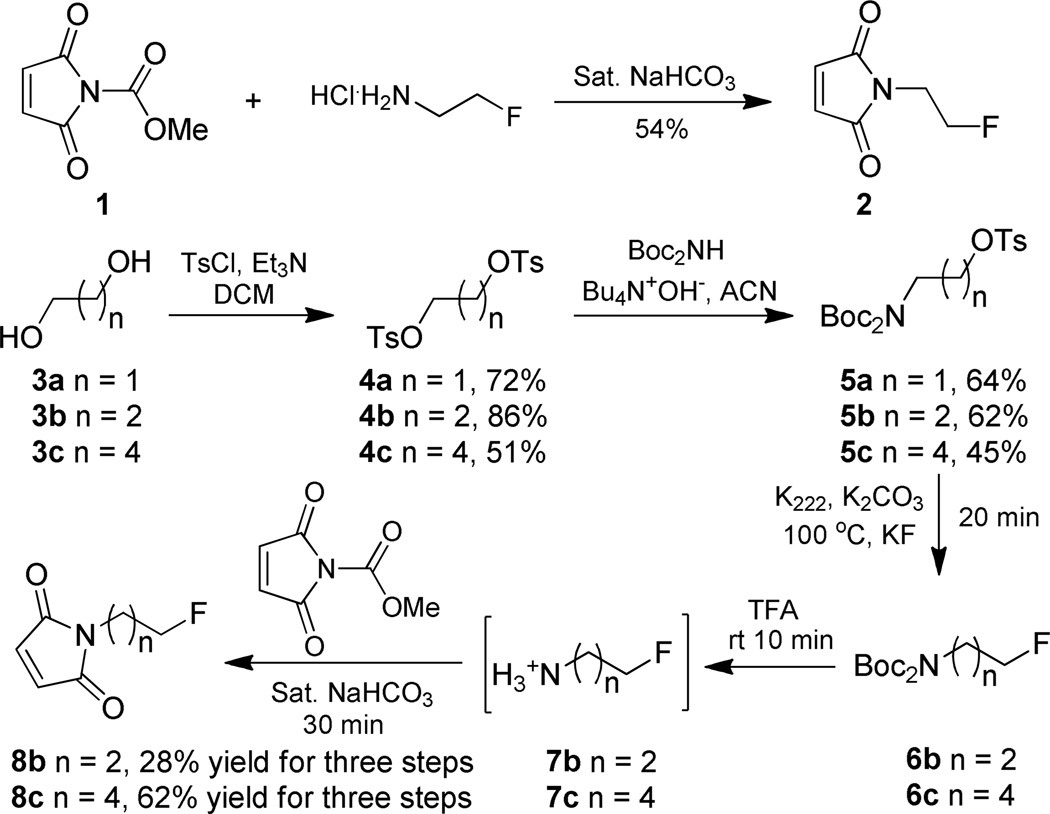
Compounds 136, 4a37, 4b38, 4c39, 5a40 (Scheme 1) are known compounds and were prepared according to literature procedures. Their physical and spectroscopic data are in agreement with those previously reported.
Scheme 1.
Synthesis of N-2-fluoroethylmaleimide 2N-3-fluoropropylmaleimide 8bN-5-fluoropentylmaleimide standards 8c respectively.
1-(2-fluoroethyl)-1H-pyrrole-2,5-dione, 2
Fluoroethylamine hydrochloride (110 mg, 2.2 mmol) was dissolved in a saturated aqueous solution of sodium bicarbonate (4 mL). The solution was cooled to 0 °C and stirred for 10 min before adding N-(methoxycarbonyl)maleimide 1 (310 mg, 2 mmol). The resulting solution was stirred at 0 °C for 10 min and at room temperature for an additional 20 min and the reaction was monitored by TLC. The mixture was directly subjected to silica gel flash chromatography using hexane/EtOAc (3/1) as the eluent to afford compound 2 (150 mg, 1.05 mmol, 53% yield) as a colorless solid. 1H NMR (300 MHz, CDCl3) δ 6.72 (s, 2H), 4.58 (t, J = 5.1 Hz, 1H), 4.42 (t, J = 4.8 Hz, 1H), 3.84 (t, J = 5.1 Hz, 1H), 3.76 (t, J = 5.1 Hz, 1H); 13C NMR (75.5 MHz, CDCl3) δ 170.5, 134.4, 80.5 (d, J = 171.4 Hz), 38.2 (d, J = 21.9 Hz); 19F NMR (282 MHz, CDCl3) δ −224.7 ∼ −225.2 (m); MS (APCI) 144.0 [M + H]+; HRMS (APCI): Calcd for C6H7NO2F, 144.0461 [M + H]+, found 144.0457.
Typical procedure for the synthesis of compounds 2-[bis(tert-butoxycarbonyl)amino]ethoxy(4-methylphenyl)-dioxo-λ6-sulfane 5a, 3-[bis(tert-butoxycarbonyl)amino]propoxy(4-methylphenyl)-dioxo-λ6-sulfane 5b, and 5-[bis(tert-butoxycarbonyl)amino]pentoxy(4-methylphenyl)-dioxo-λ6-sulfane 5c
Commercial di-tert-butyl iminodicarboxylate (1 eq.) was dissolved in ethanol and treated with 1.1 equivalents of 40% aqueous n-Bu4N+OH− at room temperature for 1 h. The solvents were removed and the residue dried by repeated evaporation with acetonitrile. To the solution of the resulting salt in acetonitrile was added the bis-tosylate of ethylene glycol (1.1 eq.). The resulting solution was stirred at room temperature and monitored by TLC until the starting material was completely consumed. The reaction solution was diluted with water and extracted with CH2Cl2. The organic layer was dried and evaporated. The residue was subjected to flash chromatography using hexane/EtOAc (4/1) as the eluent to give the title compound 5.
Compound 5a
Colorless solid, 2.44 g, yield 64%. 1H NMR (300 MHz, CDCl3) δ 7.72 (d, J = 6.6 Hz, 2H), 7.30 (d, J = 8.1 Hz, 2H), 4.07 (t, J = 5.7 Hz, 2H), 3.83 (t, J = 5.7 Hz, 2H), 2.38 (s, 3H), 1.42 (s, 18H).
Compound 5b
Colorless solid, 1.32 g, yield 62%. 1H NMR (300 MHz, CD3OD) δ 7.81 (d, J = 8.4 Hz, 2H), 7.47 (d, J = 8.1 Hz, 2H), 4.08 (t, J = 6.3 Hz, 2H), 3.64 (t, J = 6.6 Hz, 2H), 2.47 (s, 3H), 1.91 (t, J = 7.2 Hz, 2H), 1.53 (s, 18H); 13C NMR (75.5 MHz, CDCl3) δ 153.9, 146.6, 134.6, 131.3, 129.2, 84.1, 69.9, 29.9, 28.4, 21.7; MS (APCI) 447.2 [M + NH4]+; HRMS (APCI): Calcd for C20H35N2O7S, 447.2165 [M + NH4]+, found 447.2166.
Compound 5c
Liquid, 0.41g, yield 45%. 1H NMR (300 MHz, CD3OD) δ 7.92 – 7.89 (m, 2H), 7.56 (d, J = 7.8 Hz, 2H), 4.18 – 4.14 (t, J = 6.3 Hz, 2H), 3.67 – 3.61 (m, 2H), 2.57 (s, 3H), 1.83 – 1.72 (m, 2H), 1.70 – 1.65 (m, 2H), 1.64 (s, 18H), 1.57 – 1.41 (m, 2H); 13C NMR (75.5 MHz, CDCl3) δ 154.0, 146.3, 134.6, 131.2, 128.9, 83.5, 71.8, 47.0, 29.5, 29.4, 28.5, 23.7, 21.8; MS (APCI) 475.2 [M + NH4]+; HRMS (APCI): Calcd for C22H39N2O7S, 475.2478 [M + NH4]+, found 475.2478.
Typical procedure for the synthesis of 1-(3-fluoropropyl)-1H-pyrrole-2,5-dione 8b and 1-(5-fluoropentyl)-1H-pyrrole-2,5-dione 8c
To a test tube with a stirring bar was added K222 (1 eq.) in acetonitrile (1 mL), and potassium fluoride (2 eq.). The solvents were evaporated under a stream of argon while being heated in a 100 °C oil bath. Three portions of acetonitrile (1 mL) were added successively and each evaporated in turn. Then tosylate substrate 5b (or 5c) (1 eq.) in acetonitrile (1 mL) was added. The reaction solution was heated at 100 °C for 20 min. The solvent was removed under a stream of argon. The residue was treated with TFA (1 mL) at room temperature for 10 min. After evaporation of TFA with argon flow, the reaction was placed in 0 °C ice water, saturated sodium bicarbonate (4 mL) and N-(methoxycarbonyl)maleimide (2 eq.) was added successively. The solution was stirred at 0 °C for 20 min and then at room temperature for 10 min, then subjected to silica gel chromatography directly using hexane/EtOAc (4/1) as the eluent to afford compound 8b (or 8c).
Compound 8b
Colorless solid, 28% yield for three steps, 1H NMR (300 MHz, CDCl3) δ 6.73 (s, 2H), 4.56 (t, J = 5.7 Hz, 1H), 4.41 (t, J = 5.7 Hz, 1H), 3.70 (t, J = 6.9 Hz, 2H), 2.08 – 2.04 (m, 1H), 1.99 – 1.95 (m, 1H); 13C NMR (75.5 MHz, CDCl3) δ 170.9, 134.4, 81.8 (d, J = 166.1 Hz), 34.8 (d, J = 21.0 Hz), 29.6 (d, J = 78.0 Hz); 19F NMR (282 MHz, CDCl3) δ −220.9 ∼ −221.4 (m); MS (APCI) 158.1 [M + H]+; HRMS (APCI): Calcd for C7H9NO2F, 158.0617 [M + H]+, found 158.0612.
Compound 8c
Liquid, 62% yield for three steps, 1H NMR (300 MHz, CDCl3) δ 6.71 (s, 2H), 4.53 (t, J = 6.0 Hz, 1H), 4.37 (t, J = 6.0 Hz, 1H), 3.56 (t, J = 7.2 Hz, 2H), 1.81 – 1.60 (m, 4H), 1.48 – 1.28 (m, 2H); 13C NMR (75.5 MHz, CDCl3) δ 171.0, 134.3, 84.0 (d, J = 164.6 Hz), 37.8, 30.1 (d, J = 78.0 Hz), 28.4, 22.7 (d, J = 21.0 Hz); 19F NMR (282 MHz, CDCl3) δ −218.4 ∼ −218.9 (m); MS (APCI) 186.1 [M + H]+; HRMS (APCI): Calcd for C9H13NO2F, 186.0930 [M + H]+, found 186.0927.
General procedure for the synthesis of nonradioactive fluoroalkylmaleimido-[cys40]-exendin-4
[cys40]-exendin-4 was dissolved in degassed phosphate-buffered saline (PBS), N-2-fluoroethylmaleimde or N-5-fluoropentylmaleimide in EtOH was added in one portion and incubated for 1 h, then the mixture was subjected to semi-preparative HPLC chromatography to give N-2-fluoroethylmaleimido-[cys40]-exendin-4 and N-5-fluoropentylmaleimido-[cys40]-exendin-4, respectively. For semi-preparative HPLC, a Vydac C18 protein column (9.4 × 250 mm) and a gradient elution profile were used with 0.1% trifluoroacetic acid (TFA) in water (solvent A) and 0.1% TFA in CH3CN (solvent B). The elution profile was isocratic at 25% solvent B for 5 min, then a gradient to 55% solvent B over 20 min, and finally to 90% B over the next 5 min. The major peak at about 17 min was collected and lyophilized. For analytical HPLC, a Vydac C18 protein column (4.6 × 250 mm) was used with a gradient elution profile using water (solvent A), CH3CN (solvent B), 1 mM EDTANa2, and 1% TFA in water (solvent C). The gradient used was 65% water, 25% acetonitrile, 10% solvent C for 5 min, then a linear gradient to 35% water, 55% acetonitrile, 10% solvent C by 25 min, and a linear gradient to 90% acetonitrile, 10% solvent C at 30 min.
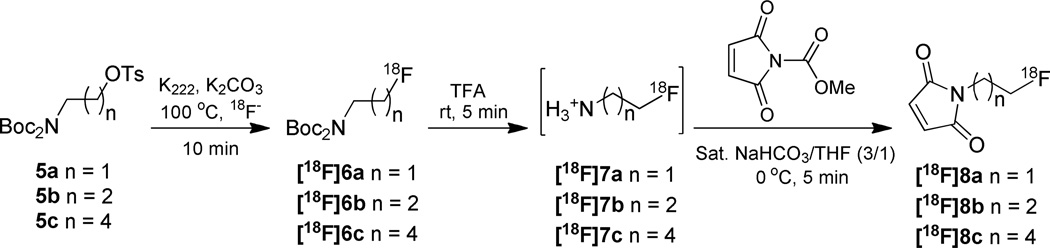
Typical procedure for the synthesis of compounds [18F]8a, [18F]8b and [18F]8c
To a test tube was added K222 (4.5 mg, 12 µmol in 100 µL acetonitrile), K2CO3 (60 µL of 0.1 M in water, 6 µmol), and [18F]fluoride (59 – 77 mCi). The solvents were evaporated under a stream of argon while being heated in a 100 °C heating block. Three portions of acetonitrile (200 µL) were added successively and each evaporated in turn, the drying procedure took about 10 min. Then tosylate 5 (2.5 – 3.0 mg, 6.0 µmol) in acetonitrile (200 µL) was added. The reaction solution was heated at 100 °C for 10 min. The reaction solution was cooled to room temperature and diluted with CH2Cl2 (200 µL); the mixture was loaded onto a short plug of silica gel and eluted with another portion of CH2Cl2 (400 µL) into a 13 × 100 mm test tube. The isolated yield for this radiolabeling step was calculated by dividing the amount of activity in the eluent by the total initial activity used. The eluate was evaporated to dryness under a stream of argon at room temperature. Trifluoroacetic acid (TFA, 200 µL) was added to the residue and allowed to stand for 5 min. The TFA was evaporated with a stream of argon. The reaction tube was then cooled in an ice bath and the following reagents were added in sequence: saturated NaHCO3 (450 µL); and N-(methoxycarbonyl)maleimide (9.3 mg, 60 µmol) in THF (150 µL). Five min later, the solution was diluted with 0.1% TFA in H2O (0.3 mL), and subjected to semi-preparative HPLC directly (Phenomenex Luna 5 µm C18 column, 250 × 10 mm, flow rate 4.0 mL/min, solvent A, 0.1% TFA in water, solvent B, 0.1% TFA in acetonitrile, using isocratic with 30% solvent B, UV detection at 210 nm and on-line radioactivity detection, product tR = 26.1 min). The collected fraction (typically 19 – 28% uncorrected yield) was diluted with 10 mL water and the product was trapped on a Sep-Pak C18 plus cartridge (360 mg sorbent per cartridge). The cartridge was washed with H2O (3 mL) and hexane (2 mL), and the product was eluted with 10% EtOH in CH2Cl2 (1.5 mL). The solvent was removed under argon flow. The three-step radiolabeling was completed in an average time of 110 min with 11–17% uncorrected yield.
For quality control, the concentrated sample was analyzed by analytical HPLC (Phenomenex Luna 3 µm C18 column, 150 × 4.6 mm, flow rate 1.0 mL/min, solvent A, 0.1% TFA in water, solvent B, 0.1% TFA in acetonitrile), using isocratic elution with 35% solvent B; product eluted at 8.1 min. Specific activity was estimated at 210 nm of wavelength by comparison with a standard curve.
Radiochemical synthesis of [18F]FPenM-[cys40]-exendin-4
To the above [18F]FPenM (8–11 mCi) was added ethanol (10 µL) and the tube was vortexed. [cys40]-exendin-4 (100–200 µg) in 100 µL 0.1% sodium ascorbate in degassed PBS was added, and the solution was vortexed followed by incubation for 30 min at room temperature. Aqueous TFA (0.1% TFA, 100 µL) was added and the solution was injected onto a semi-preparative HPLC system (as described above for nonradioactive fluoroalkylmaleimido-[cys40]-exendin-4 with the exception that the first gradient went only to 45% B over 30 min). The elution time of the radioactive product was about 29.3 min. The fraction containing [18F]FPenM-[cys40]-exendin-4 was diluted to 20 mL with water and the solution passed through a C18 BondElut cartridge (100 mg). The product that remained on the cartridge was eluted with 1 mL of 10 mM HCl in ethanol. The ethanol was concentrated on a rotary evaporator to about 100 – 200 µL. A portion of this remaining volume was used for determination of radiochemical purity. The remaining volume was diluted in PBS for in vitro and in vivo studies. For quality control, the same analytical HPLC condition as preparation of nonradioactive fluoroalkylmaleimido-[cys40]-exendin-4 was used. HPLC-MS 1119.4 [M + 4H]4+; deconvolves to 4474.0. Calculated exact mass: C196H299FN52O63S2 (4474.128).
Mouse serum stability study
[18F]FPenM-[cys40]-exendin-4 (50 µCi) was mixed with 200 µL freshly harvested mouse serum and a 50 µL aliquot was removed at 0 min. The remainder was incubated at 37 °C and additional aliquots of 50 µL were removed at 30 and 150 min. The aliquots were mixed with an equal volume of CH3CN and centrifuged. A portion of the supernatant was subjected to radioHPLC analysis using an on-line radioactivity detector.
Cell culture
INS-1 cell line was cultured in RPMI-1640 medium (GIBCO) containing 10% (v/v) fetal bovine serum (GIBCO) supplemented with penicillin (100 µg/mL), streptomycin (100 µg/mL), non-essential amino acids (100 µM) and sodium pyruvate (1 mM) at 37 °C with 5% CO2.
Animal model
All animal studies were conducted in accordance with the principles and procedures outlined in the Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Clinical Center, NIH. The INS-1 xenograft tumor models were developed in 5 to 6-week old female athymic nude mice (Harlan Laboratories) by injection of 5 × 106 cells in the left or right shoulders. Tumor growth was monitored using caliper measurements of perpendicular axes of the tumor. The tumor volume was estimated by the formula: tumor volume = a × (b2)/2, where a and b were the tumor length and width, respectively, in millimeters. The mice underwent small-animal PET studies when the tumor volume reached 100–300 mm3 (3–4 weeks after inoculation).
Receptor binding assay
The GLP-1 receptor binding assay was performed to determine binding affinities of FEtM-[cys40]-exendin-4, FPenM-[cys40]-exendin-4, FBEM-[cys40]-exendin-4, and exendin-4. GLP-1 analogs were dissolved in DMSO and diluted by cell binding buffer (1 mM CaCl2, 5 mM MgCl2, 0.5% BSA in RPMI medium) to the desired concentrations immediately before usage. At 80% confluence, the cultured cells were scraped off and suspended in binding buffer at a final concentration of 2 × 106 cells/mL. The cells were aliquoted into a 96 well plate (1 × 105 INS-1 cells/well) and incubated with 125I-GLP-1(7–36) (0.02 µCi/well, PerkinElmer, Inc) in binding buffer in the presence of different concentrations of GLP-1 analogs at room temperature for 2 h. The final volume per well is 200 µL. After incubation, the plate was washed three times with PBS containing 0.1% BSA, and the radioactivity was measured by γ-counting. The IC50 values were calculated by nonlinear regression analysis using the GraphPad Prism computer-fitting program (GraphPad Software, Inc., San Diego, CA). Each data point is a result of the average of duplicate wells.
Cell uptake and efflux studies
For cell uptake, INS-1 cells were seeded into 24-well plates at a density of 1 × 105 cells per well and incubated with 37 kBq (1 µCi) per well of [18F]FPenM-[cys40]-exendin-4 in 500 µL of serum free cell culture medium at 37 °C for 15, 30, 60, and 120 min. The cells were then washed three times with chilled PBS and lysed with 200 µL 0.1 M NaOH. For efflux studies, about 37 kBq (1 µCi) per well of [18F]FPenM-[cys40]-exendin-4 were first incubated with INS-1 cells in 24-well plates for 1 h at 37 °C. The cells were washed three times with chilled PBS and allowed to stand with fresh buffer at 37 °C. At various time points (0, 30 and 60 min), the medium was removed and the cells washed three times with chilled PBS. The cells were then lysed with 200 µL 0.1 M NaOH. The cell suspensions were collected and measured in a gamma counter. Each data point is an average of triplicate values.
MicroPET imaging
PET scans and image analysis were performed using an Inveon microPET scanner (Siemens Medical Solutions). [18F]FPenM-[cys40]-exendin-4 (∼100 µCi) was administered via tail vein injection while the animal was under isoflurane anesthesia. Five-minute and ten-minute static PET images were acquired at 30 min and 60 min post-injection (p.i.; n = 4/group), respectively. For blocking experiment, 75 µg [cys40]-exendin-4 was injected 10 min prior to the injection of [18F]FPenM-[cys40]-exendin-4. The images were reconstructed using a three-dimensional ordered-subset expectation maximization (3D OSEM) algorithm without correction for attenuation or scattering. For each scan, regions of interest (ROIs) were drawn over the tumor and major organs using Inveon Workplace 3.0 on decay-corrected whole-body coronal images. The radioactivity concentrations within the tumors, muscle, liver, and kidneys were obtained from mean pixel values within the multiple ROI volume and then converted to MBq/mL/min using the calibration factor determined for the Inveon PET system. These values were then divided by the administered activity to obtain (assuming a tissue density of 1 g/mL) an image-ROI-derived percent injected dose per gram (%ID/g).
Biodistribution studies and metabolite analysis
Immediately after PET imaging, the tumor-bearing mice were sacrificed and dissected. Blood, tumor, major organs, and tissues were collected and wet-weighed. The radioactivity in the wet whole tissue was measured with a γ-counter (Wallach Wizard, PerkinElmer). The results were expressed as percentage of injected dose per gram of tissue (%ID/g) for a group of 4 animals. For each mouse, the radioactivity of the tissue samples was calibrated against a known aliquot of the injected radiotracer. Values were expressed as mean ± SD. For a blocking study, tumor xenografted mice were pretreated with 75 µg [cys40]-exendin-4 10 min prior to injection of the corresponding [18F]FPenM-[cys40]-exendin-4. A subset of the collected tissues (homogenized tumor and kidneys, plasma, and urine) was extracted with a volume of CH3CN equal to the weight of the sample. The mixtures were centrifuged and the supernatant separated from the pellet. Supernatants were analyzed by HPLC using on-line radioactivity detection or via collection of 1 min fractions and subsequent γ-counting.
Results
Synthesis
N-2-Fluoroethylmaleimide 2 was prepared from commercially available 2-fluoroethylamine hydrochloride with a straightforward strategy (Scheme 1). N-methoxycarbonylmaleimide 1, was subjected to reaction with 2-fluoroethylamine hydrochloride in saturated sodium bicarbonate solution to give fluoroethylmaleimide standard 2 41. The condensation was performed at 0 °C, and worked up immediately upon completion as indicated by thin layer chromatography. Extended reaction time at room temperature resulted in hydrolytic decomposition of the maleimide.
The N-3-fluoropropylmaleimide and N-5-fluoropentylmaleimide were obtained from similar procedures (Scheme 1). Firstly commercially available 1,3-propanediol 3b and 1,5-pentanediol 3c were converted into the corresponding di-tosylate 4b and 4c respectively. SN2 displacement of one tosylate of 4b and 4c with di-tert-butyl iminodicarboxylate afforded compounds 5b and 5c, respectively, according to the reported procedure40. With 5b and 5c in hand, fluorine incorporation was achieved by displacement of the remaining tosylate with K222/KF. Deprotection of the Boc functional group with TFA at room temperature afforded the free fluoroalkylamine. The conjugation between resulting primary amine intermediate and N-methoxycarbonylmaleimide proceeded smoothly under aqueous conditions as described above. N-3-fluoropropylmaleimide 8b and N-5-fluoropentylmaleimide 8c were obtained in 28% and 62% yield, respectively in three steps after silica gel chromatography. Compounds 2 and 8b are colorless solids while 8c is light yellow liquid. The conjugation of 2 and 8c with [cys40]-exendin-4 proceeded smoothly in PBS buffer with 0.1% sodium ascorbate as the antioxidant.
The three synthesized compounds 5a, 5b, and 5c with different aliphatic chains (ethyl, propyl and pentyl, respectively) were evaluated for labeling with 18F (Scheme 2). For substrate 5a, the radiofluorination proceeded in low yield (20–30%, decay uncorrected), perhaps due to the volatility of the resulting product. However, when propyl analogue 5b and pentyl analogue 5c were employed as the substrates, the fluorine incorporation in the first step typically afforded 50% and 65% decay uncorrected yields, respectively. After deprotection of the amine with TFA, the resulting fluoroalkylamine salt was subjected to reaction with N-methoxycarbonylmaleimide. The optimized conditions for this exchange reaction employed saturated NaHCO3/THF (3/1, v/v) as solvent and 0 °C as the reaction temperature. A 10-fold excess of N-methoxycarbonylmaleimide 1 (60 µmol) relative to substrate 5b or 5c (6 µmol) was used to shorten the reaction time to 5 min. Poor trapping efficiency was observed for product [18F]8b, but product [18F]8c with its longer aliphatic chain was readily purified and isolated from HPLC eluate. The overall yield of the three-step production of [18F]8c based on initial [18F]fluoride was 11–17% (uncorrected) after purification and isolation by HPLC. The entire synthesis procedure was completed in an average time of 110 min with product specific activity of 20–49 GBq/µmol. [18F]FPenM was chosen as the prosthetic group for coupling with [cys40]-exendin-4 according to the typical procedure 34.
Scheme 2.
18F radiolabeling of tosylate substrates 5a, 5b, 5c.
Receptor binding assay
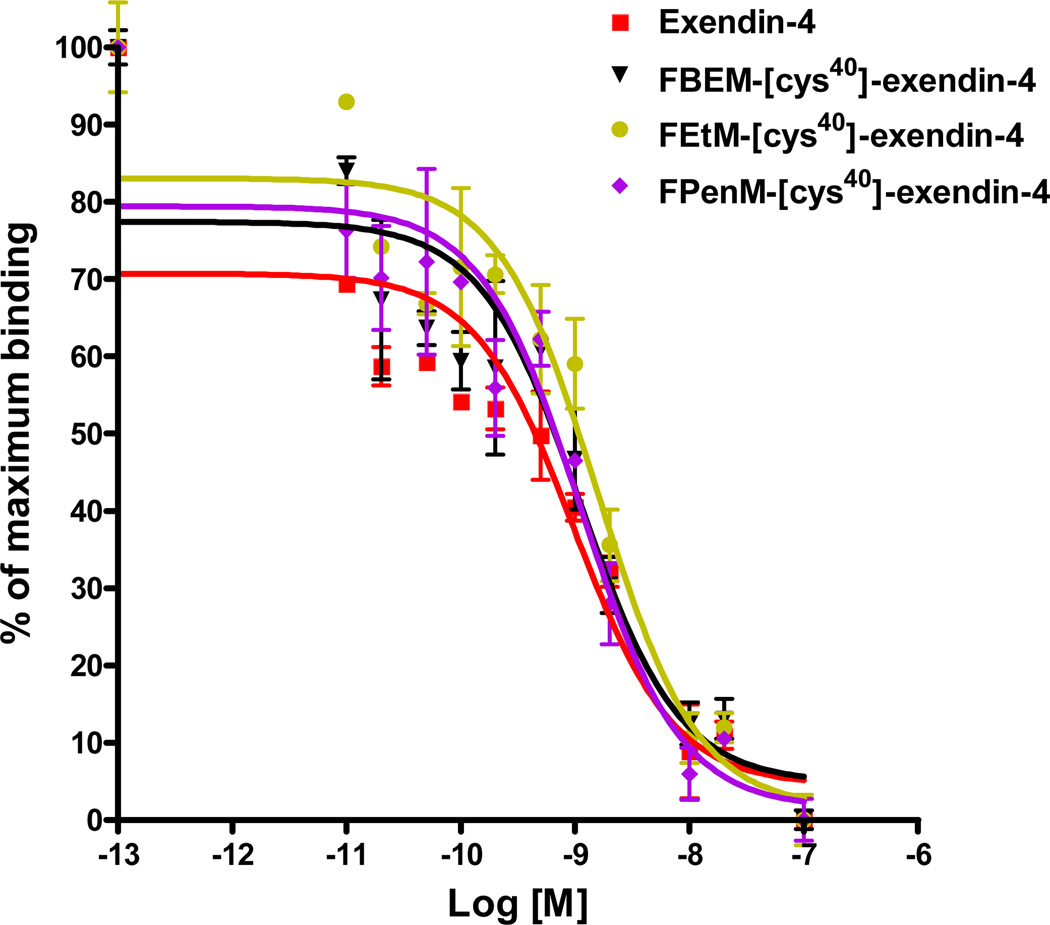
IC50 values of exendin-4, FBEM-[cys40]-exendin-4, FEtM-[cys40]-exendin-4 and FPenM-[cys40]-exendin-4 against 125I-GLP-1 in INS-1 cells are shown in Figure 3. Of the two modified tracers synthesized, FPenM-[cys40]-exendin-4 has comparable IC50 (1.11 nM) with parent exendin-4 (0.98 nM), which also showed almost the same binding affinity as FBEM-[cys40]-exendin-4 (1.10 nM). FEtM-[cys40]-exendin-4 exhibited slightly lower binding affinity (1.57 nM) compared to parent exendin-4 and FBEM-[cys40]-exendin-4. These results indicated that labeling [cys40]-exendin-4 with our newly developed FPenM did not reduce binding affinity towards INS-1 cells.
Figure 3.
Competitive binding assay (IC50) of exendin-4 ( , red), FBEM-[cys40]-exendin-4 (▼, black), FEtM-[cys40]-exendin-4 (
, red), FBEM-[cys40]-exendin-4 (▼, black), FEtM-[cys40]-exendin-4 ( , brown yellow), and FPenM-[cys40]-exendin-4 (
, brown yellow), and FPenM-[cys40]-exendin-4 ( , purple).
, purple).
Mouse serum stability
Serum stability, as analyzed by radioHPLC, showed that [18F]FPenM-[cys40]-exendin-4 was stable in mouse serum up to 2.5 h at 37 °C, with only a negligible quantity of polar component produced (Figure S1), which may be due to the oxidation of the methionine in [cys40]-exendin-434. We found that the polar component could be minimized by adding EDTANa2 to the analytical HPLC solvent as metal chelator during analysis. The extraction efficiency from the mouse serum was around 85% based on the supernatant fraction we injected for on-line radio-HPLC analysis.
Cell uptake and clearance
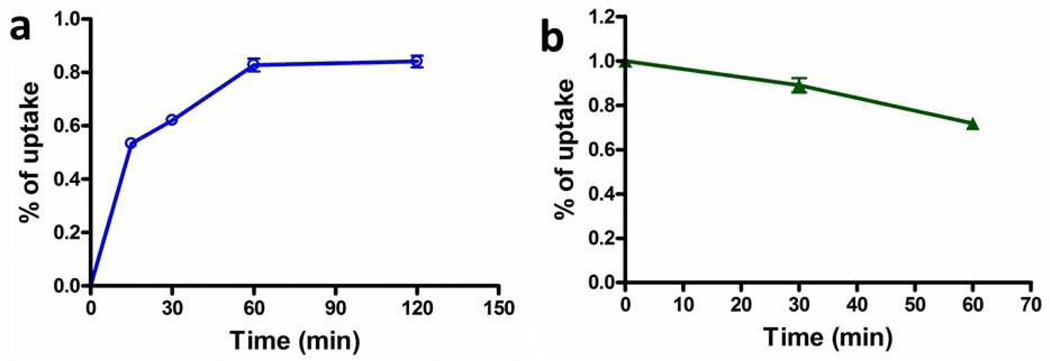
The total cell uptake kinetics of [18F]FPenM-[cys40]-exendin-4 with INS-1 tumors cells are shown in Figure 4. The cell uptake of [18F]FPenM-[cys40]-exendin-4 showed 0.62 ± 0.03 %AD at 0.5 h, increasing to 0.83 ± 0.06 %AD after 1 h, then the uptake reached a plateau. The cell efflux was performed after 60 min incubation, the cell retention was reduced to 0.89 ± 0.08 %AD at 30 min and 0.72 ± 0.06 %AD at 60 min. The specificity of the radiotracer was confirmed by minimum cell uptake of [18F]FPenM-[cys40]-exendin-4 (< 0.1%AD) in the presence of blocking dose of exendin-4 (1 µM).
Figure 4.
Total cellular uptake (a,  ) and efflux (b,
) and efflux (b,  ) of [18F]FPenM-[cys40]-exendin-4.
) of [18F]FPenM-[cys40]-exendin-4.
Imaging and biodistribution in INS-1 xenograft
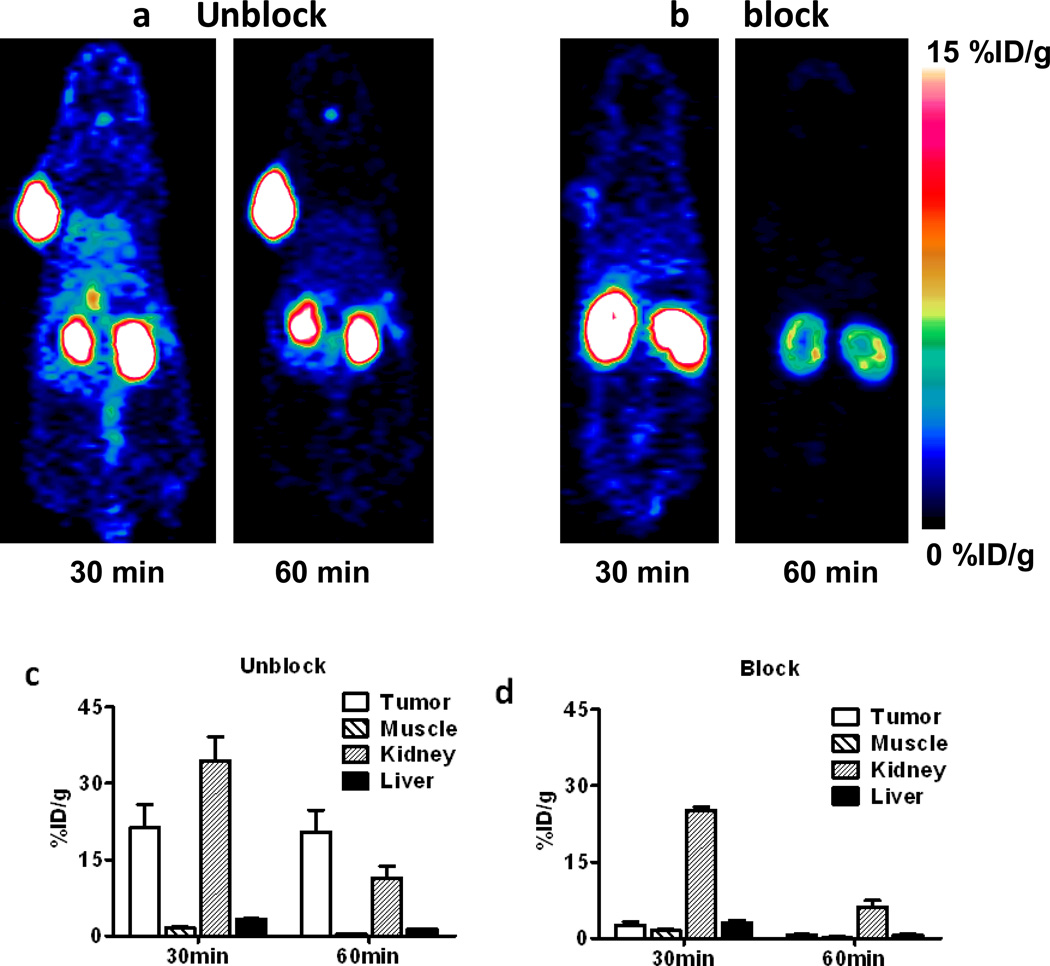
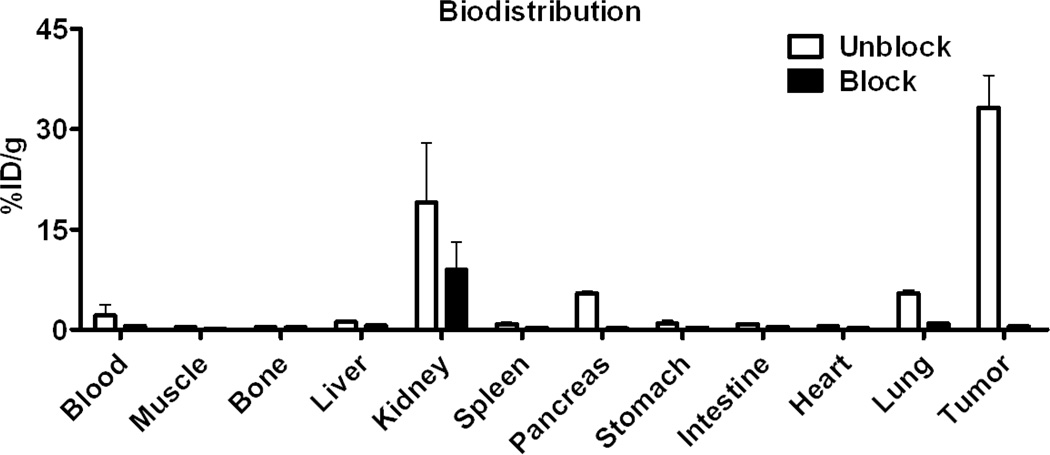
High tumor uptake was apparent by microPET imaging (Figure 5) at 30 min post-injection of [18F]FPenM-[cys40]-exendin-4 (21.30 ± 4.55 %ID/g), and remained virtually unchanged (20.32 ± 4.36% ID/g) at 60 min after tracer injection. In contrast, kidney uptake of [18F]FPenM-[cys40]-exendin-4 was high (34.41 ± 4.59 %ID/g) at 30 min and was significantly reduced by 60 min post-injection (11.30 ± 2.41 %ID/g). Similar clearance was observed from liver (3.36 ± 0.02 %ID/g at 30 min vs. 1.41 ± 0.02 %ID/g at 60 min). Negligible tracer uptake was observed in the muscle. Injection of a blocking dose of [cys40]-exendin-4 prior to tracer injection resulted in significant reduction of tumor uptake at 30 min (2.65 ± 0.63 %ID/g), but kidney and liver uptake showed slight reduction, indicating receptor specific uptake of the tracer to INS-1 tumor. Quantification of the PET data at the 1 h time point showed high tumor-to-kidney (1.80) and tumor-to-liver (8.43) ratios. The specific tumor uptake of [18F]FPenM-[cys40]-exendin-4 was further confirmed by biodistribution, which showed tumor-to-kidney and tumor-to-liver ratios of 1.75 and 28.14 respectively (Figure 6), and significant blocking was observed by injection of [cys40]-exendin-4 prior to tracer administration. Negligible uptake was observed in the bone, indicating the stability of the tracer to defluorination. The blocking dose resulted in a reduction in uptake in the kidneys, pancreas, and lung.
Figure 5.
Representative PET images of [18F]FPenM-[cys40]-exendin-4 (100 µCi) in INS-1 mouse xenograft models at 30 and 60 min post injection: (a) control; (b) INS-1 with a blocking dose of 75 µg [cys40]-exendin-4 administered 10 min prior to the radiotracer; (c) time course of uptake by selected tissues determined by quantification of PET ROIs; (d) time course of uptake by selected tissues determined by quantification of PET ROIs following blocking dose.
Figure 6.
Biodistribution of [18F]FPenM-[cys40]-exendin-4 (100 µCi) at 1 h measured by radioactive counting of dissected tissues with or without [cys40]-exendin-4 (75 µg) blocking dose prior to tracer injection.
The metabolic fate of the [18F]FPenM-[cys40]-exendin-4 in tumor, kidneys and urine were investigated by analysis of samples extracted following biodistribution studies in INS-1 tumor-bearing mice. The extracts from organs were analyzed by radio-HPLC and then compared to parent [18F]FPenM-[cys40]-exendin-4. The resulting radio-HPLC chromatograms indicated that urine contained only polar radioactive metabolites and the kidneys contained a mixture of parent [18F]FPenM-[cys40]-exendin-4 and polar radioactive metabolites; however, the tumor contained predominately parent peptide (Figure S2).
Discussion
We have developed a new thiol-specific prosthetic agent, [18F]FPenM, based on aliphatic nucleophilic substitution No additional activating functional groups are required as in the case of homoaromatic fluorination. Because the maleimide ring is not stable under typical nucleophilic radiofluorination conditions, a three-step radiolabeling procedure was employed: 1) 18F incorporation by SN2 displacement on an aliphatic tosylate substrate; 2) TFA acidolysis of amine protecting group; 3) incorporation of [18F]fluoroalkylamine into a maleimide via transamidation. Three tosylate substrates 5a, 5b, and 5c, with different lengths of aliphatic chains and hence different lipophilicity, were screened for 18F labeling.
Substrate 5a exhibited very low yield in the fluorination step, possibly due to the volatility of the resulting product. However, reaction of substrates 5b and 5c afforded higher yield for this labeling step. The subsequently produced fluoromaleimides 8a and 8b presented with an unanticipated problem.
Poor trapping efficiency was obtained for products [18F]8a and [18F]8b using solid-phase extraction of the crude reaction mixtures directly or from fractions following semi-preparative HPLC. However, product [18F]8c, with its longer aliphatic chain, was much more efficiently isolated from the HPLC eluate. The high trapping efficiency for the most lipophilic [18F]8c led us to apply this to the synthesis of [18F]FPenM-[cys40]-exendin-4.
We prepared authentic samples of FEtM-[cys40]-exendin-4 and FPenM-[cys40]-exendin-4 in order to evaluate the binding affinity relative to GLP-1. The binding affinities of exendin-4, FPenM-[cys40]-exendin-4, and FBEM-[cys40]-exendin-4 were about 1 nM and not significantly different from each other. These affinities are higher than previously reported NOTA-MAL-[cys40]-exendin-4 (2.84 nM) 35 and [Lys40(natGa-DOTA)]exendin-3 (5.7 nM) 41. [18F]FPenM-[cys40]-exendin-4 showed quite low uptake into INS-1 tumor cells. However, the efflux of radiolabeled compound was also quite slow. This cell uptake and efflux behavior was similar to that reported for FBEM-[cys40]-exendin-434.
In INS-1 tumor xenograft model, microPET imaging of [18F]FPenM-[cys40]-exendin-4 showed high tumor uptake at early time point (30 min), and remained almost the same at 60 min after tracer injection. The high tumor uptake can be significantly blocked by injection of unlabeled [cys40]-exendin-4 prior to radiolabeled tracer administration, which indicated highly specific targeting towards INS-1 tumor of the labeled peptide. Kidney uptake was higher (33 %ID/g) than tumor at 30 min, but had cleared by a factor of 3 (11 %ID/g) by 60 min. This kidney retention is similar to that of [18F]FBEM-[cys40]-exendin-4 and much lower compared to [18F]AlF-NOTA-[cys0]-exendin-4 35 and [Lys40(68Ga-DOTA)]-exendin-3 42, which showed 63 %ID/g and 120 %ID/g respectively at 60 min post-injection. We reported that kidney uptake of [18F]AlF-NOTA-MAL-[cys40]-exendin-435 increased throughout the course of a 60-min experiment. Evaluation of kidney uptake in non-tumor bearing mice using another exendin-4 analogue [18F]AlF-NOTA-[cys0]-exendin-4, in which NOTA is attached at the N-terminus of the peptide, also showed high and sustained kidney uptake in 60 min. High kidney uptake has been reported for all radiometal chelate exendin analogues43–46. The tumor-to-kidney and tumor-to-liver ratios at later time point were further confirmed by ex vivo biodistribution study. INS-1 tumor uptake of 18F-FBEM-exendin-4 is 30.27 ± 5.44% ID/g34, while that of the probe used in this study is 33.21 ± 4.79% ID/g. There is no significant difference as to tumor uptake with these two tracers. There was negligible uptake in bone, indicating the stability of the tracer to defluorination. Tracer uptake in pancreas and lung were both high and can be blocked by injection of unlabeled [cys40]-exendin-4. This is consistent with the results reported by our group34, which is due to the fact that GLP-1 receptor is mainly expressed in the pancreatic islet cell, the intestine, lung, kidneys, breast and brainstem. These results suggest that [18F]FPenM-[cys40]-exendin-4 specifically binds to GLP-1R expressing organs47.
We showed by radioHPLC that [18F]FPenM-[cys40]-exendin-4 was stable in mouse serum over 2.5 h. An ex vivo metabolite study, conducted by tissue extraction, showed that most radioactivity in tumor was intact parent compound, but the kidneys contained a great deal of polar metabolites and that the urine contained only polar metabolites. Thus the tumor has very little retention of radioactive metabolites whether they were produced or not.
GLP-1R is an important target, both for insulinoma and for imaging pancreatic beta cells, which also present GLP-1R. Beta cell transplantation currently is used for treatment of patients with type I diabetes. Since GLP-1 receptor agonists, such as exendin-4 analogs, can selectively bind to GLP-1R, they can be potentially used for detection of insulinoma and to monitor the survival of transplanted beta cells. However, immunological rejections limit the survival of the transplant. 18F labeled exendin-4 could potentially be used to monitor the viability and mass of transplanted beta cells 41. The utility of the exendin-4 analogs depends on achieving high signal to background ratio, especially in the abdomen where most insulinomas are found. In addition, beta cell transplantation is often conducted in the liver 48, 49. Our results indicated specific uptake of [18F]FPenM-[cys40]-exendin-4 in tissues containing GLP-1R. Uptake is problematic in the kidneys, but the majority of gastrointestinal tract and liver had very low background radioactivity. Thus it may prove useful as a PET imaging agent for insulinoma and pancreas imaging.
Conclusion
A new prosthetic group, [18F]FPenM, for site-specific labeling of free thiol group in proteins and peptides was developed. To the best of our knowledge, this is the first example of maleimide-containing prosthetic group synthesized based on aliphatic nucleophilic substitution and it has the smallest molecular weight among the developed prosthetic agents. Since the maleimido group was incompatible with harsh radiofluorination conditions, three synthetic steps were required for the prosthetic group synthesis. Thus, we achieved no improvement in yields or synthetic simplification compared to [18F]FBEM.. The application of [18F]FPenM was demonstrated in the synthesis and evaluation of [18F]FPenM-[cys40]-exendin-4 for imaging GLP-1R. The tracer has comparable imaging results with [18F]FBEM-[cys40]-exendin-4 developed by our group, showing high tumor-to-liver and tumor-to-kidney uptake ratios for GLP-1R imaging. INS-1 tumor xenografts, pancreas and lung showed specific uptake, indicating that [18F]FPenM-[cys40]-exendin-4 may be useful as pancreatic β-cell imaging agent. The developed tracer has higher binding affinity than NOTA-MAL-[cys40]-exendin-4 and [Lys40(natGa-DOTA)]exendin-3, superior tumor targeting and more rapid kidney clearance, showing it is a promising imaging probe for in vivo targeting of insulinomas.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by the intramural research program of the National Institute of Biomedical Imaging and Bioengineering, National Institutes of Health. The authors acknowledge the NIH Warren Grant Magnuson Clinical Center’s PET Department for radioisotope production. Lei Zhu was partially supported by the Center for Neuroscience and Regenerative Medicine (CNRM) through Henry Jackson Foundation
Footnotes
ASSOCIATED CONTENT
Supporting Information. Serum stability of [18F]FPenM-[cys40]-exendin-4. Metabolite analysis of [18F]FPenM-[cys40]-exendin-4 in the tumor, kidneys and urine. This material is available free of charge via the Internet at http://pubs.acs.org.
Notes
The authors declare no competing financial interest.
REFERENCES
- 1.Reubi JC. Peptide receptors as molecular targets for cancer diagnosis and therapy. Endocr Rev. 2003;24:389–427. doi: 10.1210/er.2002-0007. [DOI] [PubMed] [Google Scholar]
- 2.Sideris L, Dube P, Rinke A. Antitumor effects of somatostatin analogs in neuroendocrine tumors. Oncologist. 2012;17:747–755. doi: 10.1634/theoncologist.2011-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilbur DS. Radiohalogenation of proteins: an overview of radionuclides, labeling methods, and reagents for conjugate labeling. Bioconjugate Chem. 1992;3:433–470. doi: 10.1021/bc00018a001. [DOI] [PubMed] [Google Scholar]
- 4.Couturier O, Luxen A, Chatal JF, Vuillez JP, Rigo P, Hustinx R. Fluorinated tracers for imaging cancer with positron emission tomography. Eur J Nucl Med Mol Imaging. 2004;31:1182–1206. doi: 10.1007/s00259-004-1607-9. [DOI] [PubMed] [Google Scholar]
- 5.Miller PW, Long NJ, Vilar R, Gee AD. Synthesis of 11C, 18F, 15O, and 13N radiolabels for positron emission tomography. Angew Chem Int Ed. 2008;47:8998–9033. doi: 10.1002/anie.200800222. [DOI] [PubMed] [Google Scholar]
- 6.Chen K, Conti PS. Target-specific delivery of peptide-based probes for PET imaging. Adv Drug Deliv Rev. 2010;62:1005–1022. doi: 10.1016/j.addr.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Ting R, Adam MJ, Ruth TJ, Perrin DM. Arylfluoroborates and alkylfluorosilicates as potential PET imaging agents: high-yielding aqueous biomolecular 18F-labeling. J Am Chem Soc. 2005;127:13094–13095. doi: 10.1021/ja053293a. [DOI] [PubMed] [Google Scholar]
- 8.Schirrmacher R, Bradtmoller G, Schirrmacher E, Thews O, Tillmanns J, Siessmeier T, Buchholz HG, Bartenstein P, Wangler B, Niemeyer CM, Jurkschat K. 18F-labeling of peptides by means of an organosilicon-based fluoride acceptor. Angew Chem Int Ed. 2006;45:6047–6050. doi: 10.1002/anie.200600795. [DOI] [PubMed] [Google Scholar]
- 9.Mu L, Hohne A, Schubiger PA, Ametamey SM, Graham K, Cyr JE, Dinkelborg L, Stellfeld T, Srinivasan A, Voigtmann U, Klar U. Silicon-based building blocks for one-step 18F-radiolabeling of peptides for PET imaging. Angew Chem Int Ed. 2008;47:4922–4925. doi: 10.1002/anie.200705854. [DOI] [PubMed] [Google Scholar]
- 10.Becaud J, Mu L, Karramkam M, Schubiger PA, Ametamey SM, Graham K, Stellfeld T, Lehmann L, Borkowski S, Berndorff D, Dinkelborg L, Srinivasan A, Smits R, Koksch B. Direct one-step 18F-labeling of peptides via nucleophilic aromatic substitution. Bioconjugate chem. 2009;20:2254–2261. doi: 10.1021/bc900240z. [DOI] [PubMed] [Google Scholar]
- 11.Jacobson O, Zhu L, Ma Y, Weiss ID, Sun X, Niu G, Kiesewetter DO, Chen X. Rapid and simple one-step F-18 labeling of peptides. Bioconjugate chem. 2011;22:422–428. doi: 10.1021/bc100437q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mading P, Fuchtner F, Wust F. Module-assisted synthesis of the bifunctional labelling agent N-succinimidyl 4-[18F]fluorobenzoate ([18F]SFB) Appl Radiat Isot. 2005;63:329–332. doi: 10.1016/j.apradiso.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Vaidyanathan G, Zalutsky MR. Synthesis of N-succinimidyl 4-[18F]fluorobenzoate, an agent for labeling proteins and peptides with 18F. Nat Protoc. 2006;1:1655–1661. doi: 10.1038/nprot.2006.264. [DOI] [PubMed] [Google Scholar]
- 14.Jacobson O, Chen X. PET designated flouride-18 production and chemistry. Curr Top Med Chem. 2010;10:1048–1059. doi: 10.2174/156802610791384298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuchar M, Pretze M, Kniess T, Steinbach J, Pietzsch J, Loser R. Site-selective radiolabeling of peptides by 18F-fluorobenzoylation with [18F]SFB in solution and on solid phase: a comparative study. Amino Acids. 2012;43:1431–1443. doi: 10.1007/s00726-012-1216-z. [DOI] [PubMed] [Google Scholar]
- 16.Jahan M, Nag S, Krasikova R, Weber U, Muhs A, Pfeifer A, Spenger C, Willbold D, Gulyas B, Halldin C. Fluorine-18 labeling of three novel D-peptides by conjugation with N-succinimidyl-4-[18F]fluorobenzoate and preliminary examination by postmortem whole-hemisphere human brain autoradiography. Nucl Med Biol. 2012;39:315–323. doi: 10.1016/j.nucmedbio.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 17.Jacobson O, Weiss ID, Kiesewetter DO, Farber JM, Chen X. PET of tumor CXCR4 expression with 4-18F-T140. J Nucl Med. 2010;51:1796–1804. doi: 10.2967/jnumed.110.079418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang CY, Gu ZW, Yang M, Lin SN, Garcia-Prats AJ, Rogers LK, Welty SE, Smith CV. Selective modification of apoB-100 in the oxidation of low density lipoproteins by myeloperoxidase in vitro. J Lipid Res. 1999;40:686–698. [PubMed] [Google Scholar]
- 19.Fu X, Mueller DM, Heinecke JW. Generation of intramolecular and intermolecular sulfenamides, sulfinamides, and sulfonamides by hypochlorous acid: a potential pathway for oxidative cross-linking of low-density lipoprotein by myeloperoxidase. Biochemistry. 2002;41:1293–1301. doi: 10.1021/bi015777z. [DOI] [PubMed] [Google Scholar]
- 20.Beilan HS, Kenyon GL, Browne DT. Incorporation of specifically labeled cysteine into Escherichia coli protein. Anal Biochem. 1983;128:169–174. doi: 10.1016/0003-2697(83)90358-5. [DOI] [PubMed] [Google Scholar]
- 21.Kolmar H. Alternative binding proteins: biological activity and therapeutic potential of cystine-knot miniproteins. FEBS J. 2008;275:2684–2690. doi: 10.1111/j.1742-4658.2008.06440.x. [DOI] [PubMed] [Google Scholar]
- 22.Shiue CY, Wolf AP, Hainfeld JF. Synthesis of 18F-labelled N-(p-[18F]fluorophenyl)maleimide and its derivatives for labelling monoclonal antibody with 18F. J Labelled Compd Radiopharm. 1989;26:287–289. [Google Scholar]
- 23.Toyokuni T, Walsh JC, Dominguez A, Phelps ME, Barrio JR, Gambhir SS, Satyamurthy N. Synthesis of a new heterobifunctional linker, N-[4-(aminooxy)butyl]maleimide, for facile access to a thiol-reactive 18F-labeling agent. Bioconjugate chem. 2003;14:1253–1259. doi: 10.1021/bc034107y. [DOI] [PubMed] [Google Scholar]
- 24.Berndt M, Pietzsch J, Wuest F. Labeling of low-density lipoproteins using the 18F-labeled thiol-reactive reagent N-[6-(4-[18F]fluorobenzylidene)aminooxyhexyl]maleimide. Nucl Med Biol. 2007;34:5–15. doi: 10.1016/j.nucmedbio.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 25.Wuest F, Kohler L, Berndt M, Pietzsch J. Systematic comparison of two novel, thiol-reactive prosthetic groups for 18F labeling of peptides and proteins with the acylation agent succinimidyl-4-[18F]fluorobenzoate ([18F]SFB) Amino Acids. 2009;36:283–295. doi: 10.1007/s00726-008-0065-2. [DOI] [PubMed] [Google Scholar]
- 26.Wuest F, Berndt M, Bergmann R, van den Hoff J, Pietzsch J. Synthesis and application of [18F]FDG-maleimidehexyloxime ([18F]FDG-MHO): a [18F]FDG-based prosthetic group for the chemoselective 18F-labeling of peptides and proteins. Bioconjugate chem. 2008;19:1202–1210. doi: 10.1021/bc8000112. [DOI] [PubMed] [Google Scholar]
- 27.de Bruin B, Kuhnast B, Hinnen F, Yaouancq L, Amessou M, Johannes L, Samson A, Boisgard R, Tavitian B, Dolle F. 1-[3-(2-[18F]fluoropyridin-3-yloxy)propyl]pyrrole-2,5-dione: design, synthesis, and radiosynthesis of a new [18F]fluoropyridine-based maleimide reagent for the labeling of peptides and proteins. Bioconjugate Chem. 2005;16:406–420. doi: 10.1021/bc0497463. [DOI] [PubMed] [Google Scholar]
- 28.Cai W, Zhang X, Wu Y, Chen X. A thiol-reactive 18F-labeling agent, N-[2-(4-18F-fluorobenzamido)ethyl]maleimide, and synthesis of RGD peptide-based tracer for PET imaging of alpha v beta 3 integrin expression. J Nucl Med. 2006;47:1172–1180. [PMC free article] [PubMed] [Google Scholar]
- 29.Wang H, Gao H, Guo N, Niu G, Ma Y, Kiesewetter DO, Chen X. Site-specific labeling of scVEGF with fluorine-18 for positron emission tomography imaging. Theranostics. 2012;2:607–617. doi: 10.7150/thno.4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koslowsky I, Mercer J, Wuest F. Synthesis and application of 4-[18F]fluorobenzylamine: A versatile building block for the preparation of PET radiotracers. Org Biomol Chem. 2010;8:4730–4735. doi: 10.1039/c0ob00255k. [DOI] [PubMed] [Google Scholar]
- 31.Inkster JA, Liu K, Ait-Mohand S, Schaffer P, Guerin B, Ruth TJ, Storr T. Sulfonyl fluoride-based prosthetic compounds as potential 18F labelling agents. Chem Eur J. 2012;18:11079–11087. doi: 10.1002/chem.201103450. [DOI] [PubMed] [Google Scholar]
- 32.McBride WJ, D'Souza CA, Sharkey RM, Goldenberg DM. The radiolabeling of proteins by the [18F]AlF method. Appl Radiat Isot. 2012;70:200–204. doi: 10.1016/j.apradiso.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dolle F. Fluorine-18-labelled fluoropyridines: advances in radiopharmaceutical design. Curr Pharm Des. 2005;11:3221–3235. doi: 10.2174/138161205774424645. [DOI] [PubMed] [Google Scholar]
- 34.Kiesewetter DO, Gao H, Ma Y, Niu G, Quan Q, Guo N, Chen X. 18F-radiolabeled analogs of exendin-4 for PET imaging of GLP-1 in insulinoma. Eur J Nucl Med Mol Imaging. 2012;39:463–473. doi: 10.1007/s00259-011-1980-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kiesewetter DO, Guo N, Guo J, Gao H, Zhu L, Ma Y, Niu G, Chen X. Evaluation of an [18F]AlF-NOTA analog of exendin-4 for imaging of GLP-1 receptor in insulinoma. Theranostics. 2012;2:999–1009. doi: 10.7150/thno.5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keller O, Rudinger J. Preparation and some properties of maleimido acids and maleoyl derivatives of peptides. Helv Chim Acta. 1975;58:531–541. doi: 10.1002/hlca.19750580224. [DOI] [PubMed] [Google Scholar]
- 37.Mohler DL, Shen G. The synthesis of tethered ligand dimers for PPARgamma-RXR protein heterodimers. Org Biomol Chem. 2006;4:2082–2087. doi: 10.1039/b600848h. [DOI] [PubMed] [Google Scholar]
- 38.Bell TW, Choi HJ, Harte W, Drew MG. Syntheses, conformations, and basicities of bicyclic triamines. J Am Chem Soc. 2003;125:12196–12210. doi: 10.1021/ja030236d. [DOI] [PubMed] [Google Scholar]
- 39.Walczak RM, Cowart JS, Reynolds JR. Tethered PProDOTs: conformationally restricted 3,4-propylenedioxythiophene based electroactive polymers. J Mater Chem. 2007;17:254–260. [Google Scholar]
- 40.Kiesewetter DO, Sassaman MB, Robbins J, Jagoda EM, Carson RE, Appel NM, Sutkowski E, Herscovitch P, Braun A, Eckelman WC. Synthesis and evaluation of an F-18 analog of forskolin for imaging adenylyl cyclase. J Fluorine Chem. 2000;101:297–304. [Google Scholar]
- 41.Zhang Y, Chen W. Radiolabeled glucagon-like peptide-1 analogues: a new pancreatic beta-cell imaging agent. Nucl Med Commun. 2012;33:223–227. doi: 10.1097/MNM.0b013e32834e7f47. [DOI] [PubMed] [Google Scholar]
- 42.Brom M, Oyen WJ, Joosten L, Gotthardt M, Boerman OC. 68Galabelled exendin-3, a new agent for the detection of insulinomas with PET. Eur J Nucl Med Mol Imaging. 2010;37:1345–1355. doi: 10.1007/s00259-009-1363-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wild D, Wicki A, Mansi R, Behe M, Keil B, Bernhardt P, Christofori G, Ell PJ, Macke HR. Exendin-4-based radiopharmaceuticals for glucagonlike peptide-1 receptor PET/CT and SPECT/CT. J Nucl Med. 2010;51:1059–1067. doi: 10.2967/jnumed.110.074914. [DOI] [PubMed] [Google Scholar]
- 44.Wild D, Behe M, Wicki A, Storch D, Waser B, Gotthardt M, Keil B, Christofori G, Reubi JC, Macke HR. [Lys40(Ahx-DTPA-111In)NH2]exendin-4, a very promising ligand for glucagon-like peptide-1 (GLP-1) receptor targeting. J Nucl Med. 2006;47:2025–2033. [PubMed] [Google Scholar]
- 45.Wicki A, Wild D, Storch D, Seemayer C, Gotthardt M, Behe M, Kneifel S, Mihatsch MJ, Reubi JC, Macke HR, Christofori G. [Lys40(Ahx-DTPA-111In)NH2]-Exendin-4 is a highly efficient radiotherapeutic for glucagon-like peptide-1 receptor-targeted therapy for insulinoma. Clin Cancer Res. 2007;13:3696–3705. doi: 10.1158/1078-0432.CCR-06-2965. [DOI] [PubMed] [Google Scholar]
- 46.Brom M, Joosten L, Oyen WJ, Gotthardt M, Boerman OC. Radiolabelled GLP-1 analogues for in vivo targeting of insulinomas. Contrast Media Mol Imaging. 2012;7:160–166. doi: 10.1002/cmmi.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 48.Sahu S, Tosh D, Hardikar AA. New sources of beta-cells for treating diabetes. J Endocrinol. 2009;202:13–16. doi: 10.1677/JOE-09-0097. [DOI] [PubMed] [Google Scholar]
- 49.Robertson RP. Islet transplantation as a treatment for diabetes - a work in progress. N Engl J Med. 2004;350:694–705. doi: 10.1056/NEJMra032425. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.