Background: For eukaryotic cells, a direct gating of mechanosensitive channels by membrane tension has not been demonstrated.
Results: The mouse TREK-1 was purified and reconstituted in liposomes amenable to patch clamp recording.
Conclusion: The channel displayed expected electrophysiological properties, and positive pressure could reversibly close it.
Significance: TREK-1, a eukaryotic mechanosensitive channel, is directly sensitive to a modification of membrane tension.
Keywords: Liposomes, Mechanotransduction, Membrane Bilayer, Patch Clamp, Potassium Channels
Abstract
Mechanosensitive channels are detected in all cells and are speculated to play a key role in many functions including osmoregulation, growth, hearing, balance, and touch. In prokaryotic cells, a direct gating of mechanosensitive channels by membrane tension was clearly demonstrated because the purified channels could be functionally reconstituted in a lipid bilayer. No such evidence has been presented yet in the case of mechanosensitive channels from animal cells. TREK-1, a two-pore domain K+ channel, was the first animal mechanosensitive channel identified at the molecular level. It is the target of a large variety of agents such as volatile anesthetics, neuroprotective agents, and antidepressants. We have produced the mouse TREK-1 in yeast, purified it, and reconstituted the protein in giant liposomes amenable to patch clamp recording. The protein exhibited the expected electrophysiological properties in terms of kinetics, selectivity, and pharmacology. Negative pressure (suction) applied through the pipette had no effect on the channel, but positive pressure could completely and reversibly close the channel. Our interpretation of these data is that the intrinsic tension in the lipid bilayer is sufficient to maximally activate the channel, which can be closed upon modification of the tension. These results indicate that TREK-1 is directly sensitive to membrane tension.
Introduction
TREK-1 is a member of the two-pore domain (K2P)5 K+ channel family (1–3). These K2P channels are background (or leak) channels, open at rest, so that under physiological conditions, a K+ gradient across the cell membrane causes an efflux of K+, thus setting the resting potential of the cell at a negative value. In mammalian cells, the six different subfamilies of K2P channels share a common architecture. Each subunit possesses four transmembrane segments and two pore loop-forming domains, in contrast to other K+ channel families characterized by one pore loop domain per subunit. K2P channels are dimeric so that the K+ selectivity filter contains four pore loop domains as in other potassium channels. The recent determinations of the structures of two members of the K2P family, TRAAK (4) and TWIK-1 (5), are fully consistent with this view. TREK-1 is not a static open potassium pore. It is modulated by an impressive variety of factors including pressure, temperature, lipid interaction, voltage, and pH. It is the target of different classes of pharmacological agents, including volatile anesthetics, neuroprotective agents, and antidepressants (1–3).
TREK-1 is the first stretch-activated mechanosensitive channel identified at the molecular level in animal cells (6, 7). Stretch-activated channels are detected in cells belonging to the three domains of the tree of life (8, 9). They are speculated to play a key role in many functions including osmoregulation and cell volume control, growth, hearing, balance, and touch. However, except in prokaryotic cells, their mode of activation is not clearly demonstrated.
One possible mode of activation for the mechanosensitive channel, later called the tethered model by Hamill and McBride (10), proposes a direct connection between the gate of the channel and proteins located in the extracellular matrix or the cytoskeleton. Displacement of the channel relative to these elements would cause the channel to open or close. A second possible mode of activation, namely a direct gating of the channel by the membrane tension (11, 12), was clearly demonstrated in the case of prokaryotic mechanosensitive channels. These large conductance channels behave as safety valves that open during osmotic downshock and prevent the cells from bursting (13, 14). From the onset of the studies performed on these channels, it was noted that mechanosensitive channels could be recorded from giant liposomes fused with pieces of membrane of E. coli (15) or reconstituted with solubilized extract from E. coli (16). Later, the two major mechanosensitive channels in E. coli, MscL and MscS, could be purified and functionally reconstituted in liposomes (17, 18). Similar channels are also present in Archaea, where they are gated by membrane tension alone (19).
In the case of eukaryotic cells, a direct gating of mechanosensitive channels by membrane tension has not been clearly demonstrated. Plant cells harbor mechanosensitive channels belonging to the MscS family of prokaryotic cells (20). These channels are also likely to be gated directly by membrane tension, but this has yet to be shown. Piezo is a newly identified mechanosensitive channel present in animal cells. The protein was produced, purified, and reconstituted in liposomes, but no proof of a direct activation by membrane tension has yet been obtained (21). However, the yeast TRP channel remained mechanosensitive after patch excision from the yeast vacuole (22), a configuration that seems to exclude the involvement of the cytoskeleton. The NMDA receptor is a ligand-gated channel that is modulated by applied pressure. Martinac and colleagues (23) showed that this modulation is still present after purification and reconstitution of the channel in a lipid bilayer. In this study, we report the production of mouse TREK-1 in yeast and its functional reconstitution in a pure lipid bilayer.
EXPERIMENTAL PROCEDURES
Materials
Products for yeast and bacteria cultures were from Difco (BD Biosciences). Chemicals were generally from Sigma. Restriction and modification enzymes were purchased from New England Biolabs, and Phusion® high fidelity polymerase was from Finnzymes.
Recombinant Plasmid Construction
TREK-1 open reading frame was supplemented, at its 5′-end, with a sequence coding for a biotin acceptor domain (BAD) and a sequence coding for the thrombin protease cleavage site. The cleavage site was flanked by Gly-Leu-Asn-Gly-Gly toward BAD and Gly-Gly-Ser-Ala-Gly toward TREK-1. The fused gene was cloned in pYeDP60 expression plasmid (a generous gift of Dr. D. Pompon, Laboratoire d'Ingénierie des Systèmes Biologiques et des Procédés (LISBP), Toulouse, France) (24, 25), resulting in the generation of the pYeDP60-BAD-TREK-1 vector.
The mouse TREK-1 gene was a generous gift from Dr. A. Patel (Institut de Pharmacologie Moléculaire et Cellulaire (IPMC), CNRS, Valbonne, France). The plasmid pIVEX TREK-1 contains the sequence of TREK-1. To subclone TREK-1 into pYeDP60-BAD, a previous PCR was performed with Phusion® high fidelity polymerase on the plasmid pIVEX TREK-1 for adding restriction sites PmeI and FseI in 5′ and SacI in 3′ of the sequence of TREK-1. The 5′ TREK-1 primer sequence 5′-GGT TTA AAC GGT GGT TTG GTT CCA AGA GGA TCC GGT GGT TCG GCC GGC CTT GCC AGC GCC TCG CGG GAG AGA CCC GGC TAT was complementary to a region in 3′ of the cDNA of BAD in pYeDP60-BAD containing a PmeI restriction site (underlined), two codons coding for a glycine, a sequence coding for the thrombin cleavage site (in italics), two codons coding for a glycine, a linker containing a FseI restriction site (underlined, codons coding for Ser-Ala-Gly), and the 5′-end of TREK-1 (bold). The 3′ TREK-1 primer sequence 5′-ATT GAG AAC ATG AAG TAA GAG CTC GGT-3′ was complementary to a region in 3′ of the cDNA of TREK-1 containing a SacI restriction site (underlined). The resultant fragment of 1325 bp was digested with PmeI and SacI, gel-purified, inserted into a gel-purified digested pYeDP60-BAD fragment, and transformed into Escherichia coli JM109.The recombinant plasmid was colony-purified using the Wizard® Plus SV minipreps DNA purification system (Promega), and the construct was checked by DNA sequencing.
Expression and Purification of TREK-1
We used the Saccharomyces cerevisiae W303.1b/GAL4 (a, leu2-3, his3-11, trp1-1::TRP1-GAL10-GAL4, ura3-1, ade2-1, canr, cir+) yeast strain (24). Transformation was performed according to the lithium acetate/single-stranded carrier DNA/polyethylene glycol method (26). Selection of individual clones, yeast growth, induction of protein expression, and membrane fractionation by differential centrifugation were performed as described (27, 28) with the minor modification that yeast cells were broken using a Pulverisette 6 planetary mill (Fritsch) with glass beads.
The light membrane fraction was diluted to 5 mg of total protein/ml in solubilization buffer (50 mm MOPS-Tris, pH 7, 150 mm KCl, 20% (w/v) glycerol, 10 mm β-mercaptoethanol) supplemented with 1 mm PMSF and an EDTA-free protease inhibitor mixture (Roche Diagnostics). Dodecyl-β-d-maltoside (Anatrace) was then added at a detergent to protein ratio of 3 (w/w). The presence of brain lipids (Avanti Polar lipids) was maintained during all the purification steps. The suspension was homogenized using a Potter-type homogenizer and then stirred for 20 min at room temperature. Insoluble material was pelleted by centrifugation at 100,000 × g for 45 min at 6 °C.
All subsequent steps (unless otherwise specified) were performed at 4 °C. The supernatant after the centrifugation step was mixed with streptavidin-SepharoseTM high performance resin (GE Healthcare) at a ratio of 40:1 (v/v), using typically 40 ml of solubilized TREK-1 per ml of resin, and stirred overnight. The suspension was pelleted into 50-ml tubes for 5 min at 500 × g (rotor 11133, Sigma) and washed twice with a “high salt” buffer (50 mm MOPS-Tris, pH 7, 1 m KCl, 20% glycerol, 0.1 mg/ml lipids, 0.5 mg/ml DDM, 1 mm β-mercaptoethanol) (buffer:resin, 10:1 (v/v)) and then twice with a “low salt” buffer (50 mm MOPS-Tris, pH 7, 150 mm KCl, 20% glycerol, 2.5 mm CaCl2, 0.1 mg/ml lipids, 0.5 mg/ml DDM, 1 mm β-mercaptoethanol) (buffer:resin, 10:1 (v/v)). The resin was resuspended in the low salt buffer (buffer:resin, 1:1 (v/v)), thrombin (Calbiochem) was added (15 units/ml resin), and the mixture was stirred at room temperature for 10 min followed by a second addition of thrombin and stirring for another 10 min. Thrombin was inactivated with PMSF (1 mm), and the resin solution was transferred into HandeeTM centrifuge columns (Perbio Science France SAS). The proteolytically cleaved TREK-1 proteins were eluted from the column.
Protein Estimation, Immunodetection, and Mass Spectroscopy Identification
Protein concentrations were measured using the bicinchoninic acid procedure (29) in the presence of 2% (w/v) SDS, using BSA as standard. For SDS-PAGE, samples were mixed with an equal volume of denaturing buffer, heated at 90 °C for 2 min, and loaded onto Laemmli-type 10% (w/v) polyacrylamide gels. Gels were stained with Coomassie Blue or proteins were electroblotted onto polyvinylidene difluoride Immobilon P membrane (Millipore) as described previously (27). After electroblotting, membrane was blocked for 10 min in PBST (90 mm K2HPO4, 10 mm KH2PO4 (pH 7.7), 100 mm NaCl, 0.2% (v/v) Tween 20) containing 3% BSA. The membrane was washed once for 10 min in PBST. The primary antibody anti-TREK-1 (1:20,000), a generous gift from Dr. F. Lesage (IPMC, Valbonne, France), was then added to a solution PBST containing 3% BSA and incubated for 1 h at room temperature. The membrane was washed once for 10 min in PBST and then incubated with horseradish peroxidase-conjugated secondary goat anti-rabbit antibody (Bio-Rad) (1:20,000) in PBST. After three washes with PBST for 10 min each, blots were revealed by chemiluminescence with the ECL kit (GE Healthcare). For mass spectroscopy analyses, bands of interest were excised from SDS-PAGE, destained, and then digested with trypsin and analyzed by MALDI-TOF mass spectrometry using α-cyano-4-hydroxycinnamic acid as described previously (30).
Size Exclusion Chromatography
The size exclusion chromatography was performed with a Beckman Coulter Gold HPLC system. After purification, 200 μl of purified TREK-1 was applied at 1 ml/min into a silica gel filtration column (0.78 × 30-cm TosoHaas TSKgel G3000SWXL column), which was equilibrated with a buffer composed of 50 mm MOPS-Tris, pH 7; 50 mm KCl; 0.4 mg/ml DDM. To evaluate the size of purified TREK-1, the gel filtration column was calibrated under the same conditions using the Bio-Rad Gel Filtration Standards (catalogue number 151-1901; 200 μl of the mixture diluted to 0.3 mg/ml in the elution buffer was injected), with the following molecular masses and Stokes radii: thyoglobulin Mr 670,000, RS = 8.6 nm; bovine γ-globulin, Mr 158,000, RS = 5.1 nm; chicken ovalbumin, Mr 44,000, RS = 2.8 nm; equine myoglobin 17,500, RS = 1.9 nm; and vitamin B12 corresponding to the total volume.
Glycosidase Assay
Eluted TREK-1 was preincubated at 37 °C in buffer containing 50 mm sodium phosphate, pH 7.5, 1% Nonidet P-40, 35 mm DTT supplemented with 1 mm PMSF and an EDTA-free protease inhibitor mixture. Samples were incubated for 5 h at 37 °C in the absence or presence of 1500 units of N-glycosidase F (New England Biolabs) before immunodetection.
Reconstitution of Proteins and Preparation of Giant Proteoliposomes
The TREK-1 protein was reconstituted in liposomes at a lipid-to-protein ratio of 200 (w/w). We initially used a mixture of lipids (70% azolectin IV-S from soybean (Sigma)/20% lipid brain extract (Avanti Polar lipids)/10% cholesterol (Sigma)). In later experiments, azolectin alone proved to be equally effective. Reconstitution was performed in 10 mm HEPES-KOH, pH 7.4, 150 mm KCl, 10 mm β-mercaptoethanol, and detergent was removed by the addition of SM-2 Bio-Beads (Bio-Rad) as described (31). Proteoliposomes were fused into giant proteoliposomes using a cycle of dehydration-rehydration as described previously (15). Rehydration was performed in a buffer containing 150 mm KCl, 10 mm HEPES, pH 7.4.
Electrical Recording
Single channel activity was measured using standard patch clamp method. 2 μl of the giant proteoliposome suspension was deposited in the patch clamp chamber and diluted with 2 ml of the bath solution. Patch pipettes were pulled from Pyrex capillaries (Corning code 7740) and were not fire polished before use. Recordings were performed in the inside-out configuration. Pressure was applied by syringe and monitored with a piezoelectric transducer World Precision Instruments, Sarasota, FL. Unitary currents were recorded using an RK-300 patch clamp amplifier (Bio-Logic SAS, Claix, France), filtered at 1 kHz (−3-db point) through a four-pole Bessel low pass filter, sampled at 2 kHz with a Digidata 1322A digitizer (Axon), and analyzed using PClamp (Axon). The convention for the membrane potential assigns zero level to the pipette.
RESULTS
Production and Purification of TREK-1
We overexpressed BAD-TREK-1 in S. cerevisiae with the pYeDP60 vector in which the BAD (about 11 kDa) is linked to the N terminus of TREK-1 by a thrombin cleavage site, a system and construction similar to the one that allowed the purification and crystallization of rabbit SERCA1a (sarco(endo)plasmic reticulum calcium ATPase 1a) (32). The BAD was introduced in the N terminus of TREK-1 to prevent an interaction of BAD with the C-terminal tail of TREK-1, which contains sensory elements responding to heat, mechanical force, and internal pH (7, 33–36). A weak natural Kozak sequence is located downstream of the expected N terminus in the TREK-1 protein. This alternative start codon might allow the production of a truncated form of TREK-1 (37). Our purification strategy avoids the isolation of this form because in this case, BAD would be missing.
After TREK-1 expression, the light membrane fraction (Fig. 1, A and B, lane a) was obtained by differential centrifugation and solubilized with dodecyl-β-d-maltoside (Fig. 1, A and B, lane b). BAD-TREK-1 was purified in one step by affinity chromatography with a streptavidin-Sepharose resin that only binds BAD-TREK-1 that has been biotinylated in vivo (Fig. 1, A and B, lane c). TREK-1 was released from the resin by the specific thrombin cleavage (Fig. 1, A and B, lane d).
FIGURE 1.
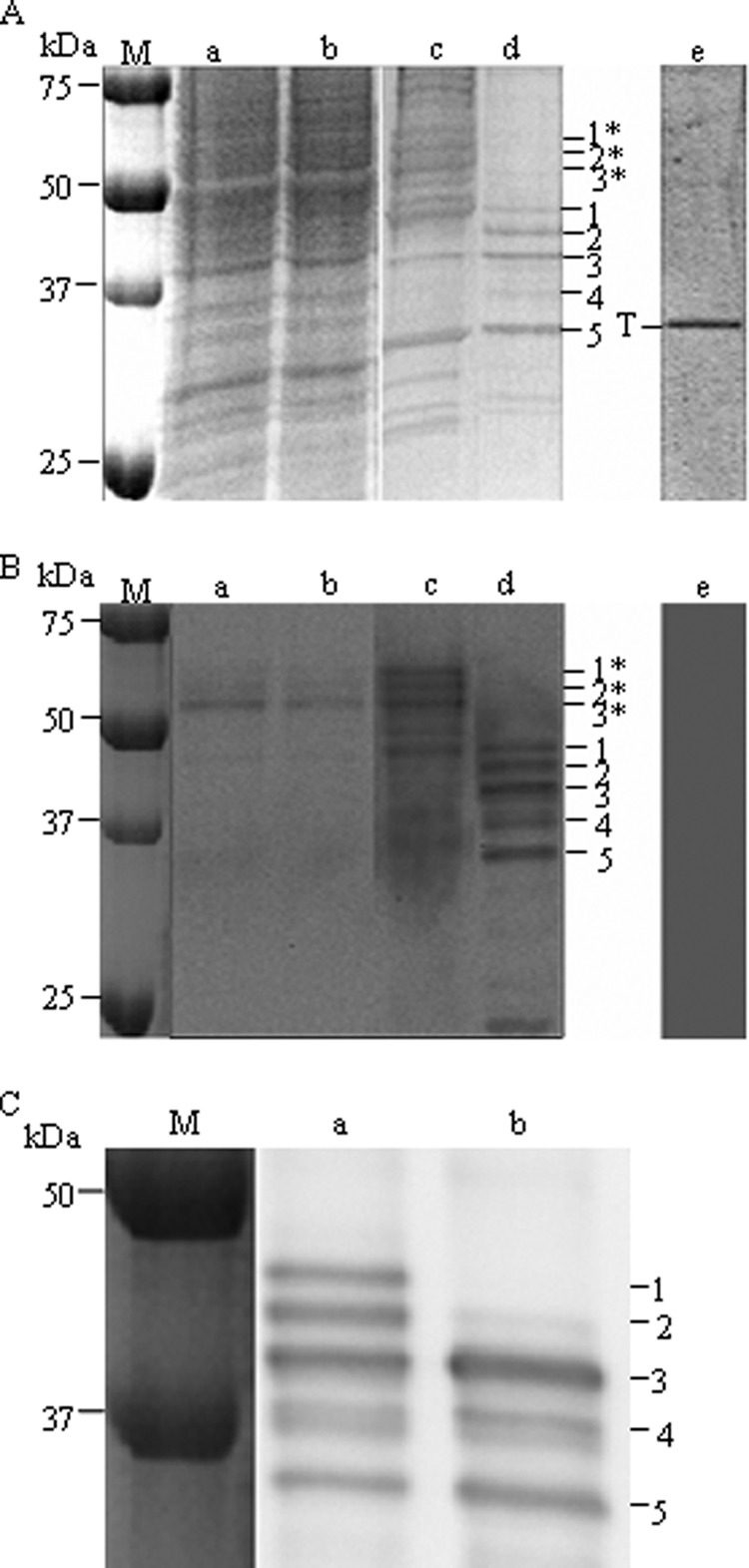
Purification of TREK-1 from yeast light membranes using streptavidin-Sepharose affinity resin. A, analysis of the solubilized and the eluted fractions by SDS-PAGE followed by Coomassie Blue staining. Lane a, yeast light membrane fraction; lane b, solubilized light membrane fraction; lane c, resin after binding and washing; lane d, elution after thrombin addition; lane e, elution as lane d but starting from yeast transformed with an empty vector. Except for the molecular mass marker (M) and the control (20 μl in lane e), 10 μl of each fraction was loaded (corresponding to 50 μg of total proteins in lane a, for example). T: thrombin. B, analyses of the same fractions as in A by Western blot with anti-TREK-1 antibodies. Only 1 μl was loaded in each lane (corresponding to 5 μg of total proteins in lane a, for example), except for 2 μl in lane e. C, effect of the N-glycosidase F on purified TREK-1. Lane a, initial sample (equivalent to lane d above); lane b, N-glycosidase-treated sample (see “Experimental Procedures”). 1*, 2*, 3*: species of BAD-TREK-1; 1–5: species of TREK-1 after removal of the BAD tag by thrombin.
TREK-1 was immunodetected with an antibody that recognizes the first 44 N-terminal amino acids of TREK-1. Three main forms of BAD-TREK-1 were detected with an apparent molecular mass of about 59, 56, and 53 kDa (Fig. 1B, lanes a–c, species 1*, 2*, and 3*). Detection with avidin peroxidase showed the same pattern (data not shown).
Beginning with 1 g of total protein in the light membrane, we typically obtained about 15 μg of purified TREK-1 distributed among three major species of sizes close to the expected subunit size of TREK-1: species 1 (47 kDa), species 2 (45 kDa), and species 3 (42 kDa), on Fig. 1, A and B (lane d) as expected after the removal of the 11-kDa BAD of the species 1*, 2*, and 3*. The three main species have been identified by peptide mass fingerprinting with a mass spectrometer MALDI-TOF as being TREK-1; the peptides are found to cover the entire protein sequence, indicating that the full protein has been purified (see supplemental Data S1). In Fig. 1C, an elution sample was treated with the N-glycosidase F to check for glycosylations. After N-glycosidase F incubation, species 1 and 2 were no longer immunodetected, and species 3 became more intense. Consequently, species 1 and 2 are glycosylated forms of species 3.
The two lower forms, species 4 (about 38 kDa) and species 5 (about 35 kDa), could not be identified by mass spectrometry due to their low abundance. Note that species 5 co-migrated with contaminant proteins such as thrombin (Fig. 1A, lane d). Species 4 and 5 probably arose from a proteolysis of the former three species due to nonspecific sites of thrombin cleavage present in TREK-1. Indeed thrombin has been reported to nonspecifically cleave at secondary sites located in some overexpressed recombinant proteins (38). Finally, and as expected, when the same purification was performed using an empty vector, the only protein revealed after elution by Coomassie Blue staining in Fig. 1A was thrombin (labeled T in lane e). None was detected by immunoblotting (Fig. 1B, lane e).
We found it important to perform a biochemical assessment of the approximate molecular mass of the purified channel by size exclusion chromatography following the experience acquired on other solubilized membrane proteins (39). To do this, an HPLC TSK silica gel column was equilibrated in a buffer containing 0.4 mg/ml DDM, and column calibration was performed in the same buffer. A sample of purified TREK-1 was applied, and aliquots of the eluted peak were deposited on SDS-PAGE and revealed by Coomassie Blue and Western blotting (Fig. 2). It is seen that TREK-1 elutes slightly ahead of bovine γ-globulin with an approximate molecular mass of 250,000, compatible with a dimeric protein with about 1 g/g of bound DDM, similar to other membrane proteins (39, 40). The optical density peak appears somewhat broader than other proteins analyzed in similar conditions, but the dispersity can probably be attributed to the slight proteolysis and an uneven glycosylation (Fig. 1). In addition to the broad peak corresponding to the TREK-1 dimer and besides a small amount of aggregated material that elutes in the void volume, we observed two other minor peaks corresponding to low molecular weight proteins (eluting after ovalbumin at elution volumes of 9.7 and 10 ml). The peak having an elution volume of 10 ml could be the result of proteolytic degradation of TREK-1 because it has about the same absorbance ratio A280/A254 as the major peak (1.39 versus 1.38). The second minor peak has an absorbance ratio A280/A254 of 1.93 and might correspond to a contaminant protein.
FIGURE 2.
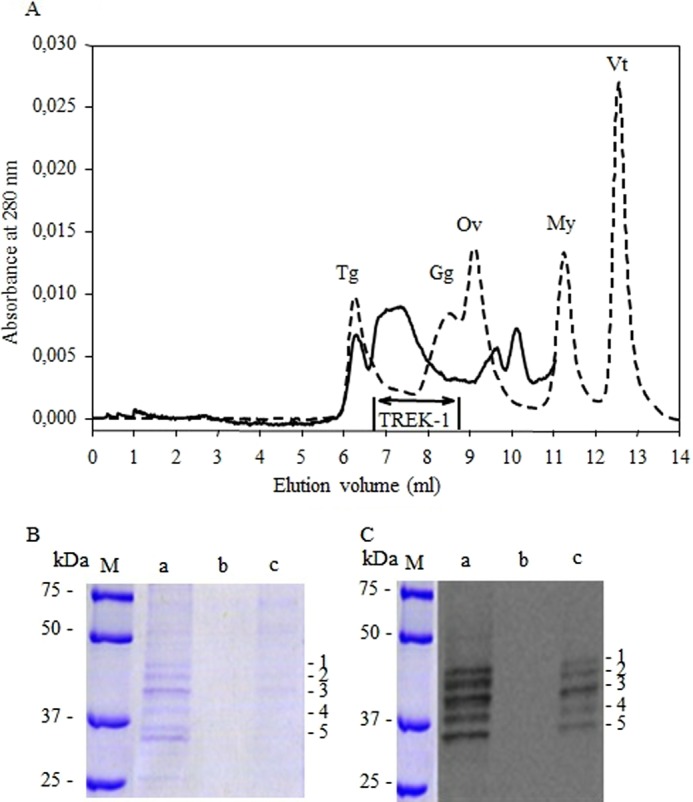
Evaluation of the size of purified TREK-1 by size exclusion chromatography. A, the figure depicts the elution profile at 280 nm from size exclusion chromatography of TREK-1 after affinity chromatography. To evaluate the size of purified TREK-1, the gel filtration column was calibrated under the same conditions using the Bio-Rad Gel Filtration Standards, with the following molecular masses and Stokes radii: thyoglobulin (Tg) Mr 670,000, RS = 8.6 nm; bovine γ-globulin (Gg), Mr 158,000, RS = 5.1 nm; chicken ovalbumin (Ov), Mr 44,000, RS = 2.8 nm; equine myoglobin (My) 17,500, RS = 1.9 nm; and vitamin B12 (Vt) corresponding to the total volume. The column elution profiles corresponding to purified TREK-1 (continuous line) and to protein standards (interrupted line) were monitored at 280 nm. The purified TREK-1 profile has been enlarged 4-fold. B, the fractions of interest (designated by the bar on the profile) were pooled and subsequently both analyzed by SDS-PAGE stained with Coomassie Blue (left panel) and revealed with anti-TREK-1 antibodies (right panel), prior or after concentration with a centrifuge concentrator. Ten microliters of each sample was loaded on respective lanes. Lane M: molecular mass marker; lane a: sample of purified TREK-1; lane b: pooled fractions after size exclusion chromatography; lane c: same sample as lane b after 10-fold concentration using a centrifuge concentrator. 1–5: species of TREK-1.
Electrophysiological Characteristics of the TREK-1 Channel Reconstituted in Giant Liposomes
The purified protein was reconstituted in lipids at a lipid-to-protein ratio of 200 (w/w) by withdrawal of the detergent. The resulting proteoliposomes were converted into giant liposomes amenable to patch clamp recording by a cycle of dehydration-rehydration. Fig. 3A displays the typical channel activity recorded in inside-out patches in the absence of pressure applied to the pipette. The channels exhibited a typical flickery bursting behavior with more active channels at positive than at negative potentials. Even under symmetrical conditions (150 mm KCl in the bath and the pipette) but with magnesium present in the pipette, a strong outward rectification was observed (Fig. 3, A and B). Under asymmetrical conditions mimicking physiological conditions (150 mm KCl in the bath versus 5 mm KCl in the pipette), the reversal potential (−81 ± 3 mV, n = 4) was very close to the Nernst potential for potassium (Fig. 3B). In a few cases, but not in the record displayed in Fig. 3, substates could be clearly observed. This type of spontaneous activity was observed in 55 patches recorded at pH 7.4 or pH 5.8. In 150 mm KCL symmetrical media, the chord conductance at +40 mV was 86 ± 11 pS, n = 14). In three patches, we observed a channel with very similar kinetics but with a conductance that was half of the previous one. This activity was not analyzed further. All other patches (∼160) were electrically silent.
FIGURE 3.
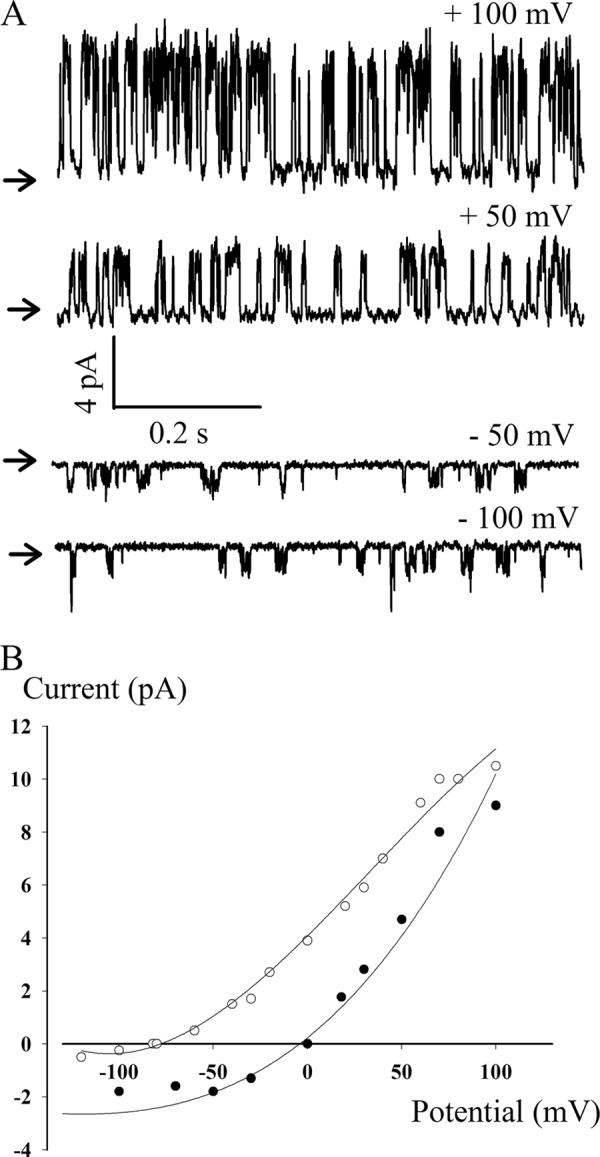
Electrophysiological characteristics of the TREK-1 protein reconstituted in giant liposomes. A, representative single channel currents recorded at different membrane potentials, in symmetrical media, as defined below. The close channel state is marked by an arrow. B, current-voltage relationship and ionic selectivity. Closed circles, I-V curves obtained in symmetrical (bath and pipette, 150 mm KCl); open circles, I-V curves obtained in asymmetrical media (bath, 150 mm KCl; pipette, 5 mm KCl). In both cases, the pH was adjusted to 7.4 with 10 mm HEPES-KOH with, in addition, 5 mm MgCl2 and 2 mm CaCl2 in the pipette. The chord conductance at 50 mV was 80 pS under symmetrical conditions and 160 pS under asymmetrical conditions.
We performed control experiments with extracts obtained from yeast transformed with an empty vector and reconstituted in azolectin lipids under the same conditions as the purified protein. No channel activity was observed in 47 patches (30 patches at pH 7.4 and 17 patches at pH 5.8).
The TREK-1 channel is insensitive to classical potassium channels inhibitors but is inhibited by chlorpromazine (7). The potassium channel produced in yeast was inhibited by chlorpromazine (n = 5). Fig. 4 shows how application of 25 μm chlorpromazine induced a complete inhibition of the channel activity that could be restored, partially if not completely, by washing out.
FIGURE 4.
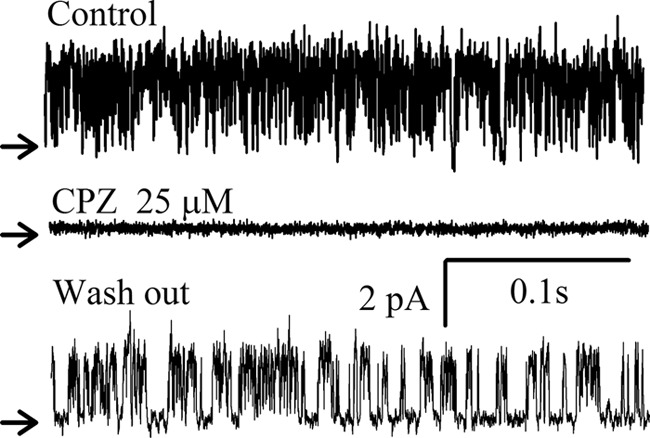
Reversible inhibition of the TREK-1 channel activity by chlorpromazine. Current recording segments obtained at +40 mV in the excised inside-out configuration under control condition, during application of 25 μm chlorpromazine (CPZ) and after washout, as indicated. The recording was performed under symmetrical ionic conditions as defined in Fig. 3.
Mechanosensitivity of the TREK-1 Channel in a Pure Lipid Bilayer
Patches were systematically subjected to mechanical force by application of positive or negative pressure to the pipette. Silent patches were all insensitive to positive or negative pressure until rupture of the membrane. The spontaneous channel activity was insensitive to negative pressure, but it could be reversibly inhibited by application of positive pressure to the pipette, at pH 7.4. A typical recording is shown in Fig. 5A. The spontaneous gating of the channel in the absence of pressure was completely inhibited by the application of positive pressure (upward deflection in the pressure trace) but resumed as soon as the pressure was released. A longer recording (Fig. 5B) summarizes most of our observations. Suction (segment a of the pressure recording, downward deflection) did not modify the open probability of the channel, whereas positive pressure (segments b, c, and d) repeatedly closed the channel. In segments b and d, positive pressure was increased or decreased by steps. The transition from a closed to an open state or vice versa occurred on a small variation of pressure. This is documented by the recordings in Fig. 6A, which show how an increase in positive pressure applied to the pipette decreases the open probability of the channels until complete closure. At pH 7.4, a reversible inhibition of the channel activity was observed repeatedly in 35 patches. In nine patches, however, no inhibition by positive pressure could be obtained until rupture of the patch.
FIGURE 5.
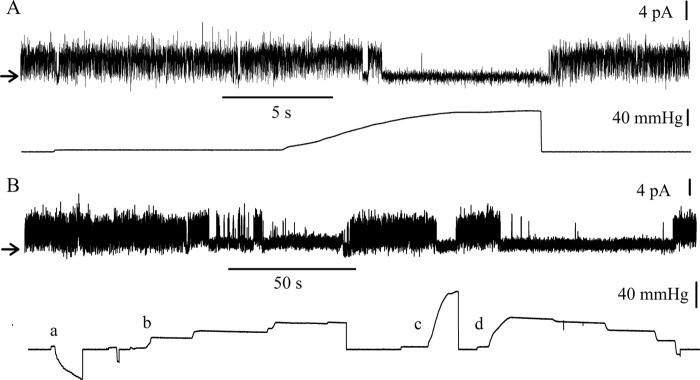
Effect of pressure, applied to the pipette, on the activity of TREK-1 reconstituted in liposomes. A and B, single-channel data recorded at +40 mV upon an application of a positive pressure to the pipette (A) and upon successive applications of negative and positive pressures (B). The upper and lower traces present single-channel currents and pressure records, respectively. The applied pressure was zero at the onset of each trace. The recording was performed under symmetrical ionic conditions as defined in Fig. 3.
FIGURE 6.
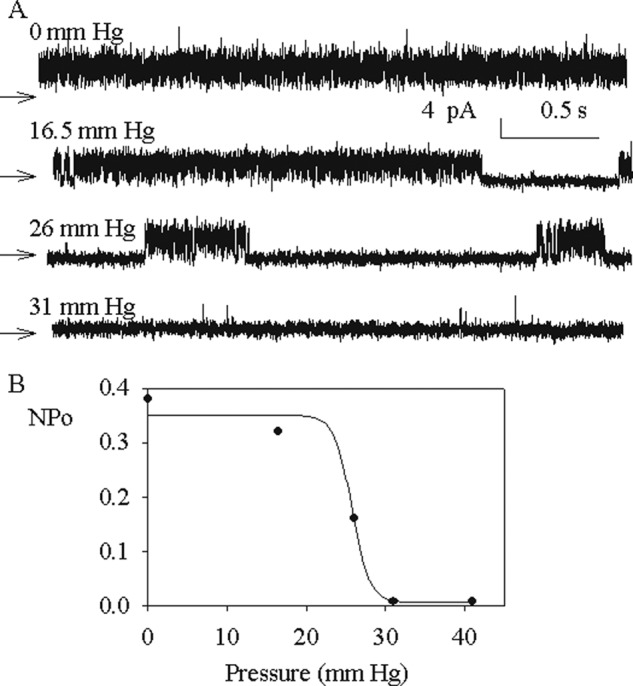
Pressure dependence of the purified TREK-1 channel. A, channel activity of TREK-1 at different applied positive pressures to the pipette, as indicated for each trace. The membrane potential was +40 mV, and the recordings were performed under symmetrical conditions as defined in Fig. 3. B, open probability (NPo) versus the applied pressure of the channels, the activity of which is shown in A. The data are obtained from 10–12 s of recording at each pressure.
Because acidic pH is an activator of TREK-1, we also performed experiments at pH 5.8. A spontaneous channel activity was observed in 11 patches out of 17 patches. At this pH, we could not inhibit channel activity by application of positive pressure.
DISCUSSION
Whether or not a channel is gated directly by membrane tension is a question that calls for a reductionist approach. The protein has to be purified, and its activity examined, after reconstitution in a lipid bilayer (41). This approach has been successfully applied to the study of prokaryotic mechanosensitive channels. In the case of eukaryotic mechanosensitive channels, the use of this approach has been delayed by the difficulty of producing functional recombinant proteins in amounts sufficient for reconstitution experiments.
In this study, we report the production, purification, and functional reconstitution of the mechanosensitive channel TREK-1. The protein was produced in yeast with a BAD extension. This procedure is supposed to select proteins that are properly folded because an unfolded BAD domain will not interact with the affinity column. Indeed only a fraction of the protein that was produced could be solubilized and purified, but the yield (∼15 μg/liter of culture) was sufficient for functional studies. The produced protein was unambiguously identified by SDS-PAGE experiments, immunoblotting, and mass spectrometry. In gel filtration experiments, the DDM-solubilized purified protein behaved as the expected dimer forming the complete channel.
The channel activity that was detected after reconstitution of the protein in giant liposomes amenable to patch clamp recording was similar to that previously documented in biological membranes for TREK-1 channels (7). The potassium channels displayed a characteristic flickery behavior and an outward rectification. In cells, this rectification is due to the presence of magnesium in the external medium (2). In our reconstituted system, it is likely to be due also to the presence of magnesium in the pipette, which is necessary for seal formation on liposomes. The potassium channels were reversibly inhibited by chlorpromazine, a well known inhibitor of TREK-1. In most cases, the observed conductance of the channels (∼80 pS at 50 mV in symmetrical potassium media) matched the conductance originally reported for TREK-1 (7). In a few cases, a potassium conductance that was nearly half that of the main conductance was recorded. It is noteworthy that after the first studies of TREK-1 channels, it was reported that a second smaller conductance could be detected in patches of biological membranes harboring TREK-1 channels (42). A similar observation (two different conductances) was also reported for TREK-2 channels (43). Whether or not these two conductances reflect a molecular modification of the protein or different states of the protein is unknown and requires further investigations. Because these were the only channel activities that were recorded and because no channel activity was detected in control experiments performed with extracts of yeast that did not produce TREK-1, we are confident that we obtained a functional protein and that the activity that we observed was a genuine TREK-1 activity.
In all our recordings, an outward rectification of the channels was noted, and not a mixture of outward and inward rectification. This means that the protein is reconstituted in liposomes with a 100% right-side orientation. This may seem surprising because it is generally assumed that purified proteins reconstitute with a 50% right-side orientation. Although this may be true for some membrane proteins, we have often observed a one-sided reconstitution for ion channels. For instance, the mechanosensitive channel MscL is reconstituted with a right-side orientation, as shown by proteolysis experiments (31). In the case of potassium channels, as well as for MscL, it is possible that the asymmetric geometry of the protein, combined with the high curvature of the membrane of the 100–200-nm liposomes in which they are reconstituted, results in a strong bias toward one orientation. Evidently, the orientation is preserved after fusion of the small proteoliposomes into giant liposomes.
In biological membranes, application of a suction (negative pressure) in the pipette, in the inside-out configuration, resulted in an increase in the open probability of the channel (7). This was the basis for the claim that TREK-1 is a mechanosensitive channel. Because the protein is reconstituted in a right-side orientation, we expected a similar behavior in our patch clamp experiments performed with proteoliposome in the inside-out configuration. Instead, we observed that suction (negative pressure) did not affect the open probability. Thus the probability of opening was probably maximal (but not equal to 1) at zero applied pressure. This is also in contrast with what has long been observed with prokaryotic mechanosensitive channels. These channels are totally closed in the native membrane as well as in liposomes, and open only when a significant negative pressure is applied to the patch. In the case of the MscL channels, opening happens close to the lytic tension of the membrane. However, in the case of proteoliposomes reconstituted with TREK-1, we consistently observed that the gating channels could be completely closed by application of a sizable positive pressure in the patch. This closure was reversible because upon a return to zero pressure in the pipette, the channels were observed to reopen. The simplest explanation for this observation is that the tension already present in a patch of liposome in the absence of applied negative pressure (44, 45) is sufficient to fully activate TREK-1. A modification of the membrane tension induced by applying a positive pressure in the pipette can then induce a transition to the closed state. This implies that the sensitivity to tension is much higher for TREK-1 than for bacterial mechanosensitive channels. More specifically, the midpoint activation tension, the tension for which the open probability is half of the maximum, must be very small. Our results also imply that TREK-1 is more sensitive to pressure in liposomes than in biological membranes. In cells, it is probable that the cytoskeleton limits the activation of the channel by membrane tension, as suggested previously (6, 7, 46).
Previous observations have shown that in biological membranes, TREK-1 could be activated by internal pH so that at pH 5.8, no further increase in channel activity could be obtained by application of a depression in the pipette (34, 36). In our experiments at pH 7.4, when channels were present in the patch, they were already maximally activated. However, when the pH of the bath was lowered to 5.8, the channels could no longer close upon application of a positive pressure. This result is fully consistent with the observations obtained in cells showing that pH is an independent activator of TREK-1.
Although a maximal activation of the TREK-1 channels by a residual membrane tension at zero pressure is a very likely explanation for our results, another possibility is that in our experiments, the channels are specifically activated by lipids present during the reconstitution procedure. In biological membranes, phospholipids were shown to be able to specifically up- or down-regulate the activity of TREK-1 (33, 47). Whatever the explanation for the maximum activity of TREK-1 channels in lipid bilayer at zero applied pressure, we believe that our main observation, the reversible closure of TREK-1 channels upon application of a positive pressure to the patch, is highly significant. It establishes for the first time that TREK-1, a eukaryotic mechanosensitive channel, is directly sensitive to a modification of membrane tension.
Supplementary Material
Acknowledgments
We thank Dr. A. Patel for the gift of the mouse gene TREK-1, Dr. F. Lesage for the gift of the TREK-1 antibody, Dr. C. Montigny for help in the protein expression experiments, and Dr. E. Honoré for many helpful discussions. We are grateful to Dr P. Decottignies for providing expertise and access to mass spectrometry. We are greatly indebted to Dr. H. McLean for the correction of the English manuscript.
This work was supported by Agence nationale de la recherche (ANR) Grant BLAN06-2_135035.

This article contains supplemental Data S1.
- K2P
- two-pore domain potassium channel
- BAD
- biotin acceptor domain
- DDM
- dodecyl-β-d-maltoside
- RS
- Stokes radius
- pS
- picosiemens.
REFERENCES
- 1. Enyedi P., Czirják G. (2010) Molecular background of leak K+ currents: two-pore domain potassium channels. Physiol. Rev. 90, 559–605 [DOI] [PubMed] [Google Scholar]
- 2. Honoré E. (2007) The neuronal background K2P channels: focus on TREK1. Nat. Rev. Neurosci. 8, 251–261 [DOI] [PubMed] [Google Scholar]
- 3. Kim D. (2003) Fatty acid-sensitive two-pore domain K+ channels. Trends Pharmacol. Sci. 24, 648–654 [DOI] [PubMed] [Google Scholar]
- 4. Brohawn S. G., del Mármol J., MacKinnon R. (2012) Crystal structure of the human K2P TRAAK, a lipid- and mechano-sensitive K+ ion channel. Science 335, 436–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Miller A. N., Long S. B. (2012) Crystal structure of the human two-pore domain potassium channel K2P1. Science 335, 432–436 [DOI] [PubMed] [Google Scholar]
- 6. Honoré E., Patel A. J., Chemin J., Suchyna T., Sachs F. (2006) Desensitization of mechano-gated K2P channels. Proc. Natl. Acad. Sci. U.S.A. 103, 6859–6864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Patel A. J., Honoré E., Maingret F., Lesage F., Fink M., Duprat F., Lazdunski M. (1998) A mammalian two pore domain mechano-gated S-like K+ channel. EMBO J. 17, 4283–4290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arnadóttir J., Chalfie M. (2010) Eukaryotic mechanosensitive channels. Annu. Rev. Biophys. 39, 111–137 [DOI] [PubMed] [Google Scholar]
- 9. Kung C., Martinac B., Sukharev S. (2010) Mechanosensitive channels in microbes. Annu. Rev. Microbiol. 64, 313–329 [DOI] [PubMed] [Google Scholar]
- 10. Hamill O. P., McBride D. W., Jr. (1997) Induced membrane hypo/hyper-mechanosensitivity: a limitation of patch-clamp recording. Annu. Rev. Physiol. 59, 621–631 [DOI] [PubMed] [Google Scholar]
- 11. Kung C. (2005) A possible unifying principle for mechanosensation. Nature 436, 647–654 [DOI] [PubMed] [Google Scholar]
- 12. Perozo E. (2006) Gating prokaryotic mechanosensitive channels. Nat. Rev. Mol. Cell Biol. 7, 109–119 [DOI] [PubMed] [Google Scholar]
- 13. Berrier C., Coulombe A., Szabo I., Zoratti M., Ghazi A. (1992) Gadolinium ion inhibits loss of metabolites induced by osmotic shock and large stretch-activated channels in bacteria. Eur. J. Biochem. 206, 559–565 [DOI] [PubMed] [Google Scholar]
- 14. Levina N., Tötemeyer S., Stokes N. R., Louis P., Jones M. A., Booth I. R. (1999) Protection of Escherichia coli cells against extreme turgor by activation of MscS and MscL mechanosensitive channels: identification of genes required for MscS activity. EMBO J. 18, 1730–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Berrier C., Coulombe A., Houssin C., Ghazi A. (1989) A patch-clamp study of ion channels of inner and outer membranes and of contact zones of E. coli, fused into giant liposomes. Pressure-activated channels are localized in the inner membrane. FEBS Lett. 259, 27–32 [DOI] [PubMed] [Google Scholar]
- 16. Sukharev S. I., Martinac B., Arshavsky V. Y., Kung C. (1993) Two types of mechanosensitive channels in the Escherichia coli cell envelope: solubilization and functional reconstitution. Biophys. J. 65, 177–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sukharev S. (2002) Purification of the small mechanosensitive channel of Escherichia coli (MscS): the subunit structure, conduction, and gating characteristics in liposomes. Biophys. J. 83, 290–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sukharev S. I., Blount P., Martinac B., Blattner F. R., Kung C. (1994) A large-conductance mechanosensitive channel in E. coli encoded by mscL alone. Nature 368, 265–268 [DOI] [PubMed] [Google Scholar]
- 19. Kloda A., Martinac B. (2001) Mechanosensitive channels in archaea. Cell Biochem. Biophys. 34, 349–381 [DOI] [PubMed] [Google Scholar]
- 20. Haswell E. S., Phillips R., Rees D. C. (2011) Mechanosensitive channels: what can they do and how do they do it? Structure 19, 1356–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Coste B., Xiao B., Santos J. S., Syeda R., Grandl J., Spencer K. S., Kim S. E., Schmidt M., Mathur J., Dubin A. E., Montal M., Patapoutian A. (2012) Piezo proteins are pore-forming subunits of mechanically activated channels. Nature 483, 176–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhou X. L., Batiza A. F., Loukin S. H., Palmer C. P., Kung C., Saimi Y. (2003) The transient receptor potential channel on the yeast vacuole is mechanosensitive. Proc. Natl. Acad. Sci. U.S.A. 100, 7105–7110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kloda A., Lua L., Hall R., Adams D. J., Martinac B. (2007) Liposome reconstitution and modulation of recombinant N-methyl-d-aspartate receptor channels by membrane stretch. Proc. Natl. Acad. Sci. U.S.A. 104, 1540–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lenoir G., Menguy T., Corre F., Montigny C., Pedersen P. A., Thinès D., le Maire M., Falson P. (2002) Overproduction in yeast and rapid and efficient purification of the rabbit SERCA1a Ca2+-ATPase. Biochim. Biophys. Acta 1560, 67–83 [DOI] [PubMed] [Google Scholar]
- 25. Pompon D., Louerat B., Bronine A., Urban P. (1996) Yeast expression of animal and plant P450s in optimized redox environments. Methods Enzymol. 272, 51–64 [DOI] [PubMed] [Google Scholar]
- 26. Gietz R. D., Schiestl R. H., Willems A. R., Woods R. A. (1995) Studies on the transformation of intact yeast cells by the LiAc/SS-DNA/PEG procedure. Yeast 11, 355–360 [DOI] [PubMed] [Google Scholar]
- 27. Cardi D., Montigny C., Arnou B., Jidenko M., Marchal E., le Maire M., Jaxel C. (2010b) Heterologous expression and affinity purification of eukaryotic membrane proteins in view of functional and structural studies: The example of the sarcoplasmic reticulum Ca2+-ATPase. Methods Mol. Biol. 601, 247–267 [DOI] [PubMed] [Google Scholar]
- 28. Cardi D., Pozza A., Arnou B., Marchal E., Clausen J. D., Andersen J. P., Krishna S., Møller J. V., le Maire M., Jaxel C. (2010a) Purified E255L mutant SERCA1a and purified PfATP6 are sensitive to SERCA-type inhibitors but insensitive to artemisinins. J. Biol. Chem. 285, 26406–26416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. (1985) Measurement of protein using bicinchoninic acid. Anal. Biochem. 150, 76–85 [DOI] [PubMed] [Google Scholar]
- 30. Hortigón-Vinagre M. P., Chardonnet S., Montigny C., Gutiérrez-Martín Y., Champeil P., Henao F. (2011) Inhibition by 4-hydroxynonenal (HNE) of Ca2+ transport by SERCA1a: low concentrations of HNE open protein-mediated leaks in the membrane. Free Radic. Biol. Med. 50, 323–336 [DOI] [PubMed] [Google Scholar]
- 31. Ajouz B., Berrier C., Besnard M., Martinac B., Ghazi A. (2000) Contributions of the different extramembranous domains of the mechanosensitive ion channel MscL to its response to membrane tension. J. Biol. Chem. 275, 1015–1022 [DOI] [PubMed] [Google Scholar]
- 32. Jidenko M., Nielsen R. C., Sørensen T. L., Møller J. V., le Maire M., Nissen P., Jaxel C. (2005) Crystallization of a mammalian membrane protein overexpressed in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U.S.A. 102, 11687–11691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chemin J., Patel A. J., Duprat F., Lauritzen I., Lazdunski M., Honoré E. (2005) A phospholipid sensor controls mechanogating of the K+ channel TREK-1. EMBO J. 24, 44–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Honoré E., Maingret F., Lazdunski M., Patel A. J. (2002) An intracellular proton sensor commands lipid- and mechano-gating of the K+ channel TREK-1. EMBO J. 21, 2968–2976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Maingret F., Lauritzen I., Patel A. J., Heurteaux C., Reyes R., Lesage F., Lazdunski M., Honoré E. (2000) TREK-1 is a heat-activated background K+ channel. EMBO J. 19, 2483–2491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Maingret F., Patel A. J., Lesage F., Lazdunski M., Honoré E. (1999) Mechano- or acid stimulation, two interactive modes of activation of the TREK-1 potassium channel. J. Biol. Chem. 274, 26691–26696 [DOI] [PubMed] [Google Scholar]
- 37. Thomas D., Plant L. D., Wilkens C. M., McCrossan Z. A., Goldstein S. A. (2008) Alternative translation initiation in rat brain yields K2P2.1 potassium channels permeable to sodium. Neuron 58, 859–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Di Cera E., Cantwell A. M. (2001) Determinants of thrombin specificity. Ann. N.Y. Acad. Sci. 936, 133–146 [DOI] [PubMed] [Google Scholar]
- 39. le Maire M., Arnou B., Olesen C., Georgin D., Ebel C., Møller J. V. (2008) Gel chromatography and analytical ultracentrifugation to determine the extent of detergent binding and aggregation, and Stokes radius of membrane proteins using sarcoplasmic reticulum Ca2+-ATPase as an example. Nat. Protoc. 3, 1782–1795 [DOI] [PubMed] [Google Scholar]
- 40. Møller J. V., le Maire M. (1993) Detergent binding as a measure of hydrophobic surface area of integral membrane proteins. J. Biol. Chem. 268, 18659–18672 [PubMed] [Google Scholar]
- 41. Ghazi A., Berrier C., Ajouz B., Besnard M. (1998) Mechanosensitive ion channels and their mode of activation. Biochimie 80, 357–362 [DOI] [PubMed] [Google Scholar]
- 42. Xian Tao L.i., Dyachenko V., Zuzarte M., Putzke C., Preisig-Müller R., Isenberg G., Daut J. (2006) The stretch-activated potassium channel TREK-1 in rat cardiac ventricular muscle. Cardiovasc. Res. 69, 86–97 [DOI] [PubMed] [Google Scholar]
- 43. Kang D., Choe C., Cavanaugh E., Kim D. (2007) Properties of single two-pore domain TREK-2 channels expressed in mammalian cells. J. Physiol. 583, 57–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Suchyna T. M., Markin V. S., Sachs F. (2009) Biophysics and structure of the patch and the gigaseal. Biophys. J. 97, 738–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ursell T., Agrawal A., Phillips R. (2011) Lipid bilayer mechanics in a pipette with glass-bilayer adhesion. Biophys. J. 101, 1913–1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lauritzen I., Chemin J., Honoré E., Jodar M., Guy N., Lazdunski M., Jane Patel A. (2005) Cross-talk between the mechano-gated K2P channel TREK-1 and the actin cytoskeleton. EMBO Rep. 6, 642–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chemin J., Patel A. J., Duprat F., Sachs F., Lazdunski M., Honore E. (2007) Up- and down-regulation of the mechano-gated K2P channel TREK-1 by PIP2 and other membrane phospholipids. Pflugers Arch. 455, 97–103 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


