Background: Altered methylation of PP2A, a major Tau phosphatase, occurs in Alzheimer disease.
Results: PP2A methylation regulates the association of PP2A with membrane rafts and plasma membrane-bound Tau levels.
Conclusion: Altered PP2A methylation inhibits targeting of PP2A and Tau to the plasma membrane.
Significance: Altered PP2A and Tau membrane distribution could promote neuronal dysfunction and phospho-Tau pathology in Alzheimer disease.
Keywords: Alzheimer Disease, Lipid Raft, Plasma Membrane, PP2A, Protein Methylation, Serine Threonine Protein Phosphatase, Tau
Abstract
Down-regulation of protein phosphatase 2A (PP2A) methylation occurs in Alzheimer disease (AD). However, the regulation of PP2A methylation remains poorly understood. We have reported that altered leucine carboxyl methyltransferase (LCMT1)-dependent PP2A methylation is associated with down-regulation of PP2A holoenzymes containing the Bα subunit (PP2A/Bα) and subsequent accumulation of phosphorylated Tau in N2a cells, in vivo and in AD. Here, we show that pools of LCMT1, methylated PP2A, and PP2A/Bα are co-enriched in cholesterol-rich plasma membrane microdomains/rafts purified from N2a cells. In contrast, demethylated PP2A is preferentially distributed in non-rafts wherein small amounts of the PP2A methylesterase PME-1 are exclusively present. A methylation-incompetent PP2A mutant is excluded from rafts. Enhanced methylation of PP2A promotes the association of PP2A and Tau with the plasma membrane. Altered PP2A methylation following expression of a catalytically inactive LCMT1 mutant, knockdown of LCMT1, or alterations in one-carbon metabolism all result in a loss of plasma membrane-associated PP2A and Tau in N2a cells. This correlates with accumulation of soluble phosphorylated Tau, a hallmark of AD and other tauopathies. Thus, our findings reveal a distinct compartmentalization of PP2A and PP2A regulatory enzymes in plasma membrane microdomains and identify a novel methylation-dependent mechanism involved in modulating the targeting of PP2A, and its substrate Tau, to the plasma membrane. We propose that alterations in the membrane localization of PP2A and Tau following down-regulation of LCMT1 may lead to PP2A and Tau dysfunction in AD.
Introduction
Typically, membrane rafts are biochemically defined as small, dynamic, heterogeneous cholesterol and sphingolipid-enriched microdomains (CEMs)2 that compartmentalize cellular processes (1, 2). Although the concept of membrane raft remains controversial based on technicalities, there is compelling evidence that membrane microdomains can serve as major signaling sorting platforms, allowing for selective compartmentalization, recruitment, and regulated interaction of key membrane proteins (3, 4). Dynamic rearrangement of membrane microdomains leading to localized protein clustering or dissociation has been linked to generation of signal transduction cascades in both normal and pathophysiological conditions (3, 4). Significantly, alterations in the composition of membrane raft and non-raft microdomains are associated with AD pathogenesis (5, 6). Both the microtubule-associated protein Tau and the amyloid precursor proteins, which play a central role in the disease, can be recruited to membrane rafts (7). Notably, AD-like phosphorylation of Tau at specific Ser/Thr residues can prevent its targeting to the plasma membrane (8–11). Although the exact role of neuronal membrane-associated Tau remains to be elucidated, it is likely to serve as a scaffold for signaling molecules (12, 13) so that altered membrane distribution of AD-Tau may underlie Tau dysfunction (14).
Significantly, Tau interacts with and is a major substrate of the Ser/Thr protein phosphatase 2A PP2A/Bα holoenzyme, a heterotrimer containing the catalytic C (PPP2CA), scaffolding A (PPP2A1A), and regulatory Bα (PPP2R2A) subunits (15–19). Notably, methylation of PP2A catalytic C subunit on the Leu-309 residue by leucine carboxyl methyltransferase 1 (LCMT1) promotes the biogenesis and stabilization of PP2A/Bα enzymes (20). We have shown that decreased LCMT1 activity and/or expression levels correlate with down-regulation of PP2A methylation and PP2A/Bα expression levels and with concomitant accumulation of phospho-Tau in AD-affected brain regions (21), in cultured N2a neuroblastoma cells and in vivo (22, 23). Conversely, PP2A C subunit is demethylated by the methylesterase PME-1. However, the precise regulation and function of LCMT1, PME-1, and PP2A methylation/demethylation processes are not completely elucidated. Current models suggest an important role of PME-1 in binding to inactive PP2A and preventing its untimely methylation (20, 24). One study has shown that PME-1 and demethylated C are enriched in the nucleus, whereas LCMT1 is abundant in the cytosol of fibroblasts, suggesting a spatial regulation of PP2A methylation/demethylation processes (25). It is likely that the compartmentalization, sequestration, and/or signal-mediated translocation of PP2A to specific subcellular domains contribute to modulate isoform-specific PP2A enzyme-substrate interactions. For instance, binding of neuronal PP2A/Bα to microtubules inhibits its catalytic activity toward Tau (26, 27); in fibroblasts, a pool of PP2A/Bα is enriched in CEMs, where it plays a critical role in regulating ERK signaling (28). Despite the major collective role of PP2A enzymes in signaling, much remains to be learned about their spatial regulation in most cells.
Here, we provide evidence that PP2A/Bα, methylated PP2A, and LCMT1 are co-enriched in CEMs/rafts purified from N2a neuroblastoma cells. We show that LCMT1-dependent PP2A methylation critically modulates the targeting of both PP2A and Tau to the plasma membrane. Our findings suggest that altered PP2A methylation in AD could lead to a significant redistribution of both Tau and PP2A from the plasma membrane to the cytosol, thereby altering the putative functions of PP2A and Tau at the neuronal plasma membrane while promoting the accumulation of cytosolic hyperphosphorylated Tau.
EXPERIMENTAL PROCEDURES
Materials and Reagents
Unless indicated, all chemicals and drugs were from Sigma.
Cell Culture and Transfection
Control Neuro-2a (N2a, American Type Culture Collection) and stable cell lines were maintained in DMEM (Invitrogen) containing 2.5 mm Hepes, pH 7.4, 10% fetal bovine serum (FBS, HyClone), and 10 μg/ml gentamycin (Invitrogen). N2a cells stably expressing SV40 small tumor antigen (St), Myc-tagged PME-1 and hemagglutinin (HA)-tagged LCMT1, Bα, wild-type C, or the methyl site L309Δ C subunit mutant have been characterized in previous studies (22, 23, 29, 30). Cells stably expressing the R71Δ catalytically inactive mutant of LCMT were generated as follows. The mutant plasmid was generated using the TransformerTM site-directed mutagenesis kit (Clontech) and pBabeHygro encoding HA-tagged wild-type LCMT1 (22) as template. Plasmids were verified by sequencing. Cells were transfected using Metafectene Pro reagent following the manufacturer's instructions (Biontex Laboratories, Munich, Germany); stable clones were generated after selection with 200 μg/ml hygromycin (Roche Applied Science). The expression levels of transfected proteins were constantly monitored by both immunoblotting and immunofluorescence. Cells mock-transfected with empty vectors were used as “controls” and behaved like nontransfected cells in our experiments. Knockdown of endogenous LCMT1 in N2a cells was performed exactly as described previously, using transient transfection with small interfering RNA (siRNA) specifically targeted to mouse LCMT1; cells transfected with mismatch siRNA were used as controls in these experiments (23).
Cell Treatment
Unless otherwise indicated, experiments were performed in 80% confluent cells cultured in regular cell culture medium. For drug treatment, cells were first starved overnight in DMEM containing 1% dialyzed FBS (HyClone) and then incubated in the same medium with the indicated concentrations of drug. To assess the regulatory role of cholesterol, cells were incubated at 37 °C for 15 min in serum-free medium with 1% methyl-β-cyclodextrin (MβCD) premixed or not with 100 μg/ml cholesterol (a gift from Dr. Joseph Goldstein, UT Southwestern, Dallas, TX) (28) prior to being harvested for fractionation.
Cell Lysis and Fractionation
To assess protein expression levels, total cell homogenates were prepared in buffer 1 (25 mm Tris, pH 7.4, 150 mm NaCl, 2 mm EDTA, 25 m sodium fluoride, 1 mm sodium orthovanadate, 2 mm dithiothreitol, 1 μm okadaic acid (OA), 5 mm PMSF, and 1% Nonidet P-40), containing a mixture of protease inhibitors (Roche Applied Science) (Sontag et al. (22)). For purification of membrane-associated proteins, cells were washed in PBS and incubated for 5 min at room temperature in buffer S (0.25 m sucrose, 1 mm imidazole, 5 mm MgCl2). The medium was aspirated, and cells were harvested in buffer S containing 1 μm OA, 1 mm DTT, and a mixture of cOmpleteTM protease inhibitors (Roche Applied Science), incubated on ice for 15 min, and then homogenized with a Dounce. Cells were centrifuged at 800 × g at 4 °C to pellet nuclei, after which the supernatant was centrifuged at 4 °C for 45 min at 100,000 × g in a Beckman TL-100.3 rotor. The supernatant (cytosol) was collected, and the remaining pellet (membrane fraction) was resuspended in buffer 1 and sonicated. Pelleted nuclear and membrane fractions were washed and recentrifuged prior to analysis by Western blot. Antibodies to nuclear and plasma membrane markers were systematically utilized to verify the purity of the fractions.
Purification of Cholesterol-enriched Plasma Membrane Microdomains
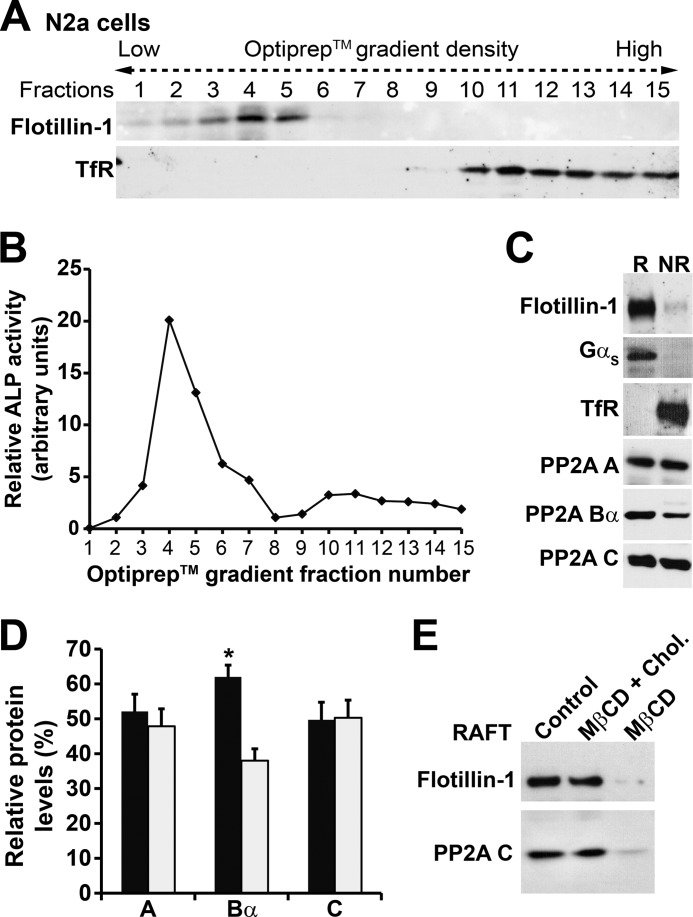
Published detergent-free procedures were utilized to purify low buoyancy CEMs (28, 31). Briefly, cells (6 × 100-mm dishes per condition) were collected in ice-cold PBS and centrifuged for 5 min at 800 × g. The pellet was homogenized in a buffer (0.25 m sucrose, 1 mm EDTA, 20 mm Tris pH 7.8) containing 1 mm DTT and cOmpleteTM protease and PhosSTOP phosphatase inhibitor cocktails (Roche Applied Science). Aliquots of total cell homogenates were kept for future analysis. Homogenates were centrifuged for 5 min at 1,000 × g to pellet nuclei. The postnuclear supernatant was loaded onto a 30% Percoll gradient and centrifuged for 30 min at 84,000 × g in a BeckmanTM TI60 ultracentrifuge to separate the plasma membrane from the cytosol and other membrane fractions. A thin layer of white particles corresponding to the purified total plasma membrane fraction (∼2 ml) was collected. After sonication, the material was resuspended in a 50% OptiPrepTM solution to give a final 23% OptiPrepTM solution that was loaded on the bottom of a 20–10% OptiPrepTM gradient mix and centrifuged for 90 min at 52,000 × g. The whole gradient was collected in 15 aliquots of 730 μl per each aliquot. The activity of alkaline phosphatase was systematically measured in 20-μl aliquots from each fraction, using the colorimetric alkaline phosphatase substrate kit (Bio-Rad). Dephosphorylation of p-nitrophenyl phosphate by alkaline phosphatase was determined by measuring absorbance at 405 nm. The protein concentration was determined in aliquots of total cell homogenates and in the purified membrane fractions using the Bradford protein assay kit (Bio-Rad). The lighter cholesterol-enriched buoyant material corresponding to membrane rafts was defined by its enrichment of flotillin-1 and alkaline phosphatase and the absence of transferrin receptor (TfR). Identified raft (R, typically fractions 1–7) and non-raft (NR, typically fractions 9–15) fractions were pooled together and analyzed by SDS-PAGE and Western blotting after trichloroacetic acid/acetone protein precipitation.
Gel Electrophoresis and Western Blotting
Protein samples were resolved either by SDS-PAGE on 12% gradient SDS-polyacrylamide gels (Amersham Biosciences) for diluted samples or NuPAGE on 4–12% Bis-Tris gels (Invitrogen) followed by Western blotting and densitometry to quantitate changes in protein expression levels (22, 23). Data were analyzed using the Student's t test. Differences with p < 0.05 were considered statistically significant. Monoclonal methylation- and demethylation-specific (Millipore) and methylation-independent (BD Biosciences) anti-C antibodies were utilized to quantify PP2A methylation levels by Western blotting and densitometry exactly as reported earlier (21–23). In some experiments, equivalent aliquots (5 μl) of cell fractions were incubated for 30 min at 37 °C in the absence or presence of 0.2 n sodium hydroxide. This alkaline treatment results in complete demethylation of PP2A at Leu-309, allowing for detection of total C expression levels with anti-demethyl C antibodies and precise quantification of demethylated PP2A levels (21, 32). Other antibodies included: rabbit anti-Tau (rPeptide, Bogart, GA); mouse anti-Tau-1 (Millipore); rabbit anti-Tau (phospho-Ser-422) (Invitrogen); mouse anti-Bα 2G9 (Millipore); mouse anti-HA 16B12 (Covance Research Products); mouse anti-LCMT and rabbit anti-Gsα (Millipore); rabbit anti-PME-1 (22); mouse anti-flotillin-1 (BD Transduction Laboratories); rabbit anti-LSD1 (Cell Signaling); mouse anti-TfR (Invitrogen); and rabbit anti-Na+/K+ ATPase (Abcam). Mouse anti-actin antibodies (Sigma) were utilized to normalize for protein loading.
RESULTS
PP2A/Bα Enzymes Are Preferentially Enriched in Membrane Rafts from N2a Cells
We have previously utilized detergent-free methods to demonstrate that PP2A/Bα is present in CEMs from fibroblasts (28). Similar procedures were carried out to isolate CEMs from N2a neuroblastoma cells and investigate the potential presence of PP2A/Bα and its regulatory enzymes. First, the plasma membrane from N2a cells was fractionated on an OptiPrepTM gradient, and aliquots of collected fractions were probed for the presence of flotillin-1, a glycosylphosphatidylinositol-linked protein that is an established raft marker, and TfR, a non-raft plasma membrane marker (Fig. 1A). Collected fractions were also analyzed for glycosylphosphatidylinositol-anchored alkaline phosphatase activity (Fig. 1B). Characterized raft fractions (defined by their enrichment in flotillin-1 and alkaline phosphatase activity) and TfR-positive non-raft fractions were separately pooled together and further analyzed by gel electrophoresis and immunoblotting. As expected, flotillin-1 was found to be enriched in, whereas TfR was excluded from purified N2a cell membrane rafts (Fig. 1C), in agreement with previous studies using similar protocols in human neuroblastoma cells (33). In addition to flotillin-1, the G-protein Gsα subunit was also concentrated in the raft fractions, as previously reported in N2a cells (34). The scaffolding A and catalytic C subunits that are common to all PP2A heterotrimers were equally present in both raft and non-raft fractions. In contrast, the PP2A regulatory Bα subunit was more concentrated in rafts (Fig. 1, C and D), an indication that PP2A/Bα holoenzymes are specifically enriched in CEMs from N2a cells, as previously observed in fibroblasts (28). Next, we assessed the effect of cholesterol depletion in N2a cells. Treatment of N2a cells for 15 min with the cholesterol-adsorbing resin, MβCD, induced the loss of flotillin-1 and PP2A from membrane rafts (Fig. 1E), as reported earlier in fibroblasts (28). Notably, this effect was abolished when cells were incubated with MβCD that had been premixed with cholesterol, indicating that the localization of PP2A in raft was cholesterol-dependent.
FIGURE 1.
Endogenous PP2A/Bα holoenzymes are enriched in raft membrane microdomains from N2a cells. Raft (R) and non-raft (NR) plasma membrane fractions were purified from N2a cells after centrifugation on OptiPrepTM gradients as described under ”Experimental Procedures.“ A, Western blot analysis of the distribution of flotillin-1, a raft marker, and TfR, a non-raft marker, in collected fractions normalized for protein content. B, relative activity of alkaline phosphatase (ALP), a glycosylphosphatidylinositol-anchored protein, in each collected fraction from the same purification shown in A. Data are shown as arbitrary units (measured absorbance corrected for protein concentration). C, equivalent aliquots of proteins (∼15 μg) from pooled raft and non-raft fractions were analyzed for the distribution of PP2A A, Bα, and C subunits. Note the preferential enrichment of flotillin-1 and the heterotrimeric G-protein Gsα subunit (Gαs) in purified rafts, which are free of TfR. In A–C, representative data are shown. Similar results were obtained in five separate experiments. D, comparative distribution of PP2A subunit levels in purified R (black bars) and NR (gray bars) fractions (n = 5; *, p < 0.001, mean ± S.D., R versus NR). E, representative distribution of PP2A-C subunit and flotillin-1 in aliquots (15 μg) of raft fractions purified from N2a cells that were incubated for 15 min in serum-deficient medium in the absence (Control) or presence of the cholesterol depletion agent MβCD, or MβCD premixed with cholesterol (MβCD + Chol). Similar results were obtained in three separate experiments.
LCMT1 and Methylated PP2A Are Concentrated in Rafts, whereas Demethylated PP2A and Small Amounts of PME-1 Are Preferentially Distributed in Non-raft Membrane Microdomains
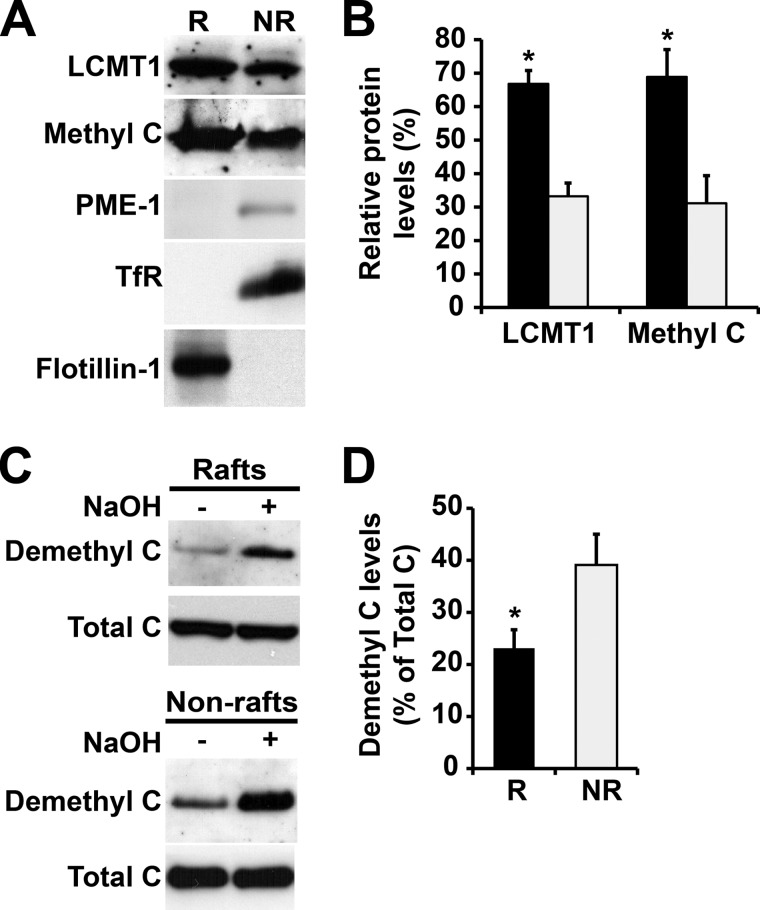
As observed with Bα, a highest proportion of methylated PP2A C subunit and LCMT1 was present in membrane rafts, relative to non-rafts (Fig. 2, A and B), supporting the hypothesis that LCMT1-dependent PP2A methylation promotes stabilization of PP2A/Bα in CEMs. In contrast, very small amounts of PME-1 were solely detected in non-rafts. Demethylated PP2A was proportionally more abundant in non-raft than in raft plasma membrane domains (Fig. 2C). Furthermore, quantitative analyses show that demethylated enzymes represented only a small fraction of total PP2A enzymes present in both raft and non-raft compartments. (Fig. 2D). Thus, our data indicate for the first time that methylated and demethylated PP2A, as well as LCMT1 and PME-1, are spatially and quantitatively segregated in distinct plasma membrane microdomains.
FIGURE 2.
Differential distribution of LCMT1, PME-1, and methylated and demethylated PP2A enzymes in N2a cell membrane microdomains. A, equivalent aliquots of proteins (∼15 μg) from pooled R and NR plasma membrane fractions purified from N2a cells were analyzed by SDS-PAGE followed by Western blotting for the presence of LCMT1, PME-1, and methylated PP2A C subunit (Methyl C). B, note the preferential enrichment of LCMT1 and methylated PP2A in R (black bars) versus NR (gray bars) fractions (n = 4, mean ± S.D.; *, p < 0.001, R versus NR). The levels of PME-1 were too low to be quantified. C, the distribution of demethylated PP2A was assessed with a specific monoclonal anti-demethyl C antibody in the same purified R and NR fractions. Parallel treatment of duplicate aliquots of R and NR fractions with sodium hydroxide (+ NaOH) induces complete demethylation of PP2A, thereby allowing for detection of total C subunit levels with the same antibody. Note that NaOH treatment does not affect the distribution of total C in R and NR fractions, as detected by a methylation-insensitive antibody. D, quantification of the total percentage of PP2A in a demethylated form in R and NR fractions (n = 4, mean ± S.D.; *, p < 0.001, R versus NR). Note the preferential concentration of demethylated C enzymes in NR fractions.
The Methylation-incompetent L309Δ Mutant of PP2A C Subunit Is Excluded from Membrane Rafts
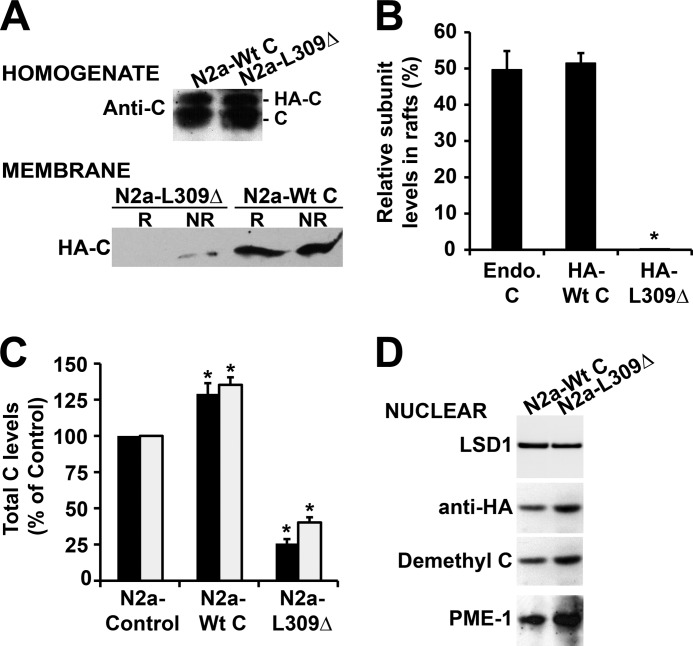
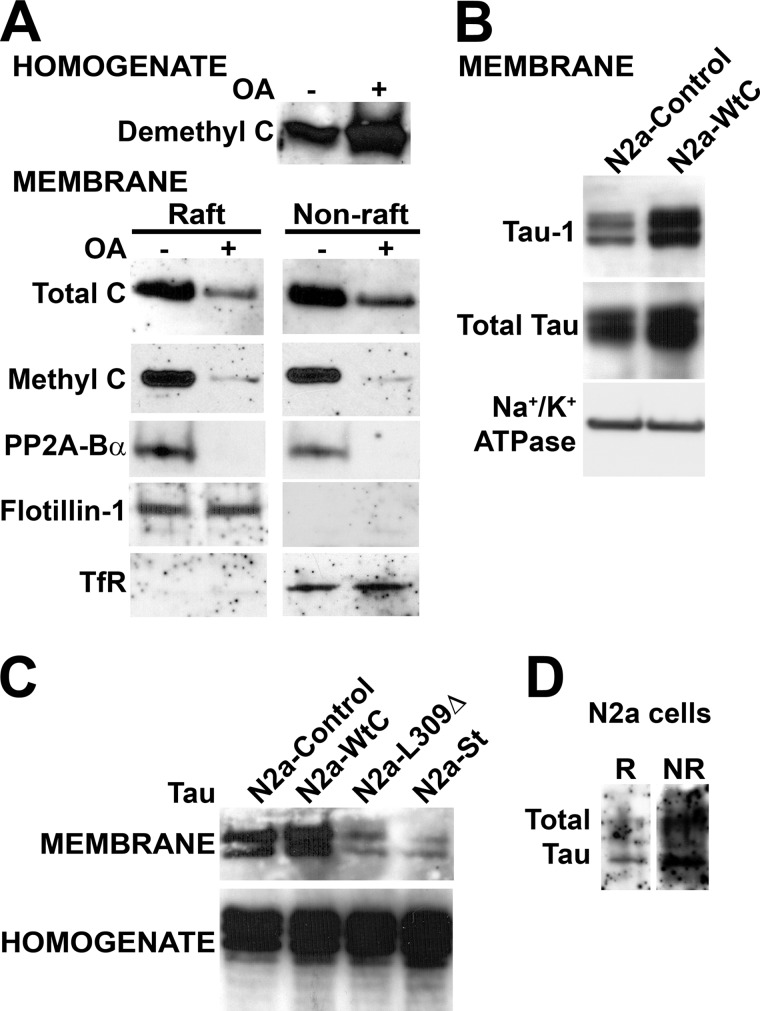
To gain further understanding of the regulation of PP2A targeting to the plasma membrane, we purified raft and non-raft fractions from well characterized N2a cell lines stably expressing either HA-tagged PP2A wild-type C subunit (N2a-Wt C) that is methylation-competent and can bind to Bα or the methylation site L309Δ C subunit mutant (N2a-L309Δ) that is methylation-incompetent and unable to associate with Bα (29, 30). As observed with endogenous C, plasma membrane-bound pools of expressed wild-type C were roughly equally distributed between rafts and non-rafts (Fig. 3, A and B). On the other hand, the L309Δ mutant was essentially absent from CEMs, and only small amounts of the mutant could be detected in non-raft membrane fractions. We have previously shown that ectopic expression of the C subunit in N2a cells only induces an ∼30% increase in total C levels, due to autoregulation of C levels and replacement of a portion of endogenous C by expressed C subunits (22). Accordingly, total C subunit amounts were increased by a similar magnitude in both raft and non-raft fractions in N2a-Wt C cells, relative to controls (Fig. 3C). In contrast, there was a dramatic decrease in total membrane-associated C subunit levels in N2a-L309Δ cells when compared with N2a and N2a-Wt C cells. We have previously reported that, relative to controls, expression of the L309Δ mutant induces an ∼35–45% reduction of methylated C and Bα subunit levels and concomitant accumulation of demethylated C in N2a cells (22). The net loss of membrane-associated PP2A in N2a-L309Δ cells could result from either inhibition of PP2A targeting to the membrane in the first place or fallout of PP2A from the plasma membrane and subsequent redistribution of the enzyme to other subcellular compartments. We observed that demethylated C and PME-1 levels were abundant in the cytosol (data not shown) and increased in nuclear fractions purified from N2a-L309Δ, relative to N2a-Wt C cells (Fig. 3D). These findings are in line with a previous study in Cos-7 and HeLa cells showing that both demethylated C and PME-1 are present in the cytoplasm and concentrated in the nucleus (25), likely as a result of complex formation between PME-1 and demethylated C (35).
FIGURE 3.
Expression of the methylation site L309Δ C subunit mutant affects the association of PP2A with the plasma membrane. Control and stable N2a cell lines expressing HA-tagged wild-type C (N2a-Wt C) or the L309Δ mutant of PP2A C subunit (N2a-L309Δ) were analyzed by Western blotting after subcellular fractionation. A, top panel, analysis of total homogenates (∼30 μg of proteins each) with anti-C antibody shows similar expression levels of ectopic proteins in N2a-Wt C and N2a-L309Δ cells. Lower panel, immunoblot analysis with anti-HA antibody of R and NR membrane fractions (∼20 μg of proteins each) purified from the same cells reveals that the L309Δ mutant is essentially excluded from rafts and present at low levels in non-rafts. B, quantitative analyses show that in contrast to the HA-tagged L309Δ mutant, the distribution of HA-tagged Wt C subunit in rafts is similar to that of endogenous C (Endo. C) (n = 4, mean ± S.D.; *, p < 0.001, relative to endogenous C). C, quantitative analyses show that relative to control cells, total raft- (black bars) and non-raft (gray bars)-associated PP2A C levels are increased in N2a-Wt C cells; in contrast, there is a significant loss of R- and NR-bound PP2A C subunit in N2a-L309Δ cells (n = 4, mean ± S.D.; *, p < 0.001, relative to controls). D, representative blot from three separate experiments showing the nuclear enrichment of demethylated C and PME-1 in N2a-L309Δ cells relative to N2a-Wt C cells. LSD-1, nuclear marker.
LCMT1-dependent Methylation Affects the Amounts of PP2A Associated with the Plasma Membrane
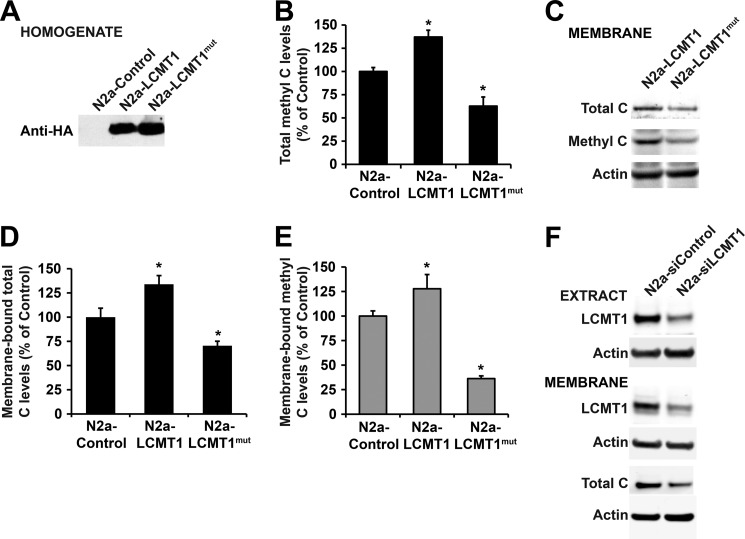
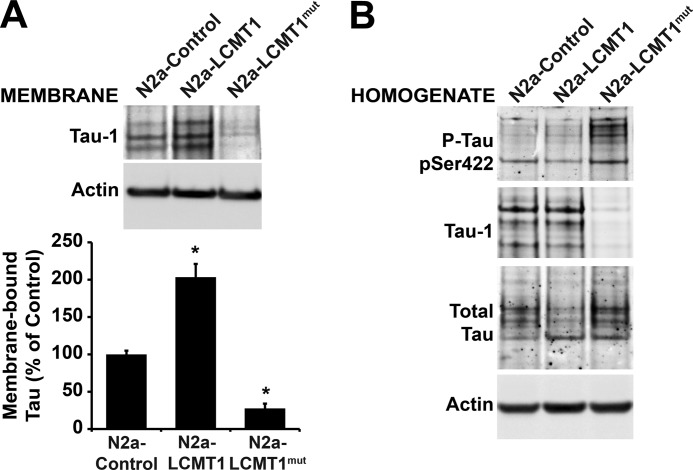
To test the hypothesis that LCMT1-dependent methylation can direct PP2A to the plasma membrane, we first compared PP2A behavior in N2a cells expressing either wild-type LCMT1 (N2a-LCMT1) or an LCMT1 mutant (N2a-LCMT1mut) bearing an R71Δ point mutation (Fig. 4A) that has previously been shown to interfere with its PP2A methyltransferase activity (36). Expectedly, the overall methylation state of endogenous PP2A was significantly reduced in total homogenates from N2a-LCMT1mut cells, relative to N2a and N2a-LCMT1 cells (Fig. 4B). This correlated with a dramatic reduction in membrane-bound total and methylated C subunit levels (Fig. 4, C–E). Conversely, expression of LCMT1 in N2a cells, which increases endogenous methylated PP2A levels by ∼30% relative to controls (22), correlated with a net increase of membrane-associated total and methylated PP2A C levels (Fig. 4, D and E). These findings support a close interrelationship between LCMT1-mediated methylation and membrane association of PP2A. To further establish the role of LCMT1 in PP2A membrane targeting, we next assessed the effects of down-regulating LCMT1 expression. We have previously reported that specific knockdown of LCMT1 in N2a cells using siRNA targeted to LCMT1 induces a significant loss in endogenous methylated PP2A C levels, relative to N2a cells or N2a cells transfected with siRNA mismatch controls (23). Experimental conditions were chosen here to only partially down-regulate LCMT1 expression to avoid potential N2a cell death associated with persistent and marked LCMT1 RNA silencing (23). An ∼42–50% reduction in cytosolic and membrane-associated LCMT1 levels was achieved following transient transfection of N2a cells with an siRNA targeted to LCMT1 (Fig. 4F). As observed with the methyltransferase-incompetent LCMT1 mutant, partial down-regulation of endogenous LCMT1 levels was also associated with an ∼42 ± 5% (p < 0.001, relative to controls) reduction in total membrane-associated PP2A C levels (Fig. 4F). Altogether, these data suggest that LCMT1-mediated PP2A methylation critically influences the amounts of PP2A present at the plasma membrane.
FIGURE 4.
Expression of the catalytically inactive LCMT1 mutant or knockdown of LCMT1 induces a loss of membrane-associated pools of PP2A. A, control N2a cells or N2a cells expressing wild-type LCMT1 (N2a-LCMT1) or the R71Δ LCMT1 mutant (N2a-LCMT1mut) were analyzed by Western blotting using anti-HA antibody to detect transfected proteins. B, comparative levels of methylated PP2A C subunit in total homogenates from control N2a, N2a-LCMT1, and N2a-LCMTmut cell lines (n = 4, mean ± S.D.; *, p < 0.001, relative to controls). C–E, representative blot (C) and quantitative analyses showing the expression levels of total (D) and methylated (E) PP2A C subunit in plasma membrane fractions purified from control N2a, N2a-LCMT1, and N2a-LCMTmut cell lines (n = 4; mean ± S.D.; *, p < 0.001, relative to controls). F, purified membrane fractions from N2a cells transfected with an siRNA targeted to LCMT1 (N2a-siLCMT1) or a mismatch siRNA control (N2a-siControl) were analyzed for LCMT1 and total C expression levels. Total cell extracts were analyzed in parallel to verify the efficacy of LCMT1 knockdown. In A, B, and F, actin blots are shown as controls.
Intact PP2A Methylation Is Required for the Targeting of Tau to the Plasma Membrane
Interestingly, Tau proteins that preferentially bind to and serve as substrate for PP2A/Bα (15–19) can also be targeted to the neuronal plasma membrane (37, 38) in a phosphorylation-dependent manner (8–11). A regulatory role for PP2A in this process has been proposed, based on the observation that incubation of cells with OA, a PP2A inhibitor, results in cytosolic Tau phosphorylation and loss of total plasma membrane-associated transfected human Tau (9, 11). We found similar effects of OA on endogenous Tau in N2a cells (data not shown). Interestingly, OA treatment can also induce the specific accumulation of demethylated PP2A in epithelial MCF7 cells (39). Likewise, we found that incubation of N2a cells with OA caused an increase in endogenous demethylated C levels (Fig. 5A). Furthermore, incubation of N2a cells with this toxin resulted in an overall decrease of membrane-bound PP2A levels and a near complete loss of raft- and non-raft-associated methyl C and PP2A/Bα. These data indicate that, by interfering with PP2A activity and/or methylation, OA can induce a net loss of both Tau and PP2A from the plasma membrane. However, a drawback of these experiments is that, at the concentrations typically used in cultured cells, OA potently inhibits all of the numerous enzymes that belong to the PP2A family, as well as other identified Ser/Thr phosphatases. This nonselectivity underlies widespread cellular effects that confound data interpretation and can even lead ultimately to cytoskeletal disruption and cytotoxicity (40). Of note, OA did not induce N2a cell toxicity under the experimental conditions used here (22). However, due to the inherent flaws of using OA, we investigated whether more specific manipulation of PP2A activity and/or PP2A methylation-dependent mechanisms can affect the membrane localization of Tau. First, we observed that total amounts of membrane-bound Tau were increased in N2a-Wt C relative to control N2a cells (Fig. 5B). As previously reported in neuroblastoma cells (8), rat PC12 cells (9), and primary neurons (11), membrane-associated Tau was primarily dephosphorylated at the Tau-1 epitope (Ser-199/Thr-202) in N2a and N2a-Wt C cells. We have previously reported that overexpression of PP2A C induces an overall dephosphorylation of Tau at many epitopes in N2a cell homogenates (22). Thus, the accumulation of dephosphorylated Tau in the plasma membrane of N2a-Wt C cells is consistent with the hypothesis that PP2A-dependent Tau dephosphorylation promotes its membrane targeting. On the other hand, relative to controls, a significant decrease in membrane-associated Tau pools was observed following expression of SV40 St (Fig. 5C), which, in contrast to OA, specifically targets PP2A. We have reported that the viral St protein markedly inhibits PP2A/Bα activity, resulting in Tau hyperphosphorylation (15). The association of Tau with the plasma membrane was also hampered in N2a-L309Δ cells. We have shown that similarly to SV40 St, expression of the methylation-incompetent L309Δ C subunit mutant enhances phosphorylation of cytosolic Tau at many AD-like phospho-epitopes, including the PHF-1 (phospho-Ser-396/404) and phospho-Ser-422 epitopes (22). Altogether, these data indicate that both PP2A activity and PP2A methylation influence the membrane distribution of Tau.
FIGURE 5.
PP2A critically regulates the translocation of Tau to the plasma membrane. A, Western blot analysis of total homogenates and R and NR membrane fractions (15 μg of proteins) prepared from N2a cells treated for 1 h with 100 nm okadaic acid (+ OA) or vehicle (− OA). B, comparative distribution of total Tau and Tau dephosphorylated at the Tau-1 epitope (Ser-199–Thr-202) in N2a and N2a-Wt C cells. Na+/K+ ATPase, plasma membrane marker. C, comparative distribution of endogenous Tau in total homogenates and plasma membrane fractions prepared from control N2a, N2a-Wt C, N2a-L309Δ, or N2a cells expressing SV40 small tumor antigen (N2a-St). D, Western blot analysis of endogenous Tau in R and NR membrane fractions (30 μg of each protein) purified from N2a cells. In A–D, representative blots are shown. Similar results were obtained in three separate experiments.
Because the phosphorylation of Tau and its association with rafts increase in response to amyloid-β treatment of human SH-SY5Y cells (33), we also intended to assess whether deregulation of PP2A could lead to changes in raft-associated Tau expression levels and/or phosphorylation. However, we were unable to reliably quantitatively compare Tau status in purified raft domains prepared from the relevant N2a cell lines due to the following technical limitations. Firstly, the bulk of endogenous Tau present at the plasma membrane in neuroblastoma cells and primary cortical neurons is present in non-raft domains, with only very small amounts detectable in rafts (33, 41). Accordingly, we found low levels of endogenous raft-associated Tau in N2a cells (Fig. 5D). Secondly, down-regulation of PP2A activity and/or methylation induces a massive loss of overall amounts of membrane-bound Tau. Thirdly, there are limitations associated with membrane raft preparations that constrain the amount of starting material that can be unfailingly fractionated without contamination from non-raft plasma proteins (33).
LCMT1 Critically Regulates the Targeting of Tau to the Plasma Membrane
In support for a role of PP2A methylation in regulating the membrane association of Tau, expression of LCMT1, which promotes Tau dephosphorylation in N2a cells (23, 29), increased the amounts of total Tau present at the plasma membrane (Fig. 6A). As expected, this was closely related to an increase in membrane-bound dephosphorylated Tau (Tau-1) pools. In contrast to its wild-type counterpart, expression of the inactive LCMT1 mutant inhibited the accumulation of dephosphorylated and total Tau at the plasma membrane, whereas concomitantly enhancing Tau phosphorylation at the Ser-422 phospho-epitope (Fig. 6B). Likewise, we found that partial knockdown of LCMT1 in N2a cells (Fig. 4F) was associated with a 42–45% decrease in total amounts of membrane-associated Tau pools relative to controls. It also increased Tau phosphorylation at the Ser-422 phospho-epitope (data not shown), as reported in our earlier study (23). Thus, our data strongly support the hypothesis that LCMT1-dependent PP2A methylation critically modulates the interaction of Tau with the plasma membrane in N2a cells.
FIGURE 6.
Altered LCMT1 activity affects the plasma membrane distribution of Tau. A, equivalent amounts of proteins (30 μg) from plasma membrane fractions purified from control N2a, N2a-LCMT1, and N2a-LCMTmut cells were analyzed for the presence of Tau dephosphorylated at the Tau-1 epitope and total Tau (n = 4; mean ± S.D.; *, p < 0.001, relative to controls). B, total homogenates from the same cells were analyzed for Tau dephosphorylated at the Tau-1 epitope or Tau phosphorylated (P-Tau) at the Ser-422 epitope. pSer422, phospho-Ser-422. In A and B, actin blots are shown as controls.
Alterations in One-carbon Metabolism Promote the Concomitant Loss of Membrane-associated PP2A and Tau
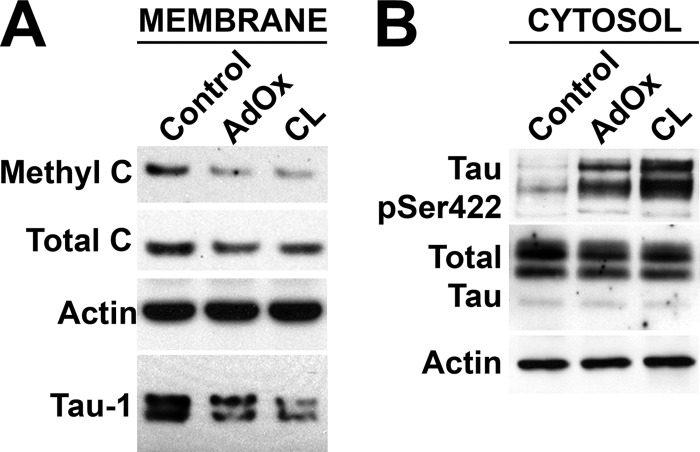
We have previously reported that LCMT1-dependent PP2A methylation modulates Tau phosphorylation in N2a cells and in vivo, by promoting an increase in PP2A/Bα expression levels (22, 23). Significantly, like all other cellular methyltransferases, the activity of LCMT1 is dependent on the availability of the universal methyl donor S-adenosylmethionine, a critical intermediate of one-carbon metabolism (42). We have shown that incubation of N2a cells with S-adenosylmethionine increases PP2A methylation and Tau dephosphorylation; conversely, decreased S-adenosylmethionine levels correlate with down-regulation of LCMT1-mediated PP2A methylation and enhanced phosphorylation of Tau at many AD-like phospho-epitopes, including PHF-1 and phospho-Ser-422 (22). Here, we assessed whether alterations in one-carbon metabolism can more specifically affect the membrane association of PP2A and Tau. To that end, N2a cells were incubated with either cycloleucine or adenosine dialdehyde, which are both known to interfere with methionine metabolism and lead to a decrease in S-adenosylmethionine levels, albeit by distinct mechanisms (43, 44). Treatment with these compounds resulted in a marked reduction of membrane pools of Tau and total and methylated PP2A levels (Fig. 7A). This was associated with concomitant cytosolic accumulation of Tau phosphorylated at the Ser-422 epitope (Fig. 7B). Thus, our results unveil new mechanisms by which alterations in one-carbon metabolism can affect the plasma membrane distribution of PP2A and Tau, and thereby influence PP2A-dependent Tau phosphorylation state.
FIGURE 7.
Alterations in one-carbon metabolism affect the distribution of endogenous PP2A and Tau at the plasma membrane. A, Western blot analysis of plasma membrane fractions (30 μg of proteins) purified from N2a cells incubated for 2 h with 50 μm adenosine dialdehyde (AdOx), 10 mm cycloleucine (CL), or vehicle alone (Control) in normal RPMI medium containing 1% dialyzed serum. B, the loss of LCMT1, methylated C, and Tau dephosphorylated at the Tau-1 epitope in the plasma membrane was associated with an increase of Tau phosphorylated at Ser-422 in cytosolic fractions purified from the same cells. pSer422, phospho-Ser-422. In A and B, representative blots from three separate experiments are shown.
DISCUSSION
The Ser/Thr PP2A family regroups a family of dozens of enzymes (45). The selective compartmentalization of PP2A isoforms, PP2A regulatory proteins, and PP2A substrates likely contributes to ensure PP2A substrate specificity. Here, we report for the first time that PP2A/Bα, methylated and demethylated PP2A enzymes, as well as the PP2A regulators LCMT1 and PME-1 are differentially distributed in discrete plasma membrane microdomains from N2a cells. Previously validated detergent-free methods were utilized here to isolate CEMs from N2a cells. This choice was based on the following observations. 1) In N2a cells, both detergent-free and classical detergent-based procedures led to the purification of low buoyant density fractions enriched in cholesterol and glycosylphosphatidylinositol-anchored proteins (46, 47); 2) concerns have been raised that detergent-based procedures may be generating protein redistribution artifacts and clusters of raft lipids that do not exist in intact cells (3, 47); and 3) although we were able to detect PP2A subunits in detergent-extracted N2a cell rafts, we have observed that when compared with detergent-free procedures, detergent-based methods induce a significant loss of PP2A Bα subunits in CEMs (data not shown). This is likely due to the fact that regulatory B subunits, which modulate PP2A intracellular signaling, often become dissociated from the core enzyme when cells are lysed with harsh detergents (48). We found that flotillin-1, but not TfR, was enriched in low buoyant density CEM fractions, and thereby defined those as membrane rafts. Using detergent-free procedures, we first show that PP2A A, Bα, and C subunits are present in membrane rafts from N2a cells. These findings are consistent with earlier studies reporting the presence of PP2A subunits in CEMs purified from non-neuronal cell types (28, 49) and in detergent-resistant membranes isolated from normal mouse cortical tissue (5). Besides Bα subunits, other regulatory B subunits not studied here may also be present in rafts. Short incubation of N2a cells with low concentrations of MβCD that selectively disrupt raft cholesterol (50, 51) decreased the amount of endogenous PP2A recovered in CEMs from N2a cells, as observed previously in fibroblasts (28) and smooth muscle cells (49).
It has been reported that PME-1 and demethylated PP2A are preferentially enriched in the nucleus, whereas LCMT1 is primarily cytoplasmic in HeLa cells (30). Likewise, we observed that these enzymes were distributed in the cytosol and nucleus of N2a cells. However, we show for the first time that pools of LCMT1, methylated C, and PP2A/Bα are present in non-rafts and concentrated in membrane rafts from N2a cells. The similar co-enrichment of these enzymes within the same plasma membrane microdomains is in line with earlier findings indicating that LCMT1 directly interacts with and methylates the demethylated AC core enzyme of PP2A, a process that favors assembly and stabilization of methylated PP2A/Bα holoenzymes (52). Conversely, demethylated C was proportionally more concentrated in non-rafts from N2a cells. In contrast to endogenous or expressed PP2A C subunit, the methylation-incompetent, Bα-binding defective L309Δ C subunit mutant was excluded from rafts, supporting the hypothesis that methylation and Bα binding promote the preferential targeting of PP2A to membrane rafts. Surprisingly, only tiny amounts of PME-1 were detected in non-raft membrane domains from N2a cells. Indeed, the preferential distribution of PME-1 in the cytosol and nucleus of N2a cells supports the notion that demethylation processes and other putative functions of PME-1 primarily occur outside of the plasma membrane. Based on current models of PP2A biogenesis (20, 24), the tiny pools of PME-1 detected in non-rafts may be bound to demethylated PP2A in this compartment and may play a role in preventing untimely local methylation of PP2A. Because PP2A/Bα enzymes appear to be protected from demethylation by PME-1 (23, 53), it is also unlikely that membrane-bound unmethylated PP2A pools occur as a result from local demethylation of pre-existing PP2A/Bα holoenzymes. So where is membrane-associated demethylated C coming from? In contrast to PP2A/Bα, methylation of PP2A C subunit enhances but is not required for stable formation of PP2A heterotrimers containing certain regulatory subunits, such as B′ subunits (54). Thus, instead we speculate that preassembled demethylated dimeric AC or trimeric PP2A enzymes can be directed to the plasma membrane. In this context, it is noteworthy that the PP2A A subunit has been identified as a palmitoylated protein in a recent global profiling screen (55). S-Palmitoylation is a universal posttranslational modification important for trafficking and localization of proteins in plasma membrane and rafts (56). In light of the well established scaffolding role of PP2A A subunit, this raises the interesting possibility that dynamic A subunit palmitoylation could contribute to modulate PP2A targeting to membrane microdomains.
The precise regulatory mechanisms by which PP2A and LCMT1 enzymes are targeted to the plasma membrane and may translocate between raft and non-raft membrane microdomains are likely to be complex and remain to be elucidated in future studies. In any case, the regulated compartmentalization of LCMT1 and its substrate PP2A could contribute to a very fine regulation of the PP2A methylation process at the level of the plasma membrane. It may also play a critical role in spatially sequestering PP2A enzymes from their substrates, such as Tau, in rafts and non-rafts.
We utilized several approaches to interfere with endogenous PP2A methylation status in N2a cells: overexpression of the L309Δ C mutant, overexpression of an inactive LCMT1 mutant, knockdown of LCMT1, and inhibition of LCMT1 activity by drugs. They all resulted in a net loss of total and membrane-associated methylated PP2A levels. The latter was also paralleled by a decrease in membrane-bound total PP2A C levels, further indicating that impaired methylation affects the spatial localization of PP2A and could impair important functions of PP2A at the plasma membrane level. Notably, we show here that altered LCMT1-dependent PP2A methylation, which induces down-regulation of PP2A/Bα holoenzymes and increases Tau phosphorylation in N2a cells (22), is associated with a concomitant loss of membrane-associated pools of both PP2A and Tau. In addition, we unveil a critical role for alterations in one-carbon metabolism and LCMT1-dependent PP2A methylation in modulating the interaction of Tau with the plasma membrane. There is increasing evidence that missorting and altered compartmentalization of key proteins such as Tau in raft and non-raft membrane microdomains play a critical role in neurodegenerative disorders, including AD pathogenesis (6). Significant alterations in cortical lipid raft composition have been recently reported in mouse models of AD (5). Of particular interest in this proteomic study is the observation that PP2A/Bα holoenzymes were identified in the group of lipid raft proteins regulating key signaling pathways and synaptic-dependent processes such as axonal guidance and long term depression, which became significantly down-regulated in AD mouse models. We have previously reported that down-regulation of LCMT1 and PP2A methylation is intimately related to the loss of PP2A/Bα and accumulation of phosphorylated Tau in AD brain tissue (22). Together with these observations, our data support a critical role for altered PP2A methylation in mediating the loss of raft-associated PP2A/Bα and contributing to deregulation of normal Tau distribution and function in AD pathogenesis.
Acknowledgments
In memoriam to Prof. Richard G. W. Anderson (1940–2011; UT Southwestern, TX) who significantly contributed to this project by providing technical support, guidance, and expertise in the purification of CEMs/rafts from N2a cells. We also thank Dr. Pingsheng Liu (UT Southwestern, Dallas, TX) in Dr. Anderson's laboratory for technical support and advice and Dr. Joseph Goldstein (UT Southwestern) for the gift of cholesterol.
This research was supported in part by a National Health and Medical Research Council (NHMRC) Australia grant (to E. S. and J. M. S.).
- CEM
- cholesterol-enriched membrane microdomain
- AD
- Alzheimer disease
- LCMT1
- leucine carboxyl methyltransferase 1
- NR
- non-raft
- R
- raft
- PME-1
- protein phosphatase methylesterase-1
- OA
- okadaic acid
- PP2A
- protein phosphatase 2A
- St
- small tumor antigen
- TfR
- transferrin receptor
- MβCD
- methyl-β-cyclodextrin
- Bis-Tris
- 2-(bis(2-hydroxyethyl)amino)-2-(hydroxymethyl)propane-1,3-diol.
REFERENCES
- 1. Pike L. J. (2006) Rafts defined: a report on the Keystone Symposium on Lipid Rafts and Cell Function. J. Lipid Res. 47, 1597–1598 [DOI] [PubMed] [Google Scholar]
- 2. Lingwood D., Simons K. (2010) Lipid rafts as a membrane-organizing principle. Science 327, 46–50 [DOI] [PubMed] [Google Scholar]
- 3. Allen J. A., Halverson-Tamboli R. A., Rasenick M. M. (2007) Lipid raft microdomains and neurotransmitter signalling. Nat. Rev. Neurosci. 8, 128–140 [DOI] [PubMed] [Google Scholar]
- 4. Staubach S., Hanisch F. G. (2011) Lipid rafts: signaling and sorting platforms of cells and their roles in cancer. Expert. Rev. Proteomics 8, 263–277 [DOI] [PubMed] [Google Scholar]
- 5. Chadwick W., Brenneman R., Martin B., Maudsley S. (2010) Complex and multidimensional lipid raft alterations in a murine model of Alzheimer's disease. Int. J. Alzheimers. Dis. 2010, 604792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Williamson R., Sutherland C. (2011) Neuronal membranes are key to the pathogenesis of Alzheimer's disease: the role of both raft and non-raft membrane domains. Curr. Alzheimer Res. 8, 213–221 [DOI] [PubMed] [Google Scholar]
- 7. Kawarabayashi T., Shoji M., Younkin L. H., Wen-Lang L., Dickson D. W., Murakami T., Matsubara E., Abe K., Ashe K. H., Younkin S. G. (2004) Dimeric amyloid-β protein rapidly accumulates in lipid rafts followed by apolipoprotein E and phosphorylated tau accumulation in the Tg2576 mouse model of Alzheimer's disease. J. Neurosci. 24, 3801–3809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arrasate M., Pérez M., Avila J. (2000) Tau dephosphorylation at tau-1 site correlates with its association to cell membrane. Neurochem. Res. 25, 43–50 [DOI] [PubMed] [Google Scholar]
- 9. Maas T., Eidenmüller J., Brandt R. (2000) Interaction of tau with the neural membrane cortex is regulated by phosphorylation at sites that are modified in paired helical filaments. J. Biol. Chem. 275, 15733–15740 [DOI] [PubMed] [Google Scholar]
- 10. Eidenmüller J., Fath T., Maas T., Pool M., Sontag E., Brandt R. (2001) Phosphorylation-mimicking glutamate clusters in the proline-rich region are sufficient to simulate the functional deficiencies of hyperphosphorylated tau protein. Biochem. J. 357, 759–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pooler A. M., Usardi A., Evans C. J., Philpott K. L., Noble W., Hanger D. P. (2012) Dynamic association of tau with neuronal membranes is regulated by phosphorylation. Neurobiol. Aging 33, 431.e27–431.e38 [DOI] [PubMed] [Google Scholar]
- 12. Lee G., Newman S. T., Gard D. L., Band H., Panchamoorthy G. (1998) Tau interacts with src-family non-receptor tyrosine kinases. J. Cell. Sci. 111, 3167–3177 [DOI] [PubMed] [Google Scholar]
- 13. Reynolds C. H., Garwood C. J., Wray S., Price C., Kellie S., Perera T., Zvelebil M., Yang A., Sheppard P. W., Varndell I. M., Hanger D. P., Anderton B. H. (2008) Phosphorylation regulates tau interactions with Src homology 3 domains of phosphatidylinositol 3-kinase, phospholipase Cγ1, Grb2, and Src family kinases. J. Biol. Chem. 283, 18177–18186 [DOI] [PubMed] [Google Scholar]
- 14. Pooler A. M., Hanger D. P. (2010) Functional implications of the association of tau with the plasma membrane. Biochem. Soc. Trans. 38, 1012–1015 [DOI] [PubMed] [Google Scholar]
- 15. Sontag E., Nunbhakdi-Craig V., Lee G., Bloom G. S., Mumby M. C. (1996) Regulation of the phosphorylation state and microtubule-binding activity of Tau by protein phosphatase 2A. Neuron 17, 1201–1207 [DOI] [PubMed] [Google Scholar]
- 16. Goedert M., Cohen E. S., Jakes R., Cohen P. (1992) p42 MAP kinase phosphorylation sites in microtubule-associated protein tau are dephosphorylated by protein phosphatase 2A1. Implications for Alzheimer's disease. FEBS Lett. 312, 95–99 [DOI] [PubMed] [Google Scholar]
- 17. Schild A., Ittner L. M., Götz J. (2006) Altered phosphorylation of cytoskeletal proteins in mutant protein phosphatase 2A transgenic mice. Biochem. Biophys. Res. Commun. 343, 1171–1178 [DOI] [PubMed] [Google Scholar]
- 18. Xu Y., Chen Y., Zhang P., Jeffrey P. D., Shi Y. (2008) Structure of a protein phosphatase 2A holoenzyme: insights into B55-mediated Tau dephosphorylation. Mol. Cell 31, 873–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sontag J. M., Nunbhakdi-Craig V., White C. L., 3rd, Halpain S., Sontag E. (2012) The protein phosphatase PP2A/Bα binds to the microtubule-associated proteins Tau and MAP2 at a motif also recognized by the kinase Fyn: implications for tauopathies. J. Biol. Chem. 287, 14984–14993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sents W., Ivanova E., Lambrecht C., Haesen D., Janssens V. (2013) The biogenesis of active protein phosphatase 2A holoenzymes: a tightly regulated process creating phosphatase specificity. FEBS J. 280, 644–661 [DOI] [PubMed] [Google Scholar]
- 21. Sontag E., Hladik C., Montgomery L., Luangpirom A., Mudrak I., Ogris E., White C. L., 3rd (2004) Downregulation of protein phosphatase 2A carboxyl methylation and methyltransferase may contribute to Alzheimer disease pathogenesis. J. Neuropathol. Exp. Neurol. 63, 1080–1091 [DOI] [PubMed] [Google Scholar]
- 22. Sontag E., Nunbhakdi-Craig V., Sontag J. M., Diaz-Arrastia R., Ogris E., Dayal S., Lentz S. R., Arning E., Bottiglieri T. (2007) Protein phosphatase 2A methyltransferase links homocysteine metabolism with tau and amyloid precursor protein regulation. J. Neurosci. 27, 2751–2759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sontag J. M., Nunbhakdi-Craig V., Montgomery L., Arning E., Bottiglieri T., Sontag E. (2008) Folate deficiency induces in vitro and mouse brain region-specific downregulation of leucine carboxyl methyltransferase-1 and protein phosphatase 2A Bα subunit expression that correlate with enhanced tau phosphorylation. J. Neurosci. 28, 11477–11487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hombauer H., Weismann D., Mudrak I., Stanzel C., Fellner T., Lackner D. H., Ogris E. (2007) Generation of active protein phosphatase 2A is coupled to holoenzyme assembly. PLoS Biol. 5, e155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Longin S., Zwaenepoel K., Martens E., Louis J. V., Rondelez E., Goris J., Janssens V. (2008) Spatial control of protein phosphatase 2A (de)methylation. Exp. Cell. Res. 314, 68–81 [DOI] [PubMed] [Google Scholar]
- 26. Sontag E., Nunbhakdi-Craig V., Lee G., Brandt R., Kamibayashi C., Kuret J., White C. L., 3rd, Mumby M. C., Bloom G. S. (1999) Molecular interactions among protein phosphatase 2A, tau, and microtubules. Implications for the regulation of tau phosphorylation and the development of tauopathies. J. Biol. Chem. 274, 25490–25498 [DOI] [PubMed] [Google Scholar]
- 27. Hiraga A., Tamura S. (2000) Protein phosphatase 2A is associated in an inactive state with microtubules through 2A1-specific interaction with tubulin. Biochem. J. 346, 433–439 [PMC free article] [PubMed] [Google Scholar]
- 28. Wang P. Y., Liu P., Weng J., Sontag E., Anderson R. G. (2003) A cholesterol-regulated PP2A/HePTP complex with dual specificity ERK1/2 phosphatase activity. EMBO J. 22, 2658–2667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nunbhakdi-Craig V., Schuechner S., Sontag J. M., Montgomery L., Pallas D. C., Juno C., Mudrak I., Ogris E., Sontag E. (2007) Expression of protein phosphatase 2A mutants and silencing of the regulatory Bα subunit induce a selective loss of acetylated and detyrosinated microtubules. J. Neurochem. 101, 959–971 [DOI] [PubMed] [Google Scholar]
- 30. Sontag J. M., Nunbhakdi-Craig V., Mitterhuber M., Ogris E., Sontag E. (2010) Regulation of protein phosphatase 2A methylation by LCMT1 and PME-1 plays a critical role in differentiation of neuroblastoma cells. J. Neurochem. 115, 1455–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Smart E. J., Ying Y. S., Mineo C., Anderson R. G. (1995) A detergent-free method for purifying caveolae membrane from tissue culture cells. Proc. Natl. Acad. Sci. U.S.A. 92, 10104–10108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bottiglieri T., Arning E., Wasek B., Nunbhakdi-Craig V., Sontag J. M., Sontag E. (2012) Acute administration of l-Dopa induces changes in methylation metabolites, reduced protein phosphatase 2A methylation, and hyperphosphorylation of Tau protein in mouse brain. J. Neurosci. 32, 9173–9181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hernandez P., Lee G., Sjoberg M., Maccioni R. B. (2009) Tau phosphorylation by cdk5 and Fyn in response to amyloid peptide Aβ25–35: involvement of lipid rafts. J. Alzheimers Dis. 16, 149–156 [DOI] [PubMed] [Google Scholar]
- 34. Oh P., Schnitzer J. E. (2001) Segregation of heterotrimeric G proteins in cell surface microdomains. Gq binds caveolin to concentrate in caveolae, whereas Gi and Gs target lipid rafts by default. Mol. Biol. Cell 12, 685–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ogris E., Du X., Nelson K. C., Mak E. K., Yu X. X., Lane W. S., Pallas D. C. (1999) A Protein phosphatase methylesterase (PME-1) is one of several novel proteins stably associating with two inactive mutants of protein phosphatase 2A. J. Biol. Chem. 274, 14382–14391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stanevich V., Jiang L., Satyshur K. A., Li Y., Jeffrey P. D., Li Z., Menden P., Semmelhack M. F., Xing Y. (2011) The structural basis for tight control of PP2A methylation and function by LCMT-1. Mol. Cell 41, 331–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brandt R., Léger J., Lee G. (1995) Interaction of tau with the neural plasma membrane mediated by tau's amino-terminal projection domain. J. Cell. Biol. 131, 1327–1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ittner L. M., Ke Y. D., Delerue F., Bi M., Gladbach A., van Eersel J., Wölfing H., Chieng B. C., Christie M. J., Napier I. A., Eckert A., Staufenbiel M., Hardeman E., Götz J. (2010) Dendritic function of tau mediates amyloid-β toxicity in Alzheimer's disease mouse models. Cell 142, 387–397 [DOI] [PubMed] [Google Scholar]
- 39. Favre B., Turowski P., Hemmings B. A. (1997) Differential inhibition and posttranslational modification of protein phosphatase 1 and 2A in MCF7 cells treated with calyculin-A, okadaic acid, and tautomycin. J. Biol. Chem. 272, 13856–13863 [DOI] [PubMed] [Google Scholar]
- 40. Swingle M., Ni L., Honkanen R. E. (2007) Small-molecule inhibitors of ser/thr protein phosphatases: specificity, use and common forms of abuse. Methods Mol. Biol. 365, 23–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Usardi A., Pooler A. M., Seereeram A., Reynolds C. H., Derkinderen P., Anderton B., Hanger D. P., Noble W., Williamson R. (2011) Tyrosine phosphorylation of tau regulates its interactions with Fyn SH2 domains, but not SH3 domains, altering the cellular localization of tau. FEBS J. 278, 2927–2937 [DOI] [PubMed] [Google Scholar]
- 42. Fowler B. (2005) Homocysteine: overview of biochemistry, molecular biology, and role in disease processes. Semin. Vasc. Med. 5, 77–86 [DOI] [PubMed] [Google Scholar]
- 43. Porter C. W., Sufrin J. R., Keith D. D. (1984) Growth inhibition by methionine analog inhibitors of S-adenosylmethionine biosynthesis in the absence of polyamine depletion. Biochem. Biophys. Res. Commun. 122, 350–357 [DOI] [PubMed] [Google Scholar]
- 44. Chen D. H., Wu K. T., Hung C. J., Hsieh M., Li C. (2004) Effects of adenosine dialdehyde treatment on in vitro and in vivo stable protein methylation in HeLa cells. J. Biochem. 136, 371–376 [DOI] [PubMed] [Google Scholar]
- 45. Virshup D. M., Shenolikar S. (2009) From promiscuity to precision: protein phosphatases get a makeover. Mol. Cell 33, 537–545 [DOI] [PubMed] [Google Scholar]
- 46. Vey M., Pilkuhn S., Wille H., Nixon R., DeArmond S. J., Smart E. J., Anderson R. G. W., Taraboulos A., Prusiner S. B. (1996) Subcellular colocalization of the cellular and scrapie prion proteins in caveolae-like membranous domains. Proc. Natl. Acad. Sci. U.S.A. 93, 14945–14949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Macdonald J. L., Pike L. J. (2005) A simplified method for the preparation of detergent-free lipid rafts. J. Lipid Res. 46, 1061–1067 [DOI] [PubMed] [Google Scholar]
- 48. Sontag E. (2001) Protein phosphatase 2A: the Trojan Horse of cellular signaling. Cell. Signal. 13, 7–16 [DOI] [PubMed] [Google Scholar]
- 49. Berrou E., Bryckaert M. (2009) Recruitment of protein phosphatase 2A to dorsal ruffles by platelet-derived growth factor in smooth muscle cells: dephosphorylation of Hsp27. Exp. Cell. Res. 315, 836–848 [DOI] [PubMed] [Google Scholar]
- 50. Furuchi T., Anderson R. G. (1998) Cholesterol depletion of caveolae causes hyperactivation of extracellular signal-related kinase (ERK). J. Biol. Chem. 273, 21099–21104 [DOI] [PubMed] [Google Scholar]
- 51. Zidovetzki R., Levitan I. (2007) Use of cyclodextrins to manipulate plasma membrane cholesterol content: evidence, misconceptions, and control strategies. Biochim. Biophys. Acta 1768, 1311–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Janssens V., Longin S., Goris J. (2008) PP2A holoenzyme assembly: in cauda venenum (the sting is in the tail). Trends Biochem. Sci. 33, 113–121 [DOI] [PubMed] [Google Scholar]
- 53. Tolstykh T., Lee J., Vafai S., Stock J. B. (2000) Carboxyl methylation regulates phosphoprotein phosphatase 2A by controlling the association of regulatory B subunits. EMBO J. 19, 5682–5691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gentry M. S., Li Y., Wei H., Syed F. F., Patel S. H., Hallberg R. L., Pallas D. C. (2005) A novel assay for protein phosphatase 2A (PP2A) complexes in vivo reveals differential effects of covalent modifications on different Saccharomyces cerevisiae PP2A heterotrimers. Eukaryot. Cell 4, 1029–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Martin B. R., Wang C., Adibekian A., Tully S. E., Cravatt B. F. (2012) Global profiling of dynamic protein palmitoylation. Nat. Methods 9, 84–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Levental I., Lingwood D., Grzybek M., Coskun U., Simons K. (2010) Palmitoylation regulates raft affinity for the majority of integral raft proteins. Proc. Natl. Acad. Sci. U.S.A. 107, 22050–22054 [DOI] [PMC free article] [PubMed] [Google Scholar]