Abstract
Objective
Prior research has documented sociodemographic disparities in the use of antiretroviral therapy (ART). Recent therapeutic developments and changing epidemiological profiles may have altered such disparities. We examine the extent to which socio-demographic differences in prescribed ART have changed between 2002 and 2008.
Methods
We analyzed data abstracted from medical records at 13 US sites participating in the Human Immunodeficiency Virus Research Network. Prescription of ART was assessed for each year in care for each patient. A total of 14,092 patients were followed up for 39,251 person-years. We examined ART use as a function of sex, race/ethnicity, human immunodeficiency virus risk group, age, and CD4 history (no test <500 cells/mm3, one or more tests between 500 and 350 cells/mm3, 1 test ≤350 cells/mm3, and 2 or more tests ≤350 cells/mm3). Using multiple logistic regression, we ascertained interactions between each of these variables and calendar year.
Results
The overall percentage prescribed ART increased from 60% to 80% between 2002 and 2008. Among those with 2 or more CD4 tests ≤350 cells/mm3, the percentage increased from 82% to 92%. ART rates were higher for those with lower CD4 counts but increased over time for all CD4 groups and for all demographic groups. Nevertheless, sex and racial/ethnic disparities persisted. Significant interactions were obtained for CD4 history by year, age by year, and age by CD4 history.
Conclusions
Although prescription of ART became more widespread from 2002 to 2008, patients who were female, black, or younger still had lower ART rates than male, white, or older patients.
Keywords: ART, disparities, time trends, utilization, sociodemographic differences
Numerous studies demonstrate the beneficial impact of combination antiretroviral therapy (ART) in the treatment of human immunodeficiency virus (HIV) infection.1–6 However, research has consistently shown sociodemographic disparities in the receipt of ART.7–15 Data from 2001 in a large multistate, multisite sample showed that women, blacks, injection drug users (IDU), and the uninsured were less likely to receive ART than their counterparts.16
Significant improvements in HIV care have occurred since these studies were conducted, including expansion of available antiretroviral drugs and regimens, simplified dosing, and greater tolerability.17,18 These advances, combined with the development of programs to identify, test, and treat patients with HIV infection, may have increased rates of ART use over time and possibly reduced sociodemographic disparities in ART receipt.
In the changing epidemiology of HIV infection, blacks and Hispanics are now disproportionally affected, accounting for 69% of new HIV infections, while only representing 29% of the US population.19–21 Similarly, the proportion of women infected with HIV has increased.20 The increasing numbers of infected patients from disadvantaged segments of society may contribute to lower rates of ART use. Factors such as distrust of the health care system, potential differences in prescribing patterns by HIV providers based on perceived likelihood of adherence, and more frequent ART discontinuation by minorities may lead to increasing disparities over time.12,22–25
Advances in HIV therapy, changing epidemiological patterns, and changing guidelines about when to start ART26 require an updated evaluation of disparities in ART use. This study examines trends in receipt of ART between 2002 and 2008, focusing on changes in sociodemographic differences (ie, sex, race/ethnicity, HIV risk group, and age) as a function of time.
Assessment of disparities requires that groups be comparable in terms of need for care or disease stage.27 Moreover, group differences in ART use may be greater at earlier disease stages, when ART guidelines allow greater clinical discretion, than at later disease stages. Therefore, analyses also examine whether sociodemographic differences in ART vary by disease stage, as reflected by CD4 cell count history.
Methods
Study Design and Participants
We conducted a serial cross-sectional analysis of ART prescription in HIV-infected adults enrolled in the HIV Research Network (HIVRN) from 2002 through 2008. The HIVRN is a consortium of sites that provide primary and subspecialty care to HIV patients, as previously described.16,28 Fifteen sites treat adult patients. Data from 13 sites, located in the northeastern (6), midwestern (2), southern (2), and western (3) United States, were included in this analysis. The remaining 2 sites discontinued participation during the study period. Eight sites have academic affiliations. All adult patients (at least 18y old) with at least 1 outpatient visit and 1 CD4 test in any calendar year between 2002 and 2008 were eligible for inclusion.
Data Collection
Data were abstracted from medical records at each site and sent to a data coordinating center after personal identifying information was removed. Data used in these analyses encompassed the period from January 1, 2002 through December 31, 2008. Problematic data elements were identified, reviewed with the site, and corrected. After this quality control and verification process, data were combined across sites to achieve a uniformly constructed multisite database. The study was approved by Institutional Review Boards at the Johns Hopkins University School of Medicine and at each participating site.
Definition of Variables
The dependent variable was combination ART prescription, defined as a prescribed regimen of multiple anti-retroviral agents, including nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs), non-nucleoside reverse transcriptase inhibitors, protease inhibitors, integrase inhibitors, a fusion or entry inhibitor, or 3 acceptable nucleoside/nucleotide reverse transcriptase inhibitors. This definition of ART was as inclusive as possible and unlikely to exclude any preferred drug combinations. Patients were considered to receive ART if they were prescribed any of these combinations at any time during a calendar year. ART receipt could vary from year to year for individual patients.
To determine disease stage and eligibility for ART, we reviewed each patient's chronological history of CD4 results and associated test dates. ART guidelines in the United States from 2002 to 2008 recommended use of ART for CD4 levels <350 cells/mm3; use of ART at higher CD4 levels was at the discretion of the provider.29 On the basis of dates of CD4 levels <500 and 350 cells/mm3, we classified each patient-year in terms of CD4 history as (1) no history of CD4 level ≤500 cells/mm3; (2) one or more CD4 levels ≤500 cells/mm3 but none ≤350 cells/mm3 in the current year or a prior year; (3) 1 CD4 level ≤350 cells/mm3 in the current year or a prior year; and (4) 2 or more CD4 levels ≤350 cells/mm3 in the current year or a prior year (defining ART eligibility). Given the inherent variability in CD4 measurement, many clinicians do not start ART until obtaining a second, confirmatory CD4 test ≤350 cells/mm3. The fourth category ensured that patients truly met the CD4≤350 cells/ mm3 threshold. Also to accommodate CD4 variability, we allowed for 2 transient drops, defined as 1 level ≤350 cells/ mm3 followed by the next test result >350 cells/mm3. If a patient had 1 drop, the date of the third CD4 level ≤350 cells/mm3 defined the year of ART eligibility; if a patient had 2 drops, the date of the fourth level ≤350 cells/mm3 defined the year of ART eligibility. Patients who met multiple criteria in the same calendar year were classified in the more advanced disease stage category. Over time, persons could transition from less severe to more severe categories (but not in the opposite direction); once in category 4, they remained there for the rest of the study period.
For each year, patients' age as of July 1 was divided into 4 groups: 18–29, 30–39, 40–49, and over 50 years old. Race/ethnicity was categorized as non-Hispanic white, non-Hispanic black, and Hispanic. Self-reported HIV transmission behavior was grouped into IDU, men who had sex with men (MSM), heterosexual transmission (HET), IDU combined with MSM (MSM/IDU), and IDU combined with heterosexual (HET/IDU). The MSM/IDU category is routinely used in national HIV surveillance, and distinguishing HET/IDU from the IDU category has been recommended.30 Insurance coverage at the first outpatient visit in each year was categorized as private, Medicaid, Medicare or dual eligible, uninsured or Ryan White funding, or unknown. Insurance status could change over time.
Data Analysis
The patient-year was the unit of analysis. The analytic sample was restricted to patient-years in which the patient was in care (defined by having at least 1 outpatient visit and 1 CD4 test in the year) and to patients who enrolled between 2002 and 2008 and were aged 18 or older. Patients entered the study in different years, and some subsequently dropped out of care or died. The mean number of years contributed to the analysis per patient was 2.8, with a maximum of 7 years.
To ensure complete data, we excluded patients who had 0 or only 1 CD4 test ever recorded (n = 2474), those with a CD4 level <501 before 2002 (n = 475), and those who were already prescribed ART in 2000 or 2001 (n = 1377). To enhance interpretability of complex interaction effects, we removed patients with race/ethnicity or risk group coded as “unknown” or “other” (n = 1594); analyses that included this group produced results similar to those presented below. The final analytic sample comprised 39,251 person-years from 14,092 individuals.
Because ART prescription in each patient-year was dichotomous, we used multivariate logistic regression analysis. Generalized estimating equations, with an unstructured correlation matrix and robust variance estimates, were used to accommodate correlation among multiple observations per patient. To focus on trends over time, calendar year was treated as a continuous rather than as a categorical variable (ie, represented by 1 linear term). All models included indicators for each HIVRN site to capture any site-specific differences in ART prescribing patterns.
Our hypotheses focus attention on 3 sets of interaction effects. First, the extent of sociodemographic disparities in ART prescription could vary over time. This could result in 4 interactions: between each demographic characteristic (sex, race/ethnicity, risk group, or age group) and year. Second, disparities could vary in terms of disease stage, yielding 4 potential interactions, between CD4 history and each demographic characteristic. Finally, ART prescription could vary as a function of the combination of year and CD4 history.
To determine the best logistic regression model, we randomly divided the sample into 2 groups of patients and performed model selection procedures separately in each random half sample (Supplemental Digital Content 1, http://links.lww.com/MLR/A279 for details of model selection procedures). The final model selected was reestimated on the entire sample to produce final estimates. To aid in interpreting the final model, we calculated predictive margins, a form of direct standardization.31
Insurance status can be viewed as mediating the effect of sociodemographic factors. For this reason, adjusting for insurance in multivariate models could reduce the magnitude of estimates of sociodemographic differences.27 We therefore initially estimated models excluding insurance. After model selection, insurance was added to the model to assess whether adjusting for insurance affected sociodemographic differences. Analyses were conducted in STATA 11 (College Station, TX).
Results
Descriptive Analyses
Table 1 shows the distribution of patients' characteristics for each year. Yearly sample sizes increased from 1674 in 2002 to 8973 in 2008, as more patients entered and remained in care than dropped out or died. Distributions of sex and race/ethnicity were stable over time, with the majority of patients being male and of minority race/ethnicity. The percentage of patients who were aged 50 or older doubled from 13% in 2002 to 26% in 2008. The majority of patients had HIV transmission risk factors of MSM or HET, with the latter rising from 36% in 2002 to 44% in 2008. The percentage of the sample who ever had 2 or more CD4 cell levels ≤350 cells/mm3 increased from 43% in 2002 to 61% in 2008, as expected because once patients entered this category they remained there.
Table 1. Sociodemographic Characteristics by Year, HIV-infected Patients at 13 HIVRN Sites.
| Variables | 2002 N=1674 (%) | 2003 N=3470 (%) | 2004 N=4925 (%) | 2005 N=5692 (%) | 2006 N=6744 (%) | 2007 N=7773 (%) | 2008 N=8973 (%) |
|---|---|---|---|---|---|---|---|
| Age (y) | |||||||
| 18–29 | 326 (19) | 593 (17) | 828 (17) | 863 (15) | 981 (15) | 1,045 (13) | 1,233 (14) |
| 30–39 | 615 (37) | 1,169 (34) | 1,530 (31) | 1,579 (28) | 1723 (26) | 1895 (24) | 2027 (22) |
| 40–49 | 509 (30) | 1218 (35) | 1754 (36) | 2178 (38) | 2609 (39) | 3014 (39) | 3408 (38) |
| ≥50 | 224 (13) | 490 (14) | 813 (17) | 1072 (19) | 1431 (21) | 1819 (23) | 2305 (26) |
| Race | |||||||
| White | 489 (29) | 991 (29) | 1432 (29) | 1611 (28) | 1848 (27) | 2181 (28) | 2417 (27) |
| Black | 826 (49) | 1689 (49) | 2375 (48) | 2799 (49) | 3417 (51) | 3903 (50) | 4582 (51) |
| Hispanic | 359 (21) | 790 (23) | 1118 (23) | 1282 (23) | 1479 (22) | 1689 (22) | 1974 (22) |
| Sex | |||||||
| Male | 1282 (77) | 2550 (73) | 3598 (73) | 4172 (73) | 4931 (73) | 5746 (74) | 6500 (72) |
| Female | 392 (23) | 920 (27) | 1327 (27) | 1520 (27) | 1813 (27) | 2027 (26) | 2473 (28) |
| HIV risk factor | |||||||
| MSM | 730 (44) | 1470 (42) | 2053 (42) | 2402 (42) | 2815 (42) | 3297 (42) | 3698 (41) |
| IDU | 130 (8) | 252 (7) | 343 (7) | 368 (6) | 401 (6) | 507 (7) | 640 (7) |
| HET | 599 (36) | 1374 (40) | 2057 (42) | 2382 (42) | 2921 (43) | 3282 (42) | 3905 (44) |
| HET/IDU | 128 (8) | 237 (7) | 296 (6) | 356 (6) | 389 (6) | 426 (5) | 479 (5) |
| MSM/IDU | 87 (5) | 137 (4) | 176 (4) | 184 (3) | 218 (3) | 261 (3) | 251 (3) |
| CD4 history (cells/mm3) | |||||||
| All >500 | 318 (19) | 549 (16) | 740 (15) | 747 (13) | 784 (12) | 914 (12) | 1047 (12) |
| Any 351–500 | 285 (17) | 561 (16) | 790 (16) | 886 (16) | 973 (14) | 1112 (14) | 1258 (14) |
| 1≤350 | 359 (21) | 652 (19) | 770 (16) | 781 (14) | 971 (14) | 1087 (14) | 1211 (14) |
| 2+≤350 | 712 (43) | 1708 (49) | 2625 (53) | 3278 (58) | 4016 (60) | 4660 (60) | 5457 (61) |
| Insurance | |||||||
| Private | 180 (11) | 277 (8) | 427 (9) | 725 (13) | 858 (13) | 1081 (14) | 1251 (14) |
| Medicaid | 523 (31) | 1010 (29) | 1378 (28) | 1939 (34) | 2299 (34) | 2502 (32) | 3213 (36) |
| Medicare/dual | 96 (6) | 225 (6) | 337 (7) | 544 (10) | 843 (12) | 1021 (13) | 1229 (14) |
| None/RW | 651 (39) | 1306 (38) | 1704 (35) | 1954 (34) | 2382 (35) | 2457 (32) | 2589 (29) |
| Unknown | 224 (13) | 652 (19) | 1079 (22) | 530 (9) | 362 (5) | 712 (9) | 690 (8) |
Cell entries are frequency, with column percentages in parentheses.
HET indicates heterosexual transmission; HIV, human immunodeficiency virus; HIVRN, HIV Research Network; IDU, injection drug use; MSM, men who have sex with men; RW, Ryan White CARE Act.
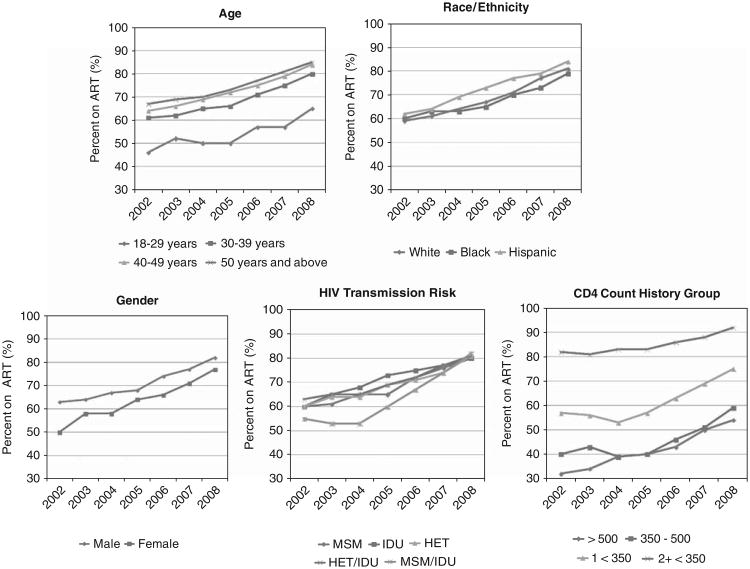
Among all patients, the observed percentage prescribed ART increased from 60% in 2002 to 81% in 2008. Among those with 2 or more CD4≤350 cells/mm3, 82% were prescribed ART in 2002, increasing to 92% in 2008. In each year, the proportion of patients prescribed ART was lower among those at earlier disease stages, although these groups also showed an increase in 2006–2008. Even among patients with no history of CD4≤500 cells/mm3, the percentage receiving ART increased from 32% to 54% (Fig. 1).
Figure 1.
Observed proportions prescribed antiretroviral therapy (ART), by year. HET indicates heterosexual transmission; HIV human immunodeficiency virus; IDU, injection drug users; MSM, men who had sex with men.
ART rates increased over time for each demographic subgroup. Nevertheless, in each year, unadjusted differences in ART prescription rates were evident. In each year, more men than women were prescribed ART. Hispanic patients were slightly more likely to be prescribed ART than white or black patients. Older patients were more likely to be prescribed ART than younger ones. Before 2007, patients with a combination of MSM and IDU risk factors were least likely to be prescribed ART.
Multivariate Analyses
The final logistic regression model included 3 interaction terms: CD4 history by year, age by year, and age by CD4 history. No 3-way interaction involving year and CD4 history was significant, and other 2-way interactions were not significant at P < 0.05. Table 2 shows the exponentiated coefficients for this model, estimated on the full sample; also shown are adjusted odds ratios (AORs) for the model including only main effects. Women were significantly less likely than men to be prescribed ART [AOR=0.83; 95% confidence interval (CI) = 0.76, 0.91]. Black patients were less likely than whites to use ART (AOR =0.79; 95% CI= 0.72, 0.86). Although patients with an MSM/IDU risk were less likely to be prescribed ART than those with MSM risk (AOR = 0.81; 95% CI = 0.67, 0.97), the overall set of risk-group indicators was not significant (χ2 = 7.74, df = 4, P = 0.10).
Table 2. GEE Logistic Regression of ART Prescription, 14,092 HIV-infected Patients, 2002–2008.
| Full Model | Main Effects Model | Model With Insurance | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Variables | Adjusted Odds Ratio | 95% CI | Adjusted Odds Ratio | 95% CI | Adjusted Odds Rati | o 95% CI |
| Age (y) | ||||||
| 18–29 | Reference | Reference | Reference | |||
| 30–39 | 1.60 | 1.26, 2.04 | 1.68 | 1.54, 1.83 | 1.59 | 1.25, 2.02 |
| 40–49 | 2.54 | 2.00, 3.22 | 2.01 | 1.84, 2.19 | 2.46 | 1.93, 3.12 |
| ≥50 | 2.46 | 1.86, 3.26 | 2.32 | 2.09, 2.58 | 2.30 | 1.74, 3.06 |
| Race | ||||||
| White | Reference | Reference | Reference | |||
| Black | 0.79 | 0.72 0.86 | 0.79 | 0.72, 0.86 | 0.78 | 0.71, 0.86 |
| Hispanic | 1.06 | 0.96, 1.18 | 1.07 | 0.97, 1.19 | 1.05 | 0.95, 1.17 |
| Sex | ||||||
| Male | Reference | Reference | Reference | |||
| Female | 0.83 | 0.76, 0.91 | 0.83 | 0.76, 0.91 | 0.83 | 0.75, 0.91 |
| HIV risk factor | ||||||
| MSM | Reference | Reference | Reference | |||
| IDU | 1.00 | 0.86, 1.16 | 0.99 | 0.85, 1.15 | 0.97 | 0.84, 1.13 |
| HET | 1.06 | 0.97, 1.17 | 1.07 | 0.97, 1.17 | 1.06 | 0.96, 1.16 |
| HET/IDU | 1.01 | 0.86, 1.18 | 0.99 | 0.85, 1.17 | 0.98 | 0.84, 1.15 |
| MSM/IDU | 0.81 | 0.67, 0.97 | 0.81 | 0.67, 0.97 | 0.78 | 0.65, 0.95 |
| CD4 history (cells/mm3) | ||||||
| All >500 | Reference | Reference | Reference | |||
| Any 351–500 | 1.31 | 1.03, 1.67 | 1.17 | 1.07, 1.27 | 1.45 | 1.06, 1.98 |
| 1≤350 | 2.20 | 1.71, 2.83 | 2.08 | 1.90, 2.29 | 2.13 | 1.53, 2.97 |
| 2+≤350 | 7.32 | 5.78, 9.28 | 7.32 | 6.70, 8.00 | 7.29 | 5.35, 9.94 |
| Year | ||||||
| Linear trend | 1.02 | 0.98, 1.07 | 1.17 | 1.15, 1.19 | 1.11 | 1.04, 1.17 |
| Age by (linear) year | ||||||
| 30–39 by year | 1.05 | 1.01, 1.10 | — | — | 1.05 | 1.01, 1.10 |
| 40–49 by year | 1.07 | 1.03, 1.12 | 1.08 | 1.03, 1.12 | ||
| ≥50 by year | 1.10 | 1.05, 1.15 | 1.10 | 1.05, 1.15 | ||
| CD4 history (cells/mm3) by (linear) year | ||||||
| Any 351–500 | 1.03 | 0.99, 1.07 | — | — | 1.02 | 0.98, 1.07 |
| 1≤350 | 1.08 | 1.04, 1.12 | 1.08 | 1.03, 1.12 | ||
| 2+≤350 | 1.15 | 1.11, 1.19 | 1.15 | 1.11, 1.19 | ||
| Age by CD4 history | ||||||
| 30–39 and 351–500 cells/mm3 | 0.96 | 0.74, 1.23 | — | — | 0.96 | 0.74, 1.24 |
| 30–39 and 1≤350 cells/mm3 | 0.90 | 0.69, 1.18 | 0.91 | 0.70, 1.18 | ||
| 30–39 and 2+≤350 cells/mm3 | 0.75 | 0.59, 0.96 | 0.76 | 0.59, 0.97 | ||
| 40–49 and 351–500 cells/mm3 | 0.71 | 0.56, 0.91 | 0.71 | 0.55, 0.91 | ||
| 40–49 and 1≤350 cells/mm3 | 0.58 | 0.45, 0.75 | 0.60 | 0.46, 0.78 | ||
| 40–49 and 2+≤350 cells/mm3 | 0.46 | 0.36, 0.58 | 0.47 | 0.37, 0.60 | ||
| 50+ and 351–500 cells/mm3 | 0.75 | 0.57, 0.99 | 0.76 | 0.58, 1.00 | ||
| 50+ and 1≤350 | 0.64 | 0.47, 0.86 | 0.68 | 0.50, 0.91 | ||
| 50+ and 2+≤350 cells/mm3 | 0.51 | 0.39, 0.68 | 0.55 | 0.41, 0.73 | ||
| Insurance | ||||||
| Private | Reference | |||||
| Medicaid | — | — | — | 2.04 | 1.59, 2.62 | |
| Medicare/dual | — | 2.46 | 1.76, 3.44 | |||
| None/Ryan White | 1.42 | 1.11, 1.82 | ||||
| Missing | 1.43 | 1.09, 1.88 | ||||
| Insurance by year | ||||||
| Medicaid by year | 0.88 | 0.84, 0.92 | ||||
| Medicare by year | — | — | — | — | 0.90 | 0.85, 0.95 |
| None/RW by year | 0.92 | 0.88, 0.96 | ||||
| Missing by year | 0.95 | 0.91, 1.00 | ||||
| Insurance by CD4 history | ||||||
| Medicaid and 351–500 cells/mm3 | 0.90 | 0.71, 1.14 | ||||
| Medicaid and 1≤350 cells/mm3 | 0.97 | 0.75, 1.25 | ||||
| Medicaid and 2≤350 cells/mm3 | 0.92 | 0.73, 1.17 | ||||
| Medicare and 351–500 cells/mm3 | 0.84 | 0.62, 1.15 | ||||
| Medicare and 1≤350 cells/mm3 | — | — | — | — | 0.75 | 0.53, 1.05 |
| Medicare and 2≤350 cells/mm3 | 0.72 | 0.53, 0.97 | ||||
| None and 351–500 cells/mm3 | 0.92 | 0.73, 1.15 | ||||
| None and 1≤350 cells/mm3 | 1.21 | 0.94, 1.57 | ||||
| None and 2≤350 cells/mm3 | 1.16 | 0.91, 1.47 | ||||
| Missing and 351–500 cells/mm3 | 0.95 | 0.72, 1.25 | ||||
| Missing and 1≤350 cells/mm3 | 0.89 | 0.67, 1.20 | ||||
| Missing and 2≤350 cells/mm3 | 0.86 | 0.66, 1.11 | ||||
The models also included indicators for each clinical site (not shown).
ART indicates antiretroviral therapy; CI, confidence interval; GEE, generalized estimating equations; HET, heterosexual transmission; HIV, human immunodeficiency virus; IDU, injection drug use; MSM, men who have sex with men; RW, Ryan White CARE Act.
Predictive margins summarize the effect of each variable (or combination of variables) by averaging predicted values from the logistic regression model over other covariates. Across all years and CD4 categories, the mean predicted probability of ART was 0.71 for men and 0.68 for women. Mean predicted probabilities were 0.72 for whites, 0.68 for blacks, and 0.73 for Hispanics. For risk group, predicted probabilities were 0.70 for MSM, 0.70 for IDU, 0.71 for HET, 0.70 for HET/IDU, and 0.66 for MSM/IDU (results not shown).
Table 3 shows mean predicted probabilities for age and CD4 history, by year, specifying a linear time trend. Overall, predicted probabilities were higher for older patients and for those with more advanced disease. For age group trends, the difference between 18 and 29 year olds and the other age groups increased over time; ART use increased for all age groups but somewhat more slowly for the youngest group. The time coefficients for those aged 30–39 (1.05), 40–49 (1.07), and 50 or older (1.09) were each significantly different from the time trend for the youngest group (1.02); the coefficients for the 3 oldest groups did not differ significantly from each other (χ2 = 3.78, df = 2, P = 0.15).
Table 3. Mean Predicted Probabilities for ART Based on Full Logistic Model, Age by Year, and CD4 History by Year.
| Year | ||||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | Overall | |
| Age (y) | ||||||||
| 18–29 | 0.52 | 0.54 | 0.57 | 0.59 | 0.61 | 0.63 | 0.65 | 0.61 |
| 30–39 | 0.58 | 0.62 | 0.65 | 0.68 | 0.71 | 0.73 | 0.76 | 0.70 |
| 40–49 | 0.60 | 0.64 | 0.68 | 0.71 | 0.74 | 0.77 | 0.79 | 0.73 |
| ≥50 | 0.61 | 0.66 | 0.69 | 0.73 | 0.76 | 0.79 | 0.81 | 0.75 |
| CD4 history (cells/mm3) | ||||||||
| All >500 | 0.37 | 0.39 | 0.41 | 0.43 | 0.45 | 0.47 | 0.49 | 0.45 |
| Any 351–500 | 0.39 | 0.41 | 0.44 | 0.47 | 0.49 | 0.52 | 0.54 | 0.49 |
| 1≤350 | 0.48 | 0.52 | 0.56 | 0.59 | 0.63 | 0.67 | 0.70 | 0.62 |
| 2+≤350 | 0.72 | 0.76 | 0.80 | 0.83 | 0.86 | 0.88 | 0.90 | 0.85 |
ART indicates antiretroviral therapy.
For the CD4 by year interaction, the time trend became stronger at more advanced disease stages; the difference between the 350–500 cells/mm3 group (AOR=1.03; 95% CI = 0.99, 1.07) and the group with 1 level ≤350 cells/mm3 (AOR = 1.08; 95% CI = 1.04, 1.12) was significant (χ2 = 6.28, df = 1, P = 0.01), as was the difference between the latter group and those with 2 or more CD4 levels ≤350 cells/mm3 (AOR=1.15; 95% CI=1.11, 1.19; χ2 = 12.41; df= 1; P = 0.001).
Age group differences in ART use were more pronounced at earlier disease stages (Table 4).
Table 4. Mean Predicted Probabilities for ART Based on Full Logistic Model, by Age, and CD4 History.
| CD4 History | ||||
|---|---|---|---|---|
|
|
||||
| All >500 | Any 350–500 | 1≤350 | 2+≤350 | |
| Age (y) | ||||
| 18–29 | 0.25 | 0.32 | 0.49 | 0.80 |
| 30–39 | 0.39 | 0.47 | 0.63 | 0.85 |
| 40–49 | 0.52 | 0.53 | 0.64 | 0.85 |
| ≥50 | 0.53 | 0.55 | 0.67 | 0.87 |
ART indicates antiretroviral therapy.
Insurance Status
A model including interactions between insurance status and (1) year and (2) CD4 history produced results virtually the same as the model excluding insurance (Table 2). Thus, adjusting for insurance did not change the observed pattern of disparities over time for other characteristics. Interactions between insurance and year (χ2 = 36.82, df = 4, P = 0.001) and insurance and CD4 (χ2 = 29.65, df = 12, P = 0.001) were significant (Supplemental Digital Content 2, http://links.lww.com/MLR/A280; Tables A and B). Mean predicted probabilities of ART were 0.68 for private, 0.72 for Medicaid, 0.73 for Medicare/Dual, 0.70 for none/Ryan White, and 0.70 for missing.
Discussion
These results, from a large multisite sample, provide updated information on trends in ART receipt. The overall proportion of patients in HIV care who were prescribed ART increased from 60% in 2002 to 81% in 2008. Among patients with a history of ≥2 CD4 levels ≤350 cells/mm3, the proportion prescribed ART increased from 82% in 2002 to 92% in 2008.
Rates of ART prescribing were about 30% higher than rates documented in the early ART era (1997–1999)14,15; more recent studies have reported rates of 70% to 89% for eligible patients (CD4≤350 cells/mm3).11,12 Of note, we examined any ART prescription during a 1-year period, whereas others examined ART use at 1 time point.15 Varying time periods for assessing ART use and differences between prescriptions and patient-reported consumption could produce noncomparable rates.
As expected, the likelihood of ART prescription increased as disease stage advanced. The current study differs from prior research16 by using a disease stage variable defined in terms of each patient's cumulative history of CD4 measurements. For all CD4 groups, the proportion prescribed ART increased steadily over time, most strongly for those with one or more CD4 tests ≤350 cells/mm3. During this period, use of ART at CD4 levels >500 cells/mm3 was at the provider's discretion. Nevertheless, by 2008 nearly half of those with no history of a CD4 tests ≤500 were prescribed ART. The element of discretion in the guidelines at higher CD4 levels suggested that other factors besides clinical necessity could affect ART receipt, resulting in possibly greater disparities for patients with less advanced HIV disease. However, interactions of CD4 history with sex, race, and risk were not significant, casting doubt on this hypothesis.
Racial/ethnic disparities in ART receipt have received considerable attention. A review of 28 studies conducted between 1984 and 1999 concluded that HIV-infected racial/ ethnic minorities had a lower rate of utilization of anti-retroviral medications than whites.13 Among participants in the Women's Interagency Health Study, both black and Hispanic patients had a lower likelihood of ART receipt than whites.11 In contrast, other studies report higher ART rates among Hispanic than white patients.14,16 This study found roughly equal adjusted odds of Hispanic and white patients' receiving ART. The proportion of non-Hispanic black patients prescribed ART remained around 4 percentage points lower than whites throughout the observation period. Future research should confirm and elucidate differences among minority groups (eg, black and Hispanic patients) in ART receipt.
Sex disparities in ART receipt have also been of concern. Prior research reported that ART receipt among female HIV patients was 5–10 percentage points lower than among male patients, without adjusting for other variables.14–16 Unadjusted sex differences in our study ranged from 4 to 13 percentage points, depending upon year, but the adjusted 3-percentage point difference was consistent across time. Interactions between sex and year were negligible in all models. Thus, sex disparities in ART receipt persist, even in a context of generally increasing use of ART among all subgroups.
Disparities in ART receipt by age have not received as much attention as sex or racial/ethnic differences. Age differences in ART prescription rates also persisted over time, with adjusted differences between the youngest and oldest groups as much as 15 percentage points. In each year, the proportion prescribed ART was greater in the older than younger age groups, although ART rates were generally similar in the 40–49 and 50 and older groups. Adjusted differences between the oldest age groups and the youngest one widened slightly over time. Prior studies have also reported higher ART rates among older patients.12,16 Older patients may be seen as leading more stable lives and as more likely to be adherent compared with the 18–29-year-old group, whose circumstances may be more disorganized and who may have less experience with HIV care. Given the growing numbers of older patients with HIV and newly infected youth, these results emphasize the importance of understanding factors producing age differences.
The association of HIV transmission risk factors with ART use is inconsistent in prior research. Some studies show that patients with an IDU risk factor are less likely, or take longer, to receive ART than MSM.7,15,16 Other studies do not find a significant association.11,12,14 In the current study, overall risk-group differences in ART use were not significant, although there is some evidence that ART rates among patients with an MSM/IDU risk are especially low. These patients may not be equivalent to those with only an IDU risk. Future research should confirm whether MSM/IDU patients differ from IDUs in terms of ART receipt, utilization of other services, or clinical outcomes.
Differences in insurance coverage are often considered as potentially mediating or explaining disparities among demographic groups.27,32 Adjusting for such mediating variables in multivariate analyses could explain part of the unadjusted sociodemographic differences, resulting in reduced magnitude of residual sociodemographic effects. For example, in 1 study significant black-white differences in ART receipt became nonsignificant when insurance status and education were controlled.15 It has been argued that assessment of demographic disparities in health care should control for health status but not for “downstream” mediating factors such as insurance or socioeconomic status.27,32 To focus on assessing demographic disparities, main analyses excluded insurance coverage and other possible mediators, such as number of outpatient visits and number of CD4 tests, unlike a prior HIVRN study.16 However, adjusting for insurance did not alter the pattern of demographic differences in ART receipt, suggesting that insurance status did not explain demographic disparities in this analysis. Indeed, unlike much research in general populations, those with private coverage had lower rates of ART use than those with public coverage. This may be due to fewer limitations in medication coverage for public plans, as well as the availability of resources provided by state AIDS Drug Assistance Programs. Future research should confirm these results and obtain more detailed information on ART restrictions in private insurance plans.
The current analyses have some limitations: First, the multivariate models represented time effects by a linear term, rather than treating calendar year as a categorical variable. The latter strategy would have required estimating many additional parameters for interaction effects involving year. Moreover, anomalous patterns for 1 or 2 years in the middle of the series might have contributed to significant interactions that would be difficult to interpret. Second, the dependent variable reflects whether ART was prescribed; data reflecting the extent of adherence to prescribed regimens were unavailable. Third, the risk-group variable was based on self-reported probable mode of HIV infection; we did not have data showing whether patients were actively engaged in substance abuse while receiving HIV care. Access to ART may depend more on current substance abuse behavior than on historical mode of HIV infection. Fourth, although multisite studies have greater generalizeability than single-site ones, the HIVRN data are not nationally representative; rates of ART prescription may be lower among providers with less experience treating HIV than the HIVRN providers. Fifth, Some CD4 counts were obtained after the last outpatient visit in a year, and the algorithm for determining the CD4 history category included these counts; however, this occurred in a relatively small number of person-years. Finally, nonreceipt of ART may arise from provider reluctance (eg, concern about adherence), from patient concerns (eg, mistrust of the medical system), or both.33,34 Unfortunately, information was not available that showed whether patient or provider decisions influenced nonreceipt of ART among those eligible, limiting our ability to explain observed disparities.
In summary, the proportion of HIV-infected patients prescribed ART increased notably between 2002 and 2008. Increases were observed in all sociodemographic subgroups, especially among those eligible for ART according to guidelines in place for most of this period. Nevertheless, racial/ ethnic and sex disparities persisted. The 2010 National Healthcare Disparities Report summarized findings for several core measures of quality of care as showing improvement from 2001 to 2007, but racial/ethnic disparity measures changed little, consistent with this study's results.35 A priori, one might expect that disparities could increase over time, if more advantaged groups had better access to new therapies. Alternatively, more widespread dissemination of ART could narrow disparities, especially if use in more advantaged groups had plateaued. The fact that ART use increased among traditionally disadvantaged groups, such as women and black patients, is encouraging and shows that improvements in care are possible for such groups. If quality is improving for disadvantaged groups, why is the gap in care not narrowing? The processes producing a pattern of fairly constant group differences in the context of overall improvement require further explication. Future research should continue to investigate factors that contribute to the persistence of sociodemographic disparities in ART receipt.
Acknowledgments
Participating Sites: Alameda County Medical Center, Oakland, CA (Howard Edelstein, MD); Children's Hospital of Philadelphia, Philadelphia, PA (Richard Rutstein, MD); Community Health Network, Rochester, NY (Roberto Corales, DO); Drexel University, Philadelphia, PA (Jeffrey Jacobson, MD, Sara Allen, CRNP); Johns Hopkins University, Baltimore, MD (Kelly Gebo, MD, Richard Moore, MD, Allison Agwu, MD); Montefiore Medical Group, Bronx, NY (Robert Beil, MD, Carolyn Chu, MD); Montefiore Medical Center, Bronx, NY (Lawrence Hanau, MD); Oregon Health and Science University, Portland, OR (P. Todd Korthuis, MD); Parkland Health and Hospital System, Dallas, TX (Laura Armas-Kolostroubis, MD); St Jude's Children's Hospital and University of Tennessee, Memphis, TN (Aditya Gaur, MD); St Luke's Roosevelt Hospital Center, New York, NY (Victoria Sharp, MD); Tampa General Health Care, Tampa, FL (Charurut Somboonwit, MD); University of California, San Diego, La Jolla, CA (Stephen Spector, MD); University of California, San Diego, CA (W. Christopher Mathews, MD); Wayne State University, Detroit, MI (Jonathan Cohn, MD).
Sponsoring Agencies: Agency for Healthcare Research and Quality, Rockville, MD (Fred Hellinger, PhD, John A. Fleishman, PhD); Health Resources and Services Administration, Rockville, MD (Robert Mills, PhD).
Data Coordinating Center: Johns Hopkins University (Richard Moore, MD, Jeanne Keruly, CRNP, Kelly Gebo, MD, Cindy Voss, MA, Bonnie Cameron, MS).
Supported by the Agency for Healthcare Research and Quality (AHRQ-06-0025). A.L.A. is supported by the National Institutes of Allergy and Infectious Diseases (K23 AI084549) and the Johns Hopkins University Richard S. Ross Clinician Scientist Award. R.D.M. is supported by NIH (K24 DA 00432, R01DA11602, R01 AA 16893).
Footnotes
Preliminary analyses presented at the Conference on Retroviruses and Opportunistic Infections, Boston, MA, February 27–March 2, 2011.
The views expressed in this paper are those of the authors. No official endorsement by DHHS, the National Institutes of Health, or the Agency for Healthcare Research and Quality is intended or should be inferred.
The authors declare no conflict of interest.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website, www.lww-medicalcare.com.
References
- 1.Ledergerber B, Egger M, Erard V, et al. AIDS-related opportunistic illnesses occurring after initiation of potent antiretroviral therapy: the Swiss HIV Cohort Study. JAMA. 1999;282:2220–2226. doi: 10.1001/jama.282.23.2220. [DOI] [PubMed] [Google Scholar]
- 2.Moore RD, Chaisson RE. Natural history of HIV infection in the era of combination antiretroviral therapy. AIDS. 1999;13:1933–1942. doi: 10.1097/00002030-199910010-00017. [DOI] [PubMed] [Google Scholar]
- 3.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 4.Gebo KA, Diener-West M, Moore RD. Hospitalization rates in an urban cohort after the introduction of highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2001;27:143–152. doi: 10.1097/00126334-200106010-00009. [DOI] [PubMed] [Google Scholar]
- 5.Torres RA, Barr M. Impact of combination therapy for HIV infection on inpatient census. N Engl J Med. 1997;336:1531–1532. doi: 10.1056/nejm199705223362118. [DOI] [PubMed] [Google Scholar]
- 6.El-Sadr WM, Lundgren JD, Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 7.Lemly DC, Shepherd BE, Hulgan T, et al. Race and sex differences in antiretroviral therapy use and mortality among HIV-infected persons in care. J Infect Dis. 2009;199:991–998. doi: 10.1086/597124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Losina E, Schackman BR, Sadownik SN, et al. Racial and sex disparities in life expectancy losses among HIV-infected persons in the united states: impact of risk behavior, late initiation, and early discontinuation of antiretroviral therapy. Clin Infect Dis. 2009;49:1570–1578. doi: 10.1086/644772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall HI, Byers RH, Ling Q, et al. Racial/ethnic and age disparities in HIV prevalence and disease progression among men who have sex with men in the United States. Am J Public Health. 2007;97:1060–1066. doi: 10.2105/AJPH.2006.087551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shapiro MF, Morton SC, McCaffrey DF, et al. Variations in the care of HIV-infected adults in the United States: results from the HIV Cost and Services Utilization Study. JAMA. 1999;281:2305–2315. doi: 10.1001/jama.281.24.2305. [DOI] [PubMed] [Google Scholar]
- 11.Lillie-Blanton M, Stone VE, Snow Jones A, et al. Association of race, substance abuse, and health insurance coverage with use of highly active antiretroviral therapy among HIV-infected women 2005. Am J Public Health. 2010;100:1493–1499. doi: 10.2105/AJPH.2008.158949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pence BW, Ostermann J, Kumar V, et al. The influence of psychosocial characteristics and race/ethnicity on the use, duration, and success of antiretroviral therapy. J Acquir Immune Defic Syndr. 2008;47:194–201. doi: 10.1097/QAI.0b013e31815ace7e. [DOI] [PubMed] [Google Scholar]
- 13.Palacio H, Kahn JG, Richards TA, et al. Effect of race and/or ethnicity in use of antiretrovirals and prophylaxis for opportunistic infection: a review of the literature. Public Health Rep. 2002;117:233–251. doi: 10.1093/phr/117.3.233. discussion 231–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McNaghten AD, Hanson DL, Dworkin MS, et al. Differences in prescription of antiretroviral therapy in a large cohort of HIV-infected patients. J Acquir Immune Defic Syndr. 2003;32:499–505. doi: 10.1097/00126334-200304150-00006. [DOI] [PubMed] [Google Scholar]
- 15.Cunningham WE, Markson LE, Andersen RM, et al. Prevalence and predictors of highly active antiretroviral therapy use in patients with HIV infection in the United States. J Acquir Immune Defic Syndr. 2000;25:115–123. doi: 10.1097/00042560-200010010-00005. [DOI] [PubMed] [Google Scholar]
- 16.Gebo KA, Fleishman JA, Conviser R, et al. Racial and gender disparities in receipt of highly active antiretroviral therapy persist in a multistate sample of HIV patients in 2001. J Acquir Immune Defic Syndr. 2005;38:96–103. doi: 10.1097/00126334-200501010-00017. [DOI] [PubMed] [Google Scholar]
- 17.Airoldi M, Zaccarelli M, Bisi L, et al. One-pill once-a-day HAART: a simplification strategy that improves adherence and quality of life of HIV-infected subjects. Patient Prefer Adherence. 2010;4:115–125. doi: 10.2147/ppa.s10330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooper V, Horne R, Gellaitry G, et al. The impact of once-nightly versus twice-daily dosing and baseline beliefs about HAART on adherence to efavirenz-based HAART over 48 weeks: the NOCTE study. J Acquir Immune Defic Syndr. 2010;53:369–377. doi: 10.1097/QAI.0b013e3181ccb762. [DOI] [PubMed] [Google Scholar]
- 19.Hall HI, Song R, Rhodes P, et al. Estimation of HIV incidence in the United States. JAMA. 2008;300:520–529. doi: 10.1001/jama.300.5.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. [Accessed March 25, 2011];HIV Surveillance Report. 2008 Available at: http://www.cdc.gov/hiv/topics/surveillance/resources/reports/
- 21.United States Census Bureau. [Accessed March 25, 2011];State and County QuickFacts. Available at: http://quickfacts.census.gov/qfd/states/00000.html.
- 22.Li X, Margolick JB, Conover CS, et al. Interruption and discontinuation of highly active antiretroviral therapy in the Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr. 2005;38:320–328. [PubMed] [Google Scholar]
- 23.Yuan Y, L’Italien G, Mukherjee J, et al. Determinants of discontinuation of initial highly active antiretroviral therapy regimens in a US HIV-infected patient cohort. HIV Med. 2006;7:156–162. doi: 10.1111/j.1468-1293.2006.00355.x. [DOI] [PubMed] [Google Scholar]
- 24.Stone VE. Physician contributions to disparities in HIV/AIDS care: the role of provider perceptions regarding adherence. Curr HIV/AIDS Rep. 2005;2:189–193. doi: 10.1007/s11904-005-0015-5. [DOI] [PubMed] [Google Scholar]
- 25.Thrasher AD, Earp JA, Golin CE, et al. Discrimination, distrust, and racial/ethnic disparities in antiretroviral therapy adherence among a national sample of HIV-infected patients. J Acquir Immune Defic Syndr. 2008;49:84–93. doi: 10.1097/QAI.0b013e3181845589. [DOI] [PubMed] [Google Scholar]
- 26.Kitahata MM, Gange SJ, Abraham AG, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009;360:1815–1826. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGuire TG, Alegria M, Cook BL, et al. Implementing the Institute of Medicine definition of disparities: an application to mental health care. Health Serv Res. 2006;41:1979–2005. doi: 10.1111/j.1475-6773.2006.00583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yehia BR, Gebo KA, Hicks PB, et al. Structures of care in the clinics of the HIV Research Network. AIDS Patient Care STDS. 2008;22:1007–1013. doi: 10.1089/apc.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. Department of Health and Human Services; 2009. [Accessed March 25, 2011]. pp. 1–161. Available at http://www.aidsinfo.nih.gov/ContentFiles/AdultAdolescentGL.pdf. [Google Scholar]
- 30.Lee LM, McKenna MT, Janssen RS. Classification of transmission risk in the National HIV/AIDS Surveillance System. Public Health Rep. 2003;118:400–407. doi: 10.1016/S0033-3549(04)50271-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Graubard BI, Korn EL. Predictive margins with survey data. Biometrics. 1999;55:652–659. doi: 10.1111/j.0006-341x.1999.00652.x. [DOI] [PubMed] [Google Scholar]
- 32.Cook BL, McGuire TG, Zuvekas SH. Measuring trends in racial/ethnic health care disparities. Med Care Res Rev. 2009;66:23–48. doi: 10.1177/1077558708323607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bogart LM, Wagner G, Galvan FH, et al. Conspiracy beliefs about HIV are related to antiretroviral treatment nonadherence among African American men with HIV. J Acquir Immune Defic Syndr. 2010;53:648–655. doi: 10.1097/QAI.0b013e3181c57dbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bogart LM, Bird ST, Walt LC, et al. Association of stereotypes about physicians to health care satisfaction, help-seeking behavior, and adherence to treatment. Soc Sci Med. 2004;58:1049–1058. doi: 10.1016/s0277-9536(03)00277-6. [DOI] [PubMed] [Google Scholar]
- 35.Agency for Healthcare Research and Quality. National Healthcare Disparities Report. Rockville, MD: AHRQ Publication No. 11-0005; 2010. [Google Scholar]