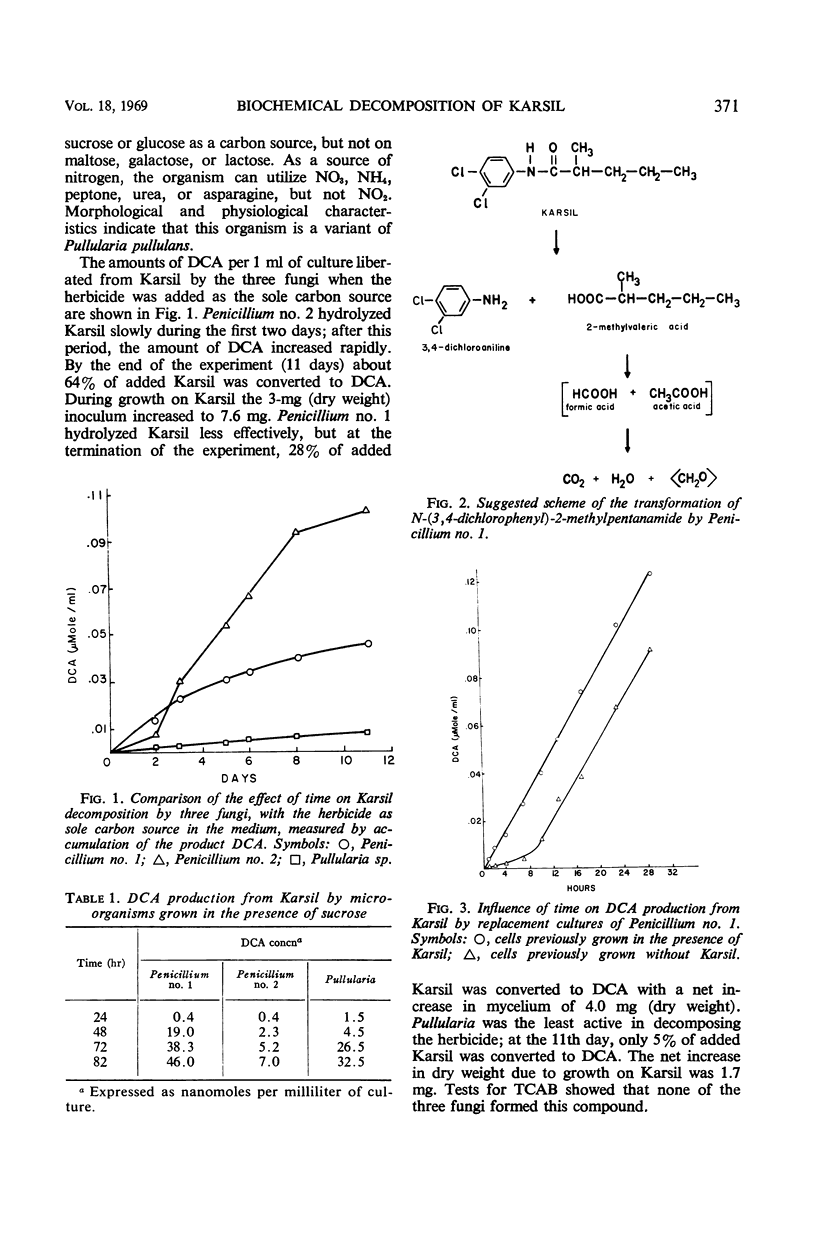
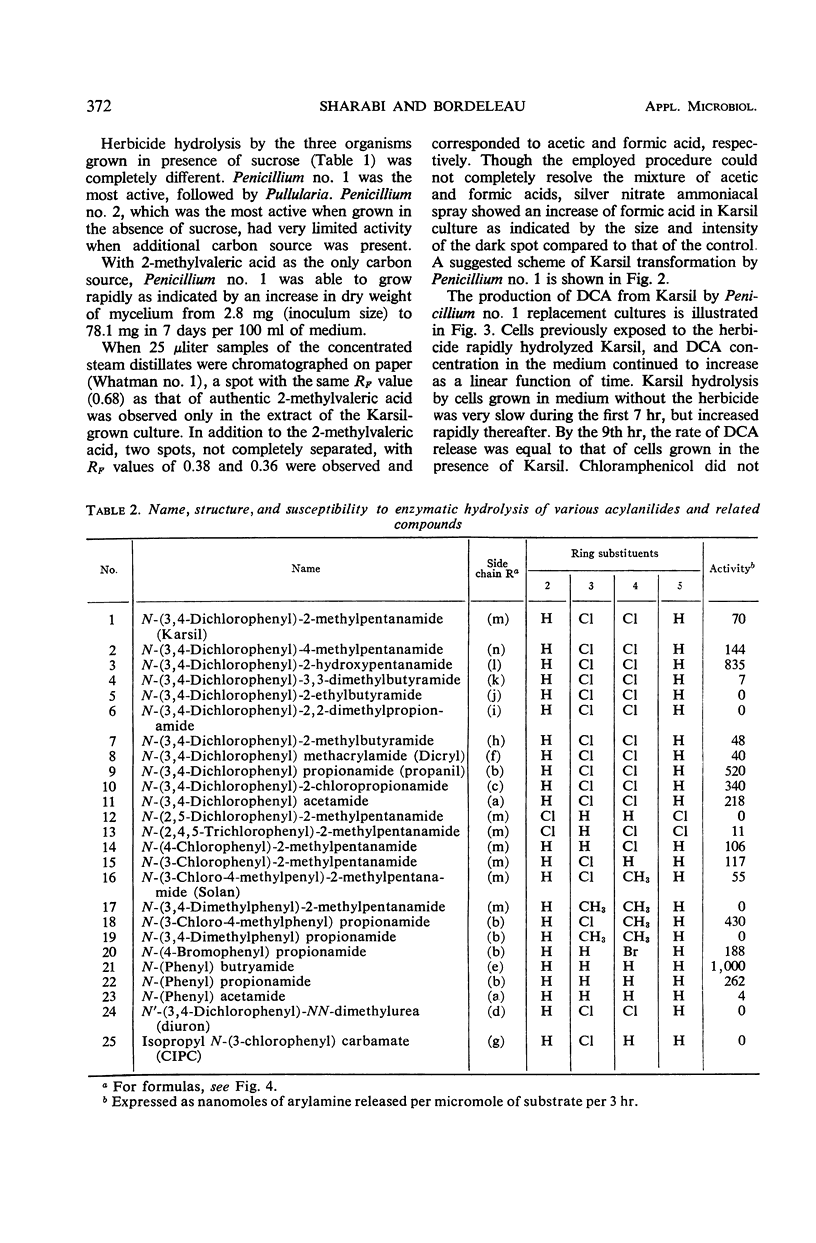
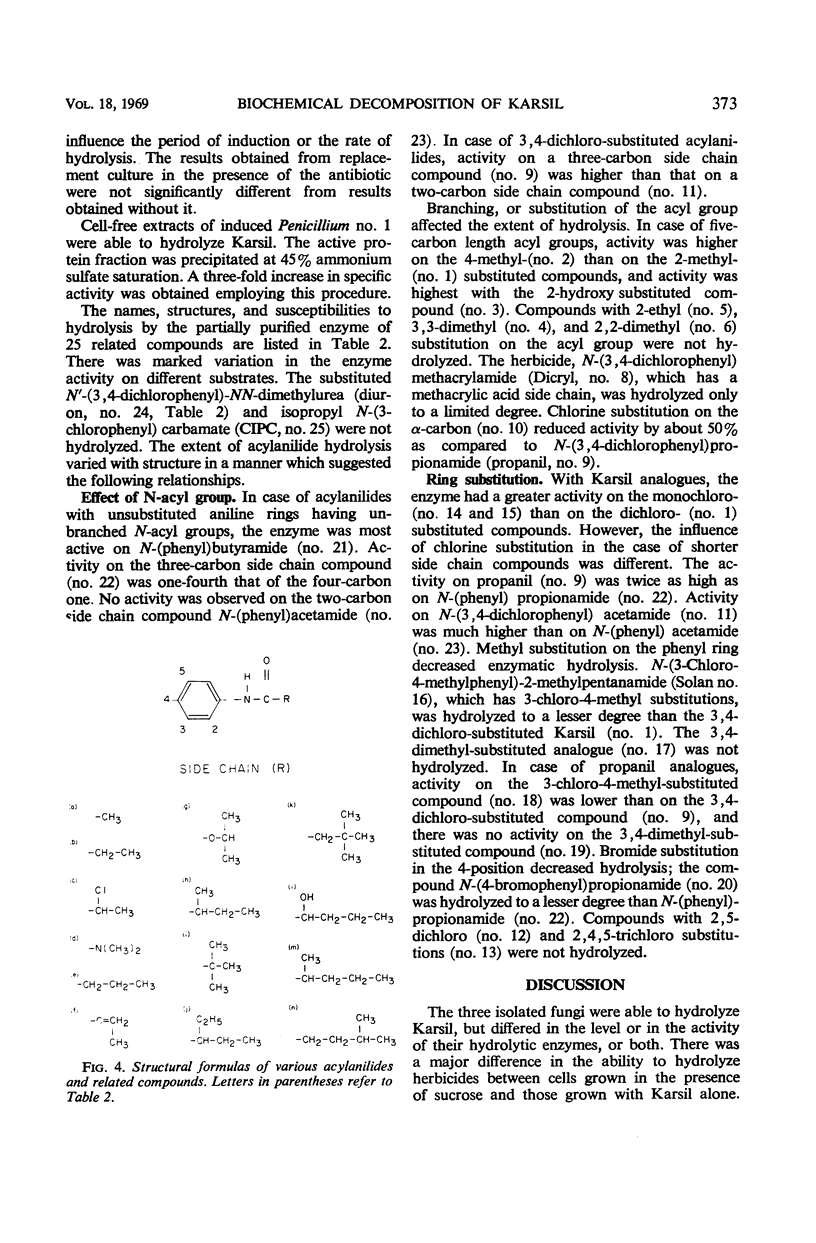
Abstract
Organisms capable of decomposing N-(3,4-dichlorophenyl)-2-methylpentanamide (Karsil) were isolated, identified, and tested for their ability to hydrolyze this herbicide. Primary products of Karsil decomposition by cells and cell-free extracts of a Penicillium sp. were identified as 2-methyl-valeric acid and 3,4-dichloroaniline. The Karsil acylamidase (EC 3.5.1.a aryl acylamine amidohydrolase) was an induced enzyme. It was partially purified and tested for its ability to hydrolyze 25 related compounds. Some relations between the structures of these compounds and their susceptibility to enzymatic hydrolysis were discerned.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartha R., Pramer D. Pesticide transformation to aniline and azo compounds in soil. Science. 1967 Jun 23;156(3782):1617–1618. doi: 10.1126/science.156.3782.1617. [DOI] [PubMed] [Google Scholar]
- NIMMO-SMITH R. H. Aromatic N-deacylation by chick-kidney mitochondria. Biochem J. 1960 May;75:284–293. doi: 10.1042/bj0750284. [DOI] [PMC free article] [PubMed] [Google Scholar]


