Abstract
Age-associated dysregulation of sleep can be worsened by Alzheimer’s disease (AD). AD and sleep restriction both impair cognition, yet it is unknown if mild chronic sleep restriction modifies the proteopathic processes involved in AD. The goal of this work was to test the hypothesis that sleep restriction worsens memory impairments, and amyloid β-peptide (Aβ) and pTau accumulations in the brain in a mouse model of AD, with a focus on a role for circulating glucocorticoids (GC). Male 3xTgAD mice were subjected to sleep restriction (SR) for 6 hrs/day for 6 weeks using the modified multiple platform technique, and behavioral (Morris water maze, fear conditioning, open field) and biochemical (immunoblot) outcomes were compared to mice undergoing daily cage transfers (large cage control; LCC) as well as control mice that remained in their home cage (control; CTL). At one week, both LCC and SR mice displayed significant elevations in plasma corticosterone compared to CTL (p<0.002). By four weeks, SR mice displayed a two-fold increase in circulating corticosterone levels compared to CTL. Behavioral data indicated deficits in contextual and cued memory in SR mice that were not present for LCC or CTL (p<0.04). Both Aβ and pTau levels increased in the cortex of SR mice compared to CTL and LCC; however these changes were not noted in the hippocampus. Significant positive correlations between cortical Aβ and pTau levels and circulating corticosterone indicate a potential role for GCs in mediating behavioral and biochemical changes observed after sleep restriction in a mouse model of AD.
Keywords: Alzheimer’s disease, sleep restriction, glucocorticoids, amyloid, Morris water maze, fear conditioning
Introduction
Disruptions to daily sleep cycles are common in modern society. Long-term insufficient sleep has adverse effects on overall health and neurological function including deficits in attention, impairments in cognition and depression (Banks and Dinges, 2007). Aging causes an increase in sleep fragmentation and restriction that can be compounded in neurodegenerative disorders such as Alzheimer’s disease (AD). Further, both aging and AD are associated with decreases in cognitive function. Yet it remains to be seen how age- and disease-related decreases in sleep affect the progression of AD including both cognitive and pathological outcomes.
Recent data show that neurodegenerative diseases are often associated with sleep disturbances beyond what is observed in normal aging. Sleep fragmentation, which can include frequent awakenings and an increase in daytime napping, is the most common sleep disturbance reported in AD patients with an incidence of roughly 30–50% (Vitiello et al., 1990). In addition to these disturbances, the latency to the first episode of REM sleep (REML) is shorter in AD patients (Bliwise et al., 1989). Several studies show disruptions of circadian rhythmicity in AD including an increase in ‘sundowning’, changes in activity patterns, and fluctuations in normal circadian changes in body temperature (van Someren et al., 1996; Volicer et al., 2001). At least one study showed a genetic predisposition to sleep disturbances in AD patients who carried a mutation in the monoamine oxidase A gene, a gene shown to play a role in maintaining circadian rhythms (Craig et al., 2006). However, despite the large number of clinical studies that outline sleep disturbance in AD patients, it is not known how sleep disturbances affect the progression of the disease.
Both clinical and experimental studies show that loss of sleep, even for only for a few hours, causes memory impairments. Using a fear conditioning paradigm in mice, Graves et al., showed that just 5 hours of sleep deprivation after the training session resulted in impairments in consolidation of contextual memory (Graves et al., 2003). A similarly short (6 hour) period of sleep restriction in mice also caused impairments in object recognition (Palchykova et al., 2006). In one study, repeated daily sleep restriction during the Morris water maze training period resulted in increases in circulating corticosterone levels and impaired learning (Hairston et al., 2005). A wide range of data demonstrate deficits in learning, memory, attention, emotional reactivity and decision making in human subjects after sleep loss (Turner et al., 2007; Chee and Chuah, 2008; Goel et al., 2009; McCoy and Strecker, 2011) yet the majority of this work utilized 1 or 2 days of total deprivation in lieu of chronic partial restriction in total sleep time which is more relevant to the most common sleep disturbances in humans. Two different controlled clinical studies showed that successive nights of sleep restriction resulted in impaired memory and mood implying that, over time, long-term mild sleep restriction can have a cumulative adverse effect on cognitive function (Dinges et al., 1997; Van Dongen et al., 2003).
The goal of this study was to test the hypothesis that long-term mild sleep restriction worsens the progression of AD using the 3xTgAD mouse model (Oddo et al., 2003). Mice (3xTgAD) were subjected to daily mild sleep restriction for 6 hours per day for 6 weeks using the MMP technique (Machado et al., 2004). The MMP technique was chosen for the current study due to its proven ability to eliminate paradoxical sleep (PS) and reduce slow wave (SWS) sleep by ~31% (Machado et al., 2004). Further, the platform method is shown to increase circulating corticosterone levels suggesting that this form of sleep restriction can be considered a stressor in mice (Palma et al., 2007). Briefly, group-housed mice were placed in a cage containing three circular platforms and water filled the cage to 1 cm below the upper surface of the platforms enabling the mice to move around the cage by jumping from one platform to another. When they reached the paradoxical phase of sleep and the onset of muscle atonia, they fell into the water and were awoken (‘sleep restriction group’; SR). A second set of mice termed ‘large cage control’ (LCC), were moved into cages identical to the sleep restriction cages described above but with bedding instead of water and returned to their home cage at the end of the 6 hour test period. A third control group remained in their cages for the duration of the study (CTL). Circulating corticosterone levels, memory, anxiety and the accumulation of Aβ and phosphorylated-tau in the cortex and hippocampus were all quantified and compared.
Results
Plasma corticosterone concentrations
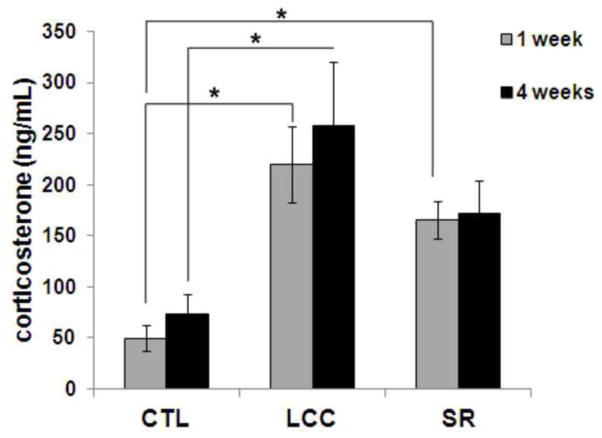
One week after beginning a course of 6 hours of sleep restriction per day, both LCC and SR mice displayed significant elevations of plasma corticosterone levels compared to non-stressed controls (CTL) (Fig 1; p<0.002). This represented a four-fold increase over normal circulating corticosterone levels. By four weeks after the start of the six week sleep restriction period, sleep restricted mice displayed a two-fold increase in circulating corticosterone levels compared to CTL. The LCC group also displayed an increase in circulating corticosterone levels compared to CTL at 4 weeks after the start of the 6 week sleep restriction period; this increase was significant (p=0.04). At 4 weeks after the start of the sleep restriction period there were no statistical differences in circulating corticosterone levels between the SR and CTL groups or between the SR and LCC groups. Further, circulating corticosterone levels were not statistically different in the SR and LCC groups at either 1 or 4 weeks.
Figure 1.
Blood plasma corticosterone in control (CTL), large cage control (LCC) and sleep restriction (SR) mice at 1 and 4 weeks after the start of the 6-week sleep restriction period.
Morris water maze
Mice were tested in the Morris water maze (MWM) prior to the beginning of the sleep restriction (pre-SR) and again at the end of the 6 week sleep restriction period (post-SR CTL, post-SR LCC, post-SR SR; Fig 2). Prior to the sleep restriction period, mice displayed a decrease in latency required to reach the target platform over time (Fig 1A). During both the 4 and 24 hour probe tests, mice spent a significantly greater amount of time in the target quadrant compared to the other three quadrants (p<0.005). After the SR period, none of the groups spent a significantly greater amount of time in the target quadrant compared to the other three quadrants (Fig 2C and 2D). No significant differences in swim speed were measured between groups (Fig 2B).
Figure 2.
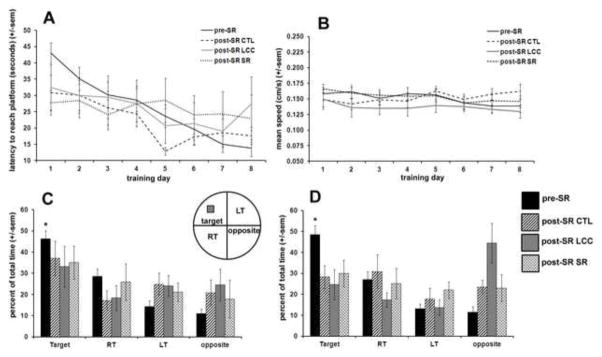
Morris water maze results for all mice prior to sleep restriction (pre-SR) and after the 6 week sleep restriction period for control (post-SR CTL), large cage control (post-SR LCC) and sleep restricted mice (post-SR SR). The latency time to reach the hidden platform decreased over time for mice tested prior to the sleep restriction period (A). Mean swim speed was not significantly different between groups (B). At both the 4 and 24 hour probe test (C&D), mice tested prior to the sleep restriction period spent a significantly greater amount of time in the target quadrant (p<0.005) whereas after the 6 week sleep restriction period no groups, including control, displayed significant preference for the target quadrant, indicating a lack of short term memory retention.
Fear conditioning
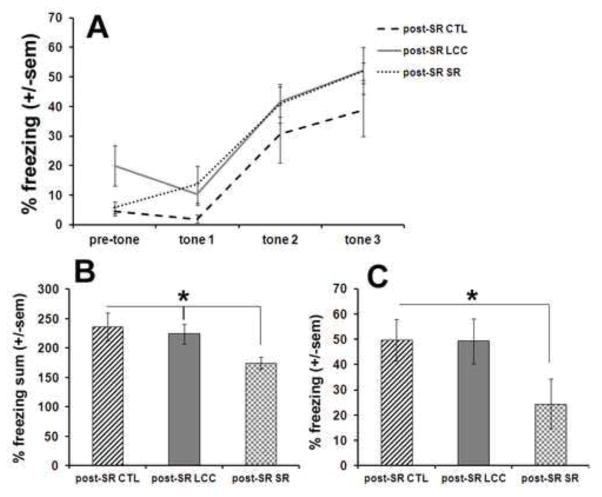
Mice were only tested in the fear conditioning paradigm once, at the end of the 6 week sleep restriction period. During the training session mice were exposed to tone-shock pairings and demonstrated an increase in percent time spent freezing between tone 1 and tone 2 and between tone 2 and tone 3 (Fig 3A). No significant differences were observed between the three groups during the training session. After 24 hours, mice were first tested in a contextual paradigm by placing mice in the apparatus for 5 minutes without any tone or shock. Mice that underwent sleep restriction (post-SR SR) displayed a significantly (p<0.04) lower percent time freezing over the 5 minute period compared to both LCC and CTL (post-SR CTL and post-SR LCC) (Fig 3B). After 3 hours, mice were tested in a cued paradigm in which they were placed in a novel context and exposed to a tone. After exposure to the tone, mice that underwent sleep restriction (post-SR SR) displayed a significantly (p=0.03) lower percentage of time freezing compared to CTL (Fig 3C).
Figure 3.
Fear conditioning testing results for all mice after the 6 week sleep restriction period for control (post-SR CTL), large cage control (post-SR LCC) and sleep restricted mice (post-SR SR). On day 1, mice were exposed to tone-shock pairings (A). On day 2, mice were first tested in a contextual paradigm (B) followed by a cued paradigm (C).
Anxiety
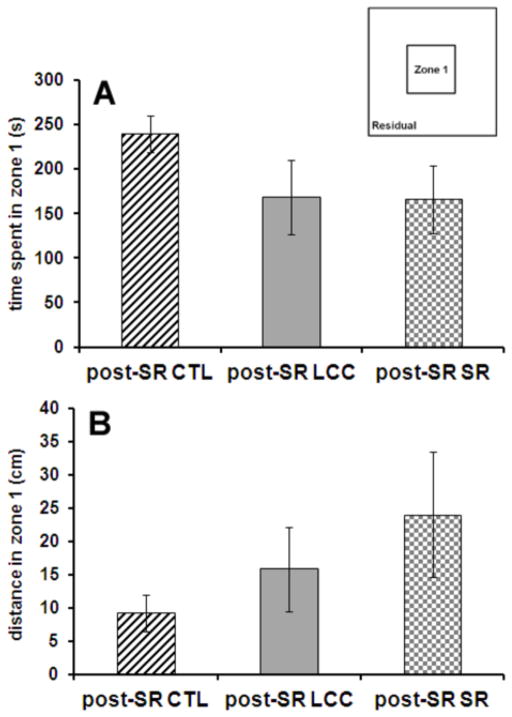
Open field testing was completed at the end of the 6 week study period as a measure of anxiety and movement. Mice were placed in an open field and the time spent in the center zone and periphery was recorded as well as the distance traveled in these two zones (Fig. 4). No significant differences between groups were observed in either time spent in zone 1 or distance traveled in zone 1.
Figure 4.
After the 6 week sleep restriction period, no differences in the time spent in zone 1 (A) or the distance traveled in zone 1 (B) are observed between groups, implying that there are no differences in anxiety between the three groups (CTL, LCC, SR).
Aβ and pTau accumulation
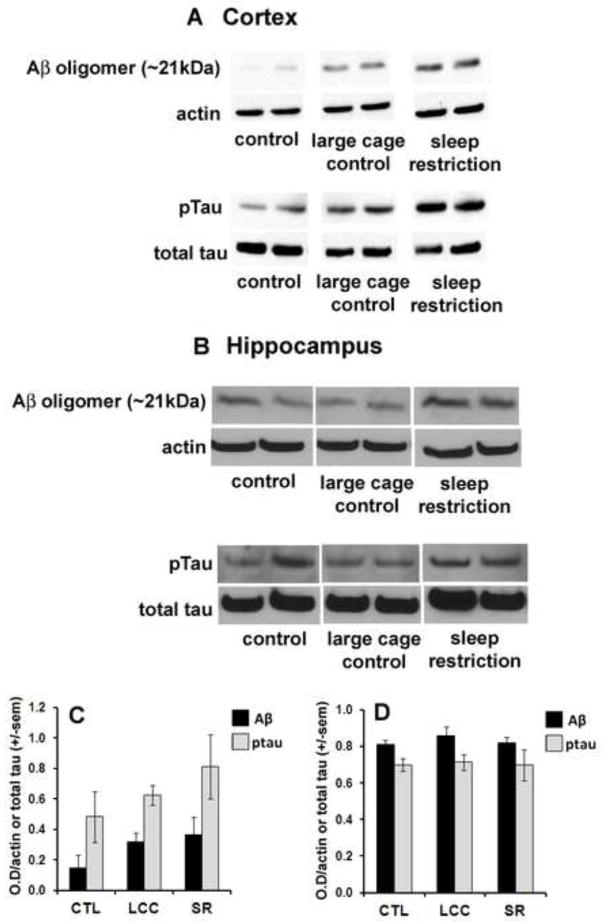
Cortical expression of Aβ increased slightly in LCC mice compared to CTL. However, a non-significant rise of ~80% in the amount of cortical Aβ oligomers was observed in SR compared to CTL (p=.22) (Fig 5A and 5C). Levels of phosphorylated Tau (pTau) in the cortex increased almost two-fold in SR compared to CTL and by a smaller amount in LCC compared to CTL (Fig 5A and 5C). Changes in both Aβ and pTau in the cortex in SR mice compared to CTL and LCC were non-significant (p>0.22). Levels of Aβ oligomers and pTau were not significantly changed in the hippocampus in either LCC or SR compared to CTL (p>0.44) or in SR compared to LCC (p>0.53) (Fig. 5B and D).
Figure 5.
Western blot results from the cortex (A) and hippocampus (B) showing increases in expression of Aβ and ptau in large cage controls compared to control with a further increase in sleep restricted mice. Quantification of western blot results indicate that cortical Aβ expression increases in LCC and SR compared to CTL whereas ptau expression increases in SR (C). Hippocampal expression levels are largely unchanged between groups (D).
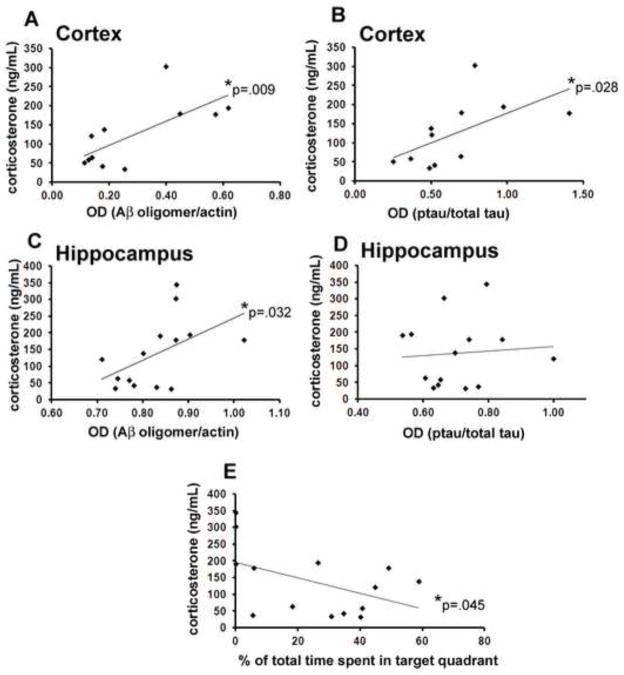
The LCC condition, which might be considered a stressful environment, resulted in significant changes or trends worsening of behavioral symptoms and Aβ and pTau pathologies in the cerebral cortex. We therefore asked whether plasma corticosterone levels, an indicator of stress, were correlated with cognitive performance and/or Aβ and pTau levels. A significant (p=0.045) negative correlation was observed between circulating corticosterone and the percent time spent in the target quadrant during the 24 hour probe test (Fig 6E). Levels of both Aβ and pTau in the cerebral cortex were positively, significantly (p<0.028; R2=0.49 Aβ; R2=0.35 pTau) correlated with circulating corticosterone levels measured 1 week after the start of the sleep restriction period. In the hippocampus, Aβ, but not pTau, was significantly correlated with circulating glucocorticoids (GC) (p=0.032; R2=0.2577). At 4 weeks after the start of the sleep restriction period, no significant correlations were observed between circulating corticosterone and Aβ or pTau in either the hippocampus (p>.34) or cortex (p>.08).
Figure 6.
Correlations between circulating glucocorticoids and hippocampal and cortical expression of Aβ and ptau. Cortical expression of both Aβ and ptau are positively, significantly (p<0.028) correlated with circulating glucocorticoids measured 1 week after the start of the sleep restriction period (A–B). In the hippocampus, Aβ, but not ptau, is significantly correlated with circulating glucocorticoids (p=0.032; C–D). Circulating corticosterone was negatively and significantly (p=0.045) correlated with the percent time spent in the target quadrant during the 24 hour probe test (E).
Discussion
Despite the prevalence of sleep disturbances in AD and the known effects of loss of sleep on cognition, no study to date has elucidated both behavioral and biochemical alterations in AD following chronic mild sleep restriction. Our data indicate a worsening of memory loss, and an accentuation of the accumulation of pTau and Aβ in the cerebral cortex in a mouse model of AD after 6 weeks of mild sleep restriction. In addition, we found significant positive correlations between plasma corticosterone levels and pTau and Aβ pathologies, suggesting a role for hyperactivation of the brain – hypothalamus – pituitary – adrenal neuroendocrine stress response in the acceleration of the aberrant molecular cascades underlying AD.
Elevated levels of circulating corticosterone were present 1 week after the start of the sleep restriction period and persisted up to 4 weeks. Studies of chronic stressors often utilize 21 day paradigms due to the ability of mice to acclimate to a stressor. Data presented here show a continued elevation of circulating corticosterone levels at 4 weeks after the start of the sleep restriction period, potentially indicating an inability of 3xTgAD mice to acclimate to the mild stressor. Interestingly, in addition to the SR mice, corticosterone levels were elevated in mice in the LCC group implying that daily transfer from one cage to another and back is sufficient to cause a sustained level of stress in 3xTgAD mice. In studies utilizing a MMP method of sleep restriction, this type of control as well as a large platform control, are generally utilized and result in only modest increases in circulating corticosterone and sleep loss (Suchecki and Tufik, 2000; Machado et al., 2004). However, the latter studies utilized wild-type mice that display normal HPA axis function. In contrast 3xTgAD mice have been shown to display an elevation of basal circulating corticosterone levels, and sensitivity to chronic mild stressors that is not present in wild-type controls (Rothman et al., 2012). It is therefore likely that, in addition to an inability to acclimate to social stress, 3xTgAD mice also display an inability to adapt to daily transfer to and from novel cages.
The current study employed two measures of cognition, MWM and fear conditioning, the latter of which suggested a worsening of memory in SR mice compared to mice in the CTL and LCC groups. Coupled with anxiety testing results which did not indicate any alterations in anxiety or exploration between groups, fear conditioning data indicate that mild sleep restriction results in a worsening of both contextual and cued memory in SR 3xTgAD mice. Several possible explanations exist for the lack of a detectable effect of SR on performance in the MWM. First, although all experimental groups were unsuccessful during the probe tests, latency to find the platform did decrease over time for the post-SR CTL group from days 1–5 potentially indicating an ability of this group to learn. Second, the MWM involves exposure to water which mice often find stressful as demonstrated by studies that show a rise in circulating corticosterone after training in the MWM (Huang et al., 2012). Because the SR protocol utilized the MMP method, SR mice were exposed to water daily over the course of 6 weeks potentially introducing adaptation or sensitization to water exposure (Machado et al., 2004). It is possible that water exposure of the SR group introduced a confounding effect in the MWM test. Additionally, it is noteworthy that although there were no differences between groups in the MWM tests after the SR period, CTL mice displayed successful reference memory prior to the SR period, as indicated by a significantly greater amount of time spent in the target quadrant during the probe test, compared to an unsuccessful probe test after the 6 week period. This could indicate that 3xTgAD mice experienced a worsening of disease symptoms due to aging during the study period. Whereas sleep restriction did not worsen spatial memory in this mouse model at the age studied in the current study, it might affect the performance of younger or older 3xTgAD mice. Finally, circulating corticosterone levels displayed a negative correlative relationship with at least one behavioral outcomes from the Morris water maze, time spent in the target quadrant during the probe test (Fig 6E), suggesting that GC levels may be directly related to cognitive performance.
Our biochemical analyses show that SR exacerbates the accumulation of Aβ and pTau in the cerebral cortex, but not the hippocampus. This is consistent with the only other study to quantify Aβ deposition after sleep restriction, which demonstrated an increase in accumulation of Aβ in the cortex after 21 days of SR in a mouse model of AD (Kang et al., 2009). In the 3xTgAD model of AD, Aβ and pTau pathologies appear first in the cortex followed by the hippocampus (Oddo et al., 2003). It is therefore likely that a longer course of SR would also worsen hippocampal pathology in this model. Further, correlations indicate a significant relationship between circulating corticosterone and Aβ and pTau levels in the cortex, but only Aβ in the hippocampus. Adrenalectomy in adult rats altered spatial distribution of APP expression and treatment with dexamethasone caused an increase in APP expression; these studies imply that GCs participate in the regulation of APP levels (Islam et al., 1998; Budas et al., 1999). Further, recent work using middle-aged rats demonstrated that both stress and GC can drive APP metabolism toward amyloidogenesis as demonstrated by an increase in APP mRNA and the levels of beta-secretase 1 (BACE) as well as an increase in the C99 fragment produced when BACE cleaves APP (Catania et al., 2009). Additional literature indicates the presence of a GC response element (GRE) in the promoter region of both the APP and BACE genes (Lahiri, 2004; Sambamurti et al., 2004). Our data showing a positive correlation between Aβ and corticoserone levels are therefore consistent with a role for corticosterone in mediating the adverse effects of SR on cognitive deficits and AD-like neuropathological changes.
It should be noted that the current study does not differentiate the effects of sleep restriction from the effects of stress and/or an increase in circulating GCs. Slow wave sleep (SWS), particularly the normal hippocampal activity that occurs during SWS, is required for learning and episodic memory. It is possible that a decrease in brain metabolism due to a loss of SWS causes increases in Aβ and ptau after sleep deprivation. However, a recent study showed that both stress and GC can drive APP metabolism toward amyloidogenesis and that a history of hypersecretion of GC, as result of chronic stress, biases APP processing toward the amyloidogenic pathway (Catania et al., 2009). Those results, taken with the current study, strongly suggest that stress can play a key role in the accumulation of Aβ in the brain and, potentially, in the progression of AD.
The goal of the current work was to test the hypothesis that, as an adverse stressor, mild sleep restriction worsens the proteopathic processes (Aβ and pTau accumulation in the brain) and cognitive cognitive deficits in a mouse model of AD. While, our data do support this hypothesis, there are several important questions that remain to be answered. For example, do wild-type mice not destined to develop Aβ and pTau pathologies, nevertheless exhibit cognitive impairment similar to that of 3xTgAD mice when subjected to chronic SR? If and how does age/disease stage influence the magnitude of the impact of SR on cognitive performance and proteopathic changes in the brain? However, despite these the questions that remain, this study provides the first evidence that chronic mild sleep restriction may worsen the progression of AD and furthers knowledge of how environmental factors affect neurodegeneration and how modulation of GCs may mediate disease processes.
Materials and Methods
Mice
Animal care and experimental procedures followed National Institutes of Health guidelines and were approved by the National Institute on Aging Animal Care and Use Committee. Fourteen month-old male triple transgenic AD (3xTgAD) mice (n=29) harboring PS1M146V, APPSwe, and tauP301L transgenes (Oddo et al., 2003) that had been backcrossed to C57BL/6 mice for seven generations were group housed (4–5 per cage) and maintained under a 12 hr light and dark cycle.
Chronic mild sleep restriction
Male 3xTgAD Mice (n=10) were subjected to daily mild sleep restriction for 6 hours per day for 6 weeks using the MMP technique (Machado et al., 2004). Group-housed mice were placed in a cage containing three circular platforms 3 cm in diameter and 2.5 cm in height. Water filled the cage to 1 cm below the upper surface of the platforms enabling the mice to move around the cage by jumping from one platform to another. When they reached the paradoxical phase of sleep and the onset of muscle atonia, they fell into the water and were awoken (SR). A second set of mice (n=10), termed ‘large cage control’ (LCC), were moved into cages identical to the sleep restriction cages described above but with bedding instead of water and returned to their home cage at the end of the 6 hour test period. A third control group (n=9) remained in their cages for the duration of the study (CTL).
Plasma corticosterone measurement
Blood samples were drawn at the end of the 6 hour sleep restriction period at 1 week and 4 weeks after the beginning of the 6 week sleep restriction paradigm. Blood was gathered using a retro-orbital bleeding technique, using a heparinized micro-hematocrit capillary tube (Fisher Scientific; Pittsburgh, PA). Blood samples were then centrifuged at 12,500 rpm for 12 minutes at 4° and plasma supernatant was removed and stored at −80°. Plasma corticosterone concentrations were quantified using an RIA kit (MP Biomedicals; Solon, OH) according to manufacturer’s instructions.
Open field
At the end of the 6-week sleep deprivation period mice were tested for anxiety behavior using an open field testing paradigm (MEDOFA-MS system; Med Associates). Briefly, mice were placed in the center of an open field, and exploration was assessed for 15 min. Cages were routinely cleaned with ethanol following each session. The dimensions of the arena were 40 cm × 40 cm, of which the peripheral 10 cm were considered as the residual zone and the central 20 cm2 were considered as zone 1.
Morris water maze
Mice were tested using a MWM paradigm prior to the beginning of the sleep restriction period and again after the 6 week period according to previously described techniques (Vorhees and Williams, 2006). Briefly, animals received 8 days of acquisition training, consisting of four trials per day, with an intertrial interval of approximately 10 min. Each trial lasted until the animal found the platform, or for a maximum of 60 s; animals that failed to find the platform within 60 s were guided there by the experimenter. On each trial mice were placed into the pool, facing the wall, with start locations varied pseudorandomly. One day after the last acquisition training session, animals were tested in a single 60 s probe trial without the platform. Only mice that spent less than fifty percent of their time floating (defined as moving at a speed of less than 2.0 cm/s) or engaging in thigmotaxis (defined as remaining within 4.0 cm of the perimeter of the pool) were used for behavioral analysis. Data were acquired and analyzed using the Anymaze software system (Stoelting; Wood Dale, IL).
Fear conditioning
Mice were tested in a fear conditioning paradigm once at the end of the six-week sleep restriction period according to previously established techniques (Okun et al., 2010). Due to the stressful nature of the test, it was not performed prior to the beginning of the 6-week sleep restriction period. Briefly, during the training session, mice were placed in a contextual conditioning chamber (model MEDVFC-NIR-M; Med Associates) and allowed to explore the chamber for 2 min. At the end of 2 min, mice were subjected to three sessions of an audio tone (5 kHz, 70 dB, 30 s), followed immediately by a 0.5-mA, 2-s foot shock from the metal grid floor. Thirty seconds separated each session. Time spent freezing during pre-tone, tone 1, tone 2 and tone 3 periods were recorded. On the following day, in the contextual fear session, mice were returned to the conditioning chamber for 5 min without undergoing any tone or shock exposure. The percentage of time freezing during each minute was recorded and used as an index of contextual memory. Three hours after the contextual condition test, mice were returned to the chamber for a cued conditioning test. Plexiglass inserts were used to create a novel environment and mice were allowed to explore the chamber for 5 min. Following this acclimation period, five audio tones were sounded for 30 s each. The percentage of time freezing before and after the audio tones was recorded and used as an index of cued memory. Cages were routinely cleaned with ethanol between tests.
Tissue collection and immunoblot analysis
Within one week of behavior testing, all mice were deeply anesthetized and decapitated for tissue harvest. The brain was exposed and separated into right and left hemispheres. The left hippocampus and cortex were dissected, immediately frozen on dry ice and stored at −80° for western blot analysis of Aβ, phosphorylated tau (pTau) and total Tau. A subset of samples were analyzed by immunoblotting for Aβ and pTau to quantify AD pathology in the hippocampus. Hippocampi (n=4 CTL, n=5 LCC, n=5 SR) and cortex (n=4 CTL, n=4 LCC, n=4 SR) were homogenized in RIPA buffer with protease inhibitor on ice. Protein concentrations were determined using a Bradford assay. Immunoblots were performed using 50 μg of total protein extract separated on 6–12% SDS-PAGE gels and then transferred electrophoretically to polyvinylidene difluoride (PVDF) (for p-Tau and total Tau) or nitrocellulose (for Aβ and actin) membranes. Membranes were blocked with 5% milk in TBS-t, washed in TBS-t and incubated overnight at 4° in a primary antibody (1:1000). The primary antibodies were: Aβ, 6E10 (Santa Cruz Biotechnology; Santa Cruz, CA); PHF-Tau, AT180 (Pierce Endogen; Rockford, IL); total Tau, T46 (Invitrogen; Carlsbad, CA); or actin (Sigma-Aldrich; St. Louis, MO). Membranes were then incubated in a secondary antibody solution for 30 minutes (1:5000, horseradish peroxidase-conjugated anti-mouse IgG) for 30 minutes. The optical density of immunoreactive bands was detected and quantified by chemiluminescence using the ECL system (Amersham).
Statistical analysis
Blood plasma corticosterone results were analyzed using a one-way ANOVA with post-hoc Bonferroni to test for differences between groups (control, large cage control, sleep restriction) at the one week and four week time points separately. MWM data (latency to reach platform, swim speed) were analyzed using a one-way ANOVA to test for differences between groups on each training day. Additionally, a repeated measures ANOVA was used to test for an effect of time on these parameters. Quantification of western blot band intensity was normalized by either actin (for Aβ) or total Tau (for pTau) and these values were tested for correlation with blood plasma corticosterone concentrations using western blot results for the hippocampus and cortex separately. Circulating plasma corticosterone data was also tested for correlation with the percent time spent in the target quadrant at the 24 hour Morris water maze probe test. For all tests significance was defined as p<0.05.
Conclusions
The current work tested the hypothesis that mild sleep restriction worsens the pathological processes (Aβ and pTau accumulation in the brain) and cognitive deficits in a mouse model of AD. Results show that sleep restriction increases circulating corticosterone levels. Behavioral data demonstrate deficits in contextual and cued memory in SR mice that were not present for controls. Further, both Aβ and pTau levels increased in the cortex of sleep restricted AD mice compared to controls and a significant positive correlation was measured between cortical Aβ and pTau levels and circulating corticosterone, potentially indicating a role for GCs in mediating behavioral and biochemical changes observed after sleep restriction in a mouse model of AD. This study provides the first evidence that chronic mild sleep restriction may worsen the progression of AD and furthers knowledge of how environmental factors affect neurodegeneration and how modulation of GCs may mediate disease processes.
Highlights.
Sleep restriction causes an increase in circulating corticosterone in 3xTg mice.
Sleep restriction causes deficits in contextual and cued memory in 3xTg mice.
Aβ and pTau levels increased in the cortex of sleep restricted 3xTg mice.
Aβ and pTau and circulating corticosterone are significantly positively correlated.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute on Aging. The authors wish to thank Ernest Dabney for assistance with retro-orbital bleeding techniques and Catherine Crews for assistance with maintenance for the in vivo portion of the study.
Abbreviations
- AD
Alzheimer’s disease
- MWM
Morris water maze
- Aβ
amyloid beta
- pTau
phosphorylated tau
- GC
glucocorticoid
- MMP
modified multiple platform
- SR
sleep restriction
- LCC
large cage control
- CTL
control
Footnotes
Conflict of Interest
The authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Banks S, Dinges DF. Behavioral and physiological consequences of sleep restriction. J Clin Sleep Med. 2007;3:519–528. [PMC free article] [PubMed] [Google Scholar]
- Bliwise DL, Tinklenberg J, Yesavage JA, Davies H, Pursley AM, Petta DE, Widrow L, Guilleminault C, Zarcone VP, Dement WC. REM latency in Alzheimer’s disease. Biol Psychiat. 1989;25:320–328. doi: 10.1016/0006-3223(89)90179-0. [DOI] [PubMed] [Google Scholar]
- Brown SA, Schmitt K, Eckert A. Aging and circadian disruption: causes and effects. Aging. 2011;3:813–817. doi: 10.18632/aging.100366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budas G, Coughlan CM, Seckl JR, Breen KC. The effect of corticosteroids on amyloid beta precursor protein/amyloid precursor-like protein expression and processing in vivo. Neurosci Lett. 1999;276:61–64. doi: 10.1016/s0304-3940(99)00790-9. [DOI] [PubMed] [Google Scholar]
- Catania C, Sotiropoulos I, Silva R, Onofri C, Breen KC, Sousa N, Almeida OF. The amyloidogenic potential and behavioral correlates of stress. Mol Psychiat. 2009;14:95–105. doi: 10.1038/sj.mp.4002101. [DOI] [PubMed] [Google Scholar]
- Chee MW, Chuah LY. Functional neuroimaging insights into how sleep and sleep deprivation affect memory and cognition. Curr Opin Neurol. 2008;21:417–423. doi: 10.1097/WCO.0b013e3283052cf7. [DOI] [PubMed] [Google Scholar]
- Craig D, Hart DJ, Passmore AP. Genetically increased risk of sleep disruption in Alzheimer’s disease. Sleep. 2006;29:1003–1007. doi: 10.1093/sleep/29.8.1003. [DOI] [PubMed] [Google Scholar]
- Crowley K. Sleep and sleep disorders in older adults. Neuropsychol Rev. 2011;21:41–53. doi: 10.1007/s11065-010-9154-6. [DOI] [PubMed] [Google Scholar]
- Csernansky JG, Dong H, Fagan AM, Wang L, Xiong C, Holtzman DM, Morris JC. Plasma cortisol and progression of dementia in subjects with Alzheimer-type dementia. Am J Psychiat. 2006;163:2164–2169. doi: 10.1176/appi.ajp.163.12.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KL, Davis BM, Greenwald BS, Mohs RC, Mathe AA, Johns CA, Horvath TB. Cortisol and Alzheimer’s disease, I: Basal studies. Am J Psychiat. 1986;143:300–305. doi: 10.1176/ajp.143.3.300. [DOI] [PubMed] [Google Scholar]
- Deltheil T, Guiard BP, Cerdan J, David DJ, Tanaka KF, Repérant C, Guilloux JP, Coudoré F, Hen R, Gardier AM. Behavioral and serotonergic consequences of decreasing or increasing hippocampus brain-derived neurotrophic factor protein levels in mice. Neuropharmacol. 2008;55:1006–1014. doi: 10.1016/j.neuropharm.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Dinges DF, Pack F, Williams K, Gillen KA, Powell JW, Ott GE, Aptowicz C, Pack AI. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 hours per night. Sleep. 1997;20:267–277. [PubMed] [Google Scholar]
- Dong H, Goico B, Martin M, Csernansky CA, Bertchume A, Csernansky JG. Modulation of hippocampal cell proliferation, memory, and amyloid plaque deposition in APPsw (Tg2576) mutant mice by isolation stress. Neuroscience. 2004;127:601–609. doi: 10.1016/j.neuroscience.2004.05.040. [DOI] [PubMed] [Google Scholar]
- Dong H, Yuede CM, Yoo HS, Martin MV, Deal C, Mace AG, Csernansky JG. Corticosterone and related receptor expression are associated with increased β-amyloid plaques in isolated Tg2576 mice. Neuroscience. 2008;155:154–163. doi: 10.1016/j.neuroscience.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel N, Rao H, Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2009;29:320–339. doi: 10.1055/s-0029-1237117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves LA, Heller EA, Pack AI, Abel T. Sleep deprivation selectively impairs memory consolidation for contextual fear conditioning. Learn Mem. 2003;10:168–176. doi: 10.1101/lm.48803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KN, Billings LM, Roozendaal B, McGaugh JL, LaFerla FM. Glucocorticoids increase amyloid-β and tau pathology in a mouse model of alzheimer’s disease. J Neurosci. 2006;26:9047–9056. doi: 10.1523/JNEUROSCI.2797-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hairston IS, Little MT, Scanlon MD, Barakat MT, Palmer TD, Sapolsky RM, Heller HC. Sleep restriction suppresses neurogenesis induced by hippocampus-dependent learning. J Neurophysiol. 2005;94:4224–4233. doi: 10.1152/jn.00218.2005. [DOI] [PubMed] [Google Scholar]
- Huang Y, Zhou W, Zhang Y. Bright lighting conditions during testing increase thigmotaxis and impair water maze performance in BALB/c mice. Behav Brain Res. 2012;226:26–31. doi: 10.1016/j.bbr.2011.08.043. [DOI] [PubMed] [Google Scholar]
- Islam A, Kalaria RN, Winblad B, Adem A. Enhanced localization of amyloid beta precursor protein in the rat hippocampus following long-term adrenalectomy. Brain Res. 1998;806:108–112. doi: 10.1016/s0006-8993(98)00711-2. [DOI] [PubMed] [Google Scholar]
- Jeong YH, Park CH, Yoo J, Shin KJ, Ahn SM, Kim HS, Lee SH, Emson PC, Suh YH. Chronic stress accelerates learning and memory impairments and increases amyloid deposition in APPV7171-CT100 transgenic mice, an Alzheimer’s disease model. FASEB. 2006;20:729–731. doi: 10.1096/fj.05-4265fje. [DOI] [PubMed] [Google Scholar]
- Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, Fujiki N, Nishino S, Holtzman DM. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326:1005–1007. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri DK. Functional characterization of amyloid beta precursor protein regulatory elements: rationale for the identification of genetic polymorphism. Ann NY Acad Sci. 2004;1030:282–288. doi: 10.1196/annals.1329.035. [DOI] [PubMed] [Google Scholar]
- Machado RB, Hipólide DC, Benedito-Silva AA, Tufik S. Sleep deprivation induced by the modified multiple platform technique: quantification of sleep loss and recovery. Brain Res. 2004;1004:45–51. doi: 10.1016/j.brainres.2004.01.019. [DOI] [PubMed] [Google Scholar]
- McCoy JG, Strecker RE. The cognitive cost of sleep lost. Neurobiol Learn Mem. 2011;96:564–582. doi: 10.1016/j.nlm.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurry SM, Logsdon RG, Teri L, Gibbons LE, Kukull WA, Bowen JD, McCormick WC, Larson EB. Characteristics of sleep disturbance in community-dwelling Alzheimer’s disease patients. J Geriatr Psychiat Neurol. 1999;12:53–59. doi: 10.1177/089198879901200203. [DOI] [PubMed] [Google Scholar]
- Mejia S, Giraldo M, Pineda D, Ardila A, Lopera F. Nongenetic factors as modifiers of the age of onset of familial alzheimer’s disease. Int Psychogeriatr. 2003;15:337–349. doi: 10.1017/s1041610203009591. [DOI] [PubMed] [Google Scholar]
- Mirescu C, Peters JD, Noiman L, Gould E. Sleep deprivation inhibits adult neurogenesis in the hippocampus by elevating glucocorticoids. PNAS. 2006;103:19170–19175. doi: 10.1073/pnas.0608644103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Okun E, Griffioen KJ, Barak B, Roberts NJ, Castro K, Pita MA, Cheng A, Mughal MR, Wan R, Ashery U, Mattson MP. Toll-like receptor 3 inhibits memory retention and constrains adult hippocampal neurogenesis. PNAS. 2010;107:15625–15630. doi: 10.1073/pnas.1005807107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palchykova S, Winsky-Sommerer R, Meerlo P, Dürr R, Tobler I. Sleep deprivation impairs object recognition in mice. Neurobiol Learn Mem. 2006;85:263–271. doi: 10.1016/j.nlm.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Palma BD, Suchecki D, Catallani B, Tufik S. Effect of sleep deprivation on the corticosterone secretion in an experimental model of autoimmune disease. Neuroimmunomodulation. 2007;14:72–77. doi: 10.1159/000107421. [DOI] [PubMed] [Google Scholar]
- Pawlyk AC, Ferber M, Shah A, Pack AI, Naidoo N. Proteomic analysis of the effects and interactions of sleep deprivation and aging in mouse cerebral cortex. J Neurochem. 2007;103:2301–2313. doi: 10.1111/j.1471-4159.2007.04949.x. [DOI] [PubMed] [Google Scholar]
- Rasmuson S, Andrew R, Näsman B, Seckl JR, Walker BR, Olsson T. Increased glucocorticoid production and altered cortisol metabolism in women with mild to moderate Alzheimer’s disease. Biol Psychiat. 2001;49:547–552. doi: 10.1016/s0006-3223(00)01015-5. [DOI] [PubMed] [Google Scholar]
- Rothman SM, Herdener N, Camandola S, Texel SJ, Mughal MR, Cong WN, Martin B, Mattson MP. 3xTgAD mice exhibit altered behavior and elevated Aβ after chronic mild social stress. Neurobiol Aging. 2012;33:830, e1–12. doi: 10.1016/j.neurobiolaging.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambamurti K, Kinsey R, Maloney B, Ge YW, Lahiri DK. Gene structure and organization of the human beta-secretase (BACE) promoter. FASEB. 2004;18:1034–1036. doi: 10.1096/fj.03-1378fje. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Lee K, Becker KG, Zhang Y, Maudsley S, Martin B, Cutler RG, Mattson MP. Hippocampal gene expression patterns underlying the enhancement of memory by running in aged mice. Neurobiol Aging. 2010;31:1937–1949. doi: 10.1016/j.neurobiolaging.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchecki D, Tufik S. Social stability attenuates the stress in the modified multiple platform method for PS deprivation in the rat. Physiol Behav. 2000;68:309–316. doi: 10.1016/s0031-9384(99)00181-x. [DOI] [PubMed] [Google Scholar]
- Turner TH, Drummond SP, Salamat JS, Brown GG. Effects of 42 hr of total sleep deprivation on component processes of verbal working memory. Neuropsychology. 2007;21:787–795. doi: 10.1037/0894-4105.21.6.787. [DOI] [PubMed] [Google Scholar]
- Umegaki H, Ikari H, Nakahata H, Endo H, Suzuki Y, Ogawa O, Nakamura A, Yamamoto T, Iguchi A. Plasma cortisol levels in elderly female subjects with Alzheimer’s disease: a cross-sectional and longitudinal study. Brain Res. 2000;881:241–243. doi: 10.1016/s0006-8993(00)02847-x. [DOI] [PubMed] [Google Scholar]
- Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–126. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Someren EJ, Hagebeuk EE, Lijzenga C, Scheltens P, de Rooij SE, Jonker C, Pot AM, Mirmiran M, Swaab DF. Circadian rest-activity rhythm disturbances in Alzheimer’s disease. Biol Psychiat. 1996;40:259–270. doi: 10.1016/0006-3223(95)00370-3. [DOI] [PubMed] [Google Scholar]
- Vitiello MV, Prinz PN, Williams DE, Frommlet MS, Ries RK. Sleep disturbances in patients with mild-stage Alzheimer’s disease. J Gerontol. 1990;45:M131–8. doi: 10.1093/geronj/45.4.m131. [DOI] [PubMed] [Google Scholar]
- Volicer L, Harper DG, Manning BC, Goldstein R, Satlin A. Sundowning and circadian rhythms in Alzheimer’s disease. Am J Psychiat. 2001;158:704–711. doi: 10.1176/appi.ajp.158.5.704. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]