Abstract
Cortical dysplasias (CDs) are highly epileptogenic lesions with a good prognosis of seizure freedom, if totally resected. However, their accurate delineation and resection can be difficult, and depend on the extent of pathology and lesion location. Intraoperative neurophysiologic assessments are valuable in these situations. We present an illustrative case of intractable epilepsy where judicious use of intraoperative neurophysiologic–techniques guided resection of precentral CD, under general anesthesia and in the absence of preoperative electrophysiologic mapping data. Ictal onset was accurately delineated using electrocorticography (ECoG). Phase reversal of the median somatosensory-evoked potentials (MSSEPs) localized the central sulcus (CS). Motor evoked potentials (MEPs) triggered by high-frequency monopolar anodal electrical cortical stimulation at the primary motor cortex (PMC) threshold delineated the PMC. Using this technique, PMC and the corticospinal tract (CST) were continuously monitored during resection. No changes in MEPs from the pre-resection baseline were seen; no residual abnormal activity was present in the postresection ECoG. The patient emerged from surgery without deficits and has been seizure free during a 10-month follow-up. Staged multimodal intraoperative neurophysiology can be used successfully under general anesthesia to guide resection of epileptogenic lesions within the precentral gyrus, as an add-on or, in certain situations, as a viable alternative to preoperative electrophysiologic mapping.
Keywords: Electrocorticography, Motor mapping, Cortical dysplasia, Central sulcus
Cortical dysplasia (CDs) are a common cause of intractable epilepsy but carry a good prognosis of seizure freedom following complete resection (Edwards et al., 2000; Wagner et al., 2011). However, total resection may be difficult to achieve because CDs may escape detection on neuro-imaging studies and can cause widespread distortion of local anatomy and neuronal networks (Guerrini & Barba, 2010). Surgical success in these situations relies upon accurate electrographic demarcation of the seizure foci and eloquent cortex (Palmini et al., 1995; Guerrini & Barba, 2010; de Oliveira et al., 2011). Unfortunately, this can be challenging to achieve preoperatively; ictal scalp electroencephalography (EEG) can be nonlocalizing, and invasive recordings may offer partial information or may not be performed. In these situations, intraoperative neurophysiologic techniques can be employed successfully to circumvent these shortcomings.
We present an example, where a staged multimodal intra-operative neurophysiologic method was used successfully under general anesthesia to guide resection of a precentral CD.
Methods
Our patient was a 40-year-old woman with medically intractable epilepsy who underwent surgical treatment. Her seizures were highly stereotyped nocturnal events with a frontal semiology.
Preoperative workup
Three-dimensional (3D) magnetic resonance imaging (MRI) of the brain showed signal changes within the right precentral regions, suggestive of CD. Functional MRI (fMRI) and diffusion tensor imaging (DTI) studies were performed to localize the right primary motor cortex (PMC) and corticospinal tract (CST). Coregistration techniques demonstrated the lesion to be in proximity to these structures (Fig. 1A,B). Long-term surface video-EEG showed frequent interictal spikes at F4 and C4, but failed to localize the seizure foci. Because of logistical and safety concerns, invasive EEG recordings were not performed. Upon reviewing the case, the multidisciplinary epilepsy surgery group agreed on proceeding with surgical resection of the right frontal lesion.
Figure 1.
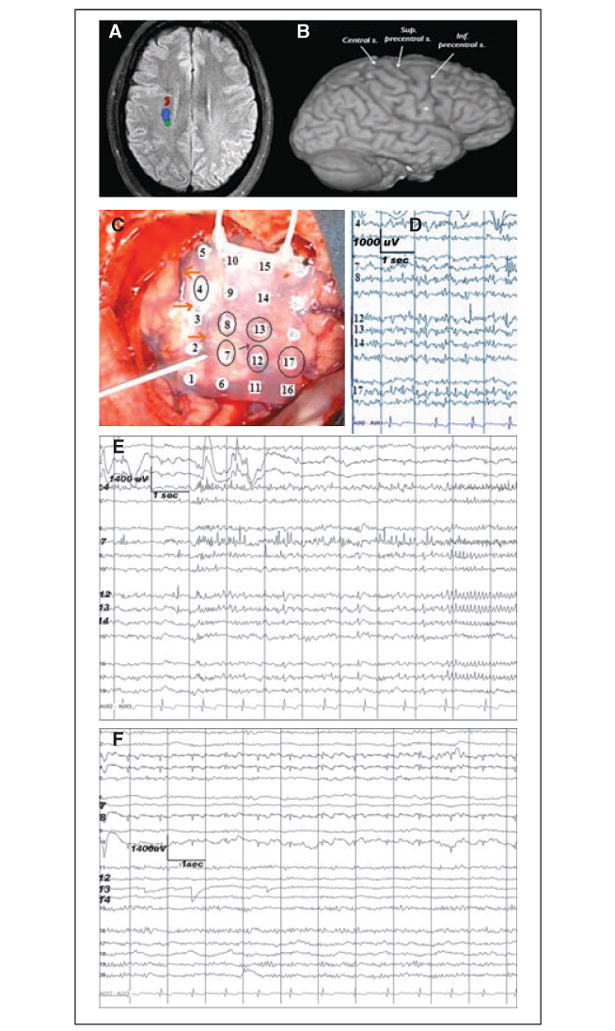
Neuroimaging and ECoG studies showing the topographic relationship among the epileptogenic dysplastic lesion, corticospinal tract, and the eloquent motor cortex. (A) fMRI showing the location of the subcortical motor (dark red and blue) and somatosensory fibers. Descending fibers of the corticospinal tract (dark red) are in proximity to the dysplastic lesion (arrow). (B) At the cortical level, the center of the lesion (dot) is located close to the precentral sulcus. (C) Preresection ECoG recording done with a 20-contact grid placed over the right frontal (contact 16 most anterior-inferior, contact 5 most posterior-superior). Circled are the contacts showing epilepti-form/epileptic activity. The reference is contralateral mastoid. The surgeon is pointing toward the central sulcus (also empha-sized by the orange arrows). The thin black arrow points towards the precentral sulcus. The recording was done using a 32-channel digital EEG machine (XLTEK/NATUS, Oakville, ON, Canada); low frequency filter = 1 Hz, high frequency filter = 70 Hz. (D) Preresection ECoG. Multifocal spikes are seen on both sides of the precentral sulcus: anterior (contacts 12, 13, and 17) and posterior (contacts 7, 8, and 4) to it. (E) Preresection ECoG. Focal ictal onset at contacts 7 and 4, within the precentral gyrus. ( F ) Postresection ECoG shows no epileptiform or epileptic activity. The recording is done via a 20-contact grid with the reference electrode on the contralateral mastoid.
Epilepsia © ILAE
Multimodal staged intraoperative neurophysiologic technique
Lesionectomy was performed under general anesthesia and neurophysiologic guidance.
The epileptogenic areas were first identified via electrocorticography (ECoG), using a 20-contact subdural grid. In preparation for functional sensorimotor mapping and monitoring, anesthesia with inhalational agents (i.e., 0.7% sevoflurane in 67% nitrous oxide with oxygen) was transitioned to total intravenous anesthesia (TIVA, i.e., 70 μg/kg/min propofol and 0.05 μg/kg/min fentanyl infusions). No neuro-muscular blocking agents were used after the induction.
Central sulcus (CS) was identified using median somatosensory evoked response (MSSEPs) phase-reversal technique. The left median nerve was electrically stimulated peripherally and somatosensory evoked potentials (SSEPs) were recorded via an eight-contact strip placed directly on the cortex. Next, using a high frequency monopolar anodal technique, the surgeon performed direct cortical electrical stimulation of the regions anterior to the identified CS. The mapping algorithm required gradual increase in the stimulus intensity in 0.5-mA increments, starting at 1 mA, up to maximum 25 mA or until positive responses or afterdischarges (ADs) were triggered. The area that triggered reliable motor evoked responses (MEPs) in the contralateral muscles when stimulated at the lowest current intensity (i.e., PMC threshold) was identified as PMC. Continuous motor monitoring during resection was employed by stimulating the identified PMC at the PMC threshold, via a contact of a second subdural strip. In the setting of stable hemodynamic and anesthetic conditions, alarm criteria were considered sudden changes from preresection baseline of neurophysiologic MEP parameters: >50% decrease of the amplitudes and/or >15% increase of latencies and/or changes in waveforms morphology (from polyphasic to biphasic/monophasic). During motor mapping and monitoring, recording for ADs was done via the strip initially used for CS localization, repositioned close to the stimulated regions. A postresection ECoG recording was repeated over areas adjacent to the resection cavity, after reversal of anesthesia from TIVA to inhalational agents, to look for residual epileptiform activity; technical details of ECoG and functional mapping/monitoring can be found in Figs. 1 and 2 respectively.
Figure 2.
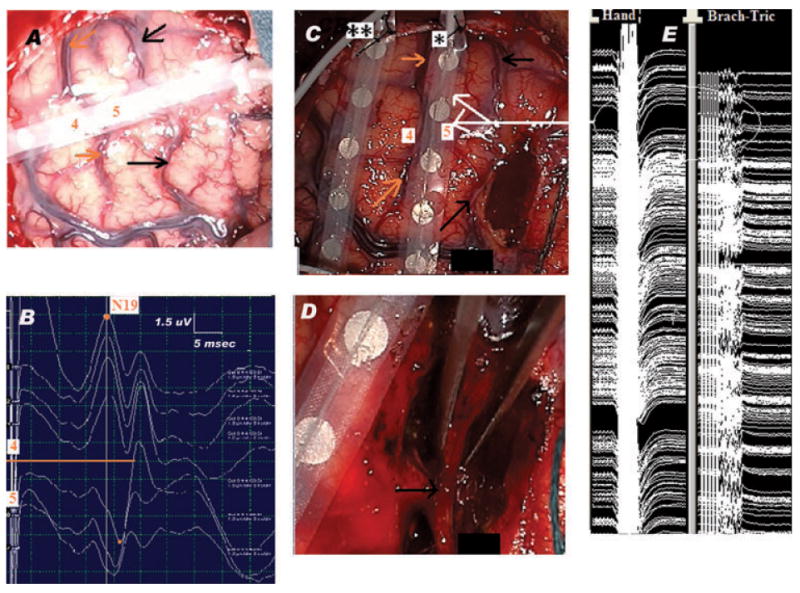
Pericentral region: Neurophysiologic-neuroanatomic correlation. Lesionectomy under continuous MEP monitoring. (A) Surgical field showing the SSEP recording strip placed across the precentral (black arrows) and central (orange arrows) sulci, with contact 5 over the hand region within the motor strip and contact 4 in the postcentral region, over the somatosensory cortex. (B) MSSEP phase reversal technique: the MSSEPs are recorded via the eight-contact subdural strip placed over the right frontoparietal cortex, perpendicular and across the presumed location of the CS, as shown in panel A. The reference electrode is located on the contralateral mastoid. The left median nerve is stimulated at the wrist with repetitive electrical pulses, at 3.17 Hz, pulse width 0.3 ms, intensity 15 mA. The orange line centers the SSEP phase reversal, which occurs at the level of CS. Note N19 recorded as a negative peak at contact 4 of the strip, in a referential montage. The recording is done using a 16-channel EP machine (XLTEK manufacturer), LFF = 30 Hz, HFF = 500 Hz. (C) Resection of the anterior portion of the lesion, situated anterior to the precentral sulcus (black arrows). Notice the placement of the two strips, one used for cortical stimulation (*), the other used for recording ADs (**). The orange arrows point toward the CS; labels 4 and 5 show the previous position of contacts 4 and 5, respectively, of the strip used for CS localization. The white arrows show the location of areas of the motor strip, which stimulated at PMC threshold (i.e., 10 mA) triggered MEPs in hand and arm regions. The stimulation was done via a handheld monopolar stimulator, connected to the anode. The cathode was a sterile needle placed at the periphery of the surgical field. Repetitive trains of six monophasic electrical pulses (250 Hz frequency of pulses within each train, each pulse of 0.5 ms duration), were delivered at 2 Hz. The recording was done using subdermal needle electrodes placed in the contralateral hemibody muscles: orbicularis oris, oculi, masseter, trapezius, deltoid, brachioradialis, triceps, abductor policis brevis (APB), abductor digiti mimini (ADM), quadriceps, anterior tibialis (AT), and abductor hallucis (AH) muscles. Two view windows allowed observation of the muscle channels and identification of MEPs locked to the stimulation as well as of self-sustained and/or spontaneous muscle activity (free EMG). This set up was important in order to easily differentiate stimulus triggered MEPs from motor seizure activity and/or muscle artifact. The PMC mapping threshold was 10 mA. The recording is done using a 16-channel EP machine (XLTEK manufacturer). LFF = 100 Hz, HFF = 2 KHz. (D) End of the resection: lesionectomy was done anterior as well as posterior to the preserved precentral sulcus (black arrow). (E) Continuous hand and arm MEPs (circled) monitoring during resection. Within the precentral gyrus, the location of the stimulated regions is indicated by the white arrows in panel C. The same recording set up was used for both motor mapping and monitoring. However, during the latter, the anodal stimulation was done via one of the contacts of a second subdural strip (*) which was placed on top of the PMC. This change in anodal stimulation resulted in a new PMC threshold (12 mA), used for the MEPs monitoring (using otherwise the same stimulus parameters).
Epilepsia © ILAE
Results
Multifocal spikes with a widespread distribution on both sides of the precentral sulcus (Fig. 1D) as well as two spontaneous electrographic seizures with a very focal onset in the precentral gyrus (Fig. 1E) were seen. Phase reversal of the cortical MSSEPs located the CS between contacts 4 (postcentral) and 5 (precentral) of the strip (Fig. 2B). Con-tralateral muscles MEPs triggered at PMC threshold enabled identification of the corresponding areas of the eloquent cortex and showed no changes from baseline during their monitoring throughout the resection (Fig. 2E). No ADs were triggered by stimulation. No residual postresection epileptiform activity was recorded (Fig. 1F). The pathology showed disorganization of the cortical layers with binucleated and dysplastic neurons. Postoperatively, the patient was neurologically intact and has remained seizure free during 10 months of follow-up.
Discussion
Mapping of the seizure foci
Invasive EEG recordings are frequently obtained in pre-surgical evaluations when surface EEG monitoring is uninformative. When invasive recordings are not obtained or provide incomplete information, intraoperative ECoG can be helpful for delineating highly epileptogenic lesions, such as CDs. These lesions often exhibit a distinct electrographic pattern that is easy to recognize (Palmini et al., 1995; Boonyapisit et al., 2003; Ferrier et al., 2006). The presence or absence of such pathognomonic electrographic features in the resected areas accurately predicted the postoperative seizure outcome (Palmini et al., 1995).
ECoG recordings are preferentially performed in awake or lightly sedated patients. However, general anesthesia offers superior airway control and is often desirable in surgeries where hypercapnia-induced brain swelling and seizures have higher risk of occurrence. In addition, lack of spontaneous movements and decreased muscle artifact in anesthetized patients increase the precision of electrophysio-logic motor mapping. Conversely, use of anesthetics such as propofol can significantly alter EEG activities. Therefore, stopping or drastically reducing its infusion is necessary for reliable ECoG recordings. To avoid intraoperative awareness and self arousals, inhalational agents can be used as a substitute in the anesthetic regimen. It is our experience that these agents provide a stable physiologic and anesthetic milieu that enables continuous ECoG background and reliable recordings. However, they can depress cortical SSEPs and MEPs triggered by cortical stimulation, to a greater extent than equipotent doses of intravenous anesthetic agents can (Sloan & Heyer, 2002).Therefore, performance of combined seizure foci and functional motor mapping remains challenging in anesthetized patients. Nevertheless, our technique shows that a timely transition from one anesthetic regimen to another allows staged and reliable use of all the necessary tests.
Motor mapping and monitoring
Low-frequency bipolar stimulation (Berger et al., 1989) has been used successfully to map motor cortex, by observing triggered movements in awake and anesthetized epilepsy patients. But these responses are difficult to quantify, and cortical electrical stimulation for at least a couple of seconds increases the risk of triggered ADs and seizures, especially in the presence of an active ECoG.
We instead used a monopolar high-frequency anodal stimulation (Taniguchi et al., 1993; Kombos et al., 1999), which enables reliable recording of MEPs that are triggered at lower thresholds, thereby allowing faster and safer mapping (Hern et al., 1962; Kombos et al., 1999). Although both methods offer comparable PMC mapping results (Kombos et al., 1999), the latter is particularly helpful under general anesthesia as it produces efficient depolarization of the motor neurons (Taniguchi et al., 1993). This is due to rapid summation of the postsynaptic potentials, following repetitive, high-frequency stimulation of the axons in regions adjacent to their cell bodies (Hern et al., 1962; Taniguchi et al., 1993). This method, therefore, offers better mapping results in areas with a high density of pyramidal cells, that is, PMC (Kombos et al., 1999) and permits continuous monitoring of the CST during resection, in the absence of disrupting movements and without surgeon’s involvement. Of note, to avoid current spread and stimulation of the CST distal to the resection site, the continuous monitoring was done at the lowest current intensity, which triggered reliable MEPs (i.e., at the new PMC threshold).
Seizure activity triggered by electrical cortical stimulation poses an increased risk to patient’s safety and can result in false localization of the motor cortex. Our method offers two ways to detect such activity: ECoG for ADs, and free run electromyography (EMG) for motor seizures. In addition, to trigger a motor response, the high-frequency anodal method requires only a brief duration (milliseconds) of direct cortical stimulation, thus further reducing the risk of triggered seizures (Szelényi et al., 2007).
Footnotes
Disclosure
None of the authors have conflicts of interest to report. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- Berger MS, Kincaid J, Ojemann GA, Lettich E. Brain mapping techniques to maximize resection, safety, and seizure control in children with brain tumors. Neurosurgery. 1989;25:786–792. doi: 10.1097/00006123-198911000-00015. [DOI] [PubMed] [Google Scholar]
- Boonyapisit K, Najm I, Klem G, Ying Z, Burrier C, LaPresto E, Nair D, Bingaman W, Prayson R, Lüders H. Epileptogenicity of focal malformations due to abnormal cortical development: direct electro-corticographic-histopathologic correlations. Epilepsia. 2003;44:69–76. doi: 10.1046/j.1528-1157.2003.08102.x. [DOI] [PubMed] [Google Scholar]
- de Oliveira RS, Santos MV, Terra VC, Sakamoto AC, Machado HR. Tailored resections for intractable rolandic cortex epilepsy in children: a single-center experience with 48 consecutive cases. Childs Nerv Syst. 2011;27:779–785. doi: 10.1007/s00381-010-1355-z. [DOI] [PubMed] [Google Scholar]
- Edwards JC, Wyllie E, Ruggeri PM, Bingaman W, Lüders H, Kotagal P, Dinner DS, Morris HH, Prayson RA, Comair YG. Seizure outcome after surgery for epilepsy due to malformation of cortical development. Neurology. 2000;55:1110–1114. doi: 10.1212/wnl.55.8.1110. [DOI] [PubMed] [Google Scholar]
- Ferrier CH, Aronica E, Leijten FS, Spliet WG, van Huffelen AC, van Rijen PC, Binnie CD. Electrocorticographic discharge patterns in glio-neuronal tumors and focal cortical dysplasia. Epilepsia. 2006;47:1477–1486. doi: 10.1111/j.1528-1167.2006.00619.x. [DOI] [PubMed] [Google Scholar]
- Guerrini R, Barba C. Malformations of cortical development and aberrant cortical networks: epileptogenesis and functional organization. J Clin Neurophysiol. 2010;27:372–379. doi: 10.1097/WNP.0b013e3181fe0585. [DOI] [PubMed] [Google Scholar]
- Hern JE, Landgren S, Phillips CG, Porter R. Selective excitation of corticofugal neurones by surface-anodal stimulation of the baboon’s motor cortex. J Physiol. 1962;161:73–90. doi: 10.1113/jphysiol.1962.sp006874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kombos T, Suess O, Kern BC, Funk T, Hoell T, Kopetsch O, Brock M. Comparison between monopolar and bipolar electrical stimulation of the motor cortex. Acta Neurochir. 1999;141:1295–1301. doi: 10.1007/s007010050433. [DOI] [PubMed] [Google Scholar]
- Palmini A, Gambardella A, Andermann F, Dubeau F, da Costa JC, Tampieri D, Gloor P, Quesney F, Andermann E. Intrinsic epileptogenicity of human dysplastic cortex as suggested by corticography and surgical results. Ann Neurol. 1995;37:476–487. doi: 10.1002/ana.410370410. [DOI] [PubMed] [Google Scholar]
- Sloan TB, Heyer EJ. Anesthesia for intraoperative neurophysiologic monitoring of the spinal cord. J Clin Neurophysiol. 2002;19:430–443. doi: 10.1097/00004691-200210000-00006. [DOI] [PubMed] [Google Scholar]
- Szelényi A, Joksimovic B, Seifert V. Intraoperative risk of seizures associated with transient direct cortical stimulation in patients with symptomatic epilepsy. J Clin Neurophysiol. 2007;24:39–43. doi: 10.1097/01.wnp.0000237073.70314.f7. [DOI] [PubMed] [Google Scholar]
- Taniguchi M, Cedzich C, Schramm J. Modification of cortical stimulation for motor evoked potentials under general anesthesia: technical description. Neurosurgery. 1993;32:219–226. doi: 10.1227/00006123-199302000-00011. [DOI] [PubMed] [Google Scholar]
- Wagner J, Urbach H, Niehusmann P, von Lehe M, Elger CE, Wellmer J. Focal cortical dysplasia type IIb: completeness of cortical, not subcortical, resection is necessary for seizure freedom. Epilepsia. 2011;52:1418–1424. doi: 10.1111/j.1528-1167.2011.03158.x. [DOI] [PubMed] [Google Scholar]


