Significance
This study shows that red cedar trees growing in the Central Appalachian Mountains of West Virginia are recovering from decades of acidic pollution. Our study shows the efficacy of the Clean Air Act in a region where acidic pollution levels were some of the highest in the United States before the Clean Air Act. We demonstrate that a large portion of the increase in water use efficiency of trees that is often attributed to increasing atmospheric CO2 over the last century may be caused by acid deposition. This study has important implications for carbon cycling in forests, showing an interaction between decreasing SO2 emissions and increasing CO2 that is not currently accounted for in biosphere–atmosphere models of climate change.
Keywords: carbon isotopes, sulfur isotopes, carbon cycle
Abstract
Using dendroisotopic techniques, we show the recovery of Juniperus virginiana L. (eastern red cedar) trees in the Central Appalachian Mountains from decades of acidic pollution. Acid deposition over much of the 20th century reduced stomatal conductance of leaves, thereby increasing intrinsic water-use efficiency of the Juniperus trees. These data indicate that the stomata of Juniperus may be more sensitive to acid deposition than to increasing atmospheric CO2. A breakpoint in the 100-y δ13C tree ring chronology occurred around 1980, as the legacy of sulfur dioxide emissions declined following the enactment of the Clean Air Act in 1970, indicating a gradual increase in stomatal conductance (despite rising levels of atmospheric CO2) and a concurrent increase in photosynthesis related to decreasing acid deposition and increasing atmospheric CO2. Tree ring δ34S shows a synchronous change in the sources of sulfur used at the whole-tree level that indicates a reduced anthropogenic influence. The increase in growth and the δ13C and δ34S trends in the tree ring chronology of these Juniperus trees provide evidence for a distinct physiological response to changes in atmospheric SO2 emissions since ∼1980 and signify the positive impacts of landmark environmental legislation to facilitate recovery of forest ecosystems from acid deposition.
A key uncertainty in coupled biosphere–atmosphere models of climate change is the long-term effects of increasing atmospheric CO2 on carbon uptake and storage in terrestrial ecosystems (1, 2). Forest ecosystems play a fundamental role in the global C cycle; they contribute ∼50% of terrestrial net primary production (3, 4), account for ∼45% of terrestrial C (5), and are a major part of the terrestrial C sink that removes nearly 30% of anthropogenic C emissions each year (6). Consequently, state-of-the-art climate models require a mechanistic understanding of how simultaneous changes in key environmental variables affect C cycling in trees and forest ecosystems. Although experiments have examined how numerous environmental factors affect tree growth and forest productivity, accurately forecasting future tree growth and C cycling requires a better understanding of complex environmental interactions across spatial and temporal gradients in natural systems.
For over a century, the combustion of fossil fuels resulted in the widespread alteration of the composition of the atmosphere. For example, though atmospheric CO2 concentrations increased by almost 40% since the mid-19th century and are continuing to increase at an unprecedented rate (7, 8), SO2 emissions in the United States also increased rapidly initially but then declined following US environmental legislation (the Clean Air Act) in 1970 and its subsequent amendment in 1990 (Fig. 1) (9, 10). Although the direct effects of elevated CO2 are positive for plants, generally increasing photosynthesis and decreasing water loss by reducing stomatal conductance (11), SO2 emissions are transformed into acidic deposition (Fig. S1), which has numerous negative impacts on ecosystem productivity (12).
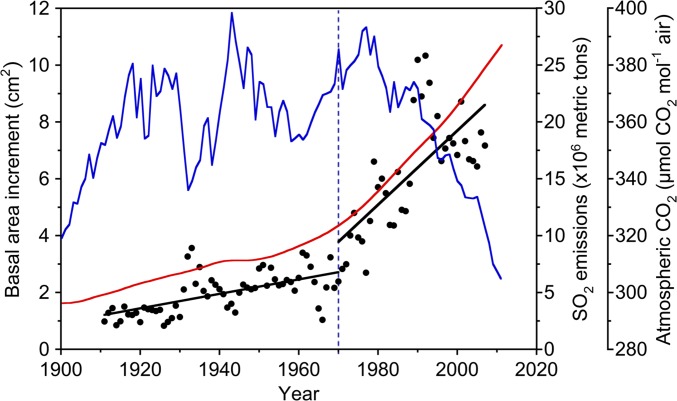
Fig. 1.
Basal growth of 118- to 480-y-old J. virginiana trees in a stand located in the Central Appalachian Mountains over the past century (n = 5 trees). The vertical blue dotted line represents the approximate date of the Clean Air Act (1970). Before 1970, y = 0.025x − 47.4, r2 = 0.38, P < 0.0001; after 1970, y = 0.130x – 253.1, r2 = 0.49, P < 0.0001. The slopes of the relationships between BAI and year before and after 1970 are different (P < 0.0001). Superimposed over BAI are trends of atmospheric CO2 concentrations (red line) (7, 8) and historical sulfur emissions in the United States (blue line) (9, 10).
The Central Appalachian Mountains have historically received some of the highest rates of acid deposition in the United States due to their downwind proximity to the abundance of coal-fired power plants in the Ohio River Valley (12). Despite these historical rates of acid deposition and the potentially long-lasting effects on ecosystems, there are many examples of tree species in the temperate deciduous forest in the eastern United States that have shown recent unexplained increases in growth (13–17). In this study, we used dendroisotopic techniques to examine simultaneous environmental influences on Juniperus virginiana trees growing in the eastern panhandle of West Virginia. Using this approach, we were able to estimate how key physiological processes have changed across the past century, and the extent to which temporal changes in atmospheric CO2 concentrations and acid deposition are responsible.
Results and Discussion
Here, we found that the basal area increment (BAI) of Juniperus in an old-growth stand in West Virginia has increased significantly since the enactment of the Clean Air Act of 1970 (Fig. 1), despite being older (118–480 y old) than the age of trees that normally exhibit rapid growth (18, 19). A recent tree ring chronology of J. virginiana, incorporating our site and others in this region, indicates an increasing growth trend for these trees since the late 20th century that is greater than any other time over the past 450 y (20). We performed a multivariate correlation analysis using historical climate variables (21), atmospheric CO2 concentrations (7, 8), and US SO2 and NOx emissions (9, 10), and found that the growth of these Juniperus trees over the last century is explained best by increases in atmospheric CO2 and NOx emissions, and decreases in SO2 emissions (Table S1).
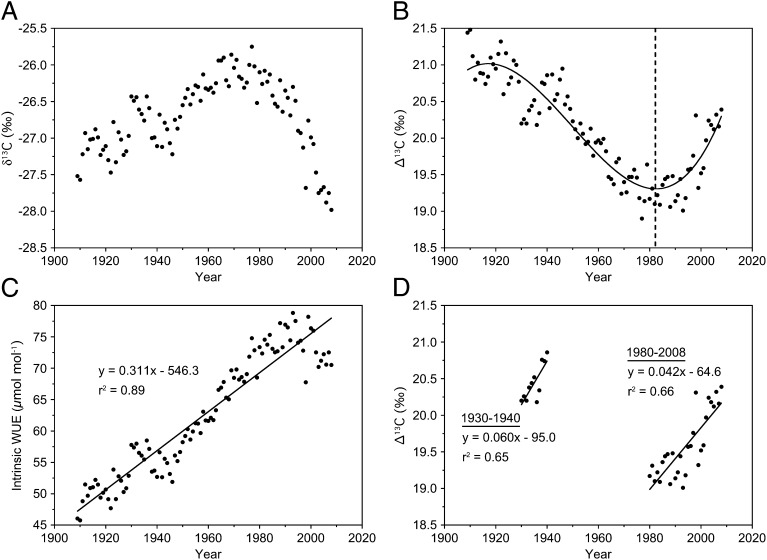
To examine changes in leaf physiology that influence tree growth over time, whole wood from tree rings from the years 1909–2008 was analyzed for δ13C (Fig. 2A), corrected to account for the differences between leaf and wood isotopic signatures, converted to ∆13C (isotopic discrimination) to account for the anthropogenic increase in isotopically light atmospheric δ13CO2, and used in standard equations to calculate leaf physiological characteristics (22). We found a striking nonlinear trend in ∆13C (Fig. 2B) that indicates a fundamental shift in foliar gas exchange of these Juniperus trees, resulting in a 66% increase in intrinsic water use efficiency (iWUE), or the ratio of carbon gained through photosynthesis (A) to the stomatal conductance to water (gw), over the 100-y time period (Fig. 2C). This isotopic signal indicates a progressive reduction in the discrimination against the heavy C isotope across the tree-ring chronology until later in the 20th century, when increased isotopic discrimination resulted in a lighter C isotopic signature being incorporated into the wood (Fig. 2B). We fit a third-order polynomial function to the sequence of ∆13C to estimate the year when the isotopic trend changed from enrichment to depletion of the heavy C isotope (y = 0.00001227x3 − 0.07179x2 + 139.9x – 90869; r2 = 0.86, P < 0.0001). The breakpoint was identified as 1982, which corresponds to the approximate time when SO2 emissions began to decline in the United States. This result was surprising because it is counter to the widely observed stomatal response of plants to increasing CO2 in the 20th century (11, 23). Here, decreasing ∆13C before ∼1980 suggest stomatal closure as if the trees were responding to increasing atmospheric CO2, yet the reversal and increases in ∆13C after 1980 suggest reduced stomatal regulation to CO2 (Fig. 2B). Similar isotopic trends in trees growing in the northern hemisphere have been observed by other investigators who have suggested that these recent shifts in δ13C are indicative of a physiological change in guard cells where stomata become uncoupled from CO2 concentration as atmospheric CO2 began to increase rapidly in the 1980s (24). After converting ∆13C to Ci/Ca (Fig. S2A), the ratio of internal leaf CO2 to atmospheric CO2, a metric of leaf physiology that provides a relative assessment of the stomatal limitation of photosynthesis, it is clear that stomatal limitations for these Juniperus trees increased until ∼1980, at which point the trend reversed and stomatal limitations to CO2 uptake are reduced. Several lines of evidence point to the declining levels of SO2 emissions around the 1980s that drive these stomatal responses in Juniperus rather than a decoupling of stomatal sensitivity to atmospheric CO2.
Fig. 2.
(A) Chronology of leaf-corrected δ13C from tree rings of J. virginiana, (B) ∆13C, (C) iWUE derived from Ca and Ci, and (D) a comparison of ∆13C from 1930 to 1940 and after 1980. The vertical dashed line at 1982 in B represents the year predicted from a third-order polynomial where the shift in ∆ occurs.
The first line of evidence is the close correspondence between the timing of the change in direction of Ci/Ca with the decline in SO2 emissions that occurred in the United States following the Clean Air Act. The directional change in the isotopic signal follows reductions in SO2 emissions that began in the 1970s, but precedes reductions in NOx emissions by over a decade (Fig. S3) (10). Although changes in tropospheric ozone might also impact Ci/Ca over time, a direct assessment of the influence of O3 is not possible because historical records of O3 are not available for the same time period as our tree-ring chronology. Regardless, because NOx is a precursor to O3 (25), it is reasonable to assume similar historical patterns of O3 and NOx, and because contemporary historical NOx emissions do not show a breakpoint around the year 1980, it is unlikely that changes in O3 explain the pattern of Ci/Ca in our tree-ring chronology. In addition, the open canopy and relatively high light levels of this Juniperus/hardwood forest suggests that canopy closure, or increased shading, around 1980 was not likely the cause for this chronology of Ci/Ca. Likewise, we found no evidence of fire, timber harvesting, or any other type of disturbance that may have increased the light levels in this forest, thereby influencing the isotopic signatures of the tree rings. There has been an increase in yearly precipitation in this region in West Virginia over 1909–2008 (∼1.2 mm⋅y−1; P < 0.012), and there has been a trend toward warmer temperatures (∼0.4 °C; P < 0.057), but year-to-year variability of both of these climatic factors were high (Fig. S4). If these changes in climate were responsible for patterns of Ci/Ca, we would expect a gradual change in the isotopic signature over the entire century rather than the observed breakpoint at ∼1980.
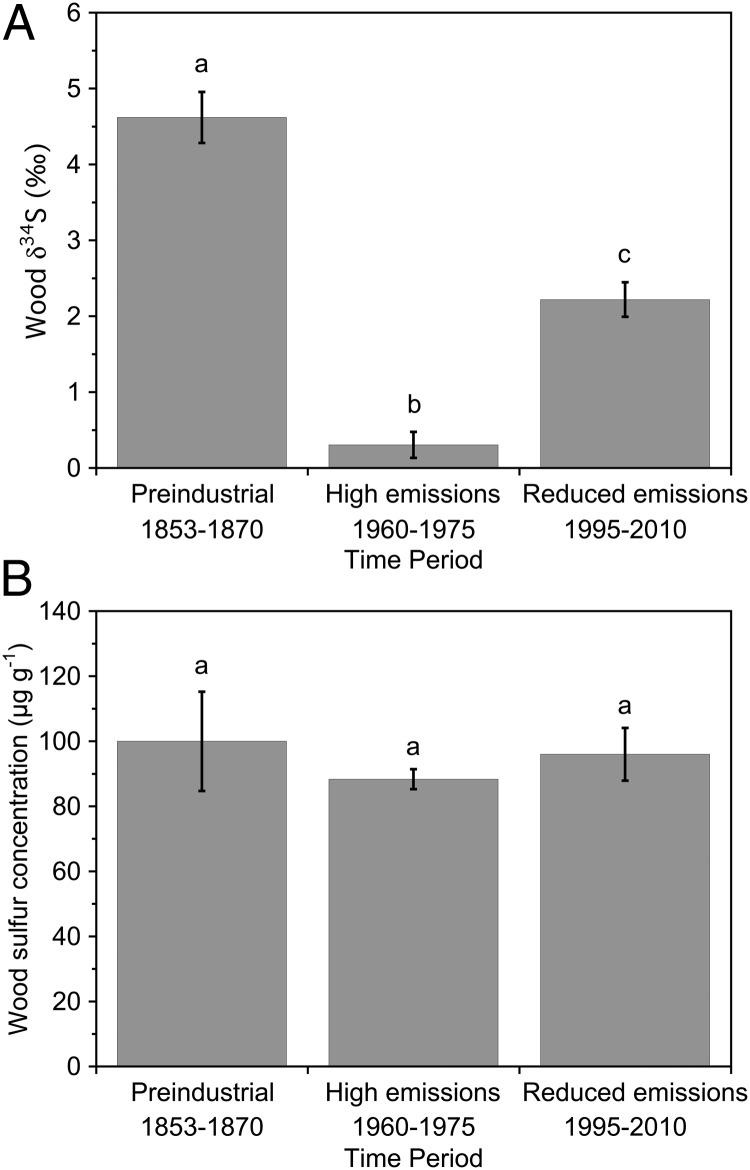
To further examine the consistency between the timing of the increase in Ci/Ca in the tree rings of Juniperus with the timing of the decline in US SO2 emissions, we compared δ34S in tree rings formed during preindustrial years to δ34S in tree rings before and after 1980 (Fig. 3). Wood δ34S is infrequently used in dendroisotopic studies because of the very low concentration of S in wood (26, 27). Sulfur can enter the plant through their roots or leaves, but either route of uptake results in very little fractionation when S is incorporated into biomass, allowing δ34S to typically reflect the sources of S incorporated by the tree (28). In this study, the sources of S used by these trees over the tree-ring chronology are unknown, but we assume that the δ34S from 1853 to 1870 shows little anthropogenic influence. The large depletion of the heavy S isotope between the preindustrial period and 1960–1975, as US SO2 emissions were increased by ∼260% compared with the mid-1800s (9), reflects a strong anthropogenic S input at the same time that δ13C indicates a decline in leaf Ci/Ca. The increase in δ34S between 1960–1975 and 1995–2010, as US SO2 emissions declined by ∼41% (9, 10) and SO4 deposition declined by ∼66% (29), indicates a reversal back toward preindustrial δ34S signatures and a reduced anthropogenic influence. Thus, just as the tree ring δ13C after ∼1980 indicates Ci/Ca increased at the leaf level, tree ring δ34S indicates a synchronous change in the sources of S used at the whole-tree level. Sulfur concentrations in wood were constant across the chronology despite large changes in SO2 emissions, reflecting the fixed physiological processes that regulate S deposition in wood, as has been shown previously (27).
Fig. 3.
Mean (A) δ34S and (B) S concentrations of J. virginiana tree rings from three time periods: 1853–1870, 1960–1975, and 1995–2010. Error bars denote ±1 SE; n = 3 for 1853–1870, and n = 5 for 1960–1975 and 1995–2010. Values designated by the same letter are not different at the 0.05 level of significance, using a Tukey–Kramer HSD post hoc test.
The second line of evidence is that the changes in the C isotopic signature of Juniperus tree rings late in the 20th century were not unique to this time period. Instead, the patterns of change in δ13C, ∆13C, and Ci/Ca after 1980 were not significantly different from the isotopic signatures found during the 1930s, when SO2 emissions were sharply reduced during the US Great Depression (Fig. 2D and Fig. S5). As with the C isotopic signatures after 1980, no other environmental factors explain the observed changes during the 1930s. In addition, the growth of these Juniperus trees during 1930–1940 was inversely correlated to SO2 emissions (y = −0.46× + 6.48; r2 = 0.72, P < 0.0009), suggesting that the trees experienced a short-term recovery from acid deposition during the Great Depression years (Fig. S6).
The third line of evidence is from the chronology of stomatal conductance to CO2 (gc) of the Juniperus foliage inferred from tree ring C isotopes. Acid misting experiments and field-based isotopic studies have indicated that SO4 deposition affects stomatal function, including reducing gc of leaves (30–32). Based on these studies, we postulated that gc would decrease before 1980 when SO2 emissions were high, but would subsequently increase in response to reductions in SO2 emissions. The chronology of Ci/Ca suggests a strong link between increased SO2 emissions and the regulation of stomata, but Ci/Ca incorporates both A and gc. To evaluate drivers of Ci/Ca independently, we performed a simulation analysis of changes in A and gc using foliar Ci derived from tree ring ∆13C of the Juniperus trees (Fig. S2B), atmospheric CO2 concentrations (7, 8), and the physiological relationship between light-saturated A and Ci of Juniperus foliage measured onsite.
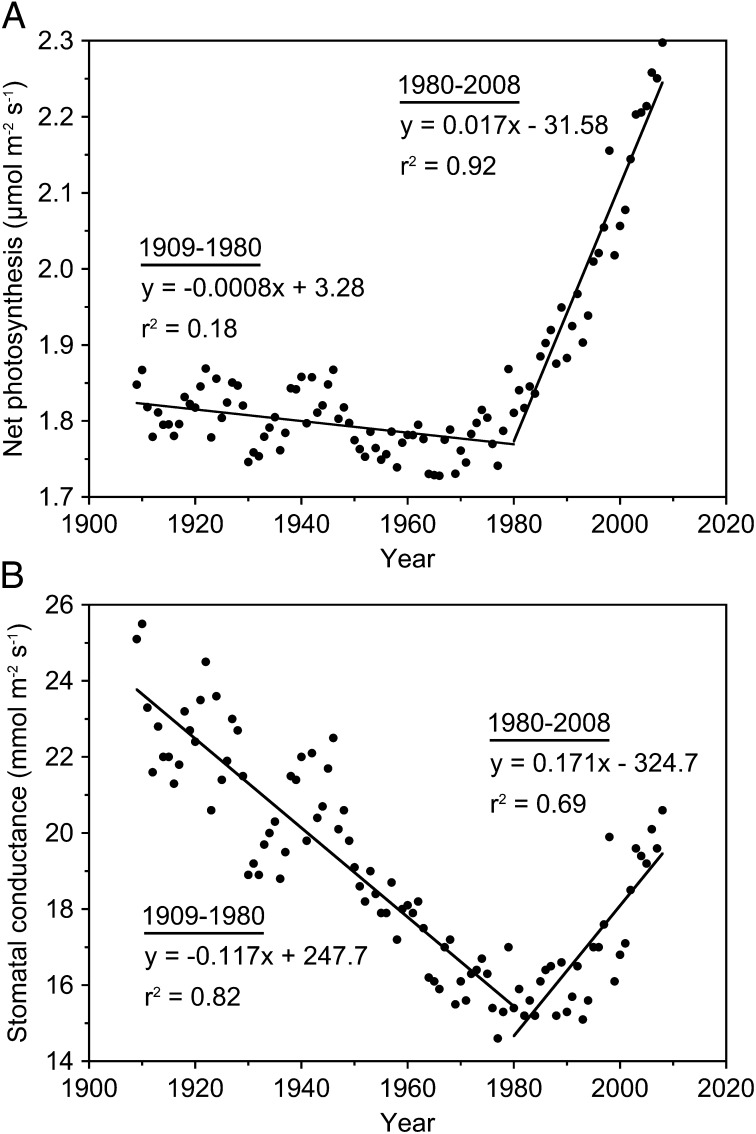
The chronology of A and gc in Juniperus shows that A changed very little from 1909–1980 (a 3% decline), whereas gc decreased by 35% over the same time period (Fig. 4 A and B). After 1980, large increases in both A (27%) and gc (33%) occurred. Increases in A after 1980 were correlated with increases in atmospheric CO2 and decreases in SO2 emissions (Fig. S7 A and B). Likewise, gc of Juniperus leaves after 1980 increased as SO2 emissions declined, despite increasing atmospheric CO2 (Fig. S7 C and D). Additionally, during 1930–1940, A increased 5.9% and gc increased 14.9% (Fig. S8), providing further support to consider that changes in the isotopic signal of Juniperus tree rings late in the 20th century and during the 1930s are both related to the same phenomenon. The chronology of A and gc binned by decade reveals that the only two time periods when there were net positive changes in A and gc of Juniperus were during the 1930s and after the 1980s (Table 1), and these correspond to time periods when increases in growth of the trees were observed (Fig. 1). During both of these time periods, gc increased to a greater extent than A, explaining why iWUE declined during the 1930s and after ∼1990 in the tree ring chronology (Table 1 and Fig. S5D).
Fig. 4.
Simulated seasonally integrated (A) net photosynthesis and (B) stomatal conductance of J. virginiana trees across the tree ring chronology.
Table 1.
Changes (%) in the photosynthetic variables (PV), net photosynthetic rates (A) and stomatal conductance to CO2 (gc) of J. virginiana trees over the 100-y chronology derived the simulation analysis using Ci from tree-ring isotopic data, atmospheric CO2 (7, 8), and the relationships between A and Ci measured on J. virginiana foliage in 2009
| PV | 1909–1920 | 1920–1930 | 1930–1940 | 1940–1950 | 1950–1960 | 1960–1970 | 1970–1980 | 1980–1990 | 1990–2000 | 2000–2008 |
| A | −1.9 | −2.0 | 5.9 | −2.5 | 0 | −1.7 | 0 | 5.9 | 10.5 | 11.1 |
| gc | −11.3 | −10.8 | 14.9 | −7.5 | −5.6 | −12.1 | −4.6 | 3.1 | 15.1 | 18.4 |
Despite decreased iWUE of Juniperus during the 1930s and after ∼1990, iWUE increased 66% over the entire chronology (Fig. 2C) due to a 23% increase in A and an 18.5% decrease in gc. Many dendroisotopic studies have concluded that the small but progressive increases in atmospheric CO2 over the last century have improved iWUE (23, 33), and suggest that a fundamental way that increasing atmospheric CO2 stimulates tree growth and forest productivity is by improving the efficiency that trees are using water. Here, acid deposition over many decades contributed to a large portion of the increase in iWUE of Juniperus, and these data signify that the effects of increasing CO2 on iWUE can be greatly overestimated if other environmental influences are not considered in these estimates. The mechanisms for increased iWUE over the entire chronology differed between 1909–1980 (small decreases in A, large decreases in gc) and 1980–2008 (greater increases in A than gc initially, but followed by greater increases in gc than A ∼1990, whereby iWUE decreases; Table 1). If we assume that the stomatal response between 1909–1980 incorporates stomatal closure due to both a CO2 response and a response to SO4 deposition (resulting in a 35% decline in gc), and after 1980 there is recovery from SO4 deposition, then the difference in gc between 1909–2008 reflects the effect of increasing CO2 (a 18.5% decline in gc; Fig. S9). Thus, if the recovery of gc from the effects of acid deposition was complete by 2008, it appears that the effects of SO4 deposition on gc of Juniperus between 1909–1980 were greater than that of atmospheric CO2 during the same time period.
SO2 emissions and acid deposition have been effectively reduced in the United States since the Clean Air Act was enacted, but despite this, acid deposition remains a considerable problem in the United States, as well as other locations globally (34). Though aquatic ecosystems in the United States have shown some improvements as acid deposition has declined (34, 35), recovery of terrestrial ecosystems is expected to be much slower than aquatic systems, and could take decades (12, 36, 37). Our findings indicate that the productivity of this stand of Juniperus growing in the Central Appalachian Mountains is recovering from acid deposition, and confirm the prediction that recovery of trees occurs over long periods of time. It is clear that it will be important to examine the generality of these results from Juniperus to understand how widespread these responses to reduced SO2 emissions are among co-occurring tree species within eastern temperate forest communities (38). Regardless, the recovery of Juniperus from long-term acid deposition involves gradual stomatal opening over several decades, and thus provides evidence for a physiological response to reductions in SO2 emissions that is distinct from the response to increased atmospheric CO2. The slower rate of increase in gc, but faster rate of increase in A, that occurred after 1980 compared with the short-term recovery during the 1930s (Fig. S8 A and B) likely reflect the widely observed effects of increased CO2 concentrations on gc and A (11). If we consider A as a diffusive process described by Fick’s law, where 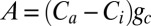 , then as the recovery from acid deposition of these Juniperus trees occurs, carbon uptake is enhanced by both increased Ca as atmospheric CO2 increases and increased gc as SO2 emissions decline. Taken together, these results highlight the complexities of significant interactions between atmospheric CO2 and acid deposition on stomatal conductance, photosynthesis, and tree growth that have important consequences for forest C cycling in many northern hemisphere forests.
, then as the recovery from acid deposition of these Juniperus trees occurs, carbon uptake is enhanced by both increased Ca as atmospheric CO2 increases and increased gc as SO2 emissions decline. Taken together, these results highlight the complexities of significant interactions between atmospheric CO2 and acid deposition on stomatal conductance, photosynthesis, and tree growth that have important consequences for forest C cycling in many northern hemisphere forests.
Materials and Methods
Tree Selection and Growth Measurements.
The study site is a stand of J. virginiana (red cedar) trees located along the south branch of the Potomac River in southern Grant County, WV, on a northwestern facing limestone outcrop located above Smoke Hole Canyon (38° 53′02′′ N 79° 14′12′′ W; 670 m above sea level). This site is in the Ridge and Valley Physiographic Province of northeastern West Virginia and has been defined as a cedar glade (39). The soil in this glade woodland has little organic matter and is primarily composed of broken limestone. In addition to Juniperus trees, the overstory vegetation is comprised of Quercus muehlenbergii, Quercus alba, Fraxinus americana, and Juglans nigra, and the open overstory allows most trees to receive high sunlight throughout the day.
Five Juniperus trees were randomly selected along a transect running parallel to the bluff at least 6 m from the edge. An exception to the random sampling was that trees with obvious strip bark were avoided. Tree rings used in this study were obtained in 2008. To minimize bias from uneven radial growth, we took two increment cores from each tree parallel to the contours of the slope and recorded the diameter at 1 m above ground level. Increment cores were returned to the laboratory, processed according to standard dendrochronological techniques (40), and cross-dated using both WinDENDRO (Regent Instruments Inc.) and a master chronology created previously for the site using >20 Juniperus trees (20). All tree-ring series correlated with the master chronology, having interseries correlations >0.50. The expressed population signal, a measure of the common variance in a chronology, was >0.75 for the current residual chronology for the duration of the 20th century, suggesting that the trees had similar growth patterns. Once each core was measured, cross-dated, and statistically validated, we assigned a calendar year to each annual tree ring. The five trees were aged to be 119, 138, 166, 308, and 480 y old. Because tree-ring width declines as trees mature, and growth trends are not readily detectable based on changes in ring widths alone, we converted the cross-dated tree-ring series into annual BAI to characterize growth trends from 1911 to 2007 using
where R is tree radius for a given year and n is the year of ring formation.
Carbon Isotopic Analysis of Tree Rings.
Tree rings for years 1909–2008 were collected for isotopic analysis by scalpel dissection of cores under a dissecting microscope at the boundary of late wood and early wood. Wood samples were cut to 1 mg and packed into tin capsules for carbon isotopic analysis. Within years, earlywood and latewood were combined for sample analysis to ensure enough material for peak detection in the isotopic analysis. Whole-wood samples were analyzed for δ13C with a ThermoFinnigan ConFlo III interface and Finnigan Delta-plus Continuous Flow Stable Isotope Ratio Mass Spectrometer. Values of δ13C in parts per million (‰) were calculated using the following formula:
where R is the ratio of 13C:12C and PeeDee belemnite (PDB) from the Pee Dee River Formation (Hemingway, SC) was the standard to which the samples were compared. In-house laboratory standards (apple leaves) had a within-run variation of <0.04‰ (n = 50), whereas the laboratory standard (flour) used to assess variability between runs analyzed over time had variation <0.07‰ (n = 25).
Wood samples were corrected by −3.2‰ to account for the differences between leaf and wood isotopic signatures. Leaf δ13C was measured in the upper and lower portions of the Juniperus trees across the growing season to attain a seasonally integrated δ13Cleaf value of −27.79 (n = 65). Our correction value was calculated from the difference between this integrated δ13Cleaf value and δ13C of the outer most ring of radial growth (2008) of the five tree ring samples (range = −2.4 to −4.3). Similar estimates of the offset between wood tissue and leaf tissue have been found (41, 42).
Carbon isotope discrimination (∆13C) accounts for the anthropogenic increase in isotopically light atmospheric δ13CO2 and was calculated according to Farquhar et al. (22),
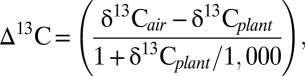 |
where δ13Cplant values were leaf-corrected values from each tree ring, and δ13Cair values were atmospheric values for the specific year of the tree ring. Values of δ13Cair from 1909 to 2003 were taken from McCarroll and Loader (43), who interpolated yearly values taken from the Law Dome ice cores (44). Values of δ13Cair from 2004 to 2008 were decreased incrementally by 0.02‰ y−1 (45).
The seasonally integrated Ci/Ca ratio (the ratio of the leaf intercellular CO2 concentration, Ci, to the CO2 concentration in the atmosphere, Ca) for each year was calculated from ∆13C according to ref. 22, using
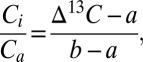 |
where a is the fractionation constant due to diffusion of CO2 through the stomatal aperture (4.4‰) (46) and b is the fractionation constant due to ribulose-1,5-bisphosphate carboxylase–oxygenase (27‰) (47). Values of Ci for each year were calculated from the Ci/Ca ratios, using Ca values from the Law Dome ice cores and direct atmospheric measurements (7, 8). Intrinsic water-use efficiency, the ratio of photosynthesis (A) to stomatal conductance of water vapor (gw), compares photosynthetic properties independent of evaporative demand and reflects integrated carbon uptake and water loss over the time of tissue development. Using Ci and Ca, iWUE was calculated as
where Fick’s law of diffusion of CO2 into the leaf, 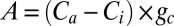 , is rearranged to solve for A/gc, and gc is converted to gw using 0.625 as the constant for differences between diffusion of CO2 and H2O (48).
, is rearranged to solve for A/gc, and gc is converted to gw using 0.625 as the constant for differences between diffusion of CO2 and H2O (48).
Simulation of Photosynthesis and Stomatal Conductance over the Chronology.
A simulation analysis of changes in net photosynthetic rates (A) and stomatal conductance (gc) of J. virginiana trees over the 100-y chronology was conducted using foliar Ci derived from ∆13C, Ca (7, 8), and the relationship between A and Ci (A–Ci curves) of Juniperus foliage measured onsite in 2009. We measured 55 A–Ci curves on 10 Juniperus trees (ages 98–481 y) from April to September 2009. Values of isotopically derived Ci were used with A–Ci curves to estimate A for each year. Stomatal conductance to CO2 (gc) was estimated for each year using the methods in ref. 49. For this simulation, we made two assumptions: first, that A was in the CO2-limited region of the A–Ci curve for the chronology, i.e., atmospheric CO2 was never at saturating concentrations (11), and second, that there was little photosynthetic acclimation to CO2 by the trees over the chronology, consistent with results from Free Air CO2 Enrichment experiments and CO2 meta-analyses (11, 50–52). The meta-analysis by Ainsworth and Rogers (11) indicates that, of all plant functional types, trees have shown the least potential for photosynthetic acclimation in experiments when CO2 is doubled from current-day concentrations. As such, it is unlikely that photosynthetic rates of these Juniperus trees have acclimatized to the ∼29% increase in CO2 between 1909 and 2008.
The A–Ci relationship was determined using an open-flow gas exchange system with an attached red/blue light-emitting diode light source (LI6400; Li-Cor, Inc.). Small branches from 10 Juniperus trees were cut from the upper canopy and immediately placed into containers filled with water (floral water picks). Foliage from the end of branches was used for these analyses. Measurements were made between 1000 and 1600 Eastern Standard Time on sunny days to minimize diurnal effects with saturating light (1,500 μmol⋅m−2⋅s−1). To generate A–Ci curves, light-saturated photosynthesis was measured at 10 CO2 concentrations between 50 and 1,500 μL CO2⋅L−1 air. The initial CO2 concentration was maintained at 380 μL⋅CO2⋅L−1 air. After an equilibration of ∼5 min at each CO2 concentration, A and Ci were recorded. After the gas-exchange analyses, all leaves were taken to the laboratory and scanned to determine projected leaf area (CanoScan 9950F; Canon), and gas exchange of Juniperus was expressed on a total leaf area basis (53).
Sulfur Isotopic Analysis of Tree Rings.
Five additional Juniperus trees from the same stand were randomly selected and cored in 2013 as described above for BAI and C isotopes. Each core was cross-dated with existing cores left over from the 2008 analysis, and we assigned a calendar year to each annual tree ring. Tree rings for years 1853–1870, 1960–1975, and 1995–2010 were collected for isotopic analysis by scalpel dissection of cores under a dissecting microscope at the boundary of late wood and early wood. Rings from each time period were pooled for each tree and then ground into a fine powder for sulfur isotopic analysis. An n = 5 was used for 1960–1975 and 1995–2010, and an n = 3 was used for 1853–1870.
Wood samples were analyzed for δ34S using a PYRO cube elemental analyzer (Elementar) operated in carbon, nitrogen, sulfur mode with extra O2 added (100 mL/min for 70 s) to account for the high C:S of wood and the small sample mass available (∼80 mg dry weight). Trapped SO2 gas was delivered to a Delta XP isotope ratio mass spectrometer (ThermoFinnigan) for analysis. Values of δ34S were corrected according to the international atomic energy standards S-1 and S-2 (−0.3 and 22.7‰, respectively) and an internal laboratory standard of argentite (S-6). Analytical precision for δ34S was ± 0.4‰. Analyses of δ34S were performed in the G. G. Hatch Stable Isotope Laboratory at the University of Ottawa in Ontario, Canada.
Statistics.
To determine if there had been any significant changes in climatic conditions, we examined the linear trends of monthly, seasonal, and yearly means of precipitation and temperature using the 100-y period from 1909 to 2008. Precipitation and temperature data were obtained from the National Climatic Data Center for West Virginia climate region 6 (21), which includes the portion of West Virginia in the Ridge and Valley physiographic province and contains our study site. We used annual average of the atmospheric CO2 concentrations reconstructed from ice cores (7) and recorded at Mauna Loa observatory since 1953 (8); overlapping values were averaged. Atmospheric CO2 concentrations increased from 299 µL⋅L−1 in 1910 to 386 µL⋅L−1 in 2008. Historical US SO2 emissions and historical US NOx emissions were taken from refs. 9 and 10; overlapping values were averaged.
Linear regression was used to identify significant temporal trends in BAI of Juniperus trees over the tree-ring chronology (1911–2007). Due to the nonnormal distribution and autocorrelation in BAI, we used Kendall’s tau, a nonparametric correlation coefficient, to identify explanatory variables with the best relationships with BAI. All statistical analyses used single-year BAI data and were not detrended or smoothed, providing interpretable yearly levels of growth rather than a standardized index. Analysis of covariance was used to test for differences in BAI trends for before (1909–1970) and after (1970–2008) the Clean Air Act legislation.
Simple regression analyses were used to examine trends in tree ring ∆13C, Ci/Ca, and iWUE, as well as simulated A and gc over the 100-y tree chronology (1909–2008). Although we only used five individual trees in our isotopic analysis, this number is typically sufficient to characterize stand-level tendencies given the typical uniformity of environmental signals reflected in tree rings for a given species and site (33). Analysis of covariance was used to test for differences in isotopic trends for 1930–1940 and 1980–2008.
One-way analysis of variance was used to examine whether S isotopes differed in tree rings from 1853–1870, 1960–1975, and 1995–2010. Differences between the means of each time category were assessed using a Tukey–Kramer honestly significant difference (HSD) test.
All statistical analyses were performed using JMP software for Macintosh v. 10.0 (SAS Institute).
Supplementary Material
Acknowledgments
We thank Stockton Maxwell and Amy Hessl for sharing their expertise in dendrochronology, as well as their tree ring laboratory and equipment; Dr. William Puffenberger, Don Rohrbaugh, and the Monongahela National Forest for access to the research site; Troy Ocheltree for technical and analytical assistance for C isotopes; Paul Middlestead for analytical assistance with S isotopes; and Clint Springer, William Peterjohn, Patrick Megonigal, and two anonymous reviewers for thoughtful and constructive comments on the manuscript. The Eberly Family Faculty Development Fund at West Virginia University supported this work.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1308115110/-/DCSupplemental.
References
- 1.Friedlingstein P, et al. Climate-carbon cycle feedback analysis: Results from the (CMIP)-M-4 model intercomparison. J Clim. 2006;19(14):3337–3353. [Google Scholar]
- 2.Matthews HD. Implications of CO2 fertilization for future climate change in a coupled climate-carbon model. Glob Change Biol. 2007;13(5):1068–1078. [Google Scholar]
- 3.Field CB, Behrenfeld MJ, Randerson JT, Falkowski P. Primary production of the biosphere: Integrating terrestrial and oceanic components. Science. 1998;281(5374):237–240. doi: 10.1126/science.281.5374.237. [DOI] [PubMed] [Google Scholar]
- 4.Saugier B, Roy J, Mooney HA. Estimations of global terrestrial productivity: Converging toward a single number. In: Roy J, Saugier B, Mooney HA, editors. Terrestrial Global Productivity. San Diego: Academic; 2001. pp. 543–557. [Google Scholar]
- 5.Bonan GB. Forests and climate change: Forcings, feedbacks, and the climate benefits of forests. Science. 2008;320(5882):1444–1449. doi: 10.1126/science.1155121. [DOI] [PubMed] [Google Scholar]
- 6.Canadell JG, et al. Contributions to accelerating atmospheric CO2 growth from economic activity, carbon intensity, and efficiency of natural sinks. Proc Natl Acad Sci USA. 2007;104(47):18866–18870. doi: 10.1073/pnas.0702737104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Etheridge DM, et al. (1998) Historical CO2 records from the Law Dome DE08, DE08-2, and DSS ice cores. Trends: A Compendium of Data on Global Change (Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, US Department of Energy, Oak Ridge, TN). Available at http://cdiac.ornl.gov/trends/co2/lawdome.html.
- 8. Keeling RF, Piper SC, Bollenbacher AF, Walker JS (2009) Atmospheric CO2 records from sites in the SIO air sampling network. Trends: A Compendium of Data on Global Change (Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, US Department of Energy, Oak Ridge, TN). Available at http://cdiac.ornl.gov/trends/co2/sio-mlo.html.
- 9.Lefohn AS, Husar JD, Husar RB. Estimating historical anthropogenic global sulfur emission patterns for the period 1850–1990. Atmos Environ. 1999;33(21):3435–3444. [Google Scholar]
- 10. US Environmental Protection Agency (2012) National Emissions Inventory (NEI) Air Pollution Emissions (US Environmental Protection Agency, Chicago). Available at www.epa.gov/ttn/chief/trends/index.html. Accessed September 8, 2012.
- 11.Ainsworth EA, Rogers A. The response of photosynthesis and stomatal conductance to rising [CO2]: Mechanisms and environmental interactions. Plant Cell Environ. 2007;30(3):258–270. doi: 10.1111/j.1365-3040.2007.01641.x. [DOI] [PubMed] [Google Scholar]
- 12.Driscoll CT, et al. Acidic deposition in the northeastern United States: Sources and inputs, ecosystem effects, and management strategies. Bioscience. 2001;51(3):180–198. [Google Scholar]
- 13.Boisvenue C, Running SW. Impacts of climate change on natural forest productivity - evidence since the middle of the 20th century. Glob Change Biol. 2006;12(5):862–882. [Google Scholar]
- 14.Johnson SE, Abrams MD. Age class, longevity and growth rate relationships: Protracted growth increases in old trees in the eastern United States. Tree Physiol. 2009;29(11):1317–1328. doi: 10.1093/treephys/tpp068. [DOI] [PubMed] [Google Scholar]
- 15.Gedalof Z, Berg AA. Tree ring evidence for limited direct CO2 fertilization of forests over the 20th century. Global Biogeochem Cycles. 2010;24 doi: 10.1029/2009GB003699. GB3027. [DOI] [Google Scholar]
- 16.McMahon SM, Parker GG, Miller DR. Evidence for a recent increase in forest growth. Proc Natl Acad Sci USA. 2010;107(8):3611–3615. doi: 10.1073/pnas.0912376107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Potter C, Klooster S, Genovese V. Net primary production of terrestrial ecosystems from 2000 to 2009. Clim Change. 2012;115(2):365–378. [Google Scholar]
- 18.Ryan MG, Yoder BJ. Hydraulic limits to tree height and tree growth. Bioscience. 1997;47(4):235–242. [Google Scholar]
- 19.Weiner J, Thomas SC. The nature of tree growth and the age-related decline in forest productivity. Oikos. 2001;94(2):374–376. [Google Scholar]
- 20.Maxwell RS, Hessl A, Cook E, Buckley B. A multicentury reconstruction of may precipitation for the mid-Atlantic region using Juniperus virginiana tree rings. J Clim. 2012;25(3):1045–1056. [Google Scholar]
- 21. National Oceanic and Atmospheric Administration (NOAA) National Climatic Data Center (2012) West Virginia Climate Region 6 Meteorological Data 1895–2009 (NOAA, Asheville, NC). Available at www.ncdc.noaa.gov/temp-and-precip/time-series/index.php?parameter=tmp&month=4&year=2008&filter=12&state=46&div=6.
- 22.Farquhar GD, O'Leary MH, Berry JA. On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves. Aust J Plant Physiol. 1982;9(2):121–137. [Google Scholar]
- 23.Franks PJ, et al. Sensitivity of plants to changing atmospheric CO2 concentration: From the geological past to the next century. New Phytol. 2013;197(4):1077–1094. doi: 10.1111/nph.12104. [DOI] [PubMed] [Google Scholar]
- 24.McCarroll D, et al. Correction of tree ring stable carbon isotope chronologies for changes in the carbon dioxide content of the atmosphere. Geochim Cosmochim Acta. 2009;73(6):1539–1547. [Google Scholar]
- 25.National Research Council . Rethinking the Ozone Problem in Urban and Regional Air Pollution. Washington, DC: National Academy Press; 1991. [Google Scholar]
- 26.Kawamura H, Matsuoka N, Momoshima N, Koike M, Takashima Y. Isotopic evidence in tree rings for historical changes in atmospheric sulfur sources. Environ Sci Technol. 2006;40(18):5750–5754. doi: 10.1021/es060321w. [DOI] [PubMed] [Google Scholar]
- 27.Novak M, et al. Controls on sulfur content in tree rings of Norway spruce and European beech at a heavily polluted site. Geochem J. 2009;43(2):e1–e4. [Google Scholar]
- 28.Trust BA, Fry B. Stable sulphur isotopes in plants: A review. Plant Cell Environ. 1992;15(9):1105–1110. [Google Scholar]
- 29. National Atmospheric Deposition (NADP) Program (NRSP-3) (2013) Illinois State Water Survey (NADP, Champaign, IL). Available at http://nadp.sws.uiuc.edu/sites/siteinfo.asp?net=NTN&id=WV18.
- 30.Borer CH, Schaberg PG, DeHayes DH. Acidic mist reduces foliar membrane-associated calcium and impairs stomatal responsiveness in red spruce. Tree Physiol. 2005;25(6):673–680. doi: 10.1093/treephys/25.6.673. [DOI] [PubMed] [Google Scholar]
- 31.Savard MM, Bégin C, Parent M, Smirnoff A, Marion J. Effects of smelter sulfur dioxide emissions: A spatiotemporal perspective using carbon isotopes in tree rings. J Environ Qual. 2004;33(1):13–26. doi: 10.2134/jeq2004.1300. [DOI] [PubMed] [Google Scholar]
- 32.Rinne KT, Loader NJ, Switsur VR, Treydte KS, Waterhouse JS. Investigating the influence of sulfur dioxide (SO2) on the stable isotope ratios (δ13C and δ18O) of tree rings. Geochim Cosmochim Acta. 2010;74(8):2327–2339. [Google Scholar]
- 33.Peñuelas J, Canadell JG, Ogaya R. Increased water use efficiency during the 20th century did not translate into enhanced tree growth. Glob Ecol Biogeogr. 2011;20(4):597–608. [Google Scholar]
- 34.Burns DA, et al. National Acid Precipitation Assessment Program Report to Congress 2011: An Integrated Assessment. Washington, DC: National Science and Technology Council; 2011. [Google Scholar]
- 35.Greaver TL, et al. Ecological effects of nitrogen and sulfur air pollution in the U.S.: What do we know? Front Ecol Environ. 2012;10(7):365–372. [Google Scholar]
- 36.Likens GE, Driscoll CT, Buso DC. Long-term effects of acid rain: Response and recovery of a forest ecosystem. Science. 1996;272(4328):244–246. [Google Scholar]
- 37.Lawrence GB, et al. Early indications of soil recovery from acidic deposition in US red spruce forests. Soil Sci Soc Am J. 2012;76(4):1407–1417. [Google Scholar]
- 38.Li L, et al. Tree-ring width and δ13C records of industrial stress and recovery in Pennsylvania and New Jersey forests: Implications for CO2 uptake by temperate forests. Chem Geol. 2010;273(3-4):250–257. [Google Scholar]
- 39.Bartgis RL. The limestone glades and barrens of West Virginia. Castanea. 1993;58(2):69–89. [Google Scholar]
- 40.Stokes MA, Smiley TL. An Introduction to Tree-Ring Dating. Tucson, AZ: Univ of Arizona Press; 1996. [Google Scholar]
- 41.Leavitt SW, Long A. Evidence for 13C/12C fractionation between tree leaves and wood. Nature. 1982;298(5876):742–744. [Google Scholar]
- 42.Ward JK, et al. Carbon starvation in glacial trees recovered from the La Brea tar pits, southern California. Proc Natl Acad Sci USA. 2005;102(3):690–694. doi: 10.1073/pnas.0408315102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCarroll D, Loader NJ. Stable isotopes in tree rings. Quat Sci Rev. 2004;23(7-8):771–801. [Google Scholar]
- 44.Francey RJ, et al. A 1000-year high precision record of δ13C in atmospheric CO2. Tellus. 1999;51(2):170–193. [Google Scholar]
- 45. Keeling RF, Piper SC, Bollenbacher AF, Walker SJ (2010) Monthly atmospheric 13C/12C isotopic ratios for 11 SIO stations. Trends: A Compendium of Data on Global Change (Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, US Department of Energy, Oak Ridge, TN). Available at http://cdiac.ornl.gov/ftp/trends/co2/iso-sio/
- 46.O’Leary MH. Carbon isotope fractionation in plants. Phytochemistry. 1981;20(4):553–567. [Google Scholar]
- 47.Farquhar GD, Richards RA. Isotopic composition of plant carbon correlates with water use efficiency of wheat genotypes. Aust J Plant Physiol. 1984;11(6):539–552. [Google Scholar]
- 48.Farquhar GD, Ehleringer JR, Hubick KT. Carbon isotope discrimination and photosynthesis. Annu Rev Plant Physiol Plant Mol Biol. 1989;40:503–537. [Google Scholar]
- 49.Farquhar GD, Sharkey TD. Stomatal conductance and photosynthesis. Annu Rev Plant Physiol Plant Mol Biol. 1982;33:317–345. [Google Scholar]
- 50.Medlyn BE, et al. Stomatal conductance of forest species after long-term exposure to elevated to CO2 concentration: A synthesis. New Phytol. 2001;149(2):247–264. doi: 10.1046/j.1469-8137.2001.00028.x. [DOI] [PubMed] [Google Scholar]
- 51.Ellsworth DS, et al. Elevated CO2 effects on photosynthetic responses to light and [CO2] over ten years: A synthesis from Duke FACE. Glob Change Biol. 2012;18(1):223–242. [Google Scholar]
- 52.Darbah JNT, et al. Will photosynthetic capacity of aspen trees acclimate after long-term exposure to elevated CO2 and O3? Environ Pollut. 2010;158(4):983–991. doi: 10.1016/j.envpol.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 53.Cregg BM. Leaf-area estimation of mature foliage of Juniperus. For Sci. 1992;38(1):61–67. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.