Significance
Prognosis of pancreatic ductal adenocarcinoma (PDAC) remains very poor, with 5-y survival less than 10%. Unfortunately, adjuvant therapies are only weakly effective, and only one molecular targeted drug, a kinase inhibitor of epidermal growth factor receptor (EGFR), has been clinically approved. Hence, we examined antibodies to EGFR and to its sibling, HER2. While single antibodies to these receptors only weakly retarded growth of human PDAC in animals, significantly stronger inhibition was observed when we combined two antibodies to EGFR or two antibodies to HER2. Similarly, simultaneous inhibition of both EGFR and HER2, using a pair of antibodies, resulted in enhanced efficacy. These observations predict that targeting EGFR and HER2, by using pairs of monoclonal antibodies, might translate to better treatment of PDAC patients.
Keywords: combination therapy, signal transduction
Abstract
Due to intrinsic aggressiveness and lack of effective therapies, prognosis of pancreatic cancer remains dismal. Because the only molecular targeted drug approved for pancreatic ductal adenocarcinoma is a kinase inhibitor specific to the epidermal growth factor receptor (EGFR), and this receptor collaborates with another kinase, called HER2 (human EGF-receptor 2), we assumed that agents targeting EGFR and/or HER2 would effectively retard pancreatic ductal adenocarcinoma. Accordingly, two immunological strategies were tested in animal models: (i) two antibodies able to engage distinct epitopes of either EGFR or HER2 were separately combined, and (ii) pairs of one antibody to EGFR and another to HER2. Unlike the respective single monoclonal antibodies, which induced weak effects, both types of antibody combinations synergized in animals in terms of tumor inhibition. Immunological cooperation may not depend on receptor density, antigenic sites, or the presence of a mutant RAS protein. Nevertheless, both types of antibody combinations enhanced receptor degradation. Future efforts will examine the feasibility of each strategy and the potential of combining them to achieve sustained tumor inhibition.
Pancreatic cancer is the fourth leading cause of cancer death in western countries, with a 5-y survival of less than 10% (1). Genomic characterization of pancreatic ductal adenocarcinoma (PDAC), which accounts for over 90% of pancreatic cancer, identified multiple significantly mutated genes, including KRAS, TP53, CDKN2A, and SMAD4, and uncovered novel mutated genes (2). Advances in neoadjuvant and adjuvant chemotherapeutic regimens have resulted in some improvement in PDAC treatment outcome, but pancreatectomy remains the single most effective treatment modality for pancreatic cancer. A distinguishing molecular feature of PDAC is the presence of activating KRAS mutations in over 90% of tumors (3). Along with an ability to overcome inflammation-induced senescence (4), mutants of the RAS gene inevitably up-regulate a plethora of growth factors (e.g., TGF-alpha) and cytokines (e.g., interleukin-8), which likely contribute to disease progression. In line with this possibility, genetically engineered mouse models indicate that development of PDACs driven by KRAS is dependent on EGFR signaling (5). In the same vein, a small-molecule inhibitor of the epidermal growth factor receptor (EGFR) has been approved for the treatment of PDAC (6). Several studies reported high expression of EGFR, ranging from 7.7% to 100% of PDACs, but the abundance of ErbB-2/HER2, the oncogenic kin of EGFR, is relatively low (7). Notably, in response to ligand binding, EGFR forms heterodimers with HER2, and these complexes are characterized by enhanced signaling due to evasion of receptor endocytosis and degradation (8). Hence, simultaneous targeting of both EGFR and HER2 is a logical extension of the biochemistry of ErbB/HER signaling.
Along with low molecular weight kinase inhibitors specific to EGFR and HER2, monoclonal antibodies (mAbs) against these receptors are routinely used in oncology wards to treat breast, gastric, colorectal, and head and neck carcinomas (9). Therapeutic antibodies may recruit the effector arm of the host cellular immune defense mechanism (10, 11). In addition, they might inhibit tumor cell proliferation by interfering with ligand binding, or by blocking receptor dimerization (12–14). An important feature of therapeutic anti-EGFR and anti-HER2 mAbs is their ability to collaborate with chemotherapeutic drugs (15, 16). However, another way to improve the efficacy of mAbs to surface receptors comprises combinations of two or more mAbs, each targeting a distinct receptor’s epitope. For example, it has been reported that certain pairs of anti-EGFR antibodies can accelerate receptor endocytosis and degradation (17), probably through a mechanism involving inhibition of receptor recycling (18). Consistent with these observations, a mixture of two anti-EGFR mAbs, called Sym004, inhibited cancer cell growth (14). Similarly, synergistic antitumor effects of mAbs directed to the rodent form of HER2 associated the therapeutic effect with enhanced receptor degradation (19), and synergistic effects mediated by the human HER2 protein were later confirmed (11, 20). However, both immune mechanisms involving recruitment of killer T cells (11) and nonimmune modes of action, involving growth arrest and receptor degradation (21), have been implicated in the mechanism underlying the antitumor effect of mAbs specific to HER2. Importantly, a mixture of two mAbs to HER2, trastuzumab and pertuzumab, in combination with chemotherapy, was found to significantly prolong progression-free survival of HER2-overexpressing breast cancer patients (22).
The present study has been motivated by the lack of effective molecular targeted drugs to treat PDAC. We applied on xenografts of PDAC two immunological strategies: the first combined two antibodies to the same receptor, either EGFR or HER2, in similarity to our recent study that applied pairs of anti-EGFR antibodies on triple negative breast cancer (23). The other strategy combined two antibodies, one to EGFR and the other to HER2, in similarity to reports by Azria, Pelegrin, and coworkers, who combined two antibodies (24) and also added a third agent, namely a tyrosine kinase inhibitor (25). Here, we compare the two types of antibody combinations and also highlight potential mechanisms of the synergy observed in animals bearing human PDAC xenografts.
Results
Synergistic Inhibition of Pancreatic Carcinoma BXPC3 Xenografts by Homocombinations of Antibodies to Either EGFR or HER2.
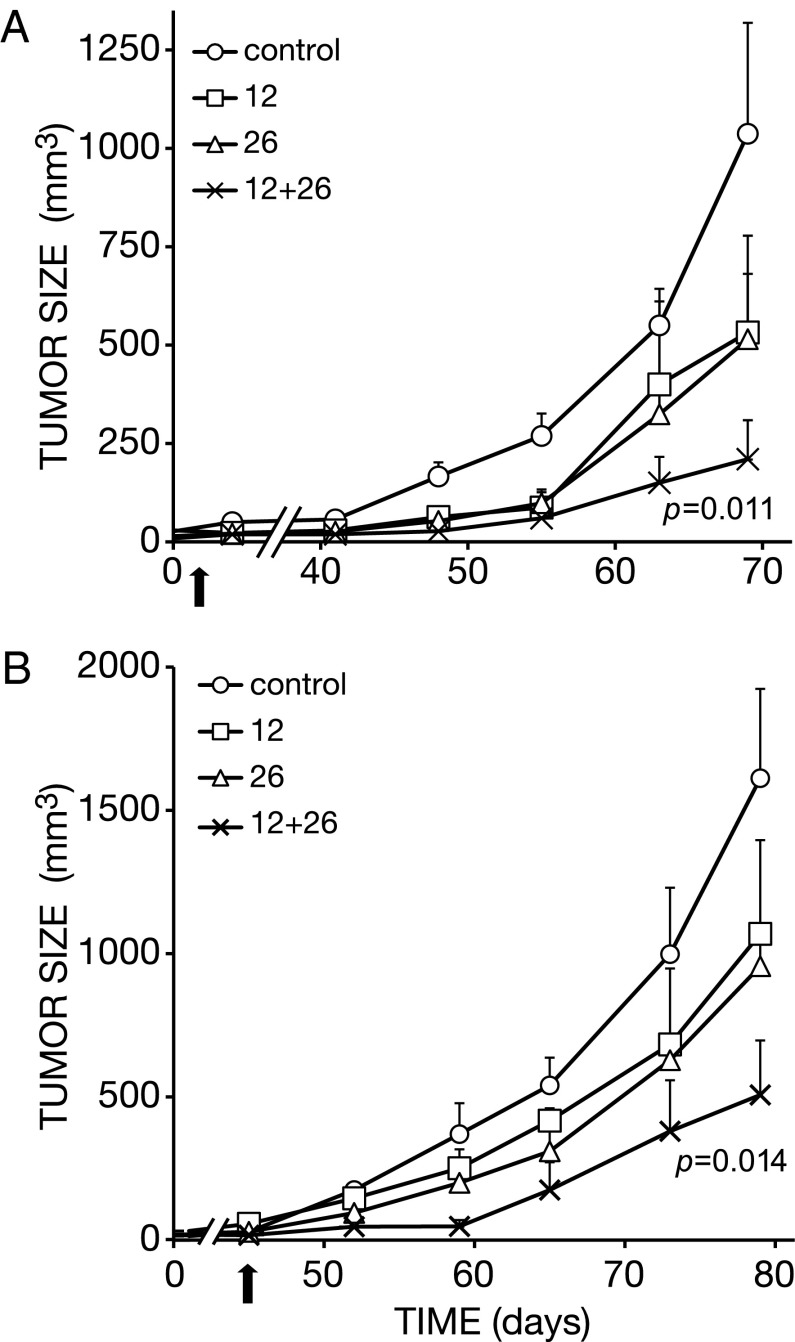
In similarity to their effects on xenografts of triple-negative breast cancer (23), when singly applied on the PDAC-derived BXPC3 human cell line, anti-EGFR mAbs 111 and 565 weakly inhibited tumorigenic growth in animals, although still better than an anti-HER2 (mAb-12) (Fig. 1A). This experiment used mice bearing palpable tumors and seven injections of mAbs (0.16 mg per animal, per injection). As expected, the combination of mAbs 111 and 565 was more effective than each mAb alone (note that the combination of mAbs used the same total amount of antibody as the single mAb treatments). The superiority of the combination reached statistical significance already after five weekly injections of the antibody mixture (P = 0.001; Fig. 1B). Another parameter that reflected the advantage of the combined treatment was the fraction of tumor-free animals: Whereas 100% of control animals and ∼75% of single mAb-treated mice developed large tumors, the majority of doubly treated mice remained tumor-free after three weekly injections (P = 0.035; Fig. 1C).
Fig. 1.
Pairs of epitope-distinct antibodies to EGFR, more than the respective single mAbs, inhibit tumorigenic growth of human pancreatic cancer cells in animals. (A) Human BXPC3 pancreatic cancer cells (5 × 106) were injected into the flank of female CD1 athymic mice. Once tumors became palpable, mice were randomized into groups of 7–8 and intraperitoneally injected seven times (once weekly), with either anti-EGFR mAbs (111 and 565; 160 µg per mouse per injection; total: 1,120 µg per mouse), or with mAb-12, an anti-HER2 antibody (160 µg per mouse per injection). The control group received saline injections. Tumor growth was monitored and the results (average volume ± SEM) are presented. The arrow marks the last injection. (B) Mice were treated as in A, except that only anti-EGFR antibodies were used and five injections were performed on days 8, 14, 18, 22, and 36 after randomization of tumor-bearing animals. Note that one group received a combination of two anti-EGFR mAbs at 1:1 ratio, but the total antibody dose per injection was kept unchanged (160 µg per injection; total: 800 µg per mouse). The P value refers to the effect of the combination compared with the control group. (C) Mice were treated as in B, except that three antibody injections were performed (on days 8, 14, and 18) and the numbers of tumor-free mice were determined on day 56 from the first injection.
We previously tested the antitumorigenic effects of single anti-HER2 mAbs, as well as pairs of antibodies to HER2, in mice bearing gastric tumors of human origin (21). The results indicated that combinations of mAbs engaging distinct, nonoverlapping epitopes of HER2 cooperatively retard tumor growth in animals. Using one such combination of epitope-distinct mAbs, namely antibodies 12 and 26, which were injected either three times (in intervals of 5–7 d) or seven times, we confirmed that synergy extends to the BXPC3 pancreatic xenografts (Fig. 2 A and B). Importantly, BXPC3 cells express ∼10-fold higher levels of EGFR relative to HER2 (24). Nevertheless, in complete analogy to anti-EGFR antibodies (Fig. 1), mAbs 12 and 26 induced relatively small effects on BXPC3 tumors when singly used, but their combination better inhibited BXPC3 tumors after three (P = 0.011; Fig. 2A) or seven (P = 0.014; Fig. 2B) injections. Likewise, we tested another pair of nonoverlapping anti-HER2 antibodies, namely 12 and 431, and observed superiority of the mixture. In conclusion, in similarity to xenografts of other cancer origins, BXPC3 tumors can be effectively inhibited in animals using mixtures of mAbs to either EGFR or HER2, despite the marked difference in surface expression displayed by these receptors.
Fig. 2.
Pairs of epitope-distinct antibodies to HER2, more than the respective single mAbs, inhibit tumorigenic growth of human pancreatic cancer cells in animals. (A) Human BXPC3 pancreatic cancer cells (5 × 106) were injected into the flank of female athymic mice, and later randomized into groups of 7–8, as described in the legend to Fig. 1. Mice were weekly injected, three times, into the peritoneum with the indicated anti-HER2 mAbs (160 µg per mouse per injection, total: 480 µg per mouse), or their combination (80 µg of each mAb per mouse per injection; total: 480 μg per mouse). Tumor volumes were monitored and the averages (±SEM) are presented, along with the P value of the comparison between single mAbs and their combination. The arrow marks the last injection. (B) Mice were treated as in A, except that seven injections of single antibodies (160 µg per mouse per injection, total: 1,120 µg per mouse ) or the combination (80 µg of each mAb per mouse per injection: total: 1,120 µg per mouse) were performed.
Synergistic Inhibition of Pancreatic Adenocarcinoma Xenografts by Mixtures of Two Antibodies, One Targeting EGFR and the Other Targeting HER2.
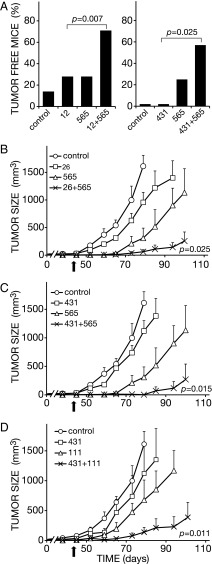
In light of the ability of EGFR and HER2 to form stable heterodimers (26, 27), and the observed antitumorigenic effects of our anti-EGFR and anti-HER2 antibodies, we assumed that mixtures comprising one of our antibodies to EGFR and another to HER2 would synergistically inhibit PDAC xenografts. Notably, a mixture of the humanized mAbs matuzumab (anti-EGFR) and trastuzumab (anti-HER2) displayed synergistic effects in animals (24), raising the possibility that synergy can be obtained with several distinct antibody mixtures. To test this prediction, we used several different mixtures: (i) mAb 565 (to EGFR) combined with mAb 12 (to HER2), (ii) mAb 565 and either mAb 431 or mAb 26 (both to HER2), and (iii) the mAbs 111 (to EGFR) and 431. Each antibody was delivered intraperitoneally into animals pretransplanted with BXPC3 cells. Antibody injections were performed seven times over a period of 45 d, and antibodies were used either alone or in combination with another mAb. Differences, in terms of the fractions of tumor-free animals, were observed already after three injections (for an example, see Fig. 3A): The majority of mice treated with antibody mixtures remained tumor-free, whereas 70–80% of mice treated with individual mAbs developed large tumors. Interestingly, when singly delivered, the HER2-specific mAb 431 conferred no protective effect, but it doubled the protective action of mAb 565 to EGFR.
Fig. 3.
Heterocombinations of antibodies to EGFR and HER2 are endowed with enhanced antitumor activities. (A) BXPC3 xenografts were implanted and mice randomized into groups of eight animals, essentially as in the legend to Fig. 1. The indicated antibodies to HER2 (431 and 12) and the 565 antibody to EGFR, were injected into the peritoneum either singly (160 µg per mouse per injection, total: 480 µg per mouse) or in combinations (80 µg of each mAb per mouse per injection; total: 480 µg per mouse). Three injections were performed on days 7, 14, and 21, and the fractions of tumor-free mice were determined on day 84. (B–D) Mice bearing palpable BXPC3 tumors were randomized and each group of eight animals was injected with the indicated mAbs seven times, at 8 d intervals, into the peritoneum. Antibodies were used either singly (160 µg per mouse per injection, total: 1,120 µg per mouse) or in combinations (80 µg of each mAb per mouse per injection; total: 1,120 µg per mouse). Tumor volumes were monitored and their average ± SEM are presented, along with the P values comparing combined treatments with single mAb injections. The arrow in each panel marks the last injection of antibodies.
The time course of tumor development provided further support to the overall superiority of antibody mixtures, which seems to be independent of the identity of partnering mAbs (Fig. 3B–D). Thus, the combination of mAbs 565 and 26 better inhibited BXPC3 tumors than each antibody alone (P = 0.025; Fig. 3B), and this was true also for the 565 plus 431 combination (P = 0.015; Fig. 3C), as well as the 111 plus 431 mixture (P = 0.011; Fig. 3D). Because all antibody mixtures we tested consistently inhibited tumor growth better than the respective single antibodies, we propose that antitumor synergy may not rely on specific epitopes of EGFR or HER2. In other words, simultaneous engagement of any site of EGFR and HER2 might fulfill the requirement for synergy, probably due to the intrinsic propensity of EGFR and HER2 to form heterodimers (Discussion).
The KRAS-Mutated PANC-1 Xenograft of Pancreatic Adenocarcinoma Is Sensitive to Mixture of Two Nonoverlapping Antibodies to HER2.
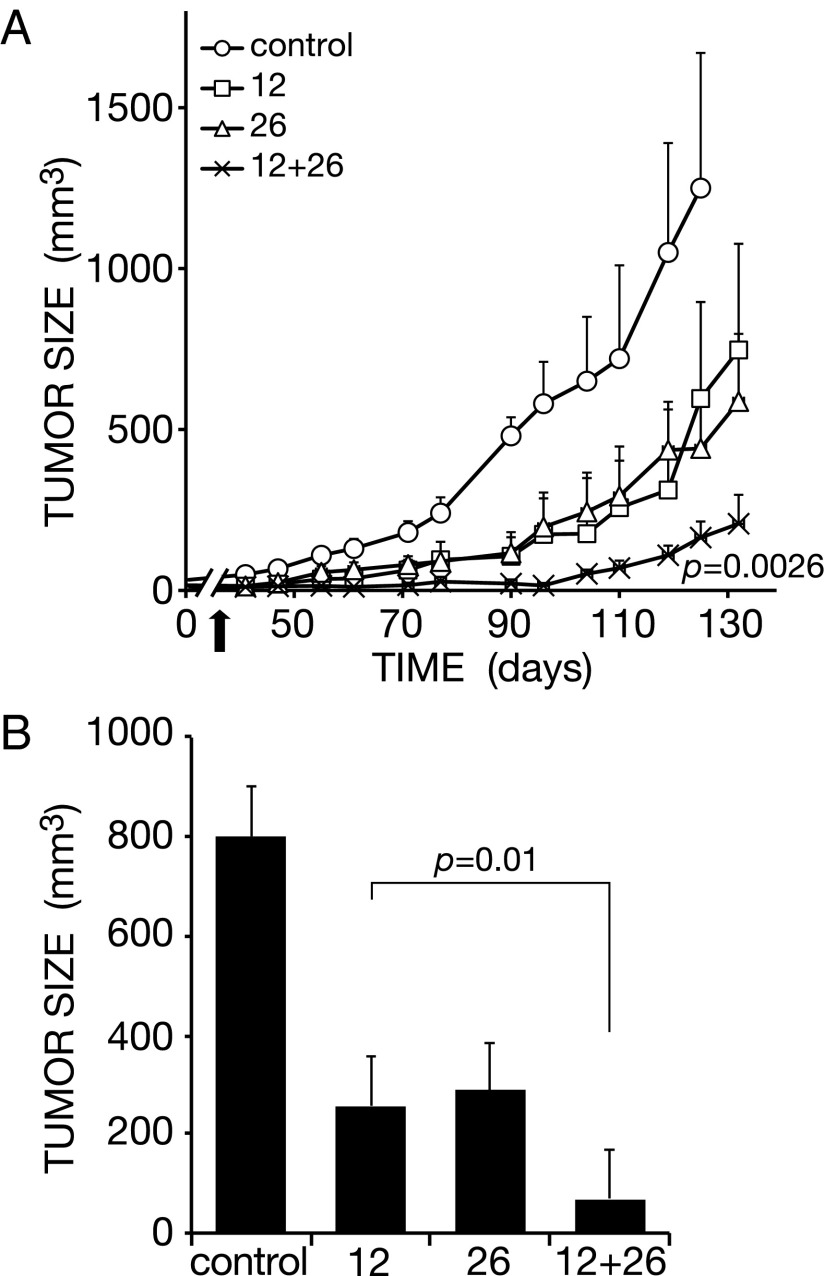
The BXPC3 xenograft model we used to this point is driven by a combination of oncogenic mutations other than the most prevalent one affecting KRAS (3). Hence, as an initial step aimed at examining relevance of our findings to human PDACs driven by mutated forms of KRAS, we tested the ability of a combination of antibodies to HER2 to inhibit tumors of PANC-1 cells, which carry a mutant KRAS allele. The experiment randomized mice bearing palpable lesions of PANC-1 cells into several groups, which were treated three times with the anti-HER2 mAbs 12 and 26, either alone or in combination. The results we obtained clearly reflected enhanced inhibitory activity of the mixture of antibodies (P = 0.0026; Fig. 4 A and B), although each mAb, when singly injected, elicited a partial effect. Because HER2 is weakly expressed at the surface of PANC-1 cells, we expect that similar combinations of epitope-distinct mAbs to EGFR, which displays higher surface expression, would similarly inhibit tumorigenic growth in animals.
Fig. 4.
A pair of epitope-distinct antibodies to HER2 inhibits tumorigenic growth of Ras-mutated pancreatic cancer xenografts in animals. (A) Human PANC-1 pancreatic cancer cells (5 × 106) expressing a mutant K-Ras were injected into the flank of athymic mice. Once tumors became palpable, mice were randomized into groups of 7–8 and intraperitoneally injected, once weekly (3 times), with the indicated anti-HER2 mAbs, either singly (160 µg per mouse per injection, total: 480 µg per mouse) or in combination (80 µg per mouse per injection for each mAb, total: 480 µg per mouse). The control group received saline injections. Tumor growth was monitored and the results (average volume ± SEM) are presented. The P values for N12, L26, and the combination vs. control are 0.03, 0.04, and 0.0026, respectively. The arrow marks the last injection of antibodies. (B) Average (±SEM) tumor sizes from A were determined on day 110 and presented as a histogram.
Homo- and Heterocombinations of Antibodies to EGFR and HER2 Might Share the Ability to Down-Regulate Surface Expression of Their Targets.
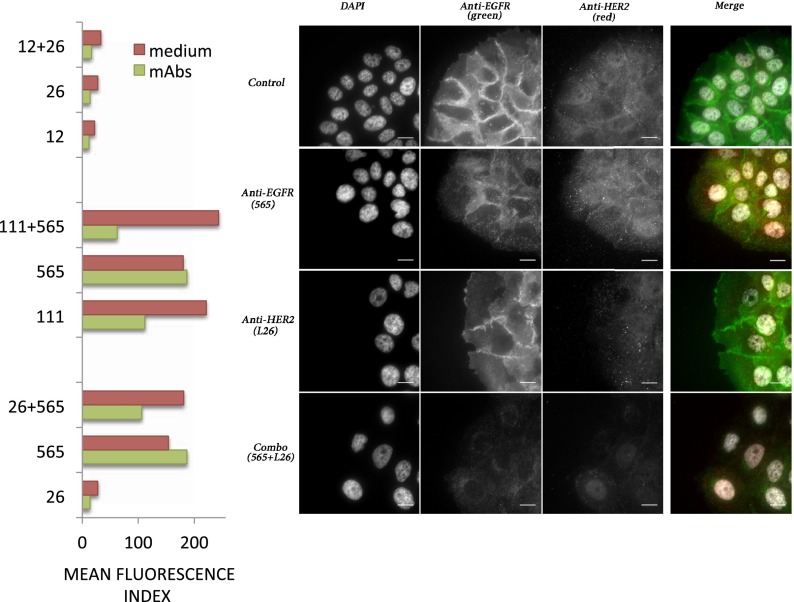
Previous studies attributed the synergistic effects of combinations of two antibodies engaging nonoverlapping epitopes on the same receptor (homocombinations) to either enhanced ability to recruit killer lymphocytes (28), or to antibody-mediated internalization and degradation of the target receptor (14, 17, 21). These observations raised the possibility that antibody-induced down-regulation of EGFR and/or HER2 underlies the synergistic action of mAb mixtures able to simultaneously target EGFR and HER2 (heterocombinations). To examine this potential mechanism of antibody collaboration, we used fluorescence-activated cell sorting (FACS) and a labeled anti-mouse secondary antibody. The results we obtained confirmed higher expression of EGFR relative to HER2 (five- to eightfold; Fig. 5A, EGFR down-regulation by mAb 565 is initiated at around 48 h and the receptor is sometimes still intact at that time point). In addition, the results raised the possibility that both the homocombinations (mAbs 12 and 26 to HER2 and mAbs 111 and 565 to EGFR) and the 26 plus 565 heterocombination can reduce surface expression of EGFR and/or HER2 (Fig. 5A).
Fig. 5.
Both homo- and heterocombinations of mAbs to EGFR and HER2 promote receptor down-regulation. (Left) BXPC3 cells were incubated for 48 h with medium alone or with medium containing the indicated mAbs (either singly or in combinations). The average mean fluorescence index of triplicate samples, as derived from cell sorting analyses, is presented (SEM < 10%). (Right) BXPC3 cells growing on fibronectin-coated coverslips were treated for 48 h with the indicated mAbs to EGFR (mAb 565) or to HER2 (mAb 26), either singly or in combination. Thereafter, cells were washed, permeabilized, and fixed. Shown are the results of staining with antibodies to EGFR (green) and HER2 (red), as well as counterstaining with DAPI. Note the low abundance of HER2 relative to EGFR.
To individually address each receptor, we followed the fate of mAb-bound receptors in BXPC3 cells incubated for 48 h in the absence or presence of single mAbs or their combinations. Following permeabilization and fixation, cells were counterstained with DAPI to visualize nuclei. Thereafter, cells were labeled with mAbs to EGFR or HER2, and this was followed by a fluorescein-tagged secondary anti-mouse Ig that tagged EGFR (green) and HER2 (red). The resulting immunofluorescence photos are depicted in Fig. 5B and Fig. S1. Unlike EGFR, which displayed strong signals at cell–cell junctions, HER2 exhibited weaker membranal and cytoplasmic signals. In accordance with their selectivity, mAb 565 reduced the junctional EGFR signal and increased punctate cytoplasmic staining, while not affecting HER2, and mAb 26 specifically reduced the HER2 signal. As expected, a marked elimination of EGFR was observed after treatment with the homocombination of mAbs 111 and 565, as is also evident from the HER2 (red) staining, now undisturbed by the green signal of EGFR. Surprisingly, however, the homocombination of anti-HER2 mAbs 12 and 26 reduced not only the HER2 signal but also the signal of EGFR, suggesting interrelationships or physical associations between EGFR and HER2. Perhaps most important, we found that the heterocombination 26 plus 565, almost completely erased the signals of both EGFR and HER2, consistent with the marked synergy observed in animals (Fig. 3B). Taken together, these observations propose that both homo- and heterocombinations of mAbs to EGFR and HER2 harness the process of receptor internalization and degradation, a physiologically important mode of negative feedback regulation (29), to inhibit tumorigenic growth of pancreatic adenocarcinomas.
In summary, we applied two different approaches of combination immunotherapy on PDAC animal models, and learned that both offer benefits beyond the efficacy achieved by the respective single antibodies. Because we observed synergistic inhibitory effects when examining several distinct antibody combinations, it seems that the underlying mechanism is independent from antibody’s and epitope’s identity, and may not require receptor overexpression. Our initial mechanistic studies imply that these therapeutic strategies share an ability to remove the target receptor from the surface of cancer cells, but they might differ in terms of the underlying mechanism of forced internalization.
Discussion
Passive cancer immunotherapies targeting specific tumor antigens, like EGFR and HER2, are designed to mimic the immune system’s response to foreign invaders. However, unlike the currently used single mAbs, the antibody arm of the immune system almost invariably employs polyclonal antibodies. In addition, several distinct antigens are often simultaneously targeted by the natural immune response. Conceivably, these features of the immune system, if properly mimicked by combinations of therapeutic mAbs, would increase the efficacy of cancer therapy. Accordingly, the present study compared single mAbs with either combinations of two antibodies to the same surface protein (i.e., homocombinations) or with combinations of two mAbs each targeting a distinct antigen (heterocombinations). It is notable that the total dose of antibody was kept equal for proper comparisons. We focused on ErbB/HER antigens and PDAC because of two reasons: First, new PDAC-targeting drugs are dreadfully needed. Second, previous reports indicated that simultaneous targeting of EGFR and HER2 in PDAC models is beneficial (24), and similarly combining two antibodies to either EGFR (14, 17) or to human HER2 (11, 20, 21) increased therapeutic efficacy in non-PDAC models of cancer.
The present study has shown that combining two mAbs to the same ErbB protein synergistically inhibits PDAC-derived xenografts. Previous studies established similar effects on gastric (20, 21) and breast cancer (11, 14, 30), including the aggressive triple negative subtype (23). Importantly, synergy requires engagement of two nonoverlapping antigenic sites (17, 18), and occupation of the ligand-binding site of EGFR or the dimerization site of HER2 might enhance synergy (14, 21). Unlike homocombinations of mAbs, which have been extensively studied and already reached clinical application (22), studies of antibody heterocombinations are relatively new. Along with the demonstration herein and previously (24) of enhanced efficacy of anti-EGFR and anti-HER2 heterocombinations, it has been reported that a conventional antibody molecule with dual ErbB-3/EGFR specificity was more broadly efficacious in multiple tumor models, compared with monospecific antibodies (31). Similarly, mixtures of an antibody to HER3 and either an anti-EGFR (32) or an anti-HER2 mAb (33), prolonged and enhanced the antitumor response. Decorating tumor cells by means of antibodies directed to two rather than one receptor type might enhance the efficacy of antibody-dependent cellular cytotoxicity (ADCC). However, the synergy observed when combining two antibodies often exceeds the predicted twofold enhancement. Another reason to suspect additional, non-ADCC or complement-mediated mechanisms derives from the observed partial effect of trastuzumab in mice lacking certain fragment crystalizable (Fc) receptor molecules (10). In the same vein, bivalent fragments of anti-EGFR and anti-HER2 antibodies retained synergy, despite absence of the Fc tails (25).
Several nonimmunological mechanisms have been proposed as key mediators of cancer immunotherapy, including interference with growth factor binding, interception of survival pathways and antibody-induced endocytosis of the surface antigens (9). The antitumor actions of homocombinations of mAbs to EGFR and HER2 have been associated with forced internalization and subsequent receptor degradation in lysosomes (14, 17, 21). Furthermore, this mechanism might entail collapse of large antigen-antibody lattices into endocytic vesicles, which use clathrin- as well as caveolin-mediated trafficking and avoid a recycling pathway that governs trafficking of EGFR bound to a single mAb (18, 23). Interestingly, the data we present in Fig. 5 propose that heterocombinations of mAbs to EGFR and HER2 also harness the endocytic machinery, probably by means of receptor crosstalk. Although single mAbs induced partial disappearance of their direct targets, the combination of a mAb to EGFR and another to HER2 almost completely eliminated both receptors. Notably, a similar observation was made using a different set of mAbs (25), and another report documented the ability of an anti-HER2 antibody to increase EGFR endocytosis, probably by dissociating preformed EGFR-HER2 complexes (34). Hence, it is conceivable that heterocombinations of mAbs interfere with the previously reported inclination of EGFR-HER2 heterodimers to avoid the degradative fate by enhancing receptor recycling (35, 36). If correct, both homo- and heterocombinations of therapeutic antibodies use the endocytic machinery to remove oncogenic receptors from the cell surface, and thereby retard tumor growth. Because our studies suggest that distinct molecular mechanisms of endocytosis are recruited by homo- and heterocombinations of antibodies, they raise an intriguing possibility: combining homo- and heterocombinations of mAbs (e.g., two nonoverlapping mAbs to EGFR and two nonoverlapping mAbs to HER2) might augment therapeutic efficacy and also overcome patient resistance to a single antibody.
Materials and Methods
Materials.
Monoclonal antibodies to EGFR/ErbB-1 or HER2/ErbB-2 were purified on protein A/G Plus-Aagarose, purchased from Jackson ImmunoResearch Labs. PE-conjugated affinity purified F(ab’)2 donkey anti-mouse IgG (heavy and light chains), were used in flow cytometry analyses. For immunostaining, we used a mAb anti-EGFR antibody from Alexis, and a rabbit polyclonal anti-HER2 antibody from Santa Cruz Biotechnology. Fluorescein-tagged secondary antibodies were purchased from Invitrogen.
Inhibition of Tumor Xenografts in Animals.
In vivo experiments used 6- to 8-wk-old athymic, female CD1 nude mice. Mice were injected intradermally with BXPC3 (5 × 106) cells. Tumor-bearing mice, randomized into groups of 7–8 mice, were treated by three or seven weekly i.p. injections of mAbs (160 µg), their combinations (80 + 80 µg), or PBS as control. Tumor growth was followed by means of weekly measurements of tumor volume with a caliper, using the equation D1 × D2 × D3 × 0.623 (D refers to dimension). Average tumor sizes in each group ± SEM are presented. For simplicity, mAbs N12, L26, and L431 are denoted 12, 26, and 431. Mice were killed when tumor size reached the authorized end-point of 1.5 cm3. All animal protocols were approved by the Institutional Animal Care and Use Committee of the Weizmann Institute.
Cell Sorter Analyses.
Cell surface levels were quantified using fluorescence-activated cell sorting (FACS). Receptor levels in untreated cells were measured by incubating cells in medium alone for 48 h at 37 °C, followed by gentle trypsinization, and pulse labeling with mAbs (1 µg per sample). A parallel group of cells was similarly incubated in the presence of single mAbs (10 µg/mL) or the combinations (each mAb at 5 µg/mL). Cell samples (0.5 × 106 per 0.05 mL) were then incubated for 30 min at 4 °C in the dark for staining with a fluorescein-tagged anti-mouse antibody (1 µg per sample). Bound antibodies were detected and cells analyzed using FACSORT (BD).
Immunofluorescent Cell Staining.
BXPC3 cells (40 × 103) were treated for 48 h at 37 °C with single mAbs (10 μg/mL), or with mAb combinations (each mAb at 5 μg/mL). Cells were washed, permeabilized (0.3% Triton X-100 for 5 min), and fixed [in 3% (vol/vol) paraformaldehyde]. Cells were stained with mouse monoclonal anti-EGFR or rabbit polyclonal anti-HER2 antibodies, followed by a fluorescein-tagged secondary antibody (Alexafluor, from Invitrogen). Images were acquired using a fluorescence microscope, and their analysis was performed using the DeltaVision System (Applied Precision) with a 60×/1.4 objective.
Statistical Analysis.
Two-way ANOVA multiple comparison, Fisher’s Exact, and Student t test (two-tailed) were used to analyze differences between groups. P values below 0.05 were considered significant.
Supplementary Material
Acknowledgments
We thank Dr. Rachel Heibloom for statistical analyses. This work was supported by grants from the US National Cancer Institute (CA072981), the European Research Council, the Seventh Framework Program of the European Commission, the German–Israeli Project Cooperation (DIP), the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation, the M.D. Moross Cancer Institute, and the Julius Baer Trust; and a Dukler Mudy Grant. G.M. acknowledges the support of the Sergio Lombroso Foundation. M.S. is the incumbent of the W. Garfield Weston Chair. Y.Y. is a Research Professor of the Israel Cancer Research fund, and the incumbent of the Harold and Zelda Goldenberg Professorial Chair.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1313857110/-/DCSupplemental.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Biankin AV, et al. Australian Pancreatic Cancer Genome Initiative Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012;491(7424):399–405. doi: 10.1038/nature11547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Almoguera C, et al. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988;53(4):549–554. doi: 10.1016/0092-8674(88)90571-5. [DOI] [PubMed] [Google Scholar]
- 4.Lee KE, Bar-Sagi D. Oncogenic KRas suppresses inflammation-associated senescence of pancreatic ductal cells. Cancer Cell. 2010;18(5):448–458. doi: 10.1016/j.ccr.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Navas C, et al. EGF receptor signaling is essential for k-ras oncogene-driven pancreatic ductal adenocarcinoma. Cancer Cell. 2012;22(3):318–330. doi: 10.1016/j.ccr.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore MJ, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: A phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25(15):1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 7.Saxby AJ, et al. Assessment of HER-2 status in pancreatic adenocarcinoma: Correlation of immunohistochemistry, quantitative real-time RT-PCR, and FISH with aneuploidy and survival. Am J Surg Pathol. 2005;29(9):1125–1134. doi: 10.1097/01.pas.0000160979.85457.73. [DOI] [PubMed] [Google Scholar]
- 8.Citri A, Yarden Y. EGF-ERBB signalling: Towards the systems level. Nat Rev Mol Cell Biol. 2006;7(7):505–516. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- 9.Ben-Kasus T, Schechter B, Sela M, Yarden Y. Cancer therapeutic antibodies come of age: Targeting minimal residual disease. Mol Oncol. 2007;1(1):42–54. doi: 10.1016/j.molonc.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6(4):443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 11.Spiridon CI, et al. Targeting multiple Her-2 epitopes with monoclonal antibodies results in improved antigrowth activity of a human breast cancer cell line in vitro and in vivo. Clin Cancer Res. 2002;8(6):1720–1730. [PubMed] [Google Scholar]
- 12.Klapper LN, et al. A subclass of tumor-inhibitory monoclonal antibodies to ErbB-2/HER2 blocks crosstalk with growth factor receptors. Oncogene. 1997;14(17):2099–2109. doi: 10.1038/sj.onc.1201029. [DOI] [PubMed] [Google Scholar]
- 13.Klapper LN, Waterman H, Sela M, Yarden Y. Tumor-inhibitory antibodies to HER-2/ErbB-2 may act by recruiting c-Cbl and enhancing ubiquitination of HER-2. Cancer Res. 2000;60(13):3384–3388. [PubMed] [Google Scholar]
- 14.Pedersen MW, et al. Sym004: A novel synergistic anti-epidermal growth factor receptor antibody mixture with superior anticancer efficacy. Cancer Res. 2010;70(2):588–597. doi: 10.1158/0008-5472.CAN-09-1417. [DOI] [PubMed] [Google Scholar]
- 15.Baselga J. Herceptin alone or in combination with chemotherapy in the treatment of HER2-positive metastatic breast cancer: pivotal trials. Oncology. 2001;61(Suppl 2):14–21. doi: 10.1159/000055397. [DOI] [PubMed] [Google Scholar]
- 16.Aboud-Pirak E, et al. Efficacy of antibodies to epidermal growth factor receptor against KB carcinoma in vitro and in nude mice. J Natl Cancer Inst. 1988;80(20):1605–1611. doi: 10.1093/jnci/80.20.1605. [DOI] [PubMed] [Google Scholar]
- 17.Friedman LM, et al. Synergistic down-regulation of receptor tyrosine kinases by combinations of mAbs: implications for cancer immunotherapy. Proc Natl Acad Sci USA. 2005;102(6):1915–1920. doi: 10.1073/pnas.0409610102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spangler JB, et al. Combination antibody treatment down-regulates epidermal growth factor receptor by inhibiting endosomal recycling. Proc Natl Acad Sci USA. 2010;107(30):13252–13257. doi: 10.1073/pnas.0913476107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drebin JA, Link VC, Greene MI. Monoclonal antibodies reactive with distinct domains of the neu oncogene-encoded p185 molecule exert synergistic anti-tumor effects in vivo. Oncogene. 1988;2(3):273–277. [PubMed] [Google Scholar]
- 20.Kasprzyk PG, Song SU, Di Fiore PP, King CR. Therapy of an animal model of human gastric cancer using a combination of anti-erbB-2 monoclonal antibodies. Cancer Res. 1992;52(10):2771–2776. [PubMed] [Google Scholar]
- 21.Ben-Kasus T, Schechter B, Lavi S, Yarden Y, Sela M. Persistent elimination of ErbB-2/HER2-overexpressing tumors using combinations of monoclonal antibodies: Relevance of receptor endocytosis. Proc Natl Acad Sci USA. 2009;106(9):3294–3299. doi: 10.1073/pnas.0812059106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baselga J, et al. CLEOPATRA Study Group Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366(2):109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferraro DA, et al. Inhibition of triple-negative breast cancer models by combinations of antibodies to EGFR. Proc Natl Acad Sci USA. 2013;110(5):1815–1820. doi: 10.1073/pnas.1220763110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larbouret C, et al. In vivo therapeutic synergism of anti-epidermal growth factor receptor and anti-HER2 monoclonal antibodies against pancreatic carcinomas. Clin Cancer Res. 2007;13(11):3356–3362. doi: 10.1158/1078-0432.CCR-06-2302. [DOI] [PubMed] [Google Scholar]
- 25.Larbouret C, et al. In pancreatic carcinoma, dual EGFR/HER2 targeting with cetuximab/trastuzumab is more effective than treatment with trastuzumab/erlotinib or lapatinib alone: implication of receptors’ down-regulation and dimers’ disruption. Neoplasia. 2012;14(2):121–130. doi: 10.1593/neo.111602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldman R, Levy RB, Peles E, Yarden Y. Heterodimerization of the erbB-1 and erbB-2 receptors in human breast carcinoma cells: a mechanism for receptor transregulation. Biochemistry. 1990;29(50):11024–11028. doi: 10.1021/bi00502a002. [DOI] [PubMed] [Google Scholar]
- 27.Wada T, Qian XL, Greene MI. Intermolecular association of the p185neu protein and EGF receptor modulates EGF receptor function. Cell. 1990;61(7):1339–1347. doi: 10.1016/0092-8674(90)90697-d. [DOI] [PubMed] [Google Scholar]
- 28.Spiridon CI, Guinn S, Vitetta ES. A comparison of the in vitro and in vivo activities of IgG and F(ab')2 fragments of a mixture of three monoclonal anti-Her-2 antibodies. Clin Cancer Res. 2004;10(10):3542–3551. doi: 10.1158/1078-0432.CCR-03-0549. [DOI] [PubMed] [Google Scholar]
- 29.Zwang Y, Yarden Y. Systems biology of growth factor-induced receptor endocytosis. Traffic. 2009;10(4):349–363. doi: 10.1111/j.1600-0854.2008.00870.x. [DOI] [PubMed] [Google Scholar]
- 30.Scheuer W, et al. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res. 2009;69(24):9330–9336. doi: 10.1158/0008-5472.CAN-08-4597. [DOI] [PubMed] [Google Scholar]
- 31.Schaefer G, et al. A two-in-one antibody against HER3 and EGFR has superior inhibitory activity compared with monospecific antibodies. Cancer Cell. 2011;20(4):472–486. doi: 10.1016/j.ccr.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Schoeberl B, et al. An ErbB3 antibody, MM-121, is active in cancers with ligand-dependent activation. Cancer Res. 2010;70(6):2485–2494. doi: 10.1158/0008-5472.CAN-09-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blackburn E, et al. A monoclonal antibody to the human HER3 receptor inhibits Neuregulin 1-beta binding and co-operates with Herceptin in inhibiting the growth of breast cancer derived cell lines. Breast Cancer Res Treat. 2012;134(1):53–59. doi: 10.1007/s10549-011-1908-1. [DOI] [PubMed] [Google Scholar]
- 34.Hughes JB, et al. Pertuzumab increases epidermal growth factor receptor down-regulation by counteracting epidermal growth factor receptor-ErbB2 heterodimerization. Mol Cancer Ther. 2009;8(7):1885–1892. doi: 10.1158/1535-7163.MCT-09-0291. [DOI] [PubMed] [Google Scholar]
- 35.Lenferink AE, et al. Differential endocytic routing of homo- and hetero-dimeric ErbB tyrosine kinases confers signaling superiority to receptor heterodimers. EMBO J. 1998;17(12):3385–3397. doi: 10.1093/emboj/17.12.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Worthylake R, Opresko LK, Wiley HS. ErbB-2 amplification inhibits down-regulation and induces constitutive activation of both ErbB-2 and epidermal growth factor receptors. J Biol Chem. 1999;274(13):8865–8874. doi: 10.1074/jbc.274.13.8865. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.