Abstract
Metabolic diseases such as obesity, type II diabetes, and dyslipidemia are a rising cause of mortality worldwide. The progression of many metabolic diseases is fundamentally regulated on the transcriptional level by a family of ligand-activated transcription factors, called nuclear receptors, which detect and respond to metabolic changes. Their role in maintaining metabolic homeostasis makes nuclear receptors an important pharmaceutical and dietary target. This review will present the growing evidence that flavonoids, natural secondary plant metabolites, are important regulators of nuclear receptor activity. Structural similarities between flavonoids and cholesterol derivatives combined with the promiscuous nature of most nuclear receptors provide a wealth of possibilities for pharmaceutical and dietary modulation of metabolism. While the challenges of bringing flavonoid-derived therapeutics to the market are significant, we consider this rapidly growing field to be an essential aspect of the functional food initiative and an important mine for pharmaceutical compounds.
Introduction
Metabolic diseases are a rapidly growing public health concern in the United States and worldwide.1,2 It is thought that the emerging sedentary lifestyles and high-calorie diets are too recent on an evolutionary time scale for human physiology to adapt.3 This incompatibility may underlie metabolic diseases such as obesity, type II diabetes, and dyslipidemia. While the fundamental dietary changes may ameliorate these disorders, life style changes are more difficult to establish and hard to sustain. It is therefore crucial to find complementary and alternative approaches to treat metabolic diseases.
Metabolism is a complex phenomenon regulated on multiple levels. In current practice, pharmaceutical inhibitors are designed to target rate-limiting enzymes, such as HMG-CoA reductase (HMGCR), which controls cholesterol synthesis. However, this strategy fails to consider the redundancy of metabolic pathways and long-term effects of such intervention. A distinctly different approach is to target the underlying transcriptional regulation of metabolic pathways, controlling the activity of dozens of enzymes, both known and unknown, in order to program well-defined metabolic phenotypes. Important targets in this approach are a family of ligand-activated transcription factors, called nuclear receptors (Table 1).
Table 1.
Human nuclear receptors and their agonists
| Name | Abbreviation | Symbol | Natural ligands | Drugs | Flavonoids |
|---|---|---|---|---|---|
| Constitutive androstane receptor | CAR | NR1I3 | Xenobiotics | Phenobarbital | |
| Estrogen receptor | ERα | NR3A1 | 17β-estradiol | Bazedoxifene | Daidzein148 |
| Lasofoxifene | Genistein149 | ||||
| Raloxifene | Naringenin104 | ||||
| Tamoxifen | |||||
| ERβ | NR3A2 | 17β-estradiol | Lasofoxifene | Daidzein148 | |
| Tamoxifen | Genistein149 | ||||
| Naringenin104 | |||||
| Farnesoid X receptor | FXR | NR1H4 | Bile acids | Fexaramine | EGCG138 |
| GW4064 | |||||
| INT-747 | |||||
| Glucocorticoid receptor | GR | NR3C1 | Cortisol | Dexamethasone | Daidzein148 |
| RU486 | Genistein149 | ||||
| Hepatocyte nuclear factor 4 | HNF4α | NR2A1 | Phospholipids | MEDICA16 | |
| HNF4γ | NR2A2 | Fatty acyl-CoAs | |||
| Liver X receptor | LXRα | NR1H3 | Oxysterols | GW3965 | Hesperetin106 |
| LXRβ | NR1H2 | Glucose | N-Acylthiadiazolines | Naringenin104 | |
| T00901317 | |||||
| Peroxisome proliferator-activated receptors | PPARα | PPARA | Fatty acids | Fibrates | Daidzein148 |
| GW9662 | Naringenin104 | ||||
| Nobiletin121 | |||||
| Quercetin130 | |||||
| Tangeretin120 | |||||
| PPARβ | PPARD | Fatty acids | GW501516 | ||
| PPARγ | PPARG | Fatty acids | BRL49653 | Apigenin118 | |
| Prostaglandin J2 | GW9662 | Cyanidin-3-O-glucoside159 | |||
| Thiazolidinediones | Daidzein148 | ||||
| ECG141 | |||||
| EGCG138 | |||||
| Hesperetin106 | |||||
| Kaempferol129 | |||||
| Luteolin116 | |||||
| Naringenin104 | |||||
| Nobiletin121 | |||||
| Quercetin130 | |||||
| Tangeretin120 | |||||
| Pregnane X receptor | PXR | NR1I2 | Xenobiotics | Rifampicin | Apigenin118 |
| Chrysin114 | |||||
| Daidzein148 | |||||
| Genistein149 | |||||
| Luteolin116 | |||||
| Retinoid X receptor | RXRα | RXRA | Retinoic acid | ||
| RXRβ | RXRB | ||||
| RXRγ | RXRG | ||||
| Thyroid hormone receptor | TRα | THRA | Thyroid | Levothyroxine | |
| TRβ | THRB | hormones | Liothyronine | ||
| Vitamin D receptor | VDR | NR1I1 | Vitamin D | Doxercalciferol | |
| Lithocholic acid |
Nuclear receptors comprise one of the largest groups of transcription factors found in humans, consisting of 48 different members.4 Their ligands include metabolites, vitamins, and hormones as well as xenobiotics. Direct ligand binding triggers a conformational change in the receptor, allowing it to recruit co-regulators and initiate transcription. Nuclear receptors play an essential regulatory role in critical processes including development and metabolic homeostasis.5,6 Direct activation of nuclear receptors by metabolites, such as glucose or fatty acids, allows cells to rapidly react to metabolic changes. Their role in metabolic homeostasis makes nuclear receptors promising pharmaceutical targets.
Flavonoids are a class of plant secondary metabolites that are widely found in vegetables, fruits, nuts, and seeds.7 Flavonoids are thought to have antiviral, anti-bacterial, anti-inflammatory, and anti-carcinogenic properties but their precise mechanism of action is largely unknown (reviewed in ref. 8–11). This review will show that flavonoids exert some of their effect via interactions with nuclear receptors, making them a promising pharmaceutical and nutraceutical source of compounds for the treatment of metabolic disorders.
Nuclear receptors: concepts and variety
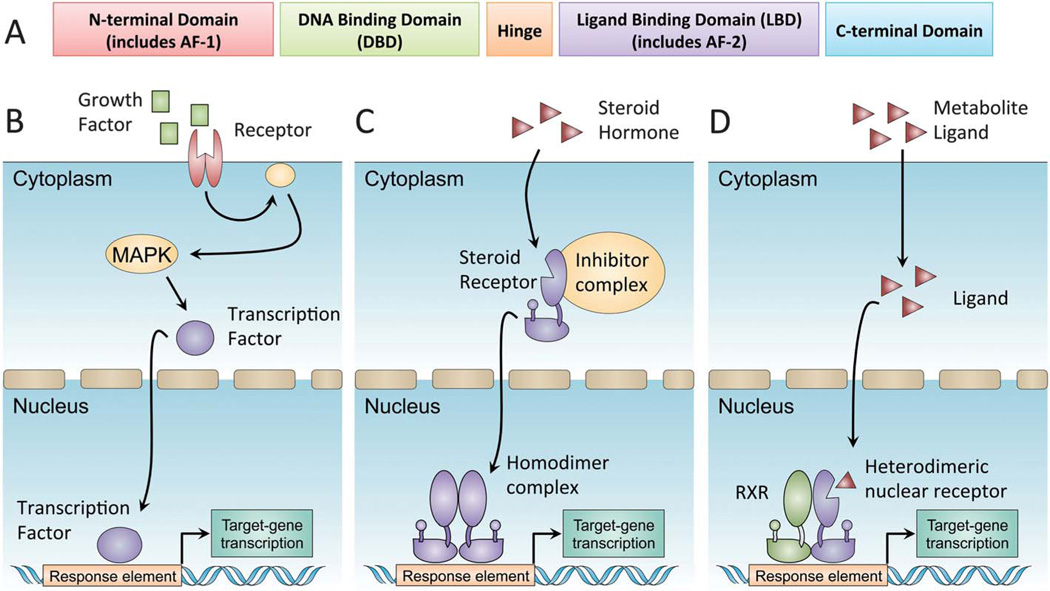
Nuclear receptors exhibit a significant variation in structure and function. A typical nuclear receptor structure can be divided into several modular segments that include a ligand-independent transactivation domain (AF-1), a DNA-binding domain (DBD), a hinge region and a ligand-binding domain (LBD) (Fig. 1A). A defining feature of many nuclear receptors is their ability to interact with different ligands, while presenting a single unique LBD, making them somewhat promiscuous receptors.12,13 Ligand binding induces a conformational change in the receptor, leading to the release of co-repressors and the recruitment of co-activators. Co-activator recruitment initiates complex formation ending with polymerase recruitment and initiation of transcription. The process is different from the classical signal transduction cascade (Fig. 1B), permitting a direct regulation of gene expression by hormones and metabolites. Two major sub-types of nuclear receptors are generally described. Type I nuclear receptors are found in the cytoplasm, appearing as a complex composed of heat shock proteins and co-repressors.14 Once activated, the complex is broken down and the nuclear receptor homodimerizes and translocates into the nucleus to initiate transcription (Fig. 1C). In contrast, type II nuclear receptors are constitutively bound to DNA, usually as heterodimers with the retinoid X receptor (RXR).15 Ligand binding induces a conformational change altering the complex composition from co-repressors to co-activators and leading to initiation of transcription (Fig. 1D).
Fig. 1.
Nuclear receptor transcriptional activation. Nuclear receptors are a family of ligand-activated transcription factors. (A) Members of the nuclear receptor superfamily have a common domain structure consisting of an amino-terminal activation domain (AF-1), a DNA-binding domain, and a carboxy-terminal ligand-binding domain (LBD). The LBD determines ligand-regulated interactions with co-activators and co-repressors through allosteric changes in a short helical region known as AF-2. (B) In a canonical signal-transduction cascade, receptor binding at the plasma membrane initiates enzymatic phosphorylation cascades culminating with transcription factor translocation into the nucleus. (C) Type I steroid nuclear receptors are synthesized in inactive forms associated with heat-shock protein (HSP) complexes in the cytoplasm. Direct hormone binding causes a conformational change, dissociation from HSP complexes and translocation into the nucleus. (D) Type II heterodimeric nuclear receptors bind constitutively to DNA with RXRs as obligate partners. Ligand binding causes a conformational change, dissociation of co-repressor complexes and recruitment of co-activators, such as PGC1α.
Nuclear receptors as pharmaceutical targets
Extensive research over the past two decades underlined nuclear receptor involvement in metabolic and inflammatory diseases, including diabetes, hyperdyslipidemia, cirrhosis and fibrosis. This prompted the pharmaceutical development of nuclear receptor agonists, such as fenofibrate and calcitriol. Fenofibrate is a peroxisome proliferator-activated receptor α(PPARα) agonist, causing a reduction in blood cholesterol levels, while calcitriol is a vitamin D receptor (VDR) agonist, increasing calcium uptake. It is thought that close to 13% of all FDA-approved drugs target the nuclear receptor family.16 This work aims to review this rapidly growing field, focusing on some of the most well described nuclear receptors.
Estrogen receptor (ER)
ERs (isoforms α and β) are type II nuclear receptors expressed in many different tissues. When activated, ER translocates into the nucleus, binding DNA either as αα homodimer or as αβ heterodimer.17,18 These combinations respond differently to different ligands, translating into tissue-selective agonistic and antagonistic effects (reviewed in ref. 19). ER natural ligands include 17β-estradiol (commonly referred to as estrogen) that binds both receptors, estrone that preferentially binds ERα, and estriol that preferentially binds ERβ.20 +ERs are expressed in most cases of breast cancer21 and are involved in ovarian, colon, and prostate cancer.22–24 Their key roles in the reproductive, musculoskeletal, and central nervous systems25–27 make ERs an attractive pharmaceutical target.
Tamoxifen was the first selective estrogen receptor modulator (SERM) approved as a cancer chemo-preventive agent. Tamoxifen binds both receptor isoforms,28 mimicking estrogen action in certain tissues while opposing it in others.29,30 It was hoped that tamoxifen would be suitable to treat menopausal symptoms as well, but its estrogenic effects on the uterus were shown to increase the risk of uterine cancer.31 Raloxifene, a second generation SERM, binds both isoforms and exhibits anti-proliferative effects in breast cancer cells alongside positive effects on osteoporosis, without uterotrophic effects.32,33 The third generation of SERMs includes bazedoxifene and lasofoxifene,34,35 which are currently approved for the treatment of osteoporosis in the European Union but not in the United States.
Peroxisome proliferator-activated receptor (PPAR)
PPARs (isoforms α, β and γ) are type II nuclear receptors that play an essential role in lipid metabolism36 and adipocyte differentiation37 as well as insulin response.38 Binding of natural ligands, such as fatty acids released during fasting, causes a conformational change in PPARs and the recruitment of co-activators, such as PGC1α.39,40 PPARα activation leads to increased fatty acid oxidation in liver and muscle,41,42 while PPARγ activation increases insulin sensitivity primarily in the adipose tissue.43 Both pathways make PPARs important targets in the treatment of dyslipidemia and diabetes. PPARα activation was found to suppress NFκB and AP1-mediated inflammatory responses in human aortic smooth muscle cells,44 making it an attractive target for anti-inflammatory treatment.
The most studied synthetic ligands for PPARs are thiazolidinediones (TZDs), a class of drugs used to increase insulin sensitivity even before their mechanism of action was understood. TZDs were found to decrease insulin resistance, modify adipocyte differentiation and induce lipoprotein lipase (LPL) through PPARγ activation.45–48 However, the clinical use of early TZDs was discontinued due to hepatotoxicity.49 A second generation of TZDs, including rosiglitazone and pioglitazone, lacked this side effect and was effectively used in the treatment of type II diabetes for over a decade.50,51 However, recent studies found that these drugs increase the risk for myocardial infarction in all patients and the risk of stroke, heart failure, and all-cause mortality in patients older than 65 years.52,53
Fibrates, such as bezafibrate and gemfibrozil, are a class of synthetic amphipathic carboxylic acids, used to treat dyslipidemia prior to the advent of statins. Fibrates increase triglyceride lipolysis by PPARα-mediated activation of LPL in the liver.54 Activation of PPARα has been suggested to increase high-density lipoprotein (HDL) levels via transcriptional changes of target genes involved in lipoprotein metabolism.55 Together these effects shift the atherogenic lipoprotein balance, reducing cardiovascular morbidity.
Farnesoid X receptor (FXR)
FXR is a bile acid receptor, which plays an important role in cholesterol metabolism. This type II nuclear receptor is highly expressed in the liver and intestine, and is activated by chenodeoxycholic acid and other bile acids.56,57 Upon activation, FXR heterodimerizes with RXR and induces the small heterodimer partner (SHP), which in turn antagonizes liver receptor homo-logue-1 (LRH-1).58 LRH-1 inhibition represses both SHP and cholesterol 7α-hydroxylase (CYP7A1), the rate-limiting enzyme in the conversion of cholesterol to bile acids, establishing a negative feedback loop.58 FXR modulation was shown to regulate lipid metabolism, possibly by interacting with PPARα and PPARγ,59,60 as well as repression of sterol regulatory element-binding protein-1c (SREBP-1c).61 The reduced triglyceride levels seen in mice after FXR activation62 could result from the combined effects of fatty acid oxidation and lipogenesis inhibition. Synthetic agonists of the FXR include GW4064, INT-747 and fexaramine.63 Some of these compounds are currently tested for the treatment of primary biliary cirrhosis and non-alcoholic fatty liver disease.
Liver X receptor (LXR)
LXRs (isoforms α and β) are type II nuclear receptors that play an important role in cholesterol, fatty acid and carbohydrate metabolism. While LXRβ is ubiquitously expressed, LXRα is predominant in the metabolic tissues such as liver, kidney, intestine and adipose tissue.64,65 The natural ligands of LXRs are oxygenated derivatives of cholesterol, such as 24(S)-hydroxy-cholesterol and 24(S),25-epoxycholesterol,66 as well as d-glucose and d-glucose-6-phosphate.67 LXR activation during feeding induces fatty acid synthesis and cholesterol transport, and its targets include ABC proteins, and the pro-lipogenic transcription factor SREBP-1c.68,69 SREBPs regulate the expression of genes involved in fatty acid and cholesterol biosynthesis.
Treatment with the synthetic LXR ligand T0901317 drastically increases hepatic lipogenesis.70 Studies suggest that selectively activating LXRβ favorably regulates the lipid profile without increasing liver triglycerides71,72 making LXRβ a potential drug target. These processes may be induced by the relatively weak LXR activator GW3965,73 or by selective LXRβ agonists such as N-acylthiadiazolines.74
Pregnane X receptor (PXR)
PXR is a steroid and xenobiotic receptor predominantly expressed in the liver. This type II nuclear receptor is activated by bile acids, such as lithocholic acid,75 and naturally occurring steroids such as progesterone.76 Activation of the PXR induces the expression of phase I and II drug-metabolizing enzymes, and drug and bile acid transporters.77 PXR is one of the main regulators of cytochrome P450 3A4 (CYP3A4), a key enzyme that catalyzes the metabolism of nearly 40% of clinically prescribed drugs.78 Differential expression of CYP3A4 can alter the therapeutic and toxicological responses to drugs, leading to adverse reactions. A semisynthetic PXR agonist named rifampicin is currently used in the treatment of cholestatic liver disease79 and its exact mechanism of action is under investigation. Like other nuclear receptors, PXR activity was found to be regulated not only by direct ligand binding but also by cell-signaling pathways such as protein kinase C (PKC) phosphorylation.80
Hepatocyte nuclear factor 4α (HNF4α)
HNF4α is an enigmatic nuclear receptor expressed in liver, kidney, pancreas, and intestine tissues.81,82 HNF4α is rather unique in that it binds DNA exclusively as a homodimer and yet behaves as a type II nuclear receptor localized primarily in the nucleus. Interestingly, fatty acids are often found in the LBD of HNF4α,83 which was considered to be constitutively active. However, recent studies suggest that linoleic acid binding does not significantly affect HNF4α transcriptional activity.84 Importantly, fatty acyl-CoA molecules were found to modulate HNF4α activity,85 leading to overall changes in lipid and carbohydrate metabolism.86,87 These metabolic effects of HNF4α transcriptional activation are mediated in part by regulation of PPARα, HNF1α, and PXR expressions.88–90 While the HNF4α knockout is embryonically lethal, mutations in this gene were shown to be associated with mature onset diabetes of the young (MODY).91 Due to its crucial role in liver homeostasis and function, many efforts have been made to synthesize specific HNF4α modulators.92
Flavonoids: dietary nuclear receptor regulators
Flavonoids are plant secondary metabolites widely found in fruits, vegetables, nuts, and seeds. They are consumed regularly with an average dietary intake of about 190 mg flavonoids per day in American diet.93 Chemically, flavonoids are polyphenolic compounds comprising of a backbone of 15-carbon molecules, with two aromatic rings connected by a three-carbon bridge. Flavonoids occur as aglycones or glycosides as well as their methylated derivatives. Based on the differences in the structure of the C ring, flavonoids can be classified into six groups (Table 2): flavanones, flavones, flavonols, flavanols (catechins), isoflavones, and anthocyanins. The basic flavonoid skeleton can have numerous substituents, with sugars and hydroxyl groups increasing water solubility of flavonoids, while other substituents, such as isopentyl and methyl groups turn flavonoids lipophilic. Over 4000 naturally occurring flavonoids have been identified to date.94
Table 2.
Backbone structure of different flavonoid subclasses
| Group | Structure | Examples |
|---|---|---|
| Flavanone | 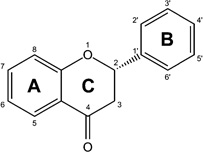 |
Hesperetin Naringenin |
| Flavone | 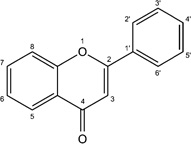 |
Apigenin Chrysin Luteolin |
| Flavonol | 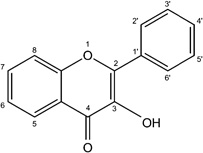 |
Kaempferol Quercetin |
| Flavanol | 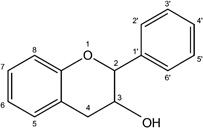 |
EGCG Epicatechin Epicatechin gallate |
| Isoflavone | 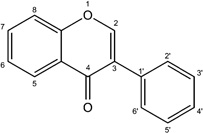 |
Daidzein Genistein |
| Anthocyanin | 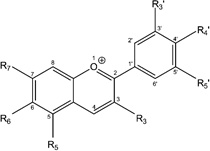 |
Cyanidin-3-O-glucoside |
The flavonoid family was shown to display some pharmacological activity, showing anti-inflammatory, anti-microbial and anti-carcinogenic properties (reviewed in ref. 8–11). While these properties may explain the success of some herbal medicine in the treatment of certain inflammatory and infectious diseases,95,96 their mechanism of action is often unresolved. Structural resemblance between flavonoids, steroids and other cholesterol derivatives suggests that flavonoids may exert some of their effects through the nuclear receptor family. The promiscuous nature of the nuclear receptor ligand-binding domain may facilitate direct transcriptional regulations of cells by dietary intake of flavonoids.
Flavanones
Flavanones are non-planar molecules with a chiral center at the C2 connecting rings B and C. High concentrations of flavanone glycosides, such as naringin and hesperidin, are found in citrus fruits. Both compounds are broken down by intestinal flora to their aglycones, naringenin and hesperetin, prior to being absorbed.
Naringenin, which is responsible for the bitter taste of grapefruits, is one of the most studied flavanones. It was found to attenuate dyslipidemia without affecting the caloric intake or fat absorption in a diabetic mouse model.97 Earlier studies showed the potential of naringenin as a normolipidemic agent, reducing lipid levels in rats and mice.98,99 This flavanone was shown to weakly bind and activate ERα and ERβ, presenting anti-estrogenic effects in rodents.100,101 Naringenin was also shown to activate phosphoinositide 3-kinase (PI3K) upstream of SREBP-1 in cultured hepatocytes,102 blocking secretion of apolipoprotein B (apoB), the main constituent protein of low-density lipoprotein (LDL).103 These myriad effects suggested that naringenin might act on an underlying transcriptional regulation of lipid metabolism. Indeed, Goldwasser and colleagues showed that naringenin is a dual agonist of PPARα and PPARγ, as well as a partial agonist inhibitor of LXRα. Naringenin was shown to directly block the association of the LXRα ligand-binding domain with the Trap220 co-activator.104 Concomitantly while naringenin did not affect the PPAR ligand-binding domain it induced PGC1α expression up-regulating a critical co-activator of both PPARs These effects translate into the induction of fatty acid oxidation genes (ACOX, CYP4A10) and inhibition of lipid and cholesterol synthesis genes (HMGCR, FAS) The net metabolic effect was the induction of a fasted-like state in rats and primary human hepatocytes
The ability of naringenin to agonize both PPARα and PPARγ suggests that it has both anti-lipogenic effects and insulin-sensitizing properties as has been clinically demonstrated for fibrates (PPARα agonists) and TZDs (PPARγ agonists) The development of multimodal drugs which can reduce triglycerides and regulate energy homeostasis and hyperglycemia, may offer valuable therapeutic options. Dual PPARα and PPARγ agonists were long sought after by the pharmaceutical industry, but their development was spurred by safety concerns. In contrast, naringenin is a dietary supplement with a clear safety record, and acts as a dual agonist. Thus, it might protect the liver from damage.
Hesperetin is another abundant flavanone found in citrus fruits. The metabolites of this compound were similarly shown to promote hypolipidemic effects in diabetic rats, lowering the expression of hepatic HMGCR and increasing the expression of the LDL receptor.105 Although hesperetin does not activate PPARα as robustly as naringenin, it can up-regulate cholesterol efflux from macrophages and adiponectin production in adipocytes by promoting LXRα and PPARγ expressions.106,107
Phloretin, found in apple tree leaves,108 is a structural analogue of flavanones94 known for inhibiting glucose transport into cells.109 The compound was shown to enhance PPARγ and C/EBPα expressions in vitro in 3T3-L1 preadipocytes, leading to increased triglyceride accumulation and adipocyte differentiation.110 It was also shown to act as a phytoestrogen and bind to ER,101 altering estrogen-responsive genes in vitro in MCF-7 human breast cancer cells.111
Flavones
Flavones, such as chrysin, apigenin and luteolin, are found mainly in honey and herbs such as parsley and celery.112,113 Flavones lack oxygenation at C3 but otherwise can have a wide range of substitutions, with polymethoxylated flavones, such as nobiletin and tangeretin, being a notable sub-family.
Interestingly the flavones, chrysin, apigenin and luteolin, were shown to affect drug metabolism, through complex interactions with the PXR. In cultured hepatocytes, these flavones strongly activated PXR-mediated CYP3A4 expression without directly binding PXR, suggesting an indirect PXR-activation pathway.114 The PXR controls the expression of CYP3A4, which is responsible for the clearance of close to 40% of drugs on the market.78 Apigenin and chrysin were also shown to activate PPARγ expression in mouse macrophages, inducing anti-inflammatory effects.115 Remarkably, PPARγ conformational changes appeared to differ from that induced by rosiglitazone suggesting apigenin and chrysin may act as allosteric effectors capable of activating PPARγ by binding at a different site.115 It has been recently suggested that luteolin potentiates insulin action in adipocytes by agonizing PPARγ and increasing the expression of PPARγ target genes such as adiponectin and leptin .116 However structural analyses suggest that luteolin acts as a weak agonist and binds PPARγ by cooperating with other ligands.117 Apigenin was additionally shown to retain both estrogenic and anti-estrogenic abilities in a dose-dependent manner At low concentrations it stimulated proliferation of breast cancer cells by enhancing ERα-mediated gene expression whereas at high concentrations it inhibited cell growth partially by reducing ERα expression .118
Polymethoxylated flavones (PMF) such as nobiletin and tangeretin are found in many citrus peels These compounds are suggested to have the most potent cholesterol-lowering effect than other citrus flavonoids.119 Tangeretin significantly decreased lipid synthesis in cultured hepatocytes, and activated PPARα, a modulator of hepatic fatty acid oxidation;120 nobiletin was shown to suppress adipogenesis in 3T3-L1 preadipocytes by inhibiting lipid accumulation and blocking PPARγ expression.121 Evidence is also emerging from animal models, where Lee showed that nobiletin improves hyperglycemia and insulin sensitivity in obese mice, possibly due to the up-regulation of PPARγ expression.122 Li and colleagues showed that a diet rich in PMF improved insulin sensitivity and dyslipidemia in insulin-resistant hamsters, showing increased expression of hepatic PPARα and PPARγ.123 Interestingly, a more recent study showed that while nobiletin decreased dyslipidemia in the Ldlr−/− mouse model, it did not activate any PPAR isoform using a luciferase reporter assay in vitro, indicating that some of its effects might be nuclear receptor-independent.124
Flavonols
Flavonols are the most widespread of the flavonoids, found throughout the plant kingdom. Their extensive distribution and structural variations in commonly consumed vegetables and fruits have been well documented.7 The common flavonol kaempferol, found in numerous vegetables and fruits such as cabbage and tomatoes, was shown to modulate PPARγ in mouse macrophages,115 and promotes bone tissue formation by inducing estrogen receptors in cultured osteoblasts.125 The binding properties of kaempferol to PPARγ seem to differ from those of rosiglitazone,115 suggesting that it is only a partial agonist and underlining once more the promiscuous binding of nuclear receptors.
Quercetin is one of the most abundant flavonols and can be found in tea, capers, lovage, apples and onion. Its glycoside rutin can also be found in citrus fruit, buckwheat, and asparagus. Quercetin was shown to ameliorate dyslipidemia, hypertension and insulin resistance in obese rats.126 These effects are thought to be the outcome of several different processes such as reduced fatty acid synthesis and inhibition of nitric oxide (NO) production in hepatocytes.127,128 Others suggested that quercetin is a weak partial agonist of PPARγ, which can be used to improve insulin-stimulated glucose uptake in mature 3T3-L1 adipocytes without promoting differentiation of preadipocytes.129 Wein and colleagues showed that quercetin failed to induce PPARγ expression in adipose tissue of rats on a high-fat diet, suggesting that its effects were PPARγ-independent.130 The same study showed only minor induction of PPARγ activity in cultured mouse embryonic fibroblasts.130 We note that in our hands quercetin is a much more potent inducer of PPARγ activity in vitro, with more recent studies showing isorhamnetin (IH), a 3′-O-methylated metabolite of quercetin, to be a potent activator of PPARγ.131 Finally, quercetin was also found to have pro-apoptotic effects in colon cancer cell lines by modulating different estrogen receptors.132,133
Flavanols
Flavanols, sometimes referred to as flavan-3-ols, include catechins and catechin gallates, which are common in tea, wine, fruits and chocolate.134,135 Epigallocatechin gallate (EGCG), a well-known antioxidant, is a member of this family found primarily in green tea. Pure EGCG prevents diet-induced obesity and hyperglycemia in mice.136,137 Recent evidence shows that EGCG suppresses adipogenesis in 3T3-L1 preadipocytes by inhibiting lipid accumulation and PPARγ expression.138 Work in high fat-fed C57BL/6J mice showed that EGCG induces fatty acid oxidation as well as an up-regulation of PPARα activity in skeletal muscle.139 Remarkably, recent work suggests that EGCG is a partial agonist of FXR.140 EGCG dose-dependently activates FXR, but fails to recruit the SRC2 co-activator in vitro. However, the compound blocks FXR activation by the potent synthetic ligand GW6064 (ref. 140).
Epicatechin gallate (ECG) is another flavanol present in green tea and red wine. The compound was found to have a similar affinity to PPARγ as rosiglitazone.141 It can by hypothesized that at least some of red wine's beneficial effects on health and metabolism are due to this PPARγ ligand; however, red wine contains other flavonoids and phenols, such as delphinidin and resveratrol, which interact with nuclear receptors, especially with estrogen receptors.142,143
Proanthocyanidins are oligomers composed of flavanol units. The grape seed proanthocyanidin extract (GSPE) increases the expression of SHP, a major FXR target, and improves the postprandial plasma lipid profile in wild-type rats.144 Further research indicated that in the murine liver, GSPE down-regulated the expression of SREBP-1 and its target lipogenic genes via the FXR pathway.145 This study suggested that proanthocyanidins are FXR ligands that hold therapeutic potential in the treatment of metabolic disorders, such as hypertriglyceridemia and type II diabetes.
Isoflavones
Isoflavones are found almost exclusively in leguminous plants, with the highest concentrations occurring in soybeans.146 Isoflavones, such as daidzein and genistein, have a significant estrogenic effect and are therefore termed phytoestrogens. Their estrogenic activity is sufficient to seriously affect the reproduction of grazing animals.147 At high concentrations, genistein and daidzein up-regulate adipogenesis and down-regulate osteogenesis in mouse mesenchymal stem cells. At lower concentrations, they act like estrogen, stimulating osteogenesis and inhibiting adipogenesis.148,149 It is possible that the presence of low-affinity phytoestrogens can diminish the effect of endogenous ER ligands such as 17β-estradiol,150 thereby antagonizing the estrogen-signaling pathway. In vitro studies suggest that genistein can promote fatty acid oxidation by activating PPARα,151 while daidzein can enhance insulin sensitization by activating PPARγ.152 In addition, equol – a daidzein metabolite – was shown to induce PXR-mediated activation of CYP3A4 in primary human hepatocytes.153 Different in vivo studies suggest that soy isoflavone intake can improve lipid metabolism and produce an anti-diabetic effect through multiple mechanisms, including PPARα and PPARγ activations.154,155
Though these results seem promising, isoflavones may become a health risk by inhibiting different thyroid functions such as thyroid peroxidase activity.156 However, early clinical trials suggest that dietary isoflavone intake does not cause abnormalities in individuals with normal thyroid function.157
Anthocyanins
Anthocyanins are water-soluble pigments that commonly appear in red, blue and purple plant tissues.158 Recent studies have found that the anthocyanin cyanidin-3-O-glucoside (C3G) and its metabolite protocatechuic acid exhibit insulin-like activity and activate PPARγ in human adipocytes.159 Another study suggests that C3G up-regulates cholesterol efflux from mouse macrophages by activating the PPARγ-LXRα-ABCA1 pathway.160 C3G was also shown to promote cholesterol efflux from human aortic endothelial cells by inducing LXRα activity,161 suggesting this anthocyanin may ameliorate the effects of atherosclerosis by enhancing reverse cholesterol transport. A diet supplemented with tart cherry, a rich source of anthocyanins, altered metabolic disorders in Dahl salt-sensitive rats with insulin resistance and hyperlipidemia.162 After 90 days on a cherry-rich diet, these rats showed reduced hyperinsulinemia and hepatic lipid accumulation accompanied by increased PPARα expression. It must be noted that while tart cherries do contain high amounts of anthocyanins (~50%) they also contain other flavonoids, such as quercetin (~25%) and isorhamnetin rutinoside (~15%), which might mediate these beneficial effects.162
Other herbal substances
There are a few examples of natural substances that contain flavonoids, among other compounds, and affect nuclear receptor activity. Guggulu, the gum resin of Commiphora mukul, has been used in traditional Indian medicine since at least 600 BC to treat a variety of disorders, including atherosclerosis and obesity.163 An extract of this resin has been shown to decrease triglyceride and LDL levels in patients with hypercholesterolemia.164,165 Guggulsterone, the active ingredient in this extract, was found to be an FXR antagonist and reduces hepatic cholesterol in mice.166
Another commonly used example is licorice (Glycyrrhiza glabra). Licorice flavonoid oil decreases the abdominal adipose tissue weight and reduces hepatic and plasma triglyceride levels in obese rats. A recent study elucidated the possible molecular mechanism underlying this metabolic amelioration: licorice flavonoid oil up-regulated hepatic expression of PPARα, which induces fatty acid oxidation, and down-regulated hepatic expression of SREBP-1c, which promotes lipid production.167 A more recent study suggests that glabridin, the major isoflavan in licorice root, is responsible for the oil's anti-obesity effects. It was found to be the functional component responsible for the reduced weight gain of high-fat-fed obese mice, in a dose-dependent manner.168 This effect was mediated, at least in part, by inhibiting PPARγ and its downstream targets in adipose tissues, as validated in vitro on 3T3-L1 preadipocytes. Glabridin was also shown to have antioxidative effects on LDL oxidation in healthy human subjects.169 Alongside glabridin, licorice roots contain a large number of flavonoids, many of which were shown to present estrogenic activity both in vivo and in vitro.170–172
Flavonoids' bioavailability and delivery
While the therapeutic potential of flavonoids is significant, their clinical utility is limited due to their poor bioavailability and rapid clearance. Despite decades of research on flavonoids, information about absorption, distribution, metabolism, and excretion of individual compounds in humans is just beginning to accumulate.173–176 Animal studies showed the bioavailability of 6% for naringenin,177 14% for EGCG, 6% for ECG,178 and 30% for luteolin.179 Clinical pharmacokinetic studies showed relatively short half-lives ranging from 2.3 hours for naringenin180 to 6.9 hours for ECG.181 In vitro studies frequently use flavonoids at concentrations higher than those that can be achieved in the plasma following dietary intake.173 However, biologically relevant plasma concentrations of flavonoids and their metabolites can be observed following oral ingestion.175
Similar to other xenobiotics, flavonoids are subjected to different chemical modifications upon absorption. In the intestinal epithelium, many flavonoids are conjugated with glucuronate and sulfate groups, becoming distinct from their original aglycone structure used in many in vitro experiments.182 Although these metabolites still possess biological activity, changes in molecular weight and polarity may affect their passive diffusion across lipid membranes.183 In an in vivo model of flavonoid bioavailability, macrophages in inflamed arteries convert quercetin-3-O-glucoronide (Q3GA) to the atheroprotective quercetin.184,185 This model suggests that Q3GA serves as a quercetin carrier in the plasma; after reaching the vascular wall, Q3GA is enzymatically deconjugated, delivering the free form of quercetin into tissues.186 Quercetin metabolites also undergo deconjugation followed by sulfation in hepatocytes, allowing the transient presence of the free aglycone form.187 Furthermore, conjugation may detrimentally affect the structure-activity relationship between flavonoids and nuclear receptors. Such an effect was demonstrated by reduced binding of sulfated isoflavones to ERs, and poor stimulation of estrogen-dependent growth of MCF-7 breast cancer cells by glucuronide isoflavones.188 In contrast, naturally occurring methylation of methoxyflavones may enhance intestinal absorption and metabolic stability over unmethylated flavonoids.189 More research is needed to determine the effect of flavonoid conjugation on their interaction with nuclear receptors.
Flavonoid–dextran complexes
One reason for the low bioavailability of flavonoids is their poor water solubility. Several studies showed that the solubility of flavonoids like naringenin,177,190 quercetin191,192 and genistein193 can be enhanced by complexation with β-cyclodextrin, an FDA approved excipient. For example, complexation of naringenin with hydroxypropoyl-β-cyclodextrin (HPβCD), a hydrophilic form of cyclodextrin, increased the bioavailability of naringenin in rats by 7.4-fold.177 Amylose can similarly be used to create flavonoid complexes. These complexes showed a high retention of genistein in simulated acidic stomach conditions and released genistein upon digestion in pancreatin solution.194 Furthermore, the amylose–genistein complexes were later shown to have increased bioavailability in rats.195
Amorphous solid dispersion
Amorphous solid dispersions are a promising new method to deliver low-solubility drugs across the gastrointestinal barrier. The higher solubility is due to the lack of the crystal structure and the rapid dissolution of the supporting matrix. Quercetin,196 silymarin,197 and nobiletin198 were recently formulated as solid dispersions. Onoue and colleagues showed that wetmilled nobiletin nanoparticles showed 13-fold higher bioavailability than the crystalline form.198
Discussion
In recent years, a family of ligand-activated transcription factors called nuclear receptors emerged as key regulators of cellular metabolism. Key metabolites were found to be the natural ligands of many nuclear receptors, previously defined as orphan receptors, and their interactions were slowly elucidated defining well-described negative and positive feedback loops. One such well-defined program is the transition from a PPARα-induced fasted state41 to a LXRα-induced fed state.199 Here fatty acids released by adipose tissues during fasting activate PPARα-controlled fatty acid oxidation, blocking LXRα-activity by competition for RXR binding partners.200,201 During feeding, glucose and cholesterol activate LXRα and SREBP-1c, respectively,67,68 blocking PPARα activity and inducing lipid synthesis for long-term storage.202,203 It is these feedback loops that dietary and pharmaceutical modulation can most readily affect.
It is long known that natural compounds found in fruits, vegetables and plants, affect human health. Ancient cultures have used these properties to form what is known as primal medicine, which is partially used today. Flavonoids, secondary plant metabolites, are emerging as key active components in many complementary and alternative treatments. Flavonoids, found in large quantities in human diet, exhibit very low toxicity compared to other active plant compounds, such as alkaloids. Much of the early excitement about flavonoids revolved around their antioxidant activity in vitro,204,205 with anti-inflammatory and anti-carcinogenic activities highlighted in many publications.206–209 In fact, the beneficial effects of fruits, vegetables, tea and red wine are already attributed to flavonoid compounds, although quantitative physiological evidence is still scarce.
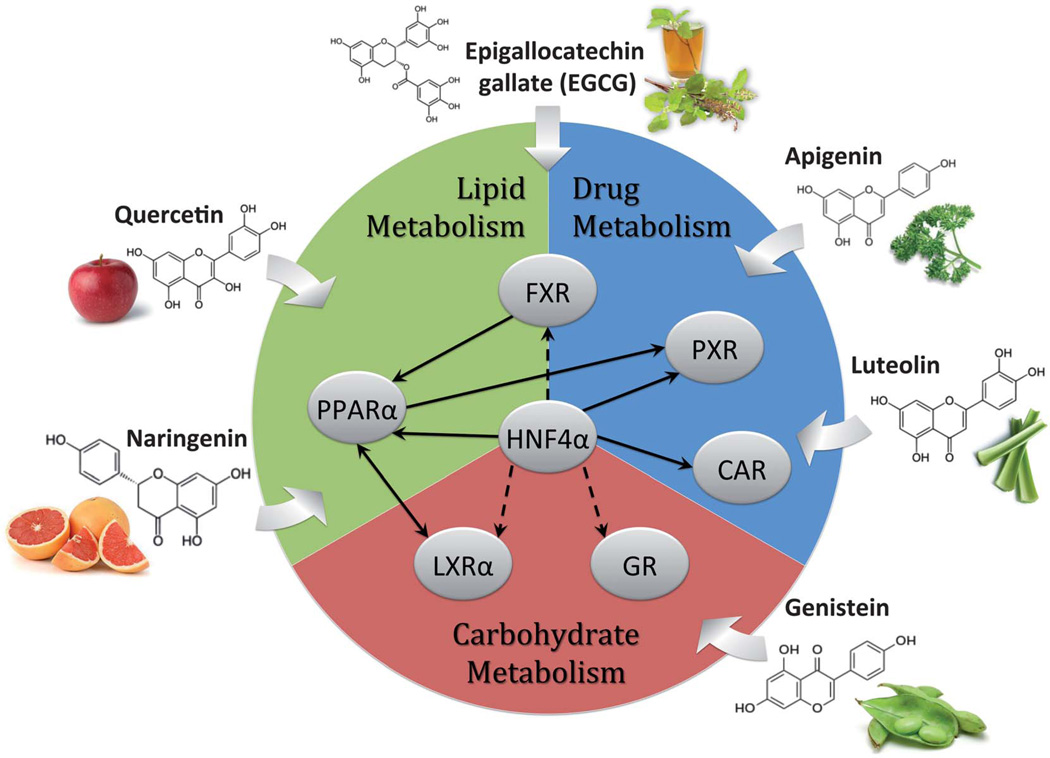
It is becoming clear that poor intestinal absorbance (5–10%) and rapid clearance (1–3 hours) of most flavonoids severely limit the clinical utility of this family. Therefore, it is important to gain a critical understanding of the mechanism of action of these natural compounds, allowing more effective flavonoid derivatives and complexes to be created. In this context, this work reviewed the growing evidence supporting flavonoids being regulators of nuclear receptor activity. It is thought that structural similarities between flavonoid and steroidal derivatives combined with the promiscuous nature of most nuclear receptors drive these interactions. In fact, it is common to find flavonoids like naringenin, which act on multiple nuclear receptors, such as ERα/β, PPARα/γ, and LXRα exerting complex metabolic responses (Fig. 2).
Fig. 2.
Nuclear receptor targeting of dietary flavonoids. Metabolic pathways are divided among the predominant nuclear receptor targets of flavonoids. Interactions between nuclear receptors were taken from BioBase® based on expression analysis and direct binding data. Solid arrows denote direct modulation of activity, while dashed arrows denote indirect interaction, through other transcription factors in the network.
Our group recently presented one example of this approach. We utilized naringenin-HPβCD complexes to increase the bioavailability of flavonoid by 11-fold.177 Complexes given to rats just prior to a fatty meal locked a PPARαHigh/LXRαLow fasted transcriptional program, reducing VLDL production by 43% and increasing insulin sensitivity by 64%.104,177 The preliminary results of a similar clinical trial were recently presented at the European Association for the Study of the Liver (EASL). It is clear that a growing understanding of the flavonoid mechanism of action can drive their clinical utility.
Although using flavonoids in vitro provides promising results, their use in patients holds many challenges. It is clear that nuclear receptor–flavonoid interactions offer many opportunities for therapeutic intervention, but the complexity of genetic and metabolic interactions (Fig. 2) is difficult to unravel. With almost every publication revealing new regulatory junctions and interactions, the potential of altering metabolic networks is being further illuminated. A combination of creativity, innovation and effort may enable the use of these interactions in order to tackle metabolic diseases – benefiting the lives of millions.
Acknowledgements
This work was supported by the European Research Council Starting Grant (TMIHCV 242699). Resources were provided by National Institute of Diabetes and Digestive and Kidney Diseases (K01DK080241) and the Harvard Clinical Nutrition Research Center (P30-DK040561). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Biographies

Yishai Avior is a graduate student in the prestigious Bioengineering program of the Hebrew University of Jerusalem; working at the microLiver Technologies Laboratory of Dr Yaakov Nahmias. He is a Magna Cum Laude graduate of the Psychology and Life Sciences program of the Hebrew University of Jerusalem. His work is focused on the role of nuclear receptors in liver development and maturation.

David Bomze is a research assistant at the microLiver Technologies Laboratory of Dr Yaakov Nahmias. He is a Summa Cum Laude graduate of the prestigious Chemistry and Life Sciences program of the Hebrew University of Jerusalem. His research interests are aimed at understanding the molecular basis of metabolic diseases with a specific focus on diabetes mellitus

Ory Ramon graduated from the Technion, Israel Institute of Technology, department of Food Engineering and Biotechnology carrying out his postdoctoral work at Rutgers, Center for Advanced Food Technology (CAFT). After serving as a World Bank consultant he worked as a Food Technology engineer, returning to academia in 1990. His work elucidated the physical properties of biopolymers and food gels, with a focus on microencapsulation of foods, drugs, and cells. He is a recipient of a Marie Curie training award, and holds several patents on microencapsulation.

Yaakov Nahmias is a Magna Cum Laude graduate of the Technion, Israel Institute of Technology. He did his PhD at the University of Minnesota and his postdoctoral training at Harvard Medical School. He is a winner of a National Institute of Health (NIH) Career Award as well as a European Research Council (ERC) Starting Grant. His work is focused on the development of microdevices for the study of liver metabolism, with an emphasis on understanding and controlling nuclear receptor activity. As of 2010 he is serving as the Director of the Center of Bioengineering at the Hebrew University of Jerusalem.
References
- 1.Mathers CD, Loncar D. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Narayan KM, Boyle JP, Geiss LS, Saaddine JB, Thompson TJ. Diabetes Care. 2006;29:2114–2116. doi: 10.2337/dc06-1136. [DOI] [PubMed] [Google Scholar]
- 3.Eaton SB, Konner M, Shostak M. Am. J. Med. 1988;84:739–749. doi: 10.1016/0002-9343(88)90113-1. [DOI] [PubMed] [Google Scholar]
- 4.Germain P, Staels B, Dacquet C, Spedding M, Laudet V. Pharmacol. Rev. 2006;58:685–704. doi: 10.1124/pr.58.4.2. [DOI] [PubMed] [Google Scholar]
- 5.Bookout AL, Jeong Y, Downes M, Yu RT, Evans RM, Mangelsdorf DJ. Cell. 2006;126:789–799. doi: 10.1016/j.cell.2006.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo X, Ikeda Y, Parker KL. Cell. 1994;77:481–490. doi: 10.1016/0092-8674(94)90211-9. [DOI] [PubMed] [Google Scholar]
- 7.Andersen OM, Markham KR. Flavonoids: Chemistry, Biochemistry, Applications. Taylor & Francis; 2005. [Google Scholar]
- 8.Cushnie TP, Lamb AJ. Int. J. Antimicrob. Agents. 2011;38:99–107. doi: 10.1016/j.ijantimicag.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 9.Galati G, O'Brien PJ. Free Radical Biol Med. 2004;37:287–303. doi: 10.1016/j.freeradbiomed.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 10.Rathee P, Chaudhary H, Rathee S, Rathee D, Kumar V, Kohli K. Inflammation Allergy: Drug Targets. 2009;8:229–235. doi: 10.2174/187152809788681029. [DOI] [PubMed] [Google Scholar]
- 11.Friedman M. Mol Nutr. Food Res. 2007;51:116–134. doi: 10.1002/mnfr.200600173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones SA, Moore LB, Shenk JL, Wisely GB, Hamilton GA, McKee DD, Tomkinson NC, LeCluyse EL, Lambert MH, Willson TM, Kliewer SA, Moore JT. Mol Endocrinol. 2000;14:27–39. doi: 10.1210/mend.14.1.0409. [DOI] [PubMed] [Google Scholar]
- 13.Seimandi M, Lemaire G, Pillon A, Perrin A, Carlavan I, Voegel JJ, Vignon F, Nicolas JC, Balaguer P. Anal Biochem. 2005;344:8–15. doi: 10.1016/j.ab.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 14.Pratt WB. BioEssays. 1992;14:841–848. doi: 10.1002/bies.950141209. [DOI] [PubMed] [Google Scholar]
- 15.Mangelsdorf DJ, Evans RM. Cell. 1995;83:841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 16.Overington JP, Al-Lazikani B, Hopkins AL. Nat Rev. Drug Discovery. 2006;5:993–996. doi: 10.1038/nrd2199. [DOI] [PubMed] [Google Scholar]
- 17.Cowley SM, Hoare S, Mosselman S, Parker MG. J. Biol Chem. 1997;272:19858–19862. doi: 10.1074/jbc.272.32.19858. [DOI] [PubMed] [Google Scholar]
- 18.Kumar V, Chambon P. Cell. 1988;55:145–156. doi: 10.1016/0092-8674(88)90017-7. [DOI] [PubMed] [Google Scholar]
- 19.Dutertre M, Smith CL. J. Pharmacol Exp. Ther. 2000;295:431–437. [PubMed] [Google Scholar]
- 20.Zhu BT, Han GZ, Shim JY, Wen Y, Jiang XR. Endocrinology. 2006;147:4132–4150. doi: 10.1210/en.2006-0113. [DOI] [PubMed] [Google Scholar]
- 21.Dunnwald LK, Rossing MA, Li CI. Breast Cancer Res. 2007;9:R6. doi: 10.1186/bcr1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonkhoff H, Fixemer T, Hunsicker I, Remberger K. Am.J. Pathol. 1999;155:641–647. doi: 10.1016/S0002-9440(10)65160-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell-Thompson M, Lynch IJ, Bhardwaj B. Cancer Res. 2001;61:632–640. [PubMed] [Google Scholar]
- 24.Rutherford T, Brown WD, Sapi E, Aschkenazi S, Munoz A, Mor G. Obstet. Gynecol. 2000;96:417–421. doi: 10.1016/s0029-7844(00)00917-0. [DOI] [PubMed] [Google Scholar]
- 25.Eddy EM, Washburn TF, Bunch DO, Goulding EH, Gladen BC, Lubahn DB, Korach KS. Endocrinology. 1996;137:4796–4805. doi: 10.1210/endo.137.11.8895349. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi S, Inoue S, Hosoi T, Ouchi Y, Shiraki M, Orimo H. J. Bone Miner. Res. 1996;11:306–311. doi: 10.1002/jbmr.5650110304. [DOI] [PubMed] [Google Scholar]
- 27.Krezel W, Dupont S, Krust A, Chambon P, Chapman PF. Proc. Natl. Acad. Sci. U. S. A. 2001;98:12278–12282. doi: 10.1073/pnas.221451898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 29.Gottardis MM, Robinson SP, Satyaswaroop PG, Jordan VC. Cancer Res. 1988;48:812–815. [PubMed] [Google Scholar]
- 30.Jordan VC, Robinson SP. Fed. Proc. 1987;46:1870–1874. [PubMed] [Google Scholar]
- 31.van Leeuwen FE, Benraadt J, Coebergh JW, Kiemeney LA, Gimbrere CH, Otter R, Schouten LJ, Damhuis RA, Bontenbal M, Diepenhorst FW, et al. Lancet. 1994;343:448–452. doi: 10.1016/s0140-6736(94)92692-1. [DOI] [PubMed] [Google Scholar]
- 32.Cummings SR, Eckert S, Krueger KA, Grady D, Powles TJ, Cauley JA, Norton L, Nickelsen T, Bjarnason NH, Morrow M, Lippman ME, Black D, Glusman JE, Costa A, Jordan VC. JAMA, J. Am. Med. Assoc. 1999;281:2189–2197. doi: 10.1001/jama.281.23.2189. [DOI] [PubMed] [Google Scholar]
- 33.Delmas PD, Bjarnason NH, Mitlak BH, Ravoux AC, Shah AS, Huster WJ, Draper M, Christiansen C. N Engl. J. Med. 1997;337:1641–1647. doi: 10.1056/NEJM199712043372301. [DOI] [PubMed] [Google Scholar]
- 34.Cummings SR, Ensrud K, Delmas PD, LaCroix AZ, Vukicevic S, Reid DM, Goldstein S, Sriram U, Lee A, Thompson J, Armstrong RA, Thompson DD, Powles T, Zanchetta J, Kendler D, Neven P, Eastell R. N. Engl. J. Med. 2010;362:686–696. doi: 10.1056/NEJMoa0808692. [DOI] [PubMed] [Google Scholar]
- 35.Silverman SL, Christiansen C, Genant HK, Vukicevic S, Zanchetta JR, de Villiers TJ, Constantine GD, Chines AA. J. Bone Miner. Res. 2008;23:1923–1934. doi: 10.1359/jbmr.080710. [DOI] [PubMed] [Google Scholar]
- 36.Keller H, Dreyer C, Medin J, Mahfoudi A, Ozato K, Wahli W. Proc. Natl. Acad. Sci. U. S. A. 1993;90:2160–2164. doi: 10.1073/pnas.90.6.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tontonoz P, Hu E, Graves RA, Budavari AI, Spiegelman BM. Genes Dev. 1994;8:1224–1234. doi: 10.1101/gad.8.10.1224. [DOI] [PubMed] [Google Scholar]
- 38.Ribon V, Johnson JH, Camp HS, Saltiel AR. Proc. Natl. Acad. Sci U. S. A. 1998;95:14751–14756. doi: 10.1073/pnas.95.25.14751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vega RB, Huss JM, Kelly DP. Mol. Cell. Biol. 2000;20:1868–1876. doi: 10.1128/mcb.20.5.1868-1876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mochizuki K, Suruga K, Fukami H, Kiso Y, Takase S, Goda T. Life Sci. 2006;80:140–145. doi: 10.1016/j.lfs.2006.08.029. [DOI] [PubMed] [Google Scholar]
- 41.Kersten S, Seydoux J, Peters JM, Gonzalez FJ, Desvergne B, Wahli W. J. Clin. Invest. 1999;103:1489–1498. doi: 10.1172/JCI6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muoio DM, Way JM, Tanner CJ, Winegar DA, Kliewer SA, Houmard JA, Kraus WE, Dohm GL. Diabetes. 2002;51:901–909. doi: 10.2337/diabetes.51.4.901. [DOI] [PubMed] [Google Scholar]
- 43.Hammarstedt A, Andersson CX, Sopasakis V Rotter, Smith U. Prostaglandins Leukotrienes Essent. Fatty Acids. 2005;73:65–75. doi: 10.1016/j.plefa.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 44.Delerive P, De Bosscher K, Besnard S, Vanden Berghe W, Peters JM, Gonzalez FJ, Fruchart JC, Tedgui A, Haegeman G, Staels B. J. Biol. Chem. 1999;274:32048–32054. doi: 10.1074/jbc.274.45.32048. [DOI] [PubMed] [Google Scholar]
- 45.Nolan JJ, Ludvik B, Beerdsen P, Joyce M, Olefsky J. N. Engl. J. Med. 1994;331:1188–1193. doi: 10.1056/NEJM199411033311803. [DOI] [PubMed] [Google Scholar]
- 46.Berger J, Bailey P, Biswas C, Cullinan CA, Doebber TW, Hayes NS, Saperstein R, Smith RG, Leibowitz MD. Endocrinology. 1996;137:4189–4195. doi: 10.1210/endo.137.10.8828476. [DOI] [PubMed] [Google Scholar]
- 47.Kobayashi J, Nagashima I, Hikita M, Bujo H, Takahashi K, Otabe M, Morisaki N, Saito Y. Br. J. Clin. Pharmacol. 1999;47:433–439. doi: 10.1046/j.1365-2125.1999.00920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Okuno A, Tamemoto H, Tobe K, Ueki K, Mori Y, Iwamoto K, Umesono K, Akanuma Y, Fujiwara T, Horikoshi H, Yazaki Y, Kadowaki T. J. Clin. Invest. 1998;101:1354–1361. doi: 10.1172/JCI1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gale EAM. Lancet. 2001;357:1870–1875. doi: 10.1016/S0140-6736(00)04960-6. [DOI] [PubMed] [Google Scholar]
- 50.Lebovitz HE, Dole JF, Patwardhan R, Rappaport EB, Freed MI. J. Clin. Endocrinol. Metab. 2001;86:280–288. doi: 10.1210/jcem.86.1.7157. [DOI] [PubMed] [Google Scholar]
- 51.Miyazaki Y, Matsuda M, DeFronzo RA. Diabetes Care. 2002;25:517–523. doi: 10.2337/diacare.25.3.517. [DOI] [PubMed] [Google Scholar]
- 52.Graham DJ, Ouellet-Hellstrom R, MaCurdy TE, Ali F, Sholley C, Worrall C, Kelman JA. JAMA, J. Am. Med. Assoc. 2010;304:411–418. doi: 10.1001/jama.2010.920. [DOI] [PubMed] [Google Scholar]
- 53.Nissen SE, Wolski K. Arch. Intern. Med. 2010;170:1191–1201. doi: 10.1001/archinternmed.2010.207. [DOI] [PubMed] [Google Scholar]
- 54.Schoonjans K, Peinado-Onsurbe J, Lefebvre AM, Heyman RA, Briggs M, Deeb S, Staels B, Auwerx J. EMBO J. 1996;15:5336–5348. [PMC free article] [PubMed] [Google Scholar]
- 55.Vu-Dac N, Schoonjans K, Laine B, Fruchart JC, Auwerx J, Staels B. J. Biol. Chem. 1994;269:31012–31018. [PubMed] [Google Scholar]
- 56.Forman BM, Goode E, Chen J, Oro AE, Bradley DJ, Perlmann T, Noonan DJ, Burka LT, McMorris T, Lamph WW, Evans RM, Weinberger C. Cell. 1995;81:687–693. doi: 10.1016/0092-8674(95)90530-8. [DOI] [PubMed] [Google Scholar]
- 57.Wang H, Chen J, Hollister K, Sowers LC, Forman BM. Mol. Cell. 1999;3:543–553. doi: 10.1016/s1097-2765(00)80348-2. [DOI] [PubMed] [Google Scholar]
- 58.Lu TT, Makishima M, Repa JJ, Schoonjans K, Kerr TA, Auwerx J, Mangelsdorf DJ. Mol. Cell. 2000;6:507–515. doi: 10.1016/s1097-2765(00)00050-2. [DOI] [PubMed] [Google Scholar]
- 59.Pineda Torra I, Claudel T, Duval C, Kosykh V, Fruchart JC, Staels B. Mol. Endocrinol. 2003;17:259–272. doi: 10.1210/me.2002-0120. [DOI] [PubMed] [Google Scholar]
- 60.Zhang Y, Castellani LW, Sinal CJ, Gonzalez FJ, Edwards PA. Genes Dev. 2004;18:157–169. doi: 10.1101/gad.1138104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Watanabe M, Houten SM, Wang L, Moschetta A, Mangelsdorf DJ, Heyman RA, Moore DD, Auwerx J. J. Clin. Invest. 2004;113:1408–1418. doi: 10.1172/JCI21025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kast HR, Nguyen CM, Sinal CJ, Jones SA, Laffitte BA, Reue K, Gonzalez FJ, Willson TM, Edwards PA. Mol. Endocrinol. 2001;15:1720–1728. doi: 10.1210/mend.15.10.0712. [DOI] [PubMed] [Google Scholar]
- 63.Pellicciari R, Gioiello A, Costantino G, Sadeghpour BM, Rizzo G, Meyer U, Parks DJ, Entrena-Guadix A, Fiorucci S. J. Med. Chem. 2006;49:4208–4215. doi: 10.1021/jm060294k. [DOI] [PubMed] [Google Scholar]
- 64.Willy PJ, Umesono K, Ong ES, Evans RM, Heyman RA, Mangelsdorf DJ. Genes Dev. 1995;9:1033–1045. doi: 10.1101/gad.9.9.1033. [DOI] [PubMed] [Google Scholar]
- 65.Apfel R, Benbrook D, Lernhardt E, Ortiz MA, Salbert G, Pfahl M. Mol. Cell. Biol. 1994;14:7025–7035. doi: 10.1128/mcb.14.10.7025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lehmann JM, Kliewer SA, Moore LB, Smith-Oliver TA, Oliver BB, Su JL, Sundseth SS, Winegar DA, Blanchard DE, Spencer TA, Willson TM. J. Biol. Chem. 1997;272:3137–3140. doi: 10.1074/jbc.272.6.3137. [DOI] [PubMed] [Google Scholar]
- 67.Mitro N, Mak PA, Vargas L, Godio C, Hampton E, Molteni V, Kreusch A, Saez E. Nature. 2007;445:219–223. doi: 10.1038/nature05449. [DOI] [PubMed] [Google Scholar]
- 68.Repa JJ, Liang G, Ou J, Bashmakov Y, Lobaccaro JM, Shimomura I, Shan B, Brown MS, Goldstein JL, Mangelsdorf DJ. Genes Dev. 2000;14:2819–2830. doi: 10.1101/gad.844900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Repa JJ, Turley SD, Lobaccaro JA, Medina J, Li L, Lustig K, Shan B, Heyman RA, Dietschy JM, Mangelsdorf DJ. Science. 2000;289:1524–1529. doi: 10.1126/science.289.5484.1524. [DOI] [PubMed] [Google Scholar]
- 70.Grefhorst A, Elzinga BM, Voshol PJ, Plosch T, Kok T, Bloks VW, van der Sluijs FH, Havekes LM, Romijn JA, Verkade HJ, Kuipers F. J. Biol. Chem. 2002;277:34182–34190. doi: 10.1074/jbc.M204887200. [DOI] [PubMed] [Google Scholar]
- 71.Lund EG, Peterson LB, Adams AD, Lam MH, Burton CA, Chin J, Guo Q, Huang S, Latham M, Lopez JC, Menke JG, Milot DP, Mitnaul LJ, Rex-Rabe SE, Rosa RL, Tian JY, Wright SD, Sparrow CP. Biochem. Pharmacol. 2006;71:453–463. doi: 10.1016/j.bcp.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 72.Quinet EM, Savio DA, Halpern AR, Chen L, Schuster GU, Gustafsson JA, Basso MD, Nambi P. Mol. Pharmacol. 2006;70:1340–1349. doi: 10.1124/mol.106.022608. [DOI] [PubMed] [Google Scholar]
- 73.Miao B, Zondlo S, Gibbs S, Cromley D, Hosagrahara VP, Kirchgessner TG, Billheimer J, Mukherjee R. J. Lipid Res. 2004;45:1410–1417. doi: 10.1194/jlr.M300450-JLR200. [DOI] [PubMed] [Google Scholar]
- 74.Molteni V, Li X, Nabakka J, Liang F, Wityak J, Koder A, Vargas L, Romeo R, Mitro N, Mak PA, Seidel HM, Haslam JA, Chow D, Tuntland T, Spalding TA, Brock A, Bradley M, Castrillo A, Tontonoz P, Saez E. J. Med. Chem. 2007;50:4255–4259. doi: 10.1021/jm070453f. [DOI] [PubMed] [Google Scholar]
- 75.Staudinger JL, Goodwin B, Jones SA, Hawkins-Brown D, MacKenzie KI, LaTour A, Liu Y, Klaassen CD, Brown KK, Reinhard J, Willson TM, Koller BH, Kliewer SA. Proc. Natl. Acad. Sci. U. S. A. 2001;98:3369–3374. doi: 10.1073/pnas.051551698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lehmann JM, McKee DD, Watson MA, Willson TM, Moore JT, Kliewer SA. J. Clin. Invest. 1998;102:1016–1023. doi: 10.1172/JCI3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kliewer SA, Goodwin B, Willson TM. Endocr. Rev. 2002;23:687–702. doi: 10.1210/er.2001-0038. [DOI] [PubMed] [Google Scholar]
- 78.Rendic S, Di Carlo FJ. Drug Metab. Rev. 1997;29:413–580. doi: 10.3109/03602539709037591. [DOI] [PubMed] [Google Scholar]
- 79.Gregorio GV, Ball CS, Mowat AP, Mieli-Vergani G. Arch. Dis. Child. 1993;69:141–143. doi: 10.1136/adc.69.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ding X, Staudinger JL. Biochem. Pharmacol. 2005;69:867–873. doi: 10.1016/j.bcp.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 81.Drewes T, Senkel S, Holewa B, Ryffel GU. Mol. Cell. Biol. 1996;16:925–931. doi: 10.1128/mcb.16.3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jiang S, Tanaka T, Iwanari H, Hotta H, Yamashita H, Kumakura J, Watanabe Y, Uchiyama Y, Aburatani H, Hamakubo T, Kodama T, Naito M. Nucl. Recept. 2003;1:5. doi: 10.1186/1478-1336-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wisely GB, Miller AB, Davis RG, Thornquest AD, Jr, Johnson R, Spitzer T, Sefler A, Shearer B, Moore JT, Willson TM, Williams SP. Structure. 2002;10:1225–1234. doi: 10.1016/s0969-2126(02)00829-8. [DOI] [PubMed] [Google Scholar]
- 84.Yuan X, Ta TC, Lin M, Evans JR, Dong Y, Bolotin E, Sherman MA, Forman BM, Sladek FM. PLoS One. 2009;4:e5609. doi: 10.1371/journal.pone.0005609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hertz R, Magenheim J, Berman I, Bar-Tana J. Nature. 1998;392:512–516. doi: 10.1038/33185. [DOI] [PubMed] [Google Scholar]
- 86.Stoffel M, Duncan SA. Proc. Natl. Acad. Sci. U. S. A. 1997;94:13209–13214. doi: 10.1073/pnas.94.24.13209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hayhurst GP, Lee YH, Lambert G, Ward JM, Gonzalez FJ. Mol. Cell. Biol. 2001;21:1393–1403. doi: 10.1128/MCB.21.4.1393-1403.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kuo CJ, Conley PB, Chen L, Sladek FM, Darnell JE, Jr, Crabtree GR. Nature. 1992;355:457–461. doi: 10.1038/355457a0. [DOI] [PubMed] [Google Scholar]
- 89.Tirona RG, Lee W, Leake BF, Lan LB, Cline CB, Lamba V, Parviz F, Duncan SA, Inoue Y, Gonzalez FJ, Schuetz EG, Kim RB. Nat. Med. 2003;9:220–224. doi: 10.1038/nm815. [DOI] [PubMed] [Google Scholar]
- 90.Pineda Torra I, Jamshidi Y, Flavell DM, Fruchart B, Staels JC. Mol. Endocrinol. 2002;16:1013–1028. doi: 10.1210/mend.16.5.0833. [DOI] [PubMed] [Google Scholar]
- 91.Yamagata K, Furuta H, Oda N, Kaisaki PJ, Menzel S, Cox NJ, Fajans SS, Signorini S, Stoffel M, Bell GI. Nature. 1996;384:458–460. doi: 10.1038/384458a0. [DOI] [PubMed] [Google Scholar]
- 92.Kiselyuk A, Lee SH, Farber-Katz S, Zhang M, Athavankar S, Cohen T, Pinkerton AB, Ye M, Bushway P, Richardson AD, Hostetler HA, Rodriguez-Lee M, Huang L, Spangler B, Smith L, Higginbotham J, Cashman J, Freeze H, Itkin-Ansari P, Dawson MI, Schroeder F, Cang Y, Mercola M, Levine F. Chem. Biol. 2012;19:806–818. doi: 10.1016/j.chembiol.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chun OK, Chung SJ, Song WO. J. Nutr. 2007;137:1244–1252. doi: 10.1093/jn/137.5.1244. [DOI] [PubMed] [Google Scholar]
- 94.Harborne JB. The flavonoids: advances in research since 1980. Chapman and Hall; 1988. [Google Scholar]
- 95.Linde K, Barrett B, Wolkart K, Bauer R, Melchart D. Cochrane Database of Systematic Reviews. 2006 doi: 10.1002/14651858.CD000530.pub2. CD000530. [DOI] [PubMed] [Google Scholar]
- 96.Ruepert L, Quartero AO, de Wit NJ, van der Heijden GJ, Rubin G, Muris JW. Cochrane Database of Systematic Reviews. 2011 doi: 10.1002/14651858.CD003460.pub3. CD003460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mulvihill EE, Allister EM, Sutherland BG, Telford DE, Sawyez CG, Edwards JY, Markle JM, Hegele RA, Huff MW. Diabetes. 2009;58:2198–2210. doi: 10.2337/db09-0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jeon SM, Kim HK, Kim HJ, Do GM, Jeong TS, Park YB, Choi MS. Transl. Res. 2007;149:15–21. doi: 10.1016/j.trsl.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 99.Mulvihill EE, Assini JM, Sutherland BG, DiMattia AS, Khami M, Koppes JB, Sawyez CG, Whitman SC, Huff MW. Arterioscler., Thromb., Vasc. Biol. 2010;30:742–748. doi: 10.1161/ATVBAHA.109.201095. [DOI] [PubMed] [Google Scholar]
- 100.Ruh MF, Zacharewski T, Connor K, Howell J, Chen I, Safe S. Biochem. Pharmacol. 1995;50:1485–1493. doi: 10.1016/0006-2952(95)02061-6. [DOI] [PubMed] [Google Scholar]
- 101.Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 102.Borradaile NM, de Dreu LE, Huff MW. Diabetes. 2003;52:2554–2561. doi: 10.2337/diabetes.52.10.2554. [DOI] [PubMed] [Google Scholar]
- 103.Wilcox LJ, Borradaile NM, de Dreu LE, Huff MW. J. Lipid Res. 2001;42:725–734. [PubMed] [Google Scholar]
- 104.Goldwasser J, Cohen PY, Yang E, Balaguer P, Yarmush ML, Nahmias Y. PLoS One. 2010;5:e12399. doi: 10.1371/journal.pone.0012399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Akiyama S, Katsumata S-i, Suzuki K, Nakaya Y, Ishimi Y, Uehara M. Biosci., Biotechnol., Biochem. 2009;73:2779–2782. doi: 10.1271/bbb.90576. [DOI] [PubMed] [Google Scholar]
- 106.Iio A, Ohguchi K, Iinuma M, Nozawa Y, Ito M. J. Nat Prod. 2012;75:563–566. doi: 10.1021/np200696r. [DOI] [PubMed] [Google Scholar]
- 107.Liu L, Shan S, Zhang K, Ning ZQ, Lu XP, Cheng YY. Phytother. Res. 2008;22:1400–1403. doi: 10.1002/ptr.2504. [DOI] [PubMed] [Google Scholar]
- 108.Hunter MD, Hull LA. Phytochemistry. 1993;34:1251–1254. [Google Scholar]
- 109.Kimmich GA, Randles J. Membr. Biochem. 1978;1:221–237. doi: 10.3109/09687687809063849. [DOI] [PubMed] [Google Scholar]
- 110.Hassan M, El Yazidi C, Landrier JF, Lairon D, Margotat A, Amiot MJ. Biochem. Biophys. Res. Commun. 2007;361:208–213. doi: 10.1016/j.bbrc.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 111.Ise R, Han D, Takahashi Y, Terasaka S, Inoue A, Tanji M, Kiyama R. FEBS Lett. 2005;579:1732–1740. doi: 10.1016/j.febslet.2005.02.033. [DOI] [PubMed] [Google Scholar]
- 112.Gheldof N, Wang XH, Engeseth NJ. J. Agric. Food Chem. 2002;50:5870–5877. doi: 10.1021/jf0256135. [DOI] [PubMed] [Google Scholar]
- 113.Justesen U, Knuthsen P, Leth T. J. Chromatogr., A. 1998;799:101–110. doi: 10.1016/s0021-9673(97)01061-3. [DOI] [PubMed] [Google Scholar]
- 114.Dong H, Lin W, Wu J, Chen T. BMC Biochem. 2010;11:23. doi: 10.1186/1471-2091-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liang Y-C, Tsai S-H, Tsai D-C, Lin-Shiau S-Y, Lin J-K. FEBS Lett. 2001;496:12–18. doi: 10.1016/s0014-5793(01)02393-6. [DOI] [PubMed] [Google Scholar]
- 116.Ding L, Jin D, Chen X. J. Nutr. Biochem. 2010;21:941–947. doi: 10.1016/j.jnutbio.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 117.Puhl AC, Bernardes A, Silveira RL, Yuan J, Campos JL, Saidemberg DM, Palma MS, Cvoro A, Ayers SD, Webb P, Reinach PS, Skaf MS, Polikarpov I. Mol. Pharmacol. 2012;81:788–799. doi: 10.1124/mol.111.076216. [DOI] [PubMed] [Google Scholar]
- 118.Long X, Fan M, Bigsby RM, Nephew KP. Mol. Cancer Ther. 2008;7:2096–2108. doi: 10.1158/1535-7163.MCT-07-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kurowska EM, Manthey JA. J. Agric. Food Chem. 2004;52:2879–2886. doi: 10.1021/jf035354z. [DOI] [PubMed] [Google Scholar]
- 120.Kurowska EM, Manthey JA, Casaschi A, Theriault AG. Lipids. 2004;39:143–151. doi: 10.1007/s11745-004-1212-8. [DOI] [PubMed] [Google Scholar]
- 121.Choi Y, Kim Y, Ham H, Park Y, Jeong HS, Lee J. J. Agric. Food Chem. 2011;59:12843–12849. doi: 10.1021/jf2033208. [DOI] [PubMed] [Google Scholar]
- 122.Lee YS, Cha BY, Choi SS, Choi BK, Yonezawa T, Teruya T, Nagai K, Woo JT. J. Nutr. Biochem. 2012 doi: 10.1016/j.jnutbio.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 123.Li RW, Theriault AG, Au K, Douglas TD, Casaschi A, Kurowska EM, Mukherjee R. Life Sci. 2006;79:365–373. doi: 10.1016/j.lfs.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 124.Mulvihill EE, Assini JM, Lee JK, Allister EM, Sutherland BG, Koppes JB, Sawyez CG, Edwards JY, Telford DE, Charbonneau A, St-Pierre P, Marette A, Huff MW. Diabetes. 2011;60:1446–1457. doi: 10.2337/db10-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Guo AJ, Choi RC, Zheng KY, Chen VP, Dong TT, Wang ZT, Vollmer G, Lau DT, Tsim KW. Chin. Med. 2012;7:10. doi: 10.1186/1749-8546-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rivera L, Moron R, Sanchez M, Zarzuelo A, Galisteo M. Obesity. 2008;16:2081–2087. doi: 10.1038/oby.2008.315. [DOI] [PubMed] [Google Scholar]
- 127.Gnoni GV, Paglialonga G, Siculella L. Eur. J. Clin. Invest. 2009;39:761–768. doi: 10.1111/j.1365-2362.2009.02167.x. [DOI] [PubMed] [Google Scholar]
- 128.Martinez-Florez S, Gutierrez-Fernandez B, Sanchez-Campos S, Gonzalez-Gallego J, Tunon MJ. J. Nutr. 2005;135:1359–1365. doi: 10.1093/jn/135.6.1359. [DOI] [PubMed] [Google Scholar]
- 129.Fang XK, Gao J, Zhu DN. Life Sci. 2008;82:615–622. doi: 10.1016/j.lfs.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 130.Wein S, Behm N, Petersen RK, Kristiansen K, Wolffram S. Eur. J. Pharm. Sci. 2010;41:16–22. doi: 10.1016/j.ejps.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 131.Ramachandran L, Manu KA, Shanmugam MK, Li F, Siveen KS, Vali S, Kapoor S, Abbasi T, Surana R, Smoot DT, Ashktorab H, Tan P, Ahn KS, Yap CW, Kumar AP, Sethi G. J. Biol. Chem. 2012 doi: 10.1074/jbc.M112.388702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bulzomi P, Galluzzo P, Bolli A, Leone S, Acconcia F, Marino M. J. Cell. Physiol. 2012;227:1891–1898. doi: 10.1002/jcp.22917. [DOI] [PubMed] [Google Scholar]
- 133.Galluzzo P, Martini C, Bulzomi P, Leone S, Bolli A, Pallottini V, Marino M. Mol. Nutr. Food Res. 2009;53:699–708. doi: 10.1002/mnfr.200800239. [DOI] [PubMed] [Google Scholar]
- 134.Arts IC, van De Putte B, Hollman PC. J. Agric. Food Chem. 2000;48:1752–1757. doi: 10.1021/jf000026+. [DOI] [PubMed] [Google Scholar]
- 135.Arts IC, van de Putte B, Hollman PC. J. Agric. Food Chem. 2000;48:1746–1751. doi: 10.1021/jf000025h. [DOI] [PubMed] [Google Scholar]
- 136.Klaus S, Pultz S, Thone-Reineke C, Wolfram S. Int. J. Obes. 2005;29:615–623. doi: 10.1038/sj.ijo.0802926. [DOI] [PubMed] [Google Scholar]
- 137.Ortsater H, Grankvist N, Wolfram S, Kuehn N, Sjoholm A. Nutr. Metab. 2012;9:11. doi: 10.1186/1743-7075-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chan CY, Wei L, Castro-Munozledo F, Koo WL. Life Sci. 2011;89:779–785. doi: 10.1016/j.lfs.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 139.Sae-Tan S, Grove KA, Kennett MJ, Lambert JD. Food Funct. 2011;2:111–116. doi: 10.1039/C0FO00155D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Li G, Lin W, Araya JJ, Chen T, Timmermann BN, Guo GL. Toxicol. Appl. Pharmacol. 2012;258:268–274. doi: 10.1016/j.taap.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zoechling A, Liebner F, Jungbauer A. Food Funct. 2011;2:28–38. doi: 10.1039/c0fo00086h. [DOI] [PubMed] [Google Scholar]
- 142.Gehm BD, McAndrews JM, Chien PY, Jameson JL. Proc. Natl. Acad. Sci. U. S. A. 1997;94:14138–14143. doi: 10.1073/pnas.94.25.14138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chalopin M, Tesse A, Martinez MC, Rognan D, Arnal JF, Andriantsitohaina R. PLoS One. 2010;5:e8554. doi: 10.1371/journal.pone.0008554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Del Bas JM, Fernández-Larrea J, Blay M, Ard`evol A, Salvadó MJ, Arola L, Bladé C. FASEB J. 2005;19:479–481. doi: 10.1096/fj.04-3095fje. [DOI] [PubMed] [Google Scholar]
- 145.Del Bas JM, Ricketts ML, Vaque M, Sala E, Quesada H, Ardevol A, Salvado MJ, Blay M, Arola L, Moore DD, Pujadas G, Fernandez-Larrea J, Blade C. Mol. Nutr. Food Res. 2009;53:805–814. doi: 10.1002/mnfr.200800364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wiseman H, Casey K, Clarke DB, Barnes KA, Bowey E. J. Agric. Food Chem. 2002;50:1404–1410. doi: 10.1021/jf011243t. [DOI] [PubMed] [Google Scholar]
- 147.Cox RI, Braden AW. Proc. Aust. Soc. Anim. Prod. 1974;10:122–129. [Google Scholar]
- 148.Dang Z, Lowik CW. J. Bone Miner. Res. 2004;19:853–861. doi: 10.1359/JBMR.040120. [DOI] [PubMed] [Google Scholar]
- 149.Dang ZC, Audinot V, Papapoulos SE, Boutin JA, Lowik CW. J. Biol. Chem. 2003;278:962–967. doi: 10.1074/jbc.M209483200. [DOI] [PubMed] [Google Scholar]
- 150.Raynaud JP, Azadian-Boulanger G, Bouton MM, Colin MC, Faure N, Fernand-Proulx L, Gautray JP, Husson JM, Jolivet A, Kelly P, et al. J. Steroid Biochem. 1984;20:981–993. doi: 10.1016/0022-4731(84)90008-6. [DOI] [PubMed] [Google Scholar]
- 151.Kim S, Shin HJ, Kim SY, Kim JH, Lee YS, Kim DH, Lee MO. Mol. Cell. Endocrinol. 2004;220:51–58. doi: 10.1016/j.mce.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 152.Cho KW, Lee OH, Banz WJ, Moustaid-Moussa N, Shay NF, Kim YC. J. Nutr. Biochem. 2010;21:841–847. doi: 10.1016/j.jnutbio.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 153.Li Y, Ross-Viola JS, Shay NF, Moore DD, Ricketts ML. J. Nutr. 2009;139:898–904. doi: 10.3945/jn.108.103572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mezei O, Banz WJ, Steger RW, Peluso MR, Winters TA, Shay N. J. Nutr. 2003;133:1238–1243. doi: 10.1093/jn/133.5.1238. [DOI] [PubMed] [Google Scholar]
- 155.Mezei O, Li Y, Mullen E, Ross-Viola JS, Shay NF. Physiol. Genomics. 2006;26:8–14. doi: 10.1152/physiolgenomics.00155.2005. [DOI] [PubMed] [Google Scholar]
- 156.Divi RL, Chang HC, Doerge DR. Biochem. Pharmacol. 1997;54:1087–1096. doi: 10.1016/s0006-2952(97)00301-8. [DOI] [PubMed] [Google Scholar]
- 157.Messina M, Redmond G. Thyroid. 2006;16:249–258. doi: 10.1089/thy.2006.16.249. [DOI] [PubMed] [Google Scholar]
- 158.Chalker-Scott L. Photochem. Photobiol. 1999;70:1–9. [Google Scholar]
- 159.Scazzocchio B, Vari R, Filesi C, D'Archivio M, Santangelo C, Giovannini C, Iacovelli A, Silecchia G, Li Volti G, Galvano F, Masella R. Diabetes. 2011;60:2234–2244. doi: 10.2337/db10-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Xia M, Hou M, Zhu H, Ma J, Tang Z, Wang Q, Li Y, Chi D, Yu X, Zhao T, Han P, Xia X, Ling W. J. Biol. Chem. 2005;280:36792–36801. doi: 10.1074/jbc.M505047200. [DOI] [PubMed] [Google Scholar]
- 161.Wang Y, Zhang Y, Wang X, Liu Y, Xia M. Atherosclerosis. 2012;223:299–305. doi: 10.1016/j.atherosclerosis.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 162.Seymour EM, Singer AA, Kirakosyan A, Urcuyo-Llanes DE, Kaufman PB, Bolling SF. J. Med. Food. 2008;11:252–259. doi: 10.1089/jmf.2007.658. [DOI] [PubMed] [Google Scholar]
- 163.Satyavati GV. Indian J. Med. Res. 1988;87:327–335. [PubMed] [Google Scholar]
- 164.Nityanand S, Srivastava JS, Asthana OP. J. Assoc. Physicians India. 1989;37:323–328. [PubMed] [Google Scholar]
- 165.Singh RB, Niaz MA, Ghosh S. Cardiovasc. Drugs Ther. 1994;8:659–664. doi: 10.1007/BF00877420. [DOI] [PubMed] [Google Scholar]
- 166.Urizar NL, Liverman AB, Dodds DT, Silva FV, Ordentlich P, Yan Y, Gonzalez FJ, Heyman RA, Mangelsdorf DJ, Moore DD. Science. 2002;296:1703–1706. doi: 10.1126/science.1072891. [DOI] [PubMed] [Google Scholar]
- 167.Honda K, Kamisoyama H, Tominaga Y, Yokota S, Hasegawa S. Anim. Sci. J. 2009;80:562–569. doi: 10.1111/j.1740-0929.2009.00670.x. [DOI] [PubMed] [Google Scholar]
- 168.Ahn J, Lee H, Jang J, Kim S, Ha T. Food Chem. Toxicol. 2013;51:439–445. doi: 10.1016/j.fct.2012.08.048. [DOI] [PubMed] [Google Scholar]
- 169.Carmeli E, Fogelman Y. Toxicol. Ind. Health. 2009;25:321–324. doi: 10.1177/0748233709103034. [DOI] [PubMed] [Google Scholar]
- 170.Tamir S, Eizenberg M, Somjen D, Stern N, Shelach R, Kaye A, Vaya J. Cancer Res. 2000;60:5704–5709. [PubMed] [Google Scholar]
- 171.Somjen D, Knoll E, Vaya J, Stern N, Tamir S. J. Steroid Biochem. Mol. Biol. 2004;91:147–155. doi: 10.1016/j.jsbmb.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 172.Tamir S, Eizenberg M, Somjen D, Izrael S, Vaya J. J. Steroid Biochem. Mol. Biol. 2001;78:291–298. doi: 10.1016/s0960-0760(01)00093-0. [DOI] [PubMed] [Google Scholar]
- 173.Hollman P. Pharm. Biol. 2004;42:74–83. [Google Scholar]
- 174.Walle T. Free Radical Biol. Med. 2004;36:829–837. doi: 10.1016/j.freeradbiomed.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 175.Manach C, Williamson G, Morand C, Scalbert A, Remesy C. Am. J. Clin. Nutr. 2005;81:230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 176.Williamson G, Manach C. Am. J. Clin. Nutr. 2005;81:243S–255S. doi: 10.1093/ajcn/81.1.243S. [DOI] [PubMed] [Google Scholar]
- 177.Shulman M, Cohen M, Soto-Gutierrez A, Yagi H, Wang H, Goldwasser J, Lee-Parsons CW, Benny-Ratsaby O, Yarmush ML, Nahmias Y. PLoS One. 2011;6:e18033. doi: 10.1371/journal.pone.0018033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhu M, Chen Y, Li RC. Planta Med. 2000;66:444–447. doi: 10.1055/s-2000-8599. [DOI] [PubMed] [Google Scholar]
- 179.Chen Z, Tu M, Sun S, Kong S, Wang Y, Ye J, Li L, Zeng S, Jiang H. Drug Metab. Pharmacokinet. 2012;27:162–168. doi: 10.2133/dmpk.dmpk-11-rg-081. [DOI] [PubMed] [Google Scholar]
- 180.Kanaze FI, Bounartzi MI, Georgarakis M, Niopas I. Eur. J. Clin. Nutr. 2007;61:472–477. doi: 10.1038/sj.ejcn.1602543. [DOI] [PubMed] [Google Scholar]
- 181.Van Amelsvoort JM, Van Hof KH, Mathot JN, Mulder TP, Wiersma A, Tijburg LB. Xenobiotica. 2001;31:891–901. doi: 10.1080/00498250110079149. [DOI] [PubMed] [Google Scholar]
- 182.Williamson G. Phytochem. Rev. 2002;1:215–222. [Google Scholar]
- 183.Williamson G, Barron D, Shimoi K, Terao J. Free Radical Res. 2005;39:457–469. doi: 10.1080/10715760500053610. [DOI] [PubMed] [Google Scholar]
- 184.Kawai Y, Nishikawa T, Shiba Y, Saito S, Murota K, Shibata N, Kobayashi M, Kanayama M, Uchida K, Terao J. J. Biol. Chem. 2008;283:9424–9434. doi: 10.1074/jbc.M706571200. [DOI] [PubMed] [Google Scholar]
- 185.Terao J, Murota K, Kawai Y. Food Funct. 2011;2:11–17. doi: 10.1039/c0fo00106f. [DOI] [PubMed] [Google Scholar]
- 186.Menendez C, Duenas M, Galindo P, Gonzalez-Manzano S, Jimenez R, Moreno L, Zarzuelo MJ, Rodriguez-Gomez I, Duarte J, Santos-Buelga C, Perez-Vizcaino F. Mol. Nutr. Food Res. 2011;55:1780–1790. doi: 10.1002/mnfr.201100378. [DOI] [PubMed] [Google Scholar]
- 187.O'Leary KA, Day AJ, Needs PW, Mellon FA, O'Brien NM, Williamson G. Biochem. Pharmacol. 2003;65:479–491. doi: 10.1016/s0006-2952(02)01510-1. [DOI] [PubMed] [Google Scholar]
- 188.Kinjo J, Tsuchihashi R, Morito K, Hirose T, Aomori T, Nagao T, Okabe H, Nohara T, Masamune Y. Biol. Pharm. Bull. 2004;27:185–188. doi: 10.1248/bpb.27.185. [DOI] [PubMed] [Google Scholar]
- 189.Wen X, Walle T. Drug Metab. Dispos. 2006;34:1786–1792. doi: 10.1124/dmd.106.011122. [DOI] [PubMed] [Google Scholar]
- 190.Wen J, Liu B, Yuan E, Ma Y, Zhu Y. Molecules. 2010;15:4401–4407. doi: 10.3390/molecules15064401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kim H, Choi J, Jung S. J. Inclusion Phenom. Macrocyclic Chem. 2009;64:43–47. [Google Scholar]
- 192.Zheng Y, Haworth IS, Zuo Z, Chow MS, Chow AH. J. Pharm. Sci. 2005;94:1079–1089. doi: 10.1002/jps.20325. [DOI] [PubMed] [Google Scholar]
- 193.Stancanelli R, Mazzaglia A, Tommasini S, Calabro ML, Villari V, Guardo M, Ficarra P, Ficarra R. J. Pharm. Biomed. Anal. 2007;44:980–984. doi: 10.1016/j.jpba.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 194.Cohen R, Orlova Y, Kovalev M, Ungar Y, Shimoni E. J. Agric. Food Chem. 2008;56:4212–4218. doi: 10.1021/jf800255c. [DOI] [PubMed] [Google Scholar]
- 195.Cohen R, Schwartz B, Peri I, Shimoni E. J. Agric. Food Chem. 2011;59:7932–7938. doi: 10.1021/jf2013277. [DOI] [PubMed] [Google Scholar]
- 196.Kakran M, Sahoo NG, Li L. Colloids Surf., B. 2011;88:121–130. doi: 10.1016/j.colsurfb.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 197.Sonali D, Tejal S, Vaishali T, Tejal G. Acta Pharm. 2010;60:427–443. doi: 10.2478/v10007-010-0038-3. [DOI] [PubMed] [Google Scholar]
- 198.Onoue S, Uchida A, Takahashi H, Seto Y, Kawabata Y, Ogawa K, Yuminoki K, Hashimoto N, Yamada S. J. Pharm. Sci. 2011;100:3793–3801. doi: 10.1002/jps.22585. [DOI] [PubMed] [Google Scholar]
- 199.Laffitte BA, Chao LC, Li J, Walczak R, Hummasti S, Joseph SB, Castrillo A, Wilpitz DC, Mangelsdorf DJ, Collins JL, Saez E, Tontonoz P. Proc. Natl. Acad. Sci. U. S. A. 2003;100:5419–5424. doi: 10.1073/pnas.0830671100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ou J, Tu H, Shan B, Luk A, DeBose-Boyd RA, Bashmakov Y, Goldstein JL, Brown MS. Proc. Natl. Acad. Sci. U. S. A. 2001;98:6027–6032. doi: 10.1073/pnas.111138698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Yoshikawa T, Shimano H, Yahagi N, Ide T, Amemiya-Kudo M, Matsuzaka T, Nakakuki M, Tomita S, Okazaki H, Tamura Y, Iizuka Y, Ohashi K, Takahashi A, Sone H, Osuga Ji J, Gotoda T, Ishibashi S, Yamada N. J. Biol. Chem. 2002;277:1705–1711. doi: 10.1074/jbc.M105711200. [DOI] [PubMed] [Google Scholar]
- 202.Ide T, Shimano H, Yoshikawa T, Yahagi N, Amemiya-Kudo M, Matsuzaka T, Nakakuki M, Yatoh S, Iizuka Y, Tomita S, Ohashi K, Takahashi A, Sone H, Gotoda T, Osuga J, Ishibashi S, Yamada N. Mol. Endocrinol. 2003;17:1255–1267. doi: 10.1210/me.2002-0191. [DOI] [PubMed] [Google Scholar]
- 203.Shimano H, Yahagi N, Amemiya-Kudo M, Hasty AH, Osuga J, Tamura Y, Shionoiri F, Iizuka Y, Ohashi K, Harada K, Gotoda T, Ishibashi S, Yamada N. J. Biol. Chem. 1999;274:35832–35839. doi: 10.1074/jbc.274.50.35832. [DOI] [PubMed] [Google Scholar]
- 204.Rice-Evans CA, Miller NJ, Paganga G. Free Radical Biol. Med. 1996;20:933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- 205.Fraga CG, Martino VS, Ferraro GE, Coussio JD, Boveris A. Biochem. Pharmacol. 1987;36:717–720. doi: 10.1016/0006-2952(87)90724-6. [DOI] [PubMed] [Google Scholar]
- 206.Garcia-Mediavilla V, Crespo I, Collado PS, Esteller A, Sanchez-Campos S, Tunon MJ, Gonzalez-Gallego J. Eur. J. Pharmacol. 2007;557:221–229. doi: 10.1016/j.ejphar.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 207.Hougee S, Sanders A, Faber J, Graus YM, van den Berg WB, Garssen J, Smit HF, Hoijer MA. Biochem. Pharmacol. 2005;69:241–248. doi: 10.1016/j.bcp.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 208.Pan MH, Chen WJ, Lin-Shiau SY, Ho CT, Lin JK. Carcinogenesis. 2002;23:1677–1684. doi: 10.1093/carcin/23.10.1677. [DOI] [PubMed] [Google Scholar]
- 209.Czyz J, Madeja Z, Irmer U, Korohoda W, Hulser DF. Int. J. Cancer. 2005;114:12–18. doi: 10.1002/ijc.20620. [DOI] [PubMed] [Google Scholar]