Abstract
Pancreatic duodenal homeobox-1 (Pdx1), a transcription factor required for pancreatic development and maintenance of β-cell function, was assessed for a possible role in postnatal β-cell formation from progenitors in the pancreatic ducts by selectively deleting Pdx1 from the ducts. Carbonic anhydrase II (CAII)Cre;Pdx1Fl mice were euglycemic for the first 2 postnatal weeks but showed moderate hyperglycemia from 3 to 7 weeks of age. By 10 weeks, they had near-normal morning fed glucose levels but showed severely impaired glucose tolerance and insulin secretion. Yet the loss of Pdx1 did not result in decreased islet and β-cell mass at 4 and 10 weeks of age. Within the same pancreas, there was a mixed population of islets, with PDX1 and MAFA protein expression normal in some cells and severely diminished in others. Even at 10 weeks, islets expressed immaturity markers. Thus, we conclude that Pdx1 is not necessary for the postnatal formation of β-cells but is essential for their full maturation to glucose-responsive β-cells.
Diabetes results from an inadequate functional β-cell mass; therefore, the possible replenishment of β-cells receives much attention. Endogenous replenishment can occur by replication and by neogenesis or differentiation of β-cells from nonendocrine progenitors or precursors (1). Neogenesis occurs during specific periods of normal embryonic and postnatal growth, after some forms of pancreatic injury (2–6), and can be induced by growth factors and/or cytokines (7–10). For example, in rodents over the first month after birth, while β-cell replication continues, significant neogenesis has been documented (11–16).
The mechanisms responsible for neogenesis are still poorly understood. A potentially important contributor is pancreatic duodenal homeobox-1 (PDX1), a transcription factor necessary for pancreatic development and maintenance of β-cell function. Global deletion of Pdx1 results in pancreatic agenesis (17,18). PDX1 function has been shown to be required for proliferation of β-cells at late gestation (19) and for maintaining the function of the mature β-cells (20,21). PDX1 is expressed in the embryonic pancreatic progenitors before becoming restricted to the β-cells and a small proportion of δ-cells. PDX1 protein is transiently expressed, however, in replicating ducts during regeneration (22–25).
We hypothesized that PDX1 was necessary for the neogenetic formation of β-cells from mature ducts and therefore generated duct-specific Pdx1-deficient mice using the Cre-lox system with Carbonic Anhydrase II (CAII)Cre (14) and Pdx1 floxed E2 mice (19) in which Pdx1 expression should be specifically deleted from ducts only starting around birth. Here, we show that Pdx1 is not necessary for formation of new β-cells from postnatal pancreatic ducts, unlike its required role for formation of all pancreatic cell types during embryonic organogenesis, but that Pdx1 is essential for these newly formed cells to mature into fully functional β-cells.
RESEARCH DESIGN AND METHODS
Animals.
Transgenic mice with floxed Pdx1 (Pdx1FL/FL) (19) and constitutive CAIICre (14) were mated. In some experiments CAIICre animals carried the reporter gene from being mated with B6.129X1-Gt(ROSA)26Sortm1(EYFP)Cos/J (ROSA26ReYFP) mice from The Jackson Laboratories. DNA extracted from tails at weaning was used for genotyping with primers recognizing the floxed Pdx1 primer 5′-AGGGTTCCGGATCGATCCCC-3′ and 5′-AGCAGCTGGAGCTAGGC-3′, the wild-type (WT) Pdx1 primers 5′-CCTTTGCGGATCCTT-3′ and 5′-GCCAACAACTGGCAGATTC, and Cre primers 5′-ACCTGAAGATGTTCGCGATTATCT-3′ and 5′-GATCATCAGCTACACCAGAGA-3′. PCR was used 40 cycles for Cre, 31 cycles for floxed Pdx1, and 37 cycles for WT Pdx1 allele.
Mice were housed in the Joslin Animal Facility on a 12-h light/12-h dark cycle and with water and food ad libitum. CAIICre+;Pdx1FL/+ mice were used for breeding to generate six genotypes: CAIICre+;Pdx1Fl/Fl, CAIICre+;Pdx1Fl/+, CAIICre+;Pdx1+/+, CAIICre-;Pdx1Fl/Fl, CAIICre-;Pdx1Fl/+ and CAIICre-;Pdx1+/+. The first two were considered bigenic experimental mice, and the others served as controls.
Body weight and morning fed glucose levels were measured weekly. Blood glucose values were measured using One-Touch glucometer (LifeScan, Milpitas, CA) on blood from tail snip. Samples for intraperitoneal glucose tolerance tests were collected from mice fasted overnight (15 h) at 0, 15, 30, 60, 90, and 120 min after an intraperitoneal injection of glucose (2 g/kg body weight). Plasma insulin was measured with a rat insulin ELISA kit (ALPCO, Salem, NH). For insulin tolerance tests, blood glucose was measured at 0, 15, 30, and 60 min after intraperitoneal insulin injection (Humulin R; Eli Lilly, Indianapolis, IN; 0.75 units/kg body weight) of fasted (9:00 a.m.–3:00 p.m.) mice.
Animals were killed under anesthesia, and the pancreas was excised for histology or islet isolation. For immunostaining, the excised pancreas was spread flat and fixed for 2 h in 4% paraformaldehyde for embedding in paraffin or for frozen blocks. For secretion studies or RNA analysis, islets were isolated by the collagenase method (26), with each mouse as a separate sample for islet studies. The Joslin Institutional Animal Care and Use Committee approved all animal procedures.
Immunochemistry.
Sections were immunostained for immunoperoxidase using the ABC kit (Vector Laboratories, Burlingame, CA) or immunofluorescence. Antigen retrieval was performed in 10 mmol/L citric acid buffer by microwave or PickCell 2100 antigen retriever (BD Biosciences). Sections were incubated overnight at 4°C with primary antibodies, followed by species-appropriate secondary antibodies (Supplementary Table 1). The tyramide (TSA) system (PerkinElmer, Waltham, MA) was used for amplification of PDX1, MAFA, and MAFB, following the manufacturer’s instruction. Images were taken in confocal mode on a Zeiss LSM 410 microscope. For comparison of the intensity of PDX1 and MAFA staining in mice of different genotypes, images were taken at the same settings on sections from littermates stained in parallel and handled identically in Adobe Photoshop. At least three animals per genotype were examined for each antigen.
Morphometric analysis of β- and non–β-cell mass.
Paraffin sections of 4- or 10-week-old male mouse pancreas stained by immunoperoxidase with a cocktail of non–β-cell islet hormones (glucagon, somatostatin, and pancreatic polypeptide [PP]) were analyzed by point counting morphometry for islet mass (27). β-cell mass was similarly determined on adjacent sections stained for insulin. Intersections with a 90-point grid were counted systematically in nonoverlapping fields to obtain β- and non–β-cell relative volumes (% total tissue) as well as the percentage of pancreatic parenchyma of total tissue; at least 150 fields were counted for each full footprint of pancreas section. Absolute mass was determined by multiplying the relative volume by pancreatic weight.
Insulin secretion.
After overnight culture in RPMI 1640 medium (11 mmol/L glucose and 10% FBS), triplicate samples of 10 equilibrated islets for each mouse placed in wells of a 24-well plate were sequentially incubated with 2.6 and 16.8 mmol/L glucose in Krebs-Ringer buffer (16 mmol/L HEPES and 0.1% BSA, pH 7.4) (28,29). Supernatant fractions and cell lysates were frozen until assayed for insulin, as above. DNA was measured on cell lysates using a Cyquant Cell Proliferation Kit (Molecular Probes, Grand Island, NY).
Quantitative real-time PCR.
Islets in excess of those needed for secretion were extracted for RNA using an Arcturus Picopure RNA isolation kit (Arcturus, Carlsbad, CA). After RT-PCR using a RT-PCR kit (Promega, Madison, WI), quantitative RT-PCR with SYBR green detection was performed using the ABI7300 real-time PCR system (Applied Biosystem, Foster City, CA) with primers (Supplementary Table 2). Samples were normalized to ribosomal 18S, an internal control gene, and the ΔΔCt method was used to calculate gene expression levels.
Statistical analysis.
Data are shown as mean ± SEM. For statistical analysis, an unpaired Student t test was used to compare two groups, and one-way ANOVA, followed by Bonferroni post hoc test, was used for more than two groups. A P value < 0.05 was considered statistically significant.
RESULTS
Pdx1 was efficiently deleted from ducts in bigenic mice.
To test if Pdx1 expression in pancreatic ducts was necessary for islet neogenesis, we generated duct-specific Pdx1-deficient mice by mating CAIICre mice and Pdx1Fl/Fl mice. Previously we showed the specificity of this promoter in that 1) CAII protein starts to be expressed in mouse pancreatic ductal cells at about embryonic day 18.5 (30), 2) lineage tracing showed the human CAII construct used in the transgenic mice followed a similar timing, 3) neither CAII nor Cre mRNA was expressed in the β-cells of the CAIICre mice, 4) hCAII-driven reporter at birth and Cre protein were only detected in ducts and ganglia in the pancreas, and 5) CAIICreERT-marked β-galactosidase background expression was about 1% of β-cells in both WT and transgenic mice (14). PDX1 protein has expression that is very low to undetectable in normally quiescent adult ductal cells but has transient (3–5 days) expression after proliferation (22). Ductal cells of 4-week-old WT and CAIICre;Pdx1Fl/Fl mice had comparable proliferation (% Ki67+) (Fig. 4F), but PDX1 protein was expressed in far fewer duct cells in CAIICre;Pdx1Fl/Fl mice than in WT mice (Fig. 1A–D), indicating efficient excision of Pdx1 in the ducts. Because PDX1 is not expressed in pancreatic ganglia, expression of the transgene in the ganglia should have no effect on the phenotype.
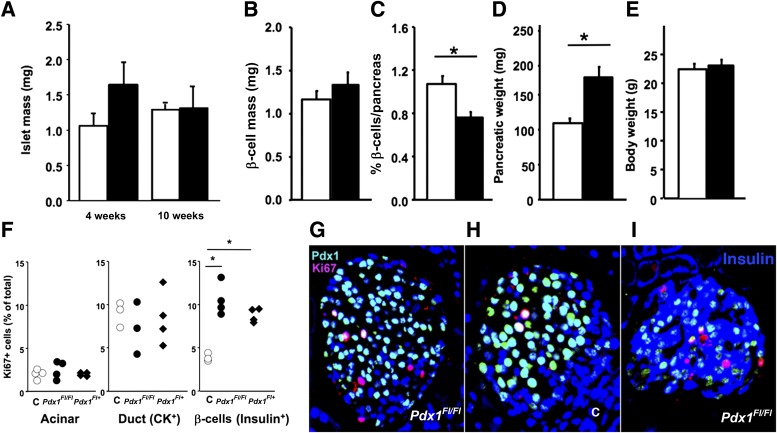
FIG. 4.
Duct-specific Pdx1-deficient mice had similar islet and β-cell mass as controls. Islet mass at 4 and 10 weeks (A) and β-cell mass at 4 weeks (B) did not differ between control (□) and CAIICre;Pdx1Fl/Fl (■) male mice (4 weeks: n = 5 control, n = 6 bigenic; 10 weeks: n = 3 both groups). At 4 weeks the relative density of β-cells (C) differed, but because the pancreatic weights (D) were increased in the bigenic (even though they had similar body weights) mice (E), the absolute β-cell mass was not reduced in the bigenic mice. F: At 4 weeks, although there was no difference in proliferation of acinar or duct (CK+) cells between control and bigenic mice, proliferation in insulin+ cells was increased in both bigenic groups (G) compared with controls (H) with Ki67+ (red), PDX1 (green), and nuclei DAPI (blue). Data for individual animals are shown in F. I: Some Ki67+insulin+ (blue) cells were PDX1−. Data are mean ± SEM. *P < 0.05.
FIG. 1.
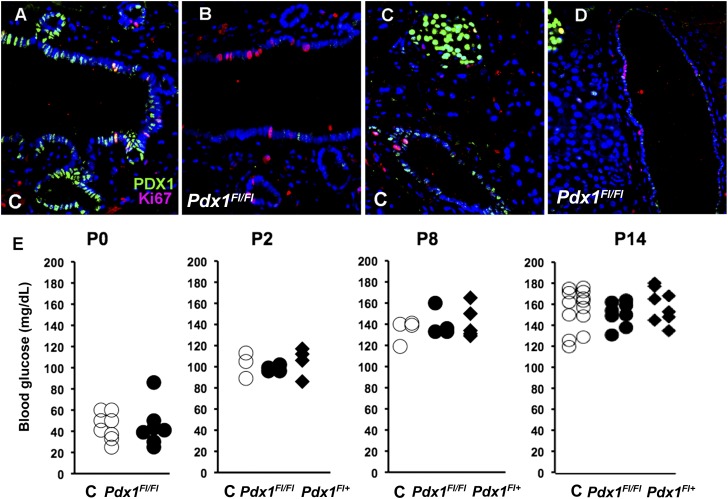
Characterization of duct-specific deletion of Pdx1 mice. A–D: Immunofluorescent evidence of effective Pdx1 excision at 4 weeks of age in CAIICre;Pdx1Fl/Fl pancreas. PDX1 protein is normally expressed transiently after replication of pancreatic duct cells. The common pancreatic ducts (A and B) and main duct (C and D) of control (C) (A and C) and bigenic CAIICre;Pdx1Fl/Fl (B and D) mice had comparable proliferation seen as Ki67+ (red). (Quantification given in Fig. 4F.) However, bigenic pancreas (B and D) had few PDX1+ (green) duct cells. PDX1+ islets are seen in upper left corner of both C and D. E: Blood glucose values over the first 2 postnatal (P) weeks did not differ between control (C) and bigenic mice (shown as Pdx1Fl/Fl and Pdx1Fl/+). Values from individual littermates are shown.
CAII starts to be expressed in ductal cells only just before birth, so embryonic development was expected to be normal. The duct-specific Pdx1-deficient mice were normal in Mendelian proportion, in body weight, and morphology of the pancreas at birth (data not shown) and had nonfasting blood glucose levels within normal reference ranges over the first 2 postnatal weeks (Fig. 1E); pancreatic weight in 2-week-old littermates did not differ (control: 29.3 ± 1.0 mg, n = 4; bigenic: 31.9 ± 1.0 mg, n = 10; P < 0.16). Together these parameters indicate appropriate embryonic development.
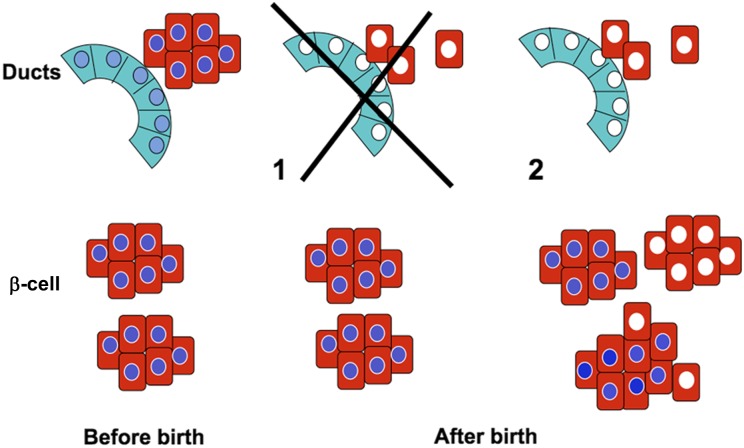
We reasoned (Fig. 2) that if PDX1 expression in the ducts were necessary for postnatal neogenesis, neonatal formation of new β-cells from ductal precursors would be impaired in the CAIICre;Pdx1Fl/Fl mice, and thus, animals at 4 weeks should have an inadequate β-cell mass and be hyperglycemic (Fig. 2 option 1). By contrast, if PDX1 in the ducts were not necessary for postnatal β-cell formation, the population of β-cells at 4 weeks would include those formed before birth expressing PDX1 plus those formed from CAII promoter-driven Cre-expressing ducts after birth without PDX1 (Fig. 2 option 2).
FIG. 2.
Schema of possible outcomes of duct-specific Pdx1 deletion. Before birth, all islets should be normal and homogeneously express PDX1 (blue nuclei). At 4 weeks, two findings are possible: 1) if PDX1 is necessary for new β-cell formation from ducts, there should be fewer islets but all should have homogeneous PDX1 expression; 2) if PDX1 is not necessary, there should be a mixed population of islets with those β-cells formed before birth with homogeneous PDX1 and those formed after birth from the Pdx1-depleted ducts, without PDX1 (white nuclei).
Impaired glucose tolerance and reduced plasma insulin in duct-specific Pdx1-deficient mice.
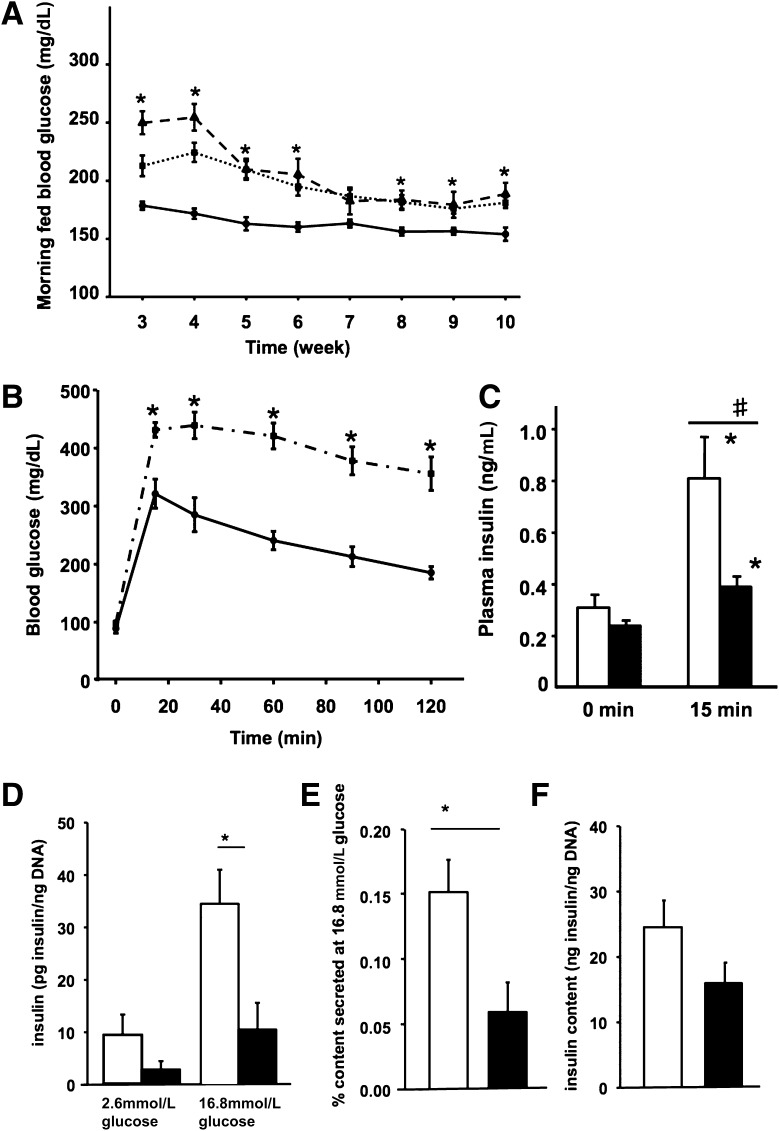
By weaning (Fig. 3A), the bigenic mice were moderately hyperglycemic (at 4 weeks CAIICre;Pdx1Fl/Fl: 254 ± 12 mg/dL, n = 23; CAIICre;Pdx1Fl/+: 224 ± 8 mg/dL, n = 26; control: 171 ± 5 mg/dL, n = 52). Yet by 10 weeks, they had near-normal morning fed blood glucose values (CAIICre;Pdx1Fl/Fl: 188 ± 10 mg/dL, n = 17; CAIICre;Pdx1Fl/+: 180 ± 5 mg/dL, n = 27; control: 153 ± 6 mg/dL, n = 33; P < 0.05 either bigenic compared with controls). Fed blood glucose values differed between CAIICre;Pdx1Fl/Fl and CAIICre;Pdx1Fl/+ mice only at 3 and 4 weeks of age. Unless specified, data from these genotypes are presented together as bigenic mice because we did not find differences between them. Despite near-normal blood glucose levels at age 10–11 weeks, duct-specific Pdx1-deficient mice had severely impaired glucose tolerance, as seen in intraperitoneal glucose tolerance tests (Fig. 3B), with significantly decreased plasma insulin levels (Fig. 3C) compared with the control littermates. Their ability to clear glucose in response to insulin, however, as seen in insulin tolerance tests (data not shown), did not differ. In a cohort taken to age 22 weeks, the morning fed blood glucose values of control and bigenic mice did not statistically differ from age 13 weeks onward, but there were elevated fasting glucose levels and still some impairment of glucose tolerance (Supplementary Fig. 1).
FIG. 3.
Duct-specific Pdx1-deficient mice had impaired glucose tolerance and impaired insulin secretion. A: Time course of morning fed blood glucose values of the controls (solid line, n = 33), CAIICre;Pdx1Fl/Fl (dashed line, n = 17), and CAIICre;Pdx1Fl/+ (dotted line, n = 23) littermates. Only at 3 and 4 weeks did the two bigenic genotypes differ from each other. B: Intraperitoneal glucose tolerance test (IPGTT) in 10-week-old animals comparing control (solid line) and bigenic mice (CAIICre;Pdx1Fl/Fl and CAIICre;Pdx1Fl/+, dashed-dotted line; n = 8–16) showed impaired glucose tolerance. C: Plasma insulin levels from the IPGTT showed significant increases in both groups at 15 min after glucose injection compared with fasting at 0 min in controls (□, n = 4) and bigenic (■, n = 9). *P < 0.025 compared with 0 min. #P < 0.004 comparing groups at 15 min. D–E: Isolated islets from 11-week-old bigenic mice (both CAIICre;Pdx1Fl/Fl and CAIICre;Pdx1Fl/+, ■, n = 10 animals) in sequential static incubation had impaired glucose-responsive insulin secretion compared with controls (□, n = 10 animals) (D) and lower percentage insulin content secreted (E) even though the islet insulin content was not significantly different (F). Data are mean ± SEM. *P < 0.007. Even if each islet aliquot with values for both glucose concentrations (n = 23 for bigenic and n = 26 for control) was used for the averaging, the basal levels and islet insulin content do not differ, but the bigenic islets showed a modest glucose-stimulated insulin release (2.6 mmol/L glucose: 3.6 ± 1.1 pg insulin/ng DNA; 16.8 mmol/L glucose: 12.5 ± 3.6 pg insulin/ng DNA; P < 0.003, paired t test).
Impaired glucose-induced insulin secretion in isolated islets of duct-specific Pdx1-deficient mice.
Islets from 11-week-old bigenic mice secreted less insulin than control islets in response to 16.8 mmol/L glucose (Fig. 3D). At high glucose, control islets secreted 0.15% of their total insulin, whereas islets from bigenic mice secreted only 0.06% of their total insulin (Fig. 3E), even though their islet insulin content was very similar (Fig. 3F). This impaired glucose responsiveness probably resulted from β-cell immaturity and a contribution from chronic mild hyperglycemia (this cohort of 11-week-old bigenic: 170 ± 6 vs. 144 ± 3 mg/dL in controls, n = 10 each group; P < 0.001), the latter known to be associated with reduced glucose-stimulated insulin secretion.
Islet and β-cell mass of duct-specific Pdx1-deficient mice were not reduced.
These physiological data support the concept of a reduced β-cell mass at 4 weeks due to a lack of postnatal neogenesis in the absence of PDX1 in the ducts offset by some hyperglycemia-driven compensation by 10 weeks. However, we found, unexpectedly, that the islet and β-cell mass did not differ between bigenic and control male mice at age 4 or 10 weeks (Fig. 4A and B). Our technique uses a cocktail of antibodies against the non–β-cell hormones glucagon, somatostatin, and PP to allow quantification of non–β-cell and β-cell mass, so the islet peripheral mantle consisting of non–β-cells is clearly defined, and even partially degranulated β-cells are still counted. At 4 and 10 weeks, although many islets of bigenic mice had a well-defined mantle, as seen in controls, we noticed a population of islets in which core cells were immunostained with both insulin and hormone-cocktail antibodies. Immunostaining for individual non–β-cell hormones showed that the PP antibody accounted for the large number of cells coexpressing insulin and non–β-cell hormones, a notable coexpression rarely seen in postnatal control mice (Supplementary Fig. 2). We therefore quantified the β-cell mass directly on adjacent insulin-stained sections from 4-week-old male animals (Fig. 4B). Although the β-cell relative volume (% of pancreatic tissue) of bigenic mice was significantly decreased (Fig. 4C), their pancreatic weight (Fig. 4D) was increased although the animals had similar body weight (Fig. 4E). The result was that absolute β-cell mass was similar for bigenic and control animals (Fig. 4B). There was no difference in acinar or duct replication (Fig. 4F). In contrast, at age 2 weeks, although pancreatic weights did not differ among genotypes, the CAIICre;Pdx1FlFl mice had significantly increased ductal proliferation (Supplementary Fig. 3). However, at 4 weeks (Fig. 4F-H) but not at 10 weeks (data not shown), more Ki67+insulin+ cells were seen in islets of bigenic mice, and some of these Ki67+ cells were PDX1nullinsulin+ (Fig. 4I), indicating that Pdx1-deficient β-cells can replicate.
Mixed population of islets in duct-specific Pdx1-deficient mice, some islets having loss of key β-cell markers.
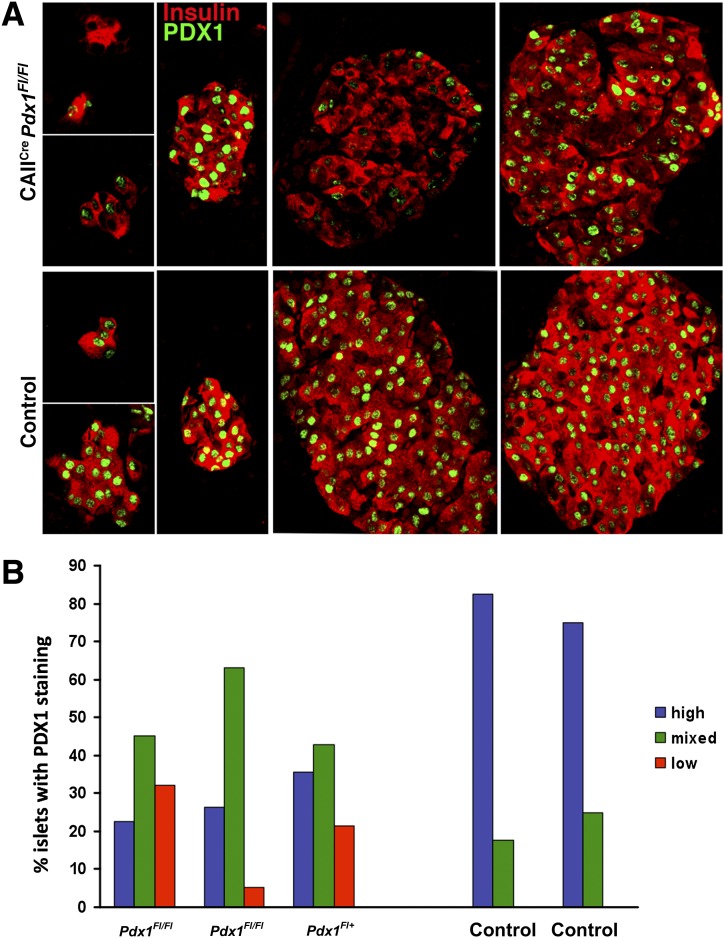
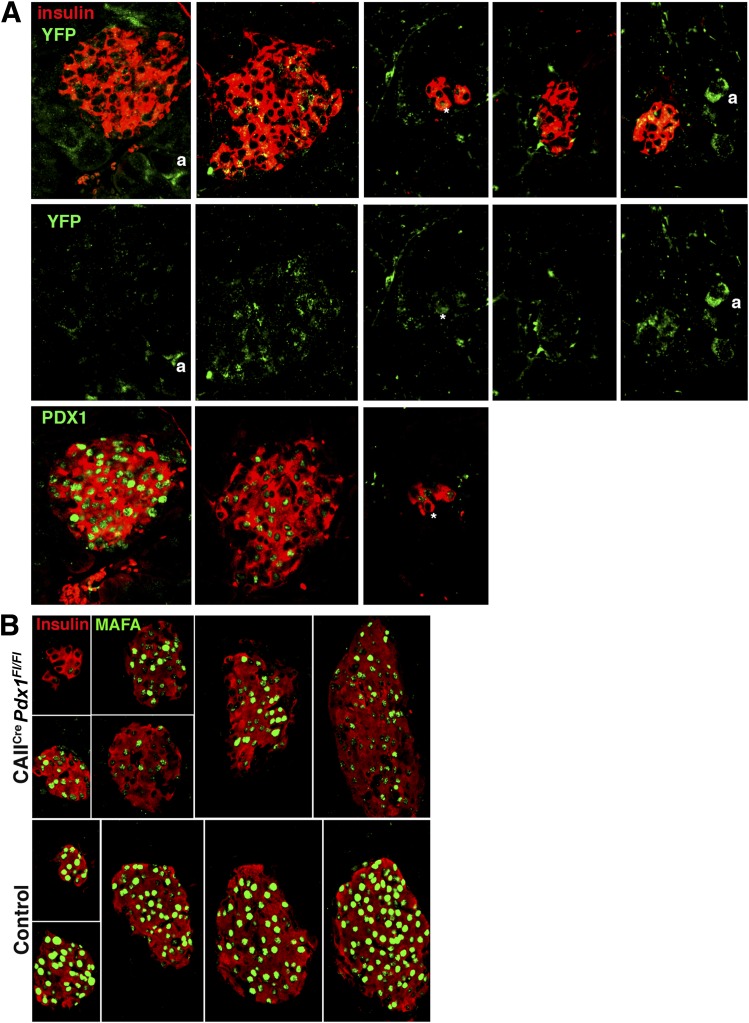
Although images for both CAIICre;Pdx1Fl/Fl and controls were taken with the same confocal settings on parallel-processed sections, there was remarkable variation in the PDX1-immunodetection signal in insulin+ cells, even within the same section of pancreas, from 10- to 12-week-old CAIICre;Pdx1Fl/Fl mice compared with strong homogeneous staining in control pancreas (Fig. 5A). Within a section of CAIICre;Pdx1Fl pancreas, some islets (whether large, small or as smaller clusters) could be found containing cells with very low to undetectable PDX1 expression. Some islets had strongly homogeneous PDX1 staining, with a minority of cells displaying little or no PDX1 staining. The intensity of insulin staining also varied similarly. Thus, there was a mixed population of islets in the CAIICre;Pdx1Fl mice (Fig. 5B): about 30% had homogeneously high or normal PDX1 expression, 20% had low to undetectable expression, and 50% displayed mixed-level expression. PDX1nullinsulin+ cells accounted for 31 ± 7.7% of all insulin+ cells (n = 3 animals with at least 18 islet/aggregates, and 625 insulin+ cells counted for each). The loss of PDX1 expression was similarly seen in the pancreas of 4-week-old CAIICre;Pdx1Fl/Fl (Supplementary Fig. 4) and of CAIICre;Pdx1Fl/+ mice at both ages (data not shown). When the ROSA26ReYFP reporter gene was introduced into the CAIICre;Pdx1 mice for lineage tracing, some lobes had YFP+ acinar and islet cells (Fig. 6A and Supplementary Fig. 5). These YFP islets have some β-cells with low to undetectable PDX1 expression, and others cells had strong PDX1 expression.
FIG. 5.
A mixed population of PDX1-expressing islets was seen in adult duct-specific Pdx1-deficient mice. A: Islets from same section of CAIICre;Pdx1Fl/Fl pancreas (12 weeks old, blood glucose at 4 weeks: 363 mg/dL, 12 weeks: 120 mg/dL) (top panel) showed variation in intensity of PDX1 (green) and insulin (red) immunostaining in contrast to those of control pancreas (12 weeks old, blood glucose at 4 weeks: 173 mg/dL, 12 weeks: 179 mg/dL) (bottom panel). B: On the basis of PDX1 immunostaining (in graph as blue: homogenous high intensity; green: mixed; red: low to undetectable intensity), bigenic mice had decreased proportion of islets with high, homogenous PDX1 expression and, importantly, the appearance of islets without PDX1 immunostaining. Data are shown for individual animals.
FIG. 6.
Islets with PDX1null β-cells show lineage tracing marker and low to undetectable MAFA expression. A: The variation of PDX1 immunostaining corresponded with the expression of lineage marker YFP in islets from a 4-week-old CAIICre;Pdx1Fl/Fl (blood glucose: 278 mg/dL) mouse. The middle panel shows YFP expression as split green channel of images shown in the top panel (insulin, red; YFP, green). The bottom panel shows same islets on adjacent section (due to antibody compatibility issues) with PDX1 (green) and insulin (red). a, lineage-marked acinar cell. *Identifies the same cell in different images. B: MAFA expression (green) showed similar variation from high intensity to low/undetectable in insulin+ (red) islets from same section of a 10-week-old CAIICre;Pdx1Fl/Fl mouse (blood glucose at 4 weeks: 272 mg/dL, 10 weeks: 189 mg/dL) compared with homogeneous high intensity of control littermate (blood glucose at 4 weeks: 172 mg/dL, 10 weeks: 178 mg/dL).
In islets of 10- to 12-week-old mice, the β-cell transcription factor MAFA had a similarly mixed expression pattern to that of PDX1. Within the same section, some islets of the bigenic mice had little to no MAFA protein expression, in a highly heterogeneous pattern, whereas others had expression indistinguishable from controls (Fig. 6B); islets with MAFAlow/null were also PDX1low/null (Supplementary Fig. 6). Because MAFA has been found to be important for the functional maturation of β-cells (29), we suspected that the β-cells with low to undetectable MAFA expression were functionally immature.
Increased neuropeptide Y and MAFB protein in β-cells of duct-specific Pdx1-deficient mice supports the concept of immaturity of some β-cells.
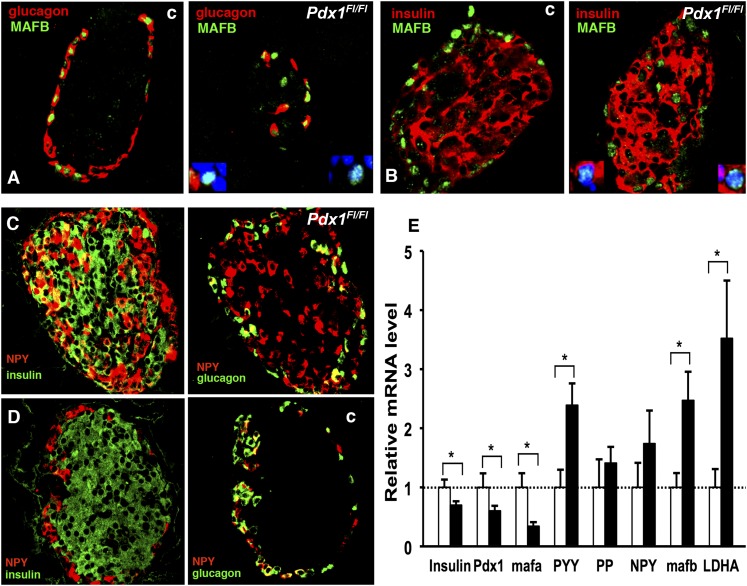
Neonatal rodent β-cells lack glucose-stimulated insulin secretion (31), with a gene expression profile different from adult β-cells (32). During early development, insulin+ cells express MAFB, followed by a switch to MAFA expression that can occur shortly after birth, but in adult mouse islets, the pattern resolves to MAFB expression restricted to glucagon+ cells and MAFA to insulin+ cells (33). Yet, in islets of 10-week-old bigenic mice, MAFB expression was detected in some insulin+ cells (Fig. 7A) and in some glucagon− cells (Fig. 7B), strongly suggesting an early stage of β-cell development.
FIG. 7.
Islets of 10- to 11-week-old bigenic mice expressed markers of immature β-cells. A and B: MAFB protein (green) was restricted to glucagon+ cells (red) in adult control (c) islets, but in bigenic (Pdx1flfl) there were both glucagon− cells (red) and insulin+ cells (red) that were MAFB+. The insets in the bigenic images show higher magnification of positive cells with DAPI-stained nuclei. In bigenic mice (C) (here blood glucose at 4 weeks: 254 mg/dL, 10 weeks: 145 mg/dL), many insulin+ cells (green) and some glucagon+ cells (green) coexpressed NPY/PYY (red), whereas in controls (D) (here blood glucose at 4 weeks: 162 mg/dL, 10 weeks: 156 mg/dL), only some glucagon+ cells coexpressed NPY/PYY (red). The same islets from adjacent sections are shown for insulin/NPY and glucagon/NPY immunostaining for bigenic and controls. E: Quantitative PCR for selected genes on RNA from islets of the same 11-week-old animals as used for insulin secretion (Fig. 3D–F) showed significant decreased expression of insulin, pdx1, and mafa mRNA and significant increased expression of PYY, mafb, and LDHA mRNA in bigenic mice (■), shown normalized to controls (□, n = 7–9). Data are mean ± SEM. *P < 0.05.
As mentioned above, the large number of cells copositive for PP and insulin were distributed throughout the pancreas. It is unlikely, however, that these cells were actually PP cells: 1) authentic PP cells are mainly localized in the head of the pancreas, 2) PP+insulin+ cells are rarely seen, even in normal early stages of pancreatic organogenesis (34), and 3) importantly, most PP, peptide YY (PYY), and neuropeptide Y (NPY) antibodies cross-react (35–37). In fact, our PP antibody stained scattered cells within the colon, so it must be considered as cross-reacting with PYY (35,36). The limited selectivity of PP or NPY antibodies leads us to consider these cells as “NPY or PYY” (NPY/PYY) cells. When anti-NPY antibody was used, islets of 4- and 10-week-old bigenic mice had many insulin+NPY/PYY+ and glucagon− NPY/PYY+ (Fig. 7C) cells in contrast to those of control mice (Fig. 7D). Bigenic mice were clearly hyperglycemic at 4 weeks, so we questioned whether the coexpression of insulin and NPY/PYY resulted from hyperglycemia. Pancreatic sections from adult rats 4 weeks after partial pancreatectomy, which showed chronic moderate hyperglycemia, had no cells with insulin-NPY/PYY copositivity (Supplementary Fig. 7), indicating that induction of NPY/PYY expression in β-cells was not caused by hyperglycemia. Recently, NPY expression was reported in adult insulin+ cells after embryonic-stage β-cell–specific deletion of NeuroD1, and these cells were characterized as immature β-cells based on expression of NPY and lactate dehydrogenase A (LDHA), plus their lack of glucose responsiveness (38). In our study, insulin+ cells with low levels of PDX1 and MAFA expression, coexpressing MAFB and NPY/PYY seen in duct-specific Pdx1-deficient pancreas, strongly suggest that the β-cells formed postnatally remained immature, even at 10 weeks of age.
Decreased expression of β-cell functional genes and increased expression of immature β-cell markers in islets of duct-specific Pdx1-deficient mice.
Consistent with our immunostaining findings, insulin, Pdx1, and mafa mRNA levels were significantly lower in islets of 11-week-old duct-specific Pdx1-deficient mice than in controls (Fig. 7E). Increased gene expression of both mafb and LDHA, the latter not expressed in adult β-cells but expressed (in rat islets) up to about 1 week postnatally (39), is consistent with our conclusion of the functional immaturity of these islets. Importantly, PYY mRNA was elevated in islets of duct-specific Pdx1-deficient mice compared with controls, in contrast to PP and NPY mRNA.
DISCUSSION
By specifically deleting Pdx1 from pancreatic ducts using duct-specific Cre-lox methods, we showed that β-cell development occurs even in the postnatal absence of PDX1 in ducts but that the resultant neogenetic insulin+PDX1null cells have characteristics of immature β-cells. Thus, we are able to arrive at the significant conclusion that Pdx1 is not necessary postnatally for formation of β-cells but is necessary for their full maturation to glucose-responsive β-cells. It is especially interesting that some islets, even within the same section, showed strong heterogeneity, with most β-cells PDX1-deficient, yet other islets showed uniformly strong PDX1 staining. These extremes probably represent, respectively, populations of newer postnatal islets and older prenatally formed islets. Importantly, we speculate that the presence of some islets with mostly strong uniform PDX1 staining, with small numbers of cells showing little or no PDX1 signal, could represent newly formed β-cells migrating to and coalescing with older islets.
Contrary to our initial hypothesis that duct-specific deletion of Pdx1 would limit postnatal islet neogenesis and result in lower islet mass at 4 weeks, with a possible “compensatory rebound” resulting from increased replication by 10 weeks, our data show that islet and β-cell mass were normal in the duct-specific Pdx1-deficient mice, with at least 30% of the β-cells lacking PDX1 protein. The lineage of such cells was verified by eYFP expression of the lineage marker. Thus, we conclude that new β-cells are able to form, in true neogenetic fashion, from postnatal ducts in which Pdx1 function is prevented. The finding that pancreatic weights were increased in bigenic mice at age 4 weeks but not at age 2 weeks was puzzling. In control mice, this 2-week period is one of an extensive expansion of the pancreas (three- to fourfold increase, from 29.3 to 110.2 mg). In bigenic mice at 2 weeks, ductal proliferation was increased above the already high level of controls, whereas at 4 weeks, the proliferation of the exocrine pancreas (acinar and duct) was similar to the controls. Analyses of Pdx1 tet-off inducible mouse model (40,41) showed that repression of Pdx1 had very different results dependent on its timing. If Pdx1 repression were initiated in mid-embryonic stage, acinar differentiation was impeded, but if initiated in the adult, exocrine (acinar and duct) proliferation was stimulated. Our data indicate that during the neonatal period of rapid pancreatic expansion, the lack of Pdx1 in the ducts resulted in a greater proliferation of duct cells that gave rise to more acinar cells and greater pancreatic weights.
With the current strong controversy over whether pancreatic ducts can give rise to new islet cells or even acinar cells postnatally (1), it is relevant to consider alternative explanations to our current findings. Could there be misexpression of carbonic anhydrase II, and thus Cre recombinase expression, in β-cells? CAII is normally expressed in rodent glucagon-expressing α-cells but not β-cells (30). In the experiments reported here, we used the human CAII promoter because CAII is limited to ductal expression in humans, and Cre immunostaining in the CAIICre pancreas was only seen in ducts and ganglia (14). With no injury involved in the current study, any misexpression would have to be significant to result in 30% labeled β-cells. Previously, however, we reported that even 40 cycles of RT-PCR failed to detect Cre or CAII mRNA in fluorescence-activated cell sorted β-cells from day 1, 2, 4, or 8-week-old CAIICre;MIPGFP mice but was easily detected in the kidneys from the same animals (14). The isolated islets used in the current study had no detectable Cre mRNA expression by quantitative PCR.
The glucose intolerance of the bigenic mice showing 70% of the β-cells as “immunofluorescently normal” was unexpected because rodents with 60% partial pancreatectomy maintain normal glucose homeostasis. Regeneration and adaptation have been found in mice and rats after 60% partial pancreatectomy, seen as the 40% β-cell mass of the remnant increasing to about 55% of sham controls (42,43) with an accompanying increase in function of individual β-cells (44,45). One must consider that the reduced glucose responsiveness partly results from glucotoxicity because chronic mild hyperglycemia was present from at least 3 weeks of age in these mice. Even slightly increased (15–20 mg/dL) blood glucose levels for at least 6 weeks can result in impaired glucose-responsive insulin secretion (42) and large alterations in gene expression (46). In our case, it is still unclear why hyperglycemia began at between 2 and 3 weeks of age. Lineage tracing experiments have suggested substantial de novo β-cell formation during this period (47). Moreover, studies of β-cell maturation in neonatal rats (13,31,32,48) show that 3-week-old pups are transiently insulin-resistant and that their β-cells are not functionally mature. In this context, a large functional impairment in 30% of the β-cells may result in modest hyperglycemia.
The presence of several markers of immature β-cells suggests that functional immaturity is partly responsible for the lack of glucose responsiveness of the isolated bigenic islets. In islets from duct-specific Pdx1-deficient mice, mafa mRNA and protein had lower than normal expression for adult β-cells, being similar to those in neonatal β-cells (29). We previously showed that although mafa overexpression could induce the maturation of glucose-responsiveness in neonatal islets, Pdx1 overexpression could not within the experiment’s timeframe (29). However, PDX1high is expressed before MAFA in insulin+ cells during development (33), suggesting that Pdx1 is an upstream regulator of mafa; thus, we expect that with longer incubation, Pdx1-infected P2 islets would have induced mafa expression and subsequently acquire glucose responsiveness. Furthermore, mafb, LDHA, and PYY mRNA were more highly expressed in bigenic islets compared with control. We conclude that the increased mafb mRNA did not reflect an increased proportion of glucagon-expressing cells, because the islet and β-cell mass were unaltered. The continued coexpression of MAFB (which is normally extinguished in mouse β-cells) and insulin in adult bigenic mice suggests that those cells remained in an early stage of β-cell development (33). Isolated islets of adult Pdx1-deficient mice also had elevated LDHA mRNA, another gene highly expressed in immature islets (39) but hardly expressed in normal adult β-cells (39,49) and induced by chronic hyperglycemia (50). Taken together, the increased expression of NPY/PYY, mafb, and LDHA and low mafa in β-cells suggest that PDX1 is necessary for the full maturation of β-cells.
We conclude that PYY is likely the specific member of the NPY/PYY/PP family that is aberrantly expressed in the duct-specific Pdx1-deficient β-cells. The cross-reactivity of most PP, PYY, and NPY antibodies has probably contributed to several previously apparently discordant conclusions. PYY and NPY were reported as markers of immature β-cells when coexpressed with insulin (34,36,38,51) and PYY as a marker of early islet precursors (35,36). After birth, NPY expression in pancreatic islets was reported as restricted to neonatal β-cells and absent from adult β-cells (52). Recently, however, NPY was reported in adult-stage insulin+ cells after embryonic β-cell–specific deletion of NeuroD1, and these cells were classified as immature based on expression of NPY protein/mRNA, LDHA, and lack of glucose-responsiveness (38). In our bigenic genetic manipulation, a large number of insulin+NPY+/PYY+ cells were detected in islets, but mRNA for only PYY, not NPY nor PP, was increased in islets from 11-week-old bigenic mice compared with controls. The discrepancy of NPY mRNA between the analyses of islets from NeuroD1-deficient mice and our Pdx1 duct-deleted mice possibly resulted from inclusion of NPY-expressing intrapancreatic ganglia in others’ islet preparations.
At 4 weeks, Pdx1-deficient mice had a higher percentage of proliferating β-cells, at least some of which were Pdx1null. This increase was likely a compensatory mechanism in response to hyperglycemia, because glucose stimulates β-cell proliferation in vivo (53–55) and in vitro (56,57). The increase was only transient, however, and by 10 weeks, there was no difference between bigenic and control mice. The finding that significant numbers of PDX1nullinsulin+ cells were proliferative indicates that PDX1 is obligatory for proliferation only under some contexts; other studies reported that Pdx1 was required for replication of β-cells at late gestation (19) or in adults (58).
Another striking finding in CAIICre;Pdx1FL mice was the mixed population of islets with varying immunofluorescent signals for PDX1, such that some islets had homogeneously normal levels, others uniformly almost none, with most consisting of a mixture of deficient and normal PDX1-expressing β-cells. The variation of PDX1 expression within and among islets is unlikely to result from hyperglycemia, because animals had only mild hyperglycemia from 7 to 8 weeks of age onward, and many β-cells had a normal PDX1 immunodetection signal that should be associated with good functional status. The variation in islet types, even within the same tissue section, suggests that besides the number of normal-level PDX1+ islets that likely represent those formed before birth, PDX1-deficient β-cells derived by neogenesis in the postnatal period from the Pdx1-depleted ducts can produce new homogeneously PDX1-depleted islets or can coalesce with older pre-existing (strongly PDX1+) islets to yield “chimeric islets.” It is unclear whether such a migration would require long-range movement or a behavior distinct from that seen in normal embryonic phases of endocrine/islet ontogeny, but the proximity of many islets to ducts does render this idea plausible.
ACKNOWLEDGMENTS
This study was supported by grants from the National Institutes of Health R01-DK-44523 (S.B.-W.), P30-DK-36836 Joslin Diabetes and Endocrinology Research Center (DERC) Advanced Microscopy Core, and by JDRF 1-2008-45 (S.B.-W.), as well as the Diabetes Research and Wellness Foundation, Daiichi Sankyo Foundation, and a grant from the Ministry of Health, Labor and Welfare of Japan (11103401), and an important group of private donors. No other potential conflicts of interest relevant to this article were reported.
L.G., A.I., C.A.-M., J.H.-L., and S.B.-W. collected data. L.G. and S.B.-W. wrote the manuscript. A.I. and Y.F. conceived the project. G.C.W., C.V.E.W., and A.S. provided critical discussions during study design and interpretation. C.V.E.W. provided mice. All authors edited and approved the manuscript. S.B.-W. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db12-1833/-/DC1.
Y.F. is currently affiliated with the Department of Medicine, Metabolism and Endocrinology, Juntendo University Faculty of Medicine, Tokyo, Japan.
REFERENCES
- 1.Bonner-Weir S, Li W-C, Ouziel-Yahalom L, Guo L, Weir GC, Sharma A. β-cell growth and regeneration: replication is only part of the story. Diabetes 2010;59:2340–2348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonner-Weir S, Baxter LA, Schuppin GT, Smith FE. A second pathway for regeneration of adult exocrine and endocrine pancreas. A possible recapitulation of embryonic development. Diabetes 1993;42:1715–1720 [DOI] [PubMed] [Google Scholar]
- 3.Li W-C, Rukstalis JM, Nishimura W, et al. Activation of pancreatic-duct-derived progenitor cells during pancreas regeneration in adult rats. J Cell Sci 2010;123:2792–2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang RN, Klöppel G, Bouwens L. Duct- to islet-cell differentiation and islet growth in the pancreas of duct-ligated adult rats. Diabetologia 1995;38:1405–1411 [DOI] [PubMed] [Google Scholar]
- 5.Xu X, D’Hoker J, Stangé G, et al. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell 2008;132:197–207 [DOI] [PubMed] [Google Scholar]
- 6.Chintinne M, Stangé G, Denys B, Ling Z, In ’t Veld P, Pipeleers D. Beta cell count instead of beta cell mass to assess and localize growth in beta cell population following pancreatic duct ligation in mice. PLoS ONE 2012;7:e43959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang TC, Bonner-Weir S, Oates PS, et al. Pancreatic gastrin stimulates islet differentiation of transforming growth factor alpha-induced ductular precursor cells. J Clin Invest 1993;92:1349–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gu D, Sarvetnick N. Epithelial cell proliferation and islet neogenesis in IFN-g transgenic mice. Development 1993;118:33–46 [DOI] [PubMed] [Google Scholar]
- 9.Xu G, Stoffers DA, Habener JF, Bonner-Weir S. Exendin-4 stimulates both β-cell replication and neogenesis, resulting in increased β-cell mass and improved glucose tolerance in diabetic rats. Diabetes 1999;48:2270–2276 [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto K, Miyagawa J, Waguri M, et al. Recombinant human betacellulin promotes the neogenesis of beta-cells and ameliorates glucose intolerance in mice with diabetes induced by selective alloxan perfusion. Diabetes 2000;49:2021–2027 [DOI] [PubMed] [Google Scholar]
- 11.Bouwens L, Wang RN, De Blay E, Pipeleers DG, Klöppel G. Cytokeratins as markers of ductal cell differentiation and islet neogenesis in the neonatal rat pancreas. Diabetes 1994;43:1279–1283 [DOI] [PubMed] [Google Scholar]
- 12.Finegood DT, Scaglia L, Bonner-Weir S. Dynamics of beta-cell mass in the growing rat pancreas. Estimation with a simple mathematical model. Diabetes 1995;44:249–256 [DOI] [PubMed] [Google Scholar]
- 13.Scaglia L, Cahill CJ, Finegood DT, Bonner-Weir S. Apoptosis participates in the remodeling of the endocrine pancreas in the neonatal rat. Endocrinology 1997;138:1736–1741 [DOI] [PubMed] [Google Scholar]
- 14.Inada A, Nienaber C, Katsuta H, et al. Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc Natl Acad Sci U S A 2008;105:19915–19919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peng SW, Zhu LY, Chen M, et al. Heterogeneity in mitotic activity and telomere length implies an important role of young islets in the maintenance of islet mass in the adult pancreas. Endocrinology 2009;150:3058–3066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chintinne M, Stangé G, Denys B, et al. Contribution of postnatally formed small beta cell aggregates to functional beta cell mass in adult rat pancreas. Diabetologia 2010;53:2380–2388 [DOI] [PubMed] [Google Scholar]
- 17.Jonsson J, Carlsson L, Edlund T, Edlund H. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature 1994;371:606–609 [DOI] [PubMed] [Google Scholar]
- 18.Offield MF, Jetton TL, Labosky PA, et al. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development 1996;122:983–995 [DOI] [PubMed] [Google Scholar]
- 19.Gannon M, Ables ET, Crawford L, et al. pdx-1 function is specifically required in embryonic beta cells to generate appropriate numbers of endocrine cell types and maintain glucose homeostasis. Dev Biol 2008;314:406–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holland AM, Hale MA, Kagami H, Hammer RE, MacDonald RJ. Experimental control of pancreatic development and maintenance. Proc Natl Acad Sci U S A 2002;99:12236–12241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahlgren U, Jonsson J, Jonsson L, Simu K, Edlund H. beta-cell-specific inactivation of the mouse Ipf1/Pdx1 gene results in loss of the beta-cell phenotype and maturity onset diabetes. Genes Dev 1998;12:1763–1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma A, Zangen DH, Reitz P, et al. The homeodomain protein IDX-1 increases after an early burst of proliferation during pancreatic regeneration. Diabetes 1999;48:507–513 [DOI] [PubMed] [Google Scholar]
- 23.Kritzik MR, Jones E, Chen Z, et al. PDX-1 and Msx-2 expression in the regenerating and developing pancreas. J Endocrinol 1999;163:523–530 [DOI] [PubMed] [Google Scholar]
- 24.Criscimanna A, Speicher JA, Houshmand G, et al. Duct cells contribute to regeneration of endocrine and acinar cells following pancreatic damage in adult mice. Gastroenterology 2011;141:1451–1462, e1–e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stanger BZ, Stiles B, Lauwers GY, et al. Pten constrains centroacinar cell expansion and malignant transformation in the pancreas. Cancer Cell 2005;8:185–195 [DOI] [PubMed] [Google Scholar]
- 26.Gotoh M, Maki T, Kiyoizumi T, Satomi S, Monaco AP. An improved method for isolation of mouse pancreatic islets. Transplantation 1985;40:437–438 [DOI] [PubMed] [Google Scholar]
- 27.Montaña E, Bonner-Weir S, Weir GC. Beta cell mass and growth after syngeneic islet cell transplantation in normal and streptozocin diabetic C57BL/6 mice. J Clin Invest 1993;91:780–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schuppin GT, Bonner-Weir S, Montana E, Kaiser N, Weir GC. Replication of adult pancreatic-beta cells cultured on bovine corneal endothelial cell extracellular matrix. In Vitro Cell Dev Biol Anim 1993;29A:339–344 [DOI] [PubMed] [Google Scholar]
- 29.Aguayo-Mazzucato C, Koh A, El Khattabi I, et al. Mafa expression enhances glucose-responsive insulin secretion in neonatal rat beta cells. Diabetologia 2011;54:583–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inada A, Nienaber C, Fonseca S, Bonner-Weir S. Timing and expression pattern of carbonic anhydrase II in pancreas. Dev Dyn 2006;235:1571–1577 [DOI] [PubMed] [Google Scholar]
- 31.Bliss CR, Sharp GW. Glucose-induced insulin release in islets of young rats: time-dependent potentiation and effects of 2-bromostearate. Am J Physiol 1992;263:E890–E896 [DOI] [PubMed] [Google Scholar]
- 32.Jermendy A, Toschi E, Aye T, et al. Rat neonatal beta cells lack the specialised metabolic phenotype of mature beta cells. Diabetologia 2011;54:594–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishimura W, Kondo T, Salameh T, et al. A switch from MafB to MafA expression accompanies differentiation to pancreatic beta-cells. Dev Biol 2006;293:526–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Myrsén-Axcrona U, Ekblad E, Sundler F. Developmental expression of NPY, PYY and PP in the rat pancreas and their coexistence with islet hormones. Regul Pept 1997;68:165–175 [DOI] [PubMed] [Google Scholar]
- 35.Upchurch BH, Aponte GW, Leiter AB. Expression of peptide YY in all four islet cell types in the developing mouse pancreas suggests a common peptide YY-producing progenitor. Development 1994;120:245–252 [DOI] [PubMed] [Google Scholar]
- 36.Jackerott M, Oster A, Larsson LI. PYY in developing murine islet cells: comparisons to development of islet hormones, NPY, and BrdU incorporation. J Histochem Cytochem 1996;44:809–817 [DOI] [PubMed] [Google Scholar]
- 37.Mulder H, Myrsén-Axcrona U, Gebre-Medhin S, Ekblad E, Sundler F. Expression of non-classical islet hormone-like peptides during the embryonic development of the pancreas. Microsc Res Tech 1998;43:313–321 [DOI] [PubMed] [Google Scholar]
- 38.Gu C, Stein GH, Pan N, et al. Pancreatic beta cells require NeuroD to achieve and maintain functional maturity. Cell Metab 2010;11:298–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thorrez L, Laudadio I, Van Deun K, et al. Tissue-specific disallowance of housekeeping genes: the other face of cell differentiation. Genome Res 2011;21:95–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hale MA, Kagami H, Shi L, et al. The homeodomain protein PDX1 is required at mid-pancreatic development for the formation of the exocrine pancreas. Dev Biol 2005;286:225–237 [DOI] [PubMed] [Google Scholar]
- 41.Holland AM, Góñez LJ, Naselli G, Macdonald RJ, Harrison LC. Conditional expression demonstrates the role of the homeodomain transcription factor Pdx1 in maintenance and regeneration of beta-cells in the adult pancreas. Diabetes 2005;54:2586–2595 [DOI] [PubMed] [Google Scholar]
- 42.Leahy JL, Bonner-Weir S, Weir GC. Minimal chronic hyperglycemia is a critical determinant of impaired insulin secretion after an incomplete pancreatectomy. J Clin Invest 1988;81:1407–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peshavaria M, Larmie BL, Lausier J, et al. Regulation of pancreatic beta-cell regeneration in the normoglycemic 60% partial-pancreatectomy mouse. Diabetes 2006;55:3289–3298 [DOI] [PubMed] [Google Scholar]
- 44.Delghingaro-Augusto V, Nolan CJ, Gupta D, et al. Islet beta cell failure in the 60% pancreatectomised obese hyperlipidaemic Zucker fatty rat: severe dysfunction with altered glycerolipid metabolism without steatosis or a falling beta cell mass. Diabetologia 2009;52:1122–1132 [DOI] [PubMed] [Google Scholar]
- 45.Liu YQ, Nevin PW, Leahy JL. beta-cell adaptation in 60% pancreatectomy rats that preserves normoinsulinemia and normoglycemia. Am J Physiol Endocrinol Metab 2000;279:E68–E73 [DOI] [PubMed] [Google Scholar]
- 46.Laybutt DR, Glandt M, Xu G, et al. Critical reduction in beta-cell mass results in two distinct outcomes over time. Adaptation with impaired glucose tolerance or decompensated diabetes. J Biol Chem 2003;278:2997–3005 [DOI] [PubMed] [Google Scholar]
- 47.Nakamura K, Minami K, Tamura K, Iemoto K, Miki T, Seino S. Pancreatic β-cells are generated by neogenesis from non-β-cells after birth. Biomed Res 2011;32:167–174 [DOI] [PubMed] [Google Scholar]
- 48.Aguayo-Mazzucato C, Sanchez-Soto C, Godinez-Puig V, Gutiérrez-Ospina G, Hiriart M. Restructuring of pancreatic islets and insulin secretion in a postnatal critical window. PLoS ONE 2006;1:e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sekine N, Cirulli V, Regazzi R, et al. Low lactate dehydrogenase and high mitochondrial glycerol phosphate dehydrogenase in pancreatic β-cells. Potential role in nutrient sensing. J Biol Chem 1994;269:4895–4902 [PubMed] [Google Scholar]
- 50.Jonas JC, Sharma A, Hasenkamp W, et al. Chronic hyperglycemia triggers loss of pancreatic β cell differentiation in an animal model of diabetes. J Biol Chem 1999;274:14112–14121 [DOI] [PubMed] [Google Scholar]
- 51.Teitelman G, Alpert S, Polak JM, Martinez A, Hanahan D. Precursor cells of mouse endocrine pancreas coexpress insulin, glucagon and the neuronal proteins tyrosine hydroxylase and neuropeptide Y, but not pancreatic polypeptide. Development 1993;118:1031–1039 [DOI] [PubMed] [Google Scholar]
- 52.Whim MD. Pancreatic beta cells synthesize neuropeptide Y and can rapidly release peptide co-transmitters. PLoS ONE 2011;6:e19478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bonner-Weir S, Deery D, Leahy JL, Weir GC. Compensatory growth of pancreatic beta-cells in adult rats after short-term glucose infusion. Diabetes 1989;38:49–53 [DOI] [PubMed] [Google Scholar]
- 54.Alonso LC, Yokoe T, Zhang P, et al. Glucose infusion in mice: a new model to induce beta-cell replication. Diabetes 2007;56:1792–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Porat S, Weinberg-Corem N, Tornovsky-Babaey S, et al. Control of pancreatic β cell regeneration by glucose metabolism. Cell Metab 2011;13:440–449 [DOI] [PubMed] [Google Scholar]
- 56.Logothetopoulos J, Valiquette N, Cvet D. Glucose stimulation of beta-cell DNA replication in the intact rat and in pancreatic islets in suspension culture. Effects of alpha-ketoisocaproic acid, dibutyryl cyclic AMP, and 3-isobutyl-1-methylxanthine in the in vitro system. Diabetes 1983;32:1172–1176 [DOI] [PubMed] [Google Scholar]
- 57.Swenne I. The role of glucose in the in vitro regulation of cell cycle kinetics and proliferation of fetal pancreatic B-cells. Diabetes 1982;31:754–760 [DOI] [PubMed] [Google Scholar]
- 58.Kulkarni RN, Jhala US, Winnay JN, Krajewski S, Montminy M, Kahn CR. PDX-1 haploinsufficiency limits the compensatory islet hyperplasia that occurs in response to insulin resistance. J Clin Invest 2004;114:828–836 [DOI] [PMC free article] [PubMed] [Google Scholar]