Abstract
OBJECTIVE
To examine the effect of dapagliflozin, a sodium-glucose cotransporter 2 (SGLT2) inhibitor, on the major components of renal glucose reabsorption (decreased maximum renal glucose reabsorptive capacity [TmG], increased splay, and reduced threshold), using the pancreatic/stepped hyperglycemic clamp (SHC) technique.
RESEARCH DESIGN AND METHODS
Subjects with type 2 diabetes (n = 12) and matched healthy subjects (n = 12) underwent pancreatic/SHC (plasma glucose range 5.5–30.5 mmol/L) at baseline and after 7 days of dapagliflozin treatment. A pharmacodynamic model was developed to describe the major components of renal glucose reabsorption for both groups and then used to estimate these parameters from individual glucose titration curves.
RESULTS
At baseline, type 2 diabetic subjects had elevated TmG, splay, and threshold compared with controls. Dapagliflozin treatment reduced the TmG and splay in both groups. However, the most significant effect of dapagliflozin was a reduction of the renal threshold for glucose excretion in type 2 diabetic and control subjects.
CONCLUSIONS
The SGLT2 inhibitor dapagliflozin improves glycemic control in diabetic patients by reducing the TmG and threshold at which glucose is excreted in the urine.
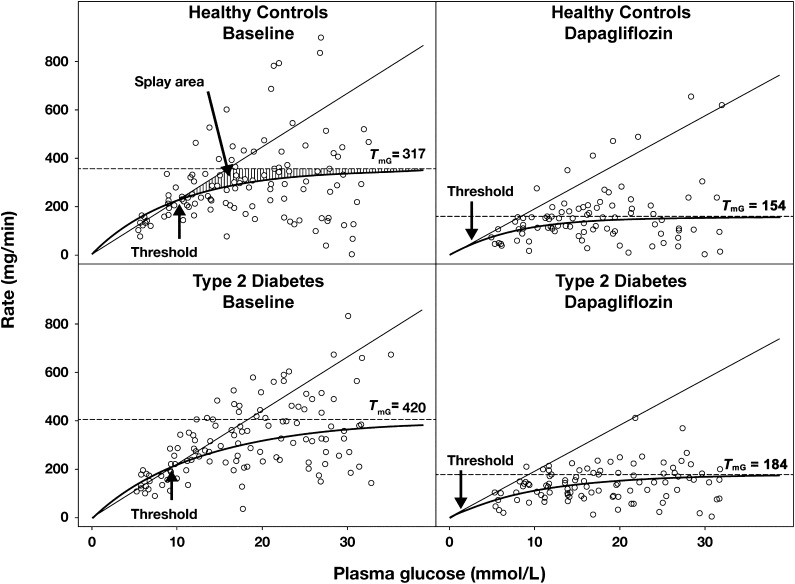
The current study was undertaken to examine the mechanism (decreased maximum renal glucose reabsorptive capacity [TmG], increased splay, and reduced threshold) through which sodium-glucose transporter 2 (SGLT2) inhibition induces glucosuria in diabetic and nondiabetic subjects. In humans, the kidney filters ∼162 g of glucose per day (glomerular filtration rate [GFR] = 180 L/day × fasting plasma glucose [FPG] = ∼5 mmol/L [90 mg/dL]), and virtually all the filtered glucose is reabsorbed (1). The high-capacity, low-affinity SGLT2 in the proximal tubule reabsorbs ∼80–90% of filtered glucose (2,3). TmG varies among individuals and averages ∼375 mg/min (2–4). Because the filtered glucose load does not exceed TmG in nondiabetic individuals, all filtered glucose is reabsorbed and returned to the circulation. If the filtered glucose load exceeds the TmG, all glucose in excess of the TmG is excreted. The plasma glucose concentration at which the filtered glucose load reaches 375 mg/min is ∼10 mmol/L (180 mg/dL) (2–4). Above the TmG, the glucose excretion rate increases linearly and parallels the increase in filtered glucose load. Glucose reabsorption and excretion curves display a nonlinear transition as TmG is approached. This rounding of the curves is termed splay (Fig. 1). The plasma glucose concentration at which glucose first appears in the urine is termed threshold and corresponds to the beginning of the splay.
Figure 1.
Relationship between the rate of urinary glucose reabsorption/renal glucose filtration and the plasma glucose concentration during SHC in type 2 diabetic and healthy subjects at baseline and after 7 days of dapagliflozin treatment. Thin line, rate of glucose filtration; ○, observed rate of reabsorption; thick line, predicted rate of reabsorption; dashed line, geometric mean of TmG.
In patients with poorly controlled type 1 or 2 diabetes, TmG is increased (5,6). Similar observations have been made in diabetic animal models (7,8). At the molecular level, increased TmG may be explained by increased SGLT2 mRNA and protein in the proximal tubule (9–11).
SGLT2 inhibitors have been developed for the treatment of type 2 diabetes (4,12–14) and have proven to be efficacious in reducing glycated hemoglobin (HbA1c) (12–16). Because their mechanism of action is independent of severity of insulin resistance and β-cell failure, they can be used at any stage of type 2 diabetes (14,16,17). Clinical trials with SGLT2 inhibitors have demonstrated that treatment in healthy subjects results in continuously excreted glucose in the absence of hyperglycemia (18,19), suggesting that factors other than a reduction in TmG must account for the drug’s glucosuric effect. Because no previous study to our knowledge has comprehensively characterized the changes in renal glucose handling through which SGLT2 inhibitors augment renal glucose excretion in humans, the current study was undertaken to examine the mechanisms through which dapagliflozin produces its glucosuric effect in individuals with type 2 diabetes and those with normal glucose tolerance.
RESEARCH DESIGN AND METHODS
Subjects
Twelve healthy subjects (41 years of age, BMI 27.0 kg/m2, HbA1c 5.5%, FPG 4.7 mmol/L [85 mg/dL], 7 males and 5 females, 11 white and 1 black) and 12 subjects with type 2 diabetes (53 years of age, BMI 29.8 kg/m2, HbA1c 6.5%, FPG 6.0 mmol/L [108 mg/dL], 7 males and 5 females, and 9 white, 2 black, and 1 Asian) participated in the study (Table 1). Other than diabetes, subjects were in good health as assessed by screening laboratory measurements, electrocardiogram, medical history, and physical examination. All subjects had an estimated GFR ≥60 mL/min by Modification of Diet in Renal Disease equation (20). No subject had microalbuminuria. Body weight was stable for ≥3 months before the study, and no subject participated in an excessively heavy exercise program. Subjects receiving antidiabetic therapy (Table 1) continued their usual regimen throughout the study except for metformin, which was withheld for 48 h before each stepped hyperglycemic clamp (SHC) procedure (because of iohexol administration). Sulfonylurea was not taken on the day of study.
Table 1.
Patient demographics and baseline characteristics

Experimental design
Eligible subjects underwent combined pancreatic/SHC (21) to determine 1) renal TmG, 2) splay in the renal glucose titration curve, 3) threshold for glucose excretion, and 4) percentage of filtered glucose load excreted at any given plasma glucose concentration. Subjects fasted overnight at the research center (10–12 h). Subjects were connected to a Biostator (Life Science Instruments, Elkhart, IN) by an intravenous line in the lower arm or dorsal hand, and arterialized blood was continuously sampled to determine blood glucose level in minute intervals. Another intravenous line for sampling of pharmacokinetic/pharmacodynamic parameters was inserted in same arm. An antecubital vein in the contralateral arm was cannulated for infusion of test substances.
A pancreatic clamp approach was used to suppress endogenous insulin secretion, reduce the volume of glucose required to achieve the maximal target glucose in healthy subjects during SHC, reduce the time required to stabilize blood glucose at each target level, and obviate potential effects of hyperinsulinemia on TmG. Type 2 diabetic subjects received an overnight insulin infusion (Novolin R 100 IU/mL; Novo Nordisk, Bagsvaerd, Denmark) before SHC to achieve and maintain an FPG of ∼100 mg/dL (target plasma glucose for the initial step of SHC [5.6 mmol/L]) without administration of glucose. Before the SHC procedure (∼2.25 h), octreotide (30 ng/kg⋅min−1) was infused to suppress endogenous pancreatic hormone secretion, and infusions of glucagon (1 ng/kg⋅min−1) and growth hormone (3 ng/kg⋅min−1) were started to restore basal plasma levels of these hormones (pancreatic clamp). An insulin infusion was started at 0.1 mU/kg⋅min−1 to replace basal insulin levels in healthy subjects. Subjects with type 2 diabetes received individualized insulin infusion rates based on the insulin infusion rate that controlled the plasma glucose level to 5.6 mmol/L (100 mg/dL) without the need for exogenous glucose administration for 60 min before the start of the pancreatic clamp. Basal insulin infusion rates were constantly maintained throughout the procedure.
A bolus of iohexol providing 45 mg organic iodine followed by a continuous infusion delivering 15 mg/h organic iodine (22) was initiated ∼1 h before the SHC to assess GFR. Plasma samples for iohexol measurement were obtained at –60, –30, and 0 min and every 40 min during SHC. Plasma samples for insulin and additional glucose measurements were collected at –30, –15, and 0 min and every 40 min during SHC. The baseline plasma glucose concentration at the start of SHC was 5.6 mmol/L (100 mg/dL). An automated SHC technique was performed with the Biostator and standard infusion pumps to infuse glucose (20% volume for volume), acutely raise plasma glucose to consecutive target levels (50 mg/dL [2.8 mmol/L] increments), and constantly maintain these levels for 40 min up to a maximum target glucose concentration of 550 mg/dL (30.5 mmol/L) (21,23).
Subjects received a water load (25 mL/kg ideal body weight) ∼2 h before the SHC, and the volume of spontaneously voided urine was replaced to initiate and sustain a water diuresis and facilitate urine collections during the procedure. Subjects voided at –30 min, and urine was collected from –30 to 0 min and every 40 min thereafter. Each urine sample volume was recorded, and concentrations of glucose and iohexol were measured.
On days 1–7 after completion of the baseline SHC, subjects ingested 10 mg of dapagliflozin each morning. On day 7, the SHC was repeated. Plasma samples for determination of dapagliflozin pharmacokinetics were obtained at 0, 40, 80, 120, 160, 200, 240, 320, 360, and 400 min and at 24 h postdosing.
Measurements
Plasma glucose was measured with the YSI 2300 Stat analyzer (YSI Life Sciences, Yellow Springs, OH) and Biostator glucose analyzer module.
Sample size determination
With the use of a one-sided test (significance of 0.05), it was determined that 10 subjects/group would provide 99% power, assuming a 40% TmG reduction with 7 days of dapagliflozin treatment. With two one-sided tests (significance of 0.05 for each test), 10 subjects/group provided 87% power to conclude that the effect of dapagliflozin on TmG would be equivalent using a 20% equivalence limit (set a priori as a reasonable estimate), assuming that TmG posttreatment-to-pretreatment ratios were log-normally distributed and an SD of the log was <0.15. To ensure that 10 subjects completed the study, 12 subjects per group were recruited.
Statistical comparisons
To characterize the reduction in TmG after dapagliflozin administration and to identify potential differences in the renal glucosuric effect of dapagliflozin between groups, absolute values were expressed as percent change in TmG, and TmG values were log-transformed. Percent changes in TmG from baseline to day 7 were analyzed by ANCOVA of logarithms of posttreatment versus pretreatment ratios in TmG, with group as the main effect and logarithm of the baseline TmG value as a covariate. From that statistical model, point estimates and 90% CIs for geometric mean percent change from baseline TmG within each group were constructed, P values were determined, and the point estimate and its 90% CI were calculated for the diabetic group-to-control group ratio of geometric means of posttreatment-to-pretreatment baseline ratios in TmG. Equivalence in dapagliflozin effect on TmG between the two groups can be concluded if the 90% CI for diabetic-to-control group ratio of geometric means for TmG at baseline is entirely contained within 80–125%. Similar analyses were performed for splay and threshold.
To identify potential differences in baseline TmG between type 2 diabetic and control subjects, the point estimate and its 90% CI were calculated for the diabetic-to-control group ratio of geometric means for TmG at baseline. These were estimated from a fitted ANOVA model for log-transformed data, with treatment group as a fixed effect. The point estimate of the difference and its 90% CI in the log scale were exponentiated to obtain the estimate and CI for the ratio of geometric means in the original scale. Similar analyses were performed for splay.
Calculations
Equations used to calculate parameters reported herein are included as Supplementary Data. Data are mean ± SD, with the exception of data presented for TmG and splay, which are adjusted geometric means.
RESULTS
Subject baseline characteristics
Twenty-four subjects (12 type 2 diabetic and 12 healthy control) were randomized, administered the study drug, and completed the study. As expected, subjects with type 2 diabetes had increased BMI, HbA1c, and FPG relative to the healthy control subjects, but they generally had very good glycemic control as evidenced by mean HbA1c (6.5%) and FPG (6.0 mmol/L [108 mg/dL]) values (Table 1).
Renal glucose threshold, TmG, and splay
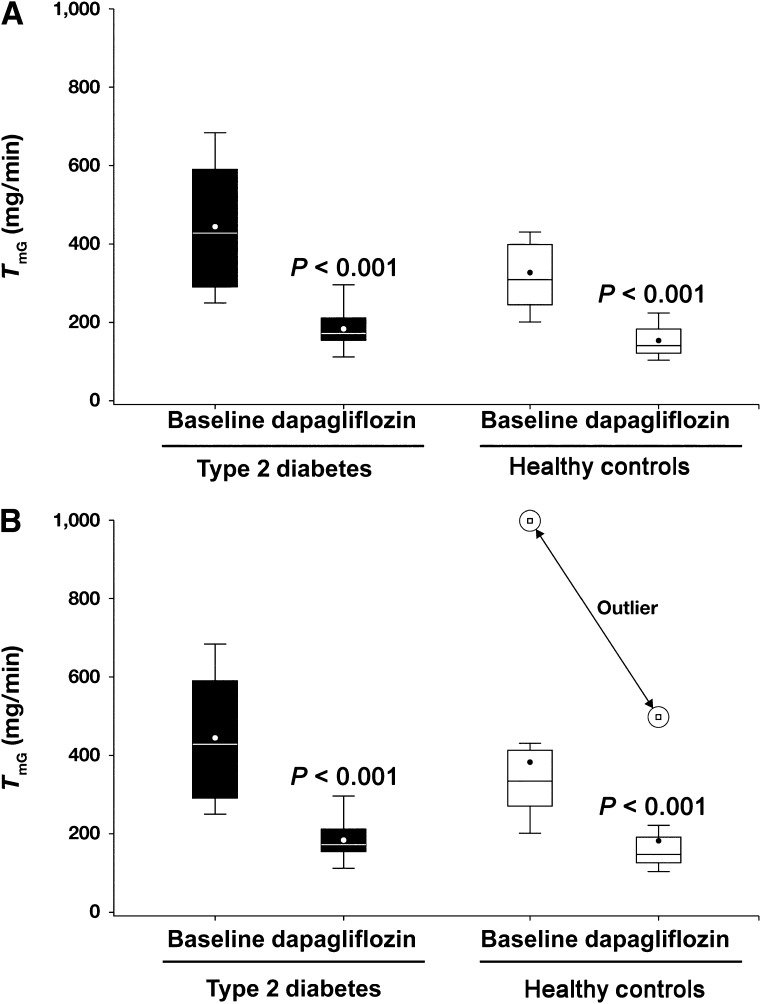
The relationship between renal glucose reabsorption and plasma glucose concentration in both groups is shown in Fig. 1. During the baseline study, the threshold, which represents the plasma glucose concentration at which glucose first appears in the urine, was slightly (15%) higher in type 2 diabetic subjects (10.9 ± 3.5 mmol/L [196 ± 63 mg/dL]) versus healthy control subjects (9.5 ± 3.7 mmol/L [171 ± 57 mg/dL]), but the difference was not significant. The calculated baseline TmG in diabetic subjects (Fig. 2A and B) was 32% higher (90% CI of ratio 1.059–1.650) than in healthy subjects (420 vs. 317 mg/min, excluding outlier; P < 0.001). In one healthy subject (outlier), little glucose excretion was detected despite plasma glucose levels >27.8 mmol/L (500 mg/dL). The calculated baseline TmG was 20% higher (90% CI of ratio 0.920–1.570) in diabetic subjects than in healthy subjects with inclusion of the outlier (420 vs. 349 mg/min, respectively, P < 0.001).
Figure 2.
TmG in type 2 diabetic and healthy subjects at baseline and after 7 days of dapagliflozin treatment. A: TmG for all subjects. B: TmG for all 12 subjects with type 2 diabetes and 11 healthy subjects minus 1 outlier, which is shown separately.
The splay in the renal glucose titration curve (expressed as the difference in area under the curve between observed data and the ideal titration curve) (Fig. 1) was 101% higher (90% CI 1.608–2.512) in type 2 diabetic versus healthy subjects at baseline (27,328 vs. 13,598 mg2/min2, excluding outlier; P < 0.001) (Supplementary Fig. 1). After 7 days of dapagliflozin treatment, both TmG and splay were reduced in both diabetic and healthy subjects (both P < 0.001). The absolute reduction in TmG was greater in diabetic versus healthy subjects as a result of a higher baseline TmG; however, the percent reduction was nearly identical (∼55%) in both groups (Fig. 2B). Splay was decreased by 34% in diabetic subjects to 16,950 mg2/min2 and by 40% in healthy subjects to 8,707 mg2/min2, but the absolute value for splay tended to be higher in diabetic subjects (Fig. 3).
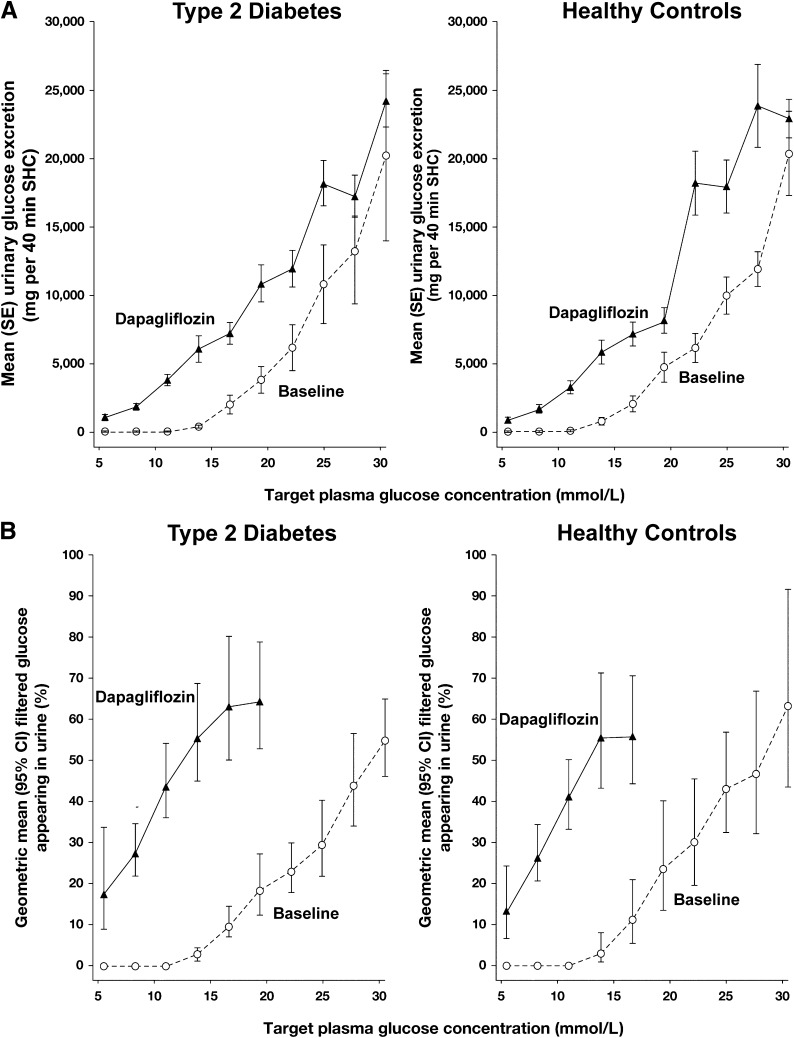
Figure 3.
A: Relationship between urinary glucose secretion (mg per 40 min SHC) and target plasma glucose concentration in type 2 diabetic and healthy subjects before and after 7 days of dapagliflozin treatment. B: Percentage of the filtered glucose load appearing in the urine of type 2 diabetic and healthy subjects before and after 7 days of dapagliflozin treatment.
At baseline, the threshold was slightly increased in type 2 diabetic versus control subjects (10.9 ± 3.5 vs. 9.5 ± 3.2 mmol/L [196 ± 63 vs. 171 ± 57 mg/dL], excluding the outlier). After dapagliflozin treatment, the threshold for glucose appearance in the urine, as determined from the model, was markedly reduced in diabetic (1.2 ± 2.6 mmol/L [21 ± 46 mg/dL]) and healthy (2.0 ± 2.2 mmol/L [37 ± 40 mg/dL] subjects (both P < 0.001). The calculated threshold (as determined by the model for the glucose titration curve) was much lower than the lowest plasma glucose studied; therefore, the actual threshold could not be precisely determined. Furthermore, the calculated threshold was well below the TmG. Thus, after dapagliflozin treatment, at plasma glucose concentrations between 5.5 and 8.3 mmol/L (100 and 150 mg/dL), none of the glucose appearing in the urine could be explained by a reduction in the TmG or splay.
Glomerular filtration rate
During the baseline SHC, mean GFR was similar in type 2 diabetic (127 ± 34 mL/min) and healthy (128 ± 36 mL/min) subjects. During the repeat SHC after 7 days of dapagliflozin, mean GFR was slightly and similarly reduced in diabetic (–18 ± 25 mL/min) and healthy (–17 ± 19 mL/min) subjects.
Dapagliflozin inhibition of renal glucose reabsorption
After dapagliflozin treatment, the amount of glucose excreted in the urine (mg per 40 min) increased in both type 2 diabetic and healthy subjects relative to their baseline values (Fig. 3A). It should be noted that after dapagliflozin treatment, at plasma glucose concentrations below the TmG, large amounts of glucose were detected in the urine in both groups. Results were not altered when urine glucose was normalized according to individual iohexol clearance (data not shown).
Dapagliflozin treatment resulted in a similar percent inhibition of renal glucose reabsorption in both groups (Fig. 3B). Of note, the percent inhibition of renal glucose reabsorption by dapagliflozin at a plasma glucose level of 5.6 mmol/L (100 mg/dL) was ∼20% in healthy subjects and 27% in type 2 diabetic subjects (Fig. 3B) and was lower than the percent inhibition (∼70%) at the elevated plasma glucose concentration of 13.9 mmol/L (250 mg/dL) in both populations. The maximal percent inhibition of renal glucose reabsorption in healthy subjects was ∼60% and in diabetic subjects,∼80% at plasma glucose concentrations of ∼16.7 mmol/L (300 mg/dL). Although the variability in urinary glucose excretion relative to the filtered glucose load made calculation of the percent inhibition of renal glucose reabsorption unreliable for plasma glucose values >22.2 mmol/L (400 mg/dL), calculation of the inhibition of renal glucose reabsorption was robust for plasma glucose concentrations of ≤16.7 mmol/L.
Pharmacokinetic profile
On day 7, plasma dapagliflozin concentrations were similar in healthy and type 2 diabetic subjects (Supplementary Fig. 2). The pharmacokinetic profile was similar in diabetic and healthy subjects (Supplementary Table 1).
Safety and tolerability
All 24 subjects completed the study. There were no discontinuations, deaths, or serious adverse events. Overall, there were 21 adverse events that occurred in 13 (54.2%) subjects, with nausea (n = 4 [16.7%]) and headache (n = 4 [16.7%]) being the most common. All adverse events were mild in intensity and resolved spontaneously. One healthy subject had elevations in aspartate transaminase (1.38-fold upper limit of normal [ULN]) and alanine aminotransferase (2.18-fold ULN) levels on day 8 (discharge day). One subject with type 2 diabetes had an elevated total bilirubin of 1.3-fold ULN on day 8. These abnormalities were not considered adverse events by the investigator, and no other abnormal laboratory values were observed.
CONCLUSIONS
In the current study, we examined for the first time in humans, to our knowledge, the effect of SGLT2 inhibition on the key parameters (TmG, threshold, and splay) of renal glucose handling. In individuals with established type 2 diabetes, multiple pathophysiologic abnormalities have been shown to contribute to the disturbance in glucose homeostasis (24). Although it long has been known that the TmG is increased in both type 1 (6) and type 2 (5) diabetes, until recently, this alteration in renal glucose handling has received little attention. With the development of SGLT2 inhibitors as a potential therapeutic modality for type 2 diabetes, there has been renewed interest in the mechanisms responsible for enhanced renal glucose reabsorption. In the current study, we used the SHC (23) with octreotide and basal hormone replacement (pancreatic clamp) to progressively increase plasma glucose concentration throughout the physiologic and pathophysiologic range while clamping plasma insulin, glucagon, and growth hormone concentrations at basal levels. This study design allowed us to quantify TmG, splay in the renal glucose reabsorptive curve, and threshold at which glucose first appears in the urine before and after treatment with an SGLT2 inhibitor.
As previously demonstrated in diabetic humans (5,6) and animals (7,8), the current results, obtained through the use of a state-of-the-art methodology, confirm that TmG is increased in type 2 diabetic versus healthy individuals (420 vs. 317 mg/min, P < 0.04) (Fig. 2) and extend the results of previous studies by excluding hyperinsulinemia as a confounding factor in measuring the TmG. This finding has important clinical implications, demonstrating that the kidney plays an active role in the pathogenesis of type 2 diabetes by excessively reabsorbing glucose and contributing to the maintenance of hyperglycemia rather than to the excretion of the excess filtered glucose load. Because 80–90% of the filtered glucose load is reabsorbed by the SGLT2 transporter (2,3), it is reasonable to assume that upregulation of this protein is responsible for the increased TmG in diabetic patients in the current study and is consistent with studies in cultured renal tubular cells in humans (11) and in vivo and in vitro studies in animals (9,10). However, in a study by Rahmoune et al. (11), the cell type used in culture experiments was not definitively identified. Farber et al. (5) demonstrated that treatment with insulin resulted in a decrease in the TmG in type 2 diabetic patients despite a constant infusion of glucose, suggesting that hyperglycemia, not hyperinsulinemia, is responsible for the increased TmG in type 2 diabetes. In the current study, the mean HbA1c in subjects with type 2 diabetes was 6.5%, and the mean FPG level was 6.0 mmol/L (108 mg/dL), indicating good glycemic control. Thus, if hyperglycemia is responsible for the increase in TmG, only a modest deterioration in glycemic control is required to increase the TmG.
The current study is the first in our knowledge to measure the splay in the renal glucose titration curve in human type 2 diabetes before and after SGLT2 inhibition. Although the splay area was increased in diabetic versus healthy subjects (Supplementary Fig. 1), the plasma glucose concentration at which glucose first appeared in the urine was not markedly different between them (10.9 vs. 9.5 mmol/L [196 vs. 171 mg/dL], respectively). After 7 days of dapagliflozin treatment, the geometric mean TmG was significantly reduced in both diabetic (420–176 mg/min) and healthy (317–150 mg/min) subjects. Of note, the percent reduction in TmG was identical (∼55%) in both groups, and the TmG after dapagliflozin treatment in diabetic subjects was lower than that in healthy subjects before dapagliflozin treatment. Dapagliflozin decreased the splay in the renal glucose titration curve in both groups. Therefore, increased splay cannot explain the glucosuric effect of the drug (Supplementary Fig. 1).
The current results demonstrate that the dramatic effect of dapagliflozin to induce glucose excretion is explained by the drug’s action to reduce the threshold at which renal glucose excretion first begins. Thus, the mean threshold was reduced in both type 2 diabetic and healthy subjects to 1.2 mmol/L (21 mg/dL) and 2.1 mmol/L (37 mg/dL), respectively. This finding can best be appreciated by examining the effect of dapagliflozin in the control group. At an FPG concentration of only 5.6 mmol/L (100 mg/dL), healthy subjects excreted 20% of the filtered glucose load (Fig. 3). This observation cannot be explained by the effect of dapagliflozin to reduce the TmG. Because the SHC started at an FPG target of 5.6 mmol/L, the precise threshold at which glucose excretion begins can only be estimated. A more precise determination of the threshold would require reduction of plasma glucose concentration <2.8 mmol/L (50 mg/dL) before starting the SHC, and this is not feasible. Sha et al. (19) indirectly examined the effect of canagliflozin on the threshold by measuring simultaneously 24-h urine glucose excretion and plasma glucose concentration at frequent intervals throughout the day. With a modeling approach, the authors concluded that SGLT2 inhibition reduced the renal threshold, which is consistent with the present results. It should be noted that the approach Sha et al. (19) used to measure the renal threshold for glucose is indirect and never has been validated by direct measurement using the gold standard renal glucose titration curve in the same subjects. Furthermore, this approach does not allow one to examine either the splay or the TmG at which glucose excretion begins.
To our knowledge, no previous study in humans has examined the effect of SGLT2 inhibition on the TmG. The current results demonstrate that dapagliflozin reduces the TmG in both diabetic and healthy subjects. The reduction in TmG would be expected to contribute to the decrease in postprandial glucose excursion after meal ingestion.
It is notable that the increase in 24-h urine glucose excretion (60–80 g/day) observed with all orally administered SGLT2 inhibitors currently in clinical trials represents only 40–50% of filtered glucose load (12,19,25). This relationship between plasma glucose concentration and the percent of glucose reabsorption that is inhibited has important implications for understanding normal renal physiology with respect to glucose handling. Because studies on renal glucose reabsorption largely have included patients with extremely elevated plasma glucose values, the percent of glucose reabsorption mediated by SGLT2 reported in the literature may overestimate the relative contribution at normal plasma glucose levels. It is possible that in humans, transporters other than SGLT2 may be capable of a much greater fraction of glucose reabsorption at lower filtered glucose loads but are saturated as tubular glucose concentrations increase, as has been demonstrated in SGLT2 knockout mice (2). It is also possible that human SGLT2 works differently from rodent SGLT2 and that the increase in plasma and urine glucose concentrations associated with the marked increase in plasma glucose levels (i.e., in excess of 13.9 mmol/L [250 mg/dL]) alters the transport characteristics of the SGLT2 transporter, rendering it more susceptible to inhibition with dapagliflozin. Finally, it is possible that after inhibition of the SGLT2 transporter, other renal tubular transporters (i.e., SGLT1) augment their reabsorption of glucose (26).
There are several limitations of the current study. First, the number of subjects in each group (n = 12) was relatively small. Second, we did not observe any significant difference in the response of any renal parameter after dapagliflozin treatment between male and female subjects. However, the number of subjects most likely was too small to detect such a difference, even if it were present. Third, the control group was slightly, although not significantly, younger. We doubt that this small, insignificant difference in age could have affected the results because the effect of dapagliflozin on TmG, threshold, and splay were similar in both groups. Fourth, it is not possible to determine whether the renal response to dapagliflozin would be different in poorly controlled diabetic patients because all individuals in the current study were reasonably well controlled. Finally, the duration of therapy was short (7 days), and with a longer treatment duration, upregulation of other transporters in the kidney could alter the quantitative findings demonstrated in the present study.
In summary, the current study examined for the first time in humans the effect of dapagliflozin on the major determinants of glucose handling (TmG, splay, and threshold) in patients with type 2 diabetes. Both the TmG and the splay in the renal glucose reabsorption curve are increased in diabetic versus healthy control subjects. Dapagliflozin induced marked glucosuria, reduced TmG, and decreased splay in subjects with and without type 2 diabetes. Most notably, dapagliflozin dramatically reduced the threshold at which glucose excretion begins to plasma glucose concentrations well below fasting levels (4.7–6.0 mmol/L [85–108 mg/dL]) in subjects with and without type 2 diabetes. From a quantitative standpoint, the reduced threshold represents the major mechanism through which SGLT2 inhibitors increase glucose excretion in humans.
Acknowledgments
This study was supported by AstraZeneca and Bristol-Myers Squibb. R.A.D. has been a member of advisory boards or consulted with Bristol-Myers Squibb, Amylin, Takeda, Boehringer Ingelheim, and Novo Nordisk; has received grant support from Takeda and Amylin; and served on the speakers’ bureau of Novo Nordisk. M.H. has been a member of advisory boards or consulted with Baxter, Boehringer Ingelheim, Pfizer, and Zealand. M.H. and L.A.M. are full-time employees of the Profil Institute for Clinical Research. S.K., X.L., Y.H., M.P., B.R.L., D.W.B., A.C., F.P.L., and S.C.G. are full-time employees of Bristol-Myers Squibb. No other potential conflicts of interest relevant to this article were reported.
R.A.D. contributed to the study concept and design, analyzed and interpreted data, and wrote and revised the article. M.H. and L.A.M. contributed to the study concept and design, supervised the study, acquired data, analyzed and interpreted data, and wrote and revised the article. S.K., X.L., Y.H., M.P., B.R.L., D.W.B., A.C., F.P.L., and S.C.G. contributed to the study concept and design, interpreted data, and wrote and revised the article. R.A.D. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
These data were presented, in part, at the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia, Pennsylvania, 8–12 June 2012, and at the 48th Annual Meeting of the European Association for the Study of Diabetes, Berlin, Germany, 1–5 October 2012.
The authors acknowledge editorial assistance provided by Alexandra Silveira, PhD, of PAREXEL and funding by AstraZeneca and Bristol-Myers Squibb.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc13-0387/-/DC1.
References
- 1.Valtin H. Renal Function: Mechanism Preserving Fluid and Solute Balance in Health Boston, Little, Brown and Company, 1983 [Google Scholar]
- 2.Vallon V, Platt KA, Cunard R, et al. SGLT2 mediates glucose reabsorption in the early proximal tubule. J Am Soc Nephrol 2011;22:104–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev 2011;91:733–794 [DOI] [PubMed] [Google Scholar]
- 4.Abdul-Ghani MA, Norton L, Defronzo RA. Role of sodium-glucose cotransporter 2 (SGLT 2) inhibitors in the treatment of type 2 diabetes. Endocr Rev 2011;32:515–531 [DOI] [PubMed] [Google Scholar]
- 5.Farber SJ, Berger EY, Earle DP. Effect of diabetes and insulin of the maximum capacity of the renal tubules to reabsorb glucose. J Clin Invest 1951;30:125–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mogensen CE. Urinary albumin excretion in early and long-term juvenile diabetes. Scand J Clin Lab Invest 1971;28:183–193 [DOI] [PubMed] [Google Scholar]
- 7.Katsuno K, Fujimori Y, Takemura Y, et al. Sergliflozin, a novel selective inhibitor of low-affinity sodium glucose cotransporter (SGLT2), validates the critical role of SGLT2 in renal glucose reabsorption and modulates plasma glucose level. J Pharmacol Exp Ther 2007;320:323–330 [DOI] [PubMed] [Google Scholar]
- 8.Noonan WT, Shapiro VM, Banks RO. Renal glucose reabsorption during hypertonic glucose infusion in female streptozotocin-induced diabetic rats. Life Sci 2001;68:2967–2977 [DOI] [PubMed] [Google Scholar]
- 9.Marks J, Carvou NJC, Debnam ES, Srai SK, Unwin RJ. Diabetes increases facilitative glucose uptake and GLUT2 expression at the rat proximal tubule brush border membrane. J Physiol 2003;553:137–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freitas HS, Anhê GF, Melo KF, et al. Na(+) -glucose transporter-2 messenger ribonucleic acid expression in kidney of diabetic rats correlates with glycemic levels: involvement of hepatocyte nuclear factor-1alpha expression and activity. Endocrinology 2008;149:717–724 [DOI] [PubMed] [Google Scholar]
- 11.Rahmoune H, Thompson PW, Ward JM, Smith CD, Hong G, Brown J. Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes. Diabetes 2005;54:3427–3434 [DOI] [PubMed] [Google Scholar]
- 12.Komoroski B, Vachharajani N, Feng Y, Li L, Kornhauser D, Pfister M. Dapagliflozin, a novel, selective SGLT2 inhibitor, improved glycemic control over 2 weeks in patients with type 2 diabetes mellitus. Clin Pharmacol Ther 2009;85:513–519 [DOI] [PubMed] [Google Scholar]
- 13.List JF, Woo V, Morales E, Tang W, Fiedorek FT. Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care 2009;32:650–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang L, Feng Y, List J, Kasichayanula S, Pfister M. Dapagliflozin treatment in patients with different stages of type 2 diabetes mellitus: effects on glycaemic control and body weight. Diabetes Obes Metab 2010;12:510–516 [DOI] [PubMed] [Google Scholar]
- 15.Bailey CJ, Gross JL, Pieters A, Bastien A, List JF. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet 2010;375:2223–2233 [DOI] [PubMed] [Google Scholar]
- 16.Ferrannini E, Ramos SJ, Salsali A, Tang W, List JF. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care 2010;33:2217–2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilding JPH, Norwood P, T’joen C, Bastien A, List JF, Fiedorek FT. A study of dapagliflozin in patients with type 2 diabetes receiving high doses of insulin plus insulin sensitizers: applicability of a novel insulin-independent treatment. Diabetes Care 2009;32:1656–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Komoroski B, Vachharajani N, Boulton D, et al. Dapagliflozin, a novel SGLT2 inhibitor, induces dose-dependent glucosuria in healthy subjects. Clin Pharmacol Ther 2009;85:520–526 [DOI] [PubMed] [Google Scholar]
- 19.Sha S, Devineni D, Ghosh A, et al. Canagliflozin, a novel inhibitor of sodium glucose co-transporter 2, dose dependently reduces calculated renal threshold for glucose excretion and increases urinary glucose excretion in healthy subjects. Diabetes Obes Metab 2011;13:669–672 [DOI] [PubMed] [Google Scholar]
- 20.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function—measured and estimated glomerular filtration rate. N Engl J Med 2006;354:2473–2483 [DOI] [PubMed] [Google Scholar]
- 21.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979;237:E214–E223 [DOI] [PubMed] [Google Scholar]
- 22.Sterner G, Frennby B, Mansson S, Nyman U, Van Westen D, Almén T. Determining ‘true’ glomerular filtration rate in healthy adults using infusion of inulin and comparing it with values obtained using other clearance techniques or prediction equations. Scand J Urol Nephrol 2008;42:278–285 [DOI] [PubMed] [Google Scholar]
- 23.Heinemann L, Ampudia-Blasco FJ. Glucose clamps with the Biostator: a critical reappraisal. Horm Metab Res 1994;26:579–583 [DOI] [PubMed] [Google Scholar]
- 24.Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009;58:773–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu JJ, Lee TW, DeFronzo RA. Why Do SGLT2 inhibitors inhibit only 30-50% of renal glucose reabsorption in humans? Diabetes 2012;61:2199–2204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Powell DR, DaCosta CM, Gay J, et al. Improved glycemic control in mice lacking Sglt1 and Sglt2. Am J Physiol Endocrinol Metab 2013;304:E117–E130 [DOI] [PubMed] [Google Scholar]