Abstract
Recent advances in basic fabrication techniques of TiO2-based nanomaterials such as nanoparticles, nanowires, nanoplatelets, and both physical- and solution-based techniques have been adopted by various research groups around the world. Our research focus has been mainly on various deposition parameters used for fabricating nanostructured materials, including TiO2-organic/inorganic nanocomposite materials. Technically, TiO2 shows relatively high reactivity under ultraviolet light, the energy of which exceeds the band gap of TiO2. The development of photocatalysts exhibiting high reactivity under visible light allows the main part of the solar spectrum to be used. Visible light-activated TiO2 could be prepared by doping or sensitizing. As far as doping of TiO2 is concerned, in obtaining tailored material with improved properties, metal and nonmetal doping has been performed in the context of improved photoactivity. Nonmetal doping seems to be more promising than metal doping. TiO2 represents an effective photocatalyst for water and air purification and for self-cleaning surfaces. Additionally, it can be used as an antibacterial agent because of its strong oxidation activity and superhydrophilicity. Therefore, applications of TiO2 in terms of photocatalytic activities are discussed here. The basic mechanisms of the photoactivities of TiO2 and nanostructures are considered alongside band structure engineering and surface modification in nanostructured TiO2 in the context of doping. The article reviews the basic structural, optical, and electrical properties of TiO2, followed by detailed fabrication techniques of 0-, 1-, and quasi-2-dimensional TiO2 nanomaterials. Applications and future directions of nanostructured TiO2 are considered in the context of various photoinduced phenomena such as hydrogen production, electricity generation via dye-sensitized solar cells, photokilling and self-cleaning effect, photo-oxidation of organic pollutant, wastewater management, and organic synthesis.
Keywords: TiO2 nanostructure, fabrication techniques, doping in TiO2, TiO2-assisted photoactivity, solar hydrogen, TiO2-based dye-sensitized solar cells, TiO2 self-cleaning, organic synthesis
Introduction
In the last century, scientists have made rapid and significant advances in the field of semiconductor physics. Semiconducting materials have been the subject of great interest due to their numerous practical applications, and they provide fundamental insights into the electronic processes involved. Similarly, material processing has become an increasingly important research field. Many new materials and devices, which possess specific properties for special purposes, have now become available, but material limitations are often the major deterrent to the achievement of new technological advances. Material scientists are now particularly interested in developing materials which maintain their required properties in extreme environments.
After the pioneering works of Efros and Efros1 and Brus2 on the size quantization effect in semiconductor nanoparticles, research on nanostructured materials has generated great interest in the scientific community. Tremendous opportunities in science and technology are now possible because of the new properties exhibited by these materials and the challenging problems in theoretical physics associated with the new properties.3–6 In general, ‘nanotechnology’ is the engineering of functional systems at the molecular scale. In its original sense, nanotechnology refers to the projected ability to construct items from the bottom up, using techniques and tools being developed today to make complete, high-performance products. As nanotechnology became an accepted concept, the meaning of the word shifted to encompass the simpler kinds of nanometer-scale technology. Formulation of a road map for development of this kind of nanotechnology is now an objective of a broadly based technology road map project of various leading nanotechnology research groups and institutes in the world. According to the US National Nanotechnology Initiative, the road map of nanotechnology can be divided into four generations (Figure 1). The first era is that of passive nanostructures, which are materials designed to perform one task. The second phase introduces active nanostructures for multitasking; for example, diodes, transistors, actuators, drug-delivery devices, and sensors. We are entering the third generation, which will feature nanosystems with thousands of interacting components. In future, we may expect the development of integrated nanosystems functioning much like a mammalian cell with hierarchical systems within systems.
Figure 1.
Road map to nanotechnology. Reproduced with permission from the US National Nanotechnology Initiative report.
The optical properties of nanocrystals are related to their size and surface chemistry and drastically differ from those of bulk materials. Preparation and study of high-quality quantum dots, nanobelts, and nanowires have been reported widely.7–9 Achievements in recent years have focused nanomaterials research on the applications in electrical and optoelectronics devices.10–12 Within the class of inorganic materials, oxide-based compounds show the most diverse range of properties. The electronic properties of these materials mainly depend on the nature of cation–oxygen bonding, which is explained either by solid-state band theory or by ionic bonding concepts from solid-state chemistry or by combining aspects of both approaches. This interplay between localized and itinerant character yields a wide range of electronic properties of metal oxides. For example, a closed-shell compound such as Al2O3 is an insulator displaying large band gaps. In many cases, these insulators can serve as effective host materials for efficient luminescence when doped with rare earth or transition metal cations. On the other hand, for closed-shell oxides based on cations with relatively high electronegativity, such as in ZnO and SnO2, the more covalent nature of bonding yields semiconductors with relatively high carrier mobilities. Electronic oxides containing transition metal cations can yield high conductivity materials, such as SrRuO3, or even superconductors, as with YBa2Cu3O7. Collective phenomenon involving electric dipole interactions in insulators yields ferroelectrics such as BaTiO3. Unpaired electron spin in some oxides results in ferromagnetism, as in CrO2, or ferrimagnetism, as in Fe3O4. In addition, many oxides display interesting metal–insulator transitions that are dependent on temperature (eg, V2O3), pressure (eg, NiO), or magnetic fields (eg, (La,Sr)MnO3).13–19 Because of their fundamental properties and obvious utility in applications, significant efforts have been invested in the growth of oxides as epitaxial thin films. Various classes of metal oxides are schematically represented in Figure 2.
Figure 2.
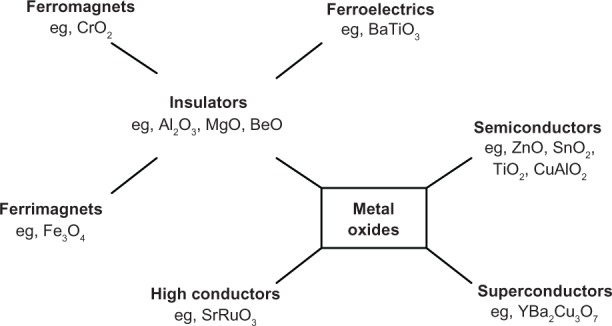
Classifications of metal oxides.
Among these oxides, materials scientists are overwhelmingly interested in the fundamental aspects and applications of semiconducting wide band-gap oxide materials. These types of materials show a wide range of electrical and optical properties. They can be transparent in the visible and infrared (IR) range and can be found in insulators as well as semiconductors. Among these wide-gap oxide materials, we will focus mainly on nanostructured titanium dioxide (TiO2), also known as titanium (IV) oxide or titania, which is a naturally occurring oxide of titanium. Because of its wide range of applications, from paint to sunscreen to food coloring to photocatalyst, hydrogen production, storage medium, sensors, solar cells, and various biological and health-related applications, this technologically important material is the subject of ongoing research and development in design, syntheses, and applications.
Properties of TiO2
Overview
TiO2 is a chemically stable, nontoxic, biocompatible, inexpensive material with very high dielectric constant and interesting photocatalytic activities. It is a wide-gap semiconductor, and depending on its chemical composition, it shows a large range of electrical conductivity. In general, TiO2 has two stable crystalline structures: anatase and rutile.20 Usually, natural rutile crystals are impure, and, therefore, early investigations were limited to ceramic samples only, but later (around 1950s), a colorless, large, single crystal of synthetic rutile was grown by the Boule technique.21,22 Thereafter, most of the research was done on the electro-optical characterization and defect chemistry of rutile single crystals.21–26 Stoichiometry of the rutile TiO2 is highly dependent on its deposition parameters, especially on annealing conditions and atmosphere.24,27–30 The charge transport phenomenon in rutile is described by small polaron model.31–34 Earlier, it was assumed that the properties of anatase would be similar to those of rutile35 until a new solar cell concept was reported using anatase, which cannot be realized by rutile structure.36 With the possibility of growing synthetic anatase single crystals,37 considerable research to systematically investigate the electronic and optical properties of anatase TiO2 has begun.35,38–43
In general, titanium metal oxidizes in ambient conditions and a thin layer of native oxide grows on the surface. This surface oxide layer is about 4 nm thick and protects the metal from further oxidation and consists mostly of rutile and anatase TiO2, but very small amounts of Ti2O3 and TiO can also be found.44 This native oxide layer not only passivates the surface, but also resists corrosion from a harsh environment and is useful as an implant material in orthopedic and dental applications.44 TiO2 powder is commonly used as a pigment in paints, coatings, plastics, papers, inks, fibers, nutrients, toothpaste, and cosmetics.45,46 Due to its high refractive index, it has found important applications in antireflection coatings, narrow-band filters, and optical waveguides.47–52 Often, the properties can be tuned by creating multilayers of TiO2 and SiO253 in a stacked conformation, where the high refractive index of TiO2 is combined with the low refractive index of SiO2 to show interesting optical properties. TiO2 has also been used as NOx, oxygen, and hydrocarbon sensors.54–58 The catalytic activities of TiO2 surface under visible irradiation led to interesting applications in water purification (by dissociating pollutants in dissolved organic molecules), water decomposition for hydrogen production,59–65 dye-sensitized solar cells (DSSCs) and electrochromic devices,36,66,67 solid-state photovoltaic solar cells,68–70 photoelectrochemical anticorrosion coatings, self-cleaning properties,71 and superhydrophilicity.72 Moreover, because it is a highly dielectric material, there are interesting electronic applications for rutile TiO2.73 Local oxidation of titanium thin films by scanning probe microscopes yields a convenient lithographic patterning technique, which does not require any etching,74 thus creating microelectronic elements.75 Silver (Ag)-incorporated TiO2 showed multicolor photochromism under visible irradiation.76 Some new applications can be possible with this material, such as rewritable color copy paper or high-density multiwavelength optical memories.77 Rutile is preferred to anatase for optical applications because of its higher refractive index. On the other hand, anatase is prefered for all the applications related to photocatalytic activity, gas sensing, and solar cells, due to its higher mobility and its catalytic properties.36,55,61 Fabrication techniques of doped or undoped TiO2 thin films with a wide variety of properties and morphologies include both wet-chemical and vacuum-based physical techniques. An overview of the available techniques can be found in in the literature.78–83 It is noteworthy that the terms ‘TiO2’ and ‘titanium dioxide’ are generally used for slightly substoichiometric films of the composition TiO2–x with x < 0.1, which exists in a mixture of rutile, anatase, and amorphous crystalline phases.
Crystal structure of TiO2
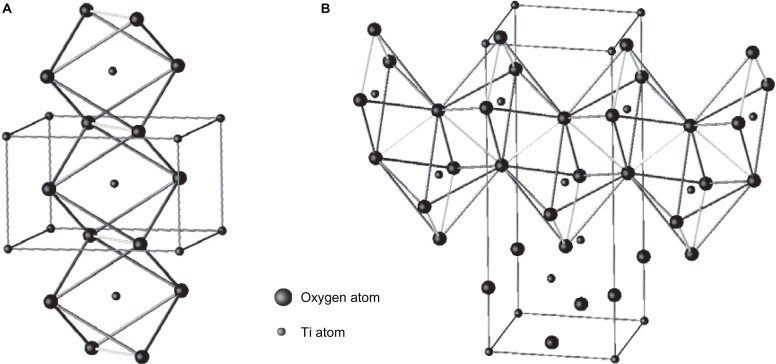
As mentioned earlier, naturally occurring TiO2 crystal has three common polymorphs: rutile (tetragonal), anatase (tetragonal), and brookite (orthorhombic),17,84,85 along with some less common structures like TiO2 II (columbite),86 TiO2 III (baddeleyite),87,88 TiO2 (H) (hollandite),89 TiO2 (R) (ramsdellite),90 and TiO2 (B) (monoclinic)91 many of which occur only at very particular conditions. Among these structures, rutile is the most stable phase,92 whereas anatase is metastable at room temperature and transforms irreversibly into rutile upon heating above a threshold temperature around 1000°C (which is in atmospheric conditions, but the threshold temperature can vary from 400°C to 1200°C depending on the grain size, ambient conditions, and impurities).93 Natural rutile crystals exhibit predominantly (110) surface,17 which is considered to be the most stable surface of stoichiometric rutile,94,95 whereas anatase is the most stable with (101) surface.83Figure 3 shows the schematics of the unit cells of the TiO2 crystal structures, and Table 1 shows the unit cell parameters (at room temperature). The rutile unit cell contains two Ti atoms (at [0, 0, 0] and [½, ½, ½]) positions, respectively) and four oxygen atoms (that form a distorted octahedron around Ti). The anatase unit cells contains four Ti atoms (at [0, 0, 0], [½, ½, ½], [0, ½, ¼], and [−½, 0, −¼]) and eight oxygen atoms (that form a distorted TiO6 octahedron around each Ti cation).82,96 The TiO6 octahedra constitute the basic building units for the various polymorphic structures of TiO2 and differ from each other by the arrangement and the distortion of the octahedra (Figure 4). In the rutile crystal, each octahedron is connected to two edge-sharing and eight corner-sharing neighboring octahedra, in which the edge-shared octahedra are aligned along the (001) surface direction.21 In the case of anatase, there are four edge-sharing neighbors, which are aligned along the (100) and (010) surface direction forming zigzag double chains perpendicular to the c-axis, thus creating open channels parallel to the c-axis in rutile and perpendicular to the c-axis in anatase.82,94
Figure 3.
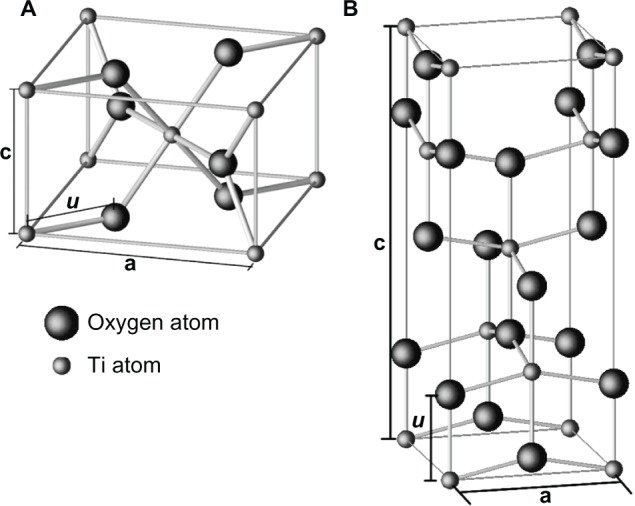
Crystallographic unit cell structure of TiO2 with A) rutile and B) anatase structures. Copyright © 2003, Cangiani. Adapted with permission from Cangiani G. Ab Initio Study of the Properties of TiO2 Rutile and Anatase Polytypes. Lausanne, France: Faculté des sciences de base, Ecole polytechnique fédérale de Lausanne EPFL; 2003.
Table 1.
Comparison of the structural, optical, and electrical properties of rutile and anatase
| Polymorphs | Rutile
|
Anatase
|
||||
|---|---|---|---|---|---|---|
| ||c | ⊥c | ||c | ⊥c | |||
| Crystal structure | Tetragonal | Tetragonal | ||||
| c = 2.9587Å [470[ | a = 4.5937Å [470[ | c = 9.5146Å [93[ | a = 3.7842Å [93[ | |||
| Space group | P42/mnm (136) [84[ | 141/amd (141) [84[ | ||||
| Most stable state | (110) [94[ | (101) [102[ | ||||
| Density | 4.25 g/cc [84[ | 3.89 g/cc [84[ | ||||
| Band gap at 10 K | 3.051 ev [471,472] | 3.035 ev [471,472] | 3.46 ev [35[ | 3.42 ev [35[ | ||
| Spectral dependence | E1/2 [35[ | E3/2 [35[ | Urbach [40[ | |||
| Nature of gap | Indirect [471,472] | Direct [471,472] | Indirect [35[ | Direct [35[ | ||
| Static dielectric constant (ɛ0, in MHz range) | 173 [13,473] | 89 [13,473] | 48 [474[ | 31 [475[ | ||
| High frequency dielelectric constant, ɛ∞ (λ = 600 nm) | 8.35 [103[ | 6.76 [103[ | 6.25 [41[ | 6.50 [41[ | ||
| Refractive index (at λ = 600 nm) | 2.89 [103[ | 2.60 [103[ | 2.50 [41[ | 2.55 [41[ | ||
| Nature of conductivity at room temperature (undoped) | n-Type semiconductor [21,112] | |||||
| Mott transition | Not observed [118[ | Observed [35[ | ||||
| Room temperature mobility in crystal | 0.1–1 cm2/vs [22,81] 0.01 cm2/vs (high impurity concentration) [81[ μ||c ≈ (2−5) * μ⊥c [22,476] |
15 cm2/vs [35,112] | ||||
| 0.6–1.5 cm2/vs [476[ | 0.6–1.5 cm2/vs [476[ | |||||
| Room temperature mobility in polycrystalline thin film | 0.1 cm2/vs [22,38] CUA | 0.1–4 cm2/vs [35[ | ||||
| Electron effective mass | 9–13 me [477[ 10–30 me [22[ 12–32 me [26[ |
~1 me [35[ | ||||
Copyright © 2003, Springer. Adapted with permission from Springer S. Free carriers in nanocrystalline titanium dioxide thin films. Ecole polytechnique fédérale de Lausanne EPFL; 2004; Lausanne, France; Thèse no 2934. For further properties please refer Diebold U. The surface science of titanium dioxide. Surf Sci Rep. 2003;48(5–8):53–229.
Figure 4.
Arrangement of TiO6 octahedra in relation to the unit cells in A) rutile and B) anatase. Only one chain is shown for each structure. Highlighted bonds are the O–O bonds. Copyright © 2003, Cangiani. Adapted with permission from Cangiani G. Ab Initio Study of the Properties of TiO2 Rutile and Anatase Polytypes. Lausanne, France: Faculté des sciences de base, Ecole polytechnique fédérale de Lausanne EPFL; 2003.
Phase diagram of the oxides of titanium shows that by varying the oxygen content from 0 to 2 oxygen per titanium atom, the main phases that can be found at room temperature are Ti, Ti2O, TiO, Ti2O3, Ti3O5, and finally TiO2.97 Additionally, a series of TinO2n−1 (with n ≥ 4) phases can be found in between Ti3O5 and TiO2 phase, which is called the Magnéli series of homologous compounds,30,98 where physical properties are changed dramatically, ranging from metallic to insulating depending on n. The formation of such reduced oxide phases can be described in terms of the elimination of a plane of oxygen atoms.30 Magnéli phases with n up to about 38 (TiO1.974)82 and 61 (TiO1.984)30 have been reported thus creating a very narrow homogeneity range for TiO2 before the lattice tends to break down and the first Magnéli phase appears.82
Optical properties of TiO2
The dielectric functions of anatase and rutile single crystals for the electric field perpendicular and parallel to the c-axis (marked with ⊥c and ||c, respectively) show that in the IR to visible spectral range, anatase is less anisotropic than rutile, whereas in the band-gap region, anatase shows important anisotropy41,99–105 It has been found that depending on the degree of reduction of rutile TiO2, a blue color arises from the visible tail of an IR absorption band peaking at about 0.75–1.18 eV.22,24,31,99–101 Similarly, in anatase too, the blue color has been observed43,102 which is caused by a wide absorption band with its maximum in the IR. In addition, a color center at 3 eV due to an oxygen vacancy has been identified giving rise to a yellow color.43 Various optical parameters of TiO2 can be found in several literatures.41,103–106 Table 1 shows various optical properties of TiO2.
Electrical/electronic properties of TiO2
The valence band (VB) of wide-gap rutile and anatase consist of O 2p states, whereas the conduction band (CB) is formed by Ti 3d states,17,29 and detailed calculations on these states can be found in the reports of Tang35 and Cangiani.107Table 1 presents various electrical properties of TiO2, and shows that these properties are dependent on the crystallographic direction. As a wide band-gap semiconductor, TiO2 crystals have a high resistivity (~1015 Ωcm),30 and bulk oxygen vacancies, titanium interstitials, and reduced crystal surfaces are considered to generate shallow electron donor levels that contribute to the electric conductivity of TiO2.30,108 In addition, it has been reported that replacement of oxygen by water vapor remarkably increases the electrical conductivity compared to films deposited with oxygen as reactive gas.109 Reduced TiO2 is an n-type semiconductor, and the n-type conductivity increases with the extent of oxygen loss within the crystal lattice. Point defects in terms of doubly charged oxygen vacancies and interstitial titanium ions with three or four charges affect the conductivity and ionization energy of the rutile crystal.28,82 The ionization energies have been reported to be around 0.007–0.08 eV (depending on temperature) for titanium interstitials,26,110 whereas oxygen vacancies contribute to the electronic conduction as double donors with a shallow donor level (0–200 meV) and a deep donor level (600–750 meV).22,30,111 For anatase, the dominant crystal defects are not yet fully understood: some researchers suggested Ti interstitials caused the dominant defects, whereas others suggested oxygen vacancies for the same, but several reports suggested the existence of both phenomena, with an activation energy of carrier generation around 4 meV.30,83,112,113 In general, oxygen vacancies are observed to be the dominant phenomenon under weakly reducing conditions or low annealing temperatures (below 870 K in vacuum), whereas in the more reducing conditions and higher annealing temperatures (above 1070 K in vacuum), titanium interstitials become more predominant in effect.30,81 Enthalpy of oxygen vacancy formation is calculated to be around 4.55 eV/vacancy, whereas the same for the triply charged interstitial titanium atom is around 9.11–9.24 eV/interstitial titanium.82,114,115 Due to the low value of carrier mobility in rutile TiO2, the transport phenomenon is assumed to follow either small polaron (an electron or hole self-trapped by the local lattice polarization which itself is generating16) hopping mechanism32,116 or phonon scattering.34 Especially, the mobility is shown to decrease in higher temperature, suggesting the presence of large polarons (and not localized small polarons, which may become more mobile at higher temperatures due to thermally activated hopping between the atoms13). However, the mobility may decrease if the small polarons diffuse by tunneling,13 and hence, some interesting charge transport models (such as multiband conduction model117) have been proposed for rutile TiO2. On the other hand, the anatase TiO2 shows higher carrier mobility, suggesting Arrhenius-type thermally activated conduction rather than small polaron hopping in rutile.35,118 However, in both the crystal structures, high concentrations of donors lead to the formation of impurity bands,35,118 and a transition from nonmetallic to metallic behavior is observed when the donor concentration exceeds a critical value.16,119
Fabrication of nanostructured TiO2
With the rapid development of nanotechnology, TiO2 nanostructures in various forms are fnding wider applications than before because of their specifically size-related properties. The energy band structure becomes discrete for nanometer-scale TiO2, and its photophysical, photochemical, and surface properties are quite different from those of the bulk ones due to the quantum size effect, and therefore, many works have focused on the synthesis of nanocrystalline TiO2 with a large specific surface area. Syntheses of 0-, 1-, and 2-dimensional nanostructures of TiO2 have been reported widely.
TiO2 nanoparticles
TiO2 nanoparticles have specific advantages in the enhancement of light absorption due to the large fraction of surface atoms. Interband electron transition is the primary mechanism of light absorption in pure semiconductors. These transitions are direct as the momentum gain by the electron from light wave is small in comparison with πh/a (‘a’ is the lattice constant). This absorption is small in direct-forbidden gap semiconductors, as in the case for TiO2, for which the direct electron transitions between the band centers are prohibited by the crystal symmetry. However, momentum is not conserved if the absorption takes place at the boundary of the crystal, for example, at the surface or at the interface between two crystals, which leads to the indirect electron transitions that can result in the essential enhancement of light absorption. This means that considerable enhancement of the absorption can be observed in small nanocrystals where the surface to volume ratio is very high and the fraction of the surface atoms is sufficiently large. The particle size at which the interface enhancement of the absorption becomes significant is around 20 nm. An additional advantage obtained in nanoparticles in the few nanometer size regimes is that the large surface-to-volume ratio makes possible the timely utilization of photogenerated carriers in interfacial processes.120–122
Vacuum-based physical techniques
Both vacuum-based and solution-based techniques have been adopted for fabrication of TiO2 nanoparticle. One of the early reported nonsolution techniques is metalorganic chemical vapor deposition (MOCVD). Okuyama and colleagues123–125 reported the formation of aerosol particles by gas-phase chemical reaction. They have used titanium tetraisopropoxide (TTIP) vapor containing ultrafine TiO2 seed particles in a laminar flow aerosol reactor, and the thermal decomposition of TTIP vapor in the controlled cylindrical furnace produced ultrafine TiO2 nanoparticles of size in the range 10–60 nm. They have also studied the effects of the initial concentration of TTIP vapor, the reaction temperature, the temperature profle of the furnace, and properties of seed particles on the particle size distribution to control the nanoparticle formation. Similarly, Ding and colleagues126 synthesized TiO2 nanoparticles supported on porous silica gel (60–100 mesh) via MOCVD process, using TTIP as precursor. The chemical vapor deposition (CVD) reactor was a quartz tube with a porous quartz disk at one end. The reactor temperature was controlled by a tubular furnace. TTIP was introduced into the CVD reactor using nitrogen as the carrier gas. The synthesis of TiO2 nanoparticle/silica gel photocatalyst involved three steps: pretreatment of the support materials, CVD reaction, and calcination. On the other hand, Li and colleagues127–129 reported the fabrication of transition metal and lanthanide ion (Nd−3, Pd−2, Pt−4, Fe−3)-doped TiO2 nanoparticles via MOCVD process using a TTIP precursor. The CVD system is similar to that given in Ding et al,126 except for a horizontal reactor. In addition, unlike other works,126 this group used Ar-diluted O2 as the carrier plus reactant gas with an elevated reaction temperature for the formation of TiO2 nanoparticles, thus avoiding the additional calcination step. The average particle size reported was 20–25 nm. For doping of TiO2, neodymium (III) acetylacetonate, palladium (II) acetylacetonate, platinum (IV) acetylacetonate, and iron (III) acetylacetonate precursors were used, and the effect of these dopants on the photocatalytic activity of TiO2 nanoparticle is discussed.
Instead of using costly metalorganic precursors, several groups used inorganic materials in conventional CVD system to fabricate silica-supported 10–20 nm TiO2 nanoparticles.130–134 Leboda and colleagues reported the CVD syntheses of titania/silica gel130 and titania/fumed silica.131 In addition, CVD preparations of titania/silica gel and titania/ZSM-5 were also reported by Schrijnemakers and collleagues132 and Stakheev and colleagues,133 respectively. Xia and colleagues134 reported the gas-phase/CVD synthesis of TiO2 nanopowder and investigated the influence of various deposition parameters on particle size. In all these reports, titanium tetrachloride (TiCl4) was applied as the precursor for CVD reaction and a two-step synthesis procedure was used. TiCl4 was first introduced and adsorbed onto the supports, and then oxygen/water vapor was brought through to start the oxidation/hydrolysis of TiCl4 species. Especially, oxidation of TiCl4 vapor, also known as the ‘chloride’ process in titania industry,135–137 now becomes one of the main gas-phase routes for commercial production of titania nanopowder. TiCl4 is an inorganic low-cost precursor, which can be readily oxidized or hydrolyzed to prepare TiO2 powders according to the following equations:
| (1) |
| (2) |
The oxidation method is an important route in a typical gas-phase/CVD method. In gas-phase processes, greater equilibrium constant (Kp) favors the formation of smaller particles, and the Kp value that exceeds 102.3 is necessary for preparation of nanoscale particles.134 Vemury and colleagues138 reported the synthesis of TiO2 nanoparticles via oxidation of TiCl4 vapors in electrically assisted hydrocarbon flames using needle-shaped or plate electrodes. A laminar premixed burner-stabilized flame reactor is used to make titania nanoparticles in the presence of externally controlled electric field. TiCl4 vapor premixed with nitrogen, oxygen, and methane were sent through the burner with argon as carrier gas. Oxide particles are formed in the flame by oxidation/hydrolysis of the precursors. External electrodes are used to create the electric field across the flame.139 Vemury and colleagues observed that the particle size decreases with increasing field strength across the flame. In addition, it charged the newly formed particles, resulting in electrostatic repulsion and dispersion, which decreased particle growth by coagulation, thus retaining the nanostructure of synthesized particles. A laser-induced CVD process to prepare TiO2 nanopowders of crystallite size around 20–30 nm has also been attempted by Casey and Haggerty.140 Titanium alkoxide vapor was heated with 10.591 μm IR radiation from a CO2 laser. Among other physical techniques, Epperson and colleagues141 used an inert gas condensation technique, where the metallic Ti is evaporated in He pressure and then slowly exposed to O2 atmosphere to obtain TiO2 nanoparticles.
Solution-based chemical techniques
Among wet-chemical processes, one of the classical methods was a sulfate process,142 where spherical titania nanoparticles were prepared from an aqueous solution of TiOSO4 by homogeneous precipitation using urea at 70°C–90°C. Especially, the presence of urea dictates the spherical shape of the nanoparticles. As-synthesized powders were amorphous hydrates of titania containing a sulfate group and crystallize by calcination into anatase (>500°C) and rutile (>900°C). In calcination, the particles shrink, but hold their original spherical shapes. Other wet-chemical processes include the sol–gel method, which is one of the widely used methods for TiO2 nanoparticle formation,143–161 because of its advantage of controlling the particle size and morphology through experimental conditions.154 Most of the reports on sol–gel syntheses of TiO2 nanoparticles involved titanium alkoxide (titanium isopropoxide [TIPO], titanium butoxide, titanium ethylhexoxide, tetra-n-butyl titanate) as the Ti source.143–146,148–158,160,161 Few used TiCl4 as the Ti precursor solution.147,159 The process generally starts with the hydrolysis of the source solution to form the complex sol, followed by heat treatment and aging to obtain the gel, and finally, annealing at elevated temperature to obtain the required oxide. A typical sol-gel synthesis process of TiO2 nanoparticles using TIPO as Ti source is as follows: first, TIPO is mixed with tetraethanolamine (TEOA) with TEOA:TIPO = 2:1 ratio, which produces a sol of organometallic complex, and then refluxed and aged under H2O (+HClO4/NaOH) solution for 24 h (at 100°C) to obtain Ti-contained gel, which is subsequently aged for 72 h (at 140°C) to obtain the TiO2 nanoparticle.154 Phase-pure anatase nanoparticles are generally prepared from titanium (IV) isopropoxide, TEOA, and/or mild acid-like acetic acid.154,162 When stronger acids are used a fraction of the product usually consists of brookite nanoparticles.163,164 The synthesis of brookite nanoparticles has been reported by thermolysis of TiCl4 in aqueous HCl solution.165 The composition of the reaction product was found to be strongly dependent on the Ti:Cl concentration ratio (~17–35). Phase-pure rutile nanoparticles have been prepared from TiCl4 or TiCl3 in HCl solution or from titanium (IV) isopropoxide in nitric acid at pH = 0.5.166–170 Several authors have compared synthesis methods for the three phases, in order to determine the effect of crystal structure on the physical properties.171–174 Reyes-Coronado and colleagues174 reported the syntheses of phase-pure anatase, rutile, and brookite TiO2 nanoparticles via a combinatorial approach using both sol-gel and hydrothermal treatment. Amorphous TiO2 nanoparticles were first prepared by sol-gel technique using Ti alkoxide as the source solution followed by hydrothermal treatment at three different acid concentrations and temperatures in order to establish the conditions for obtaining phase-pure products of anatase, rutile, and brookite phases separately. Typically, the hydrothermal condition for the synthesis of anatase phase is reported to consist of heat treatment at 200°C for 6 h under 1.5M acetic acid solution. For obtaining rutile phase, these parameters are 200°C for 8 h under 4M HCl environment, whereas the same for obtaining brookite phase is 175°C for 7 h under 3M HCl solution. Details can be found in the Reyes-Coronado et al.174
Another chemical process used to synthesize TiO2 nanoparticle is hydrothermal synthesis,175–177 where TiCl4 was used as the starting materials for Ti source. Rao and Dutta178 used Ti alkoxide precursor in toluene solvent to hydrothermally synthesize TiO2 nanoparticle. Palmisano and colleagues179 used a coprecipitation method using TiCl3 and aqueous ammonia to form titanium hydroxides followed by fring in air at elevated temperature to obtain TiO2 submicron particles of size 100–300 nm. Akhtar et al180 and Shi et al181 used direct vapor-phase oxidation of TiCl4 in an aerosol reactor at elevated temperatures to obtain TiO2 nanoparticles. Jagadale et al160 and Gao et al182 used a wet-chemical peroxide-based synthesis route, where H2TiO3 (or TTIP) dissolved in H2O2 and/or ammonia solution were used to form peroxotitanate, which was subsequently transformed to TiO2 nanoparticle via low-temperature aging or calcination. Mahshid and colleagues183 used peptization method to form TiO2 nanoparticles. Initially, TiO2 colloids in the nanometer range were prepared by hydrolysis and condensation of titanium alkoxide in aqueous media. In the presence of water, alkoxide hydrolyzes and subsequently polymerizes to form a 3-dimensional oxide network. These reactions can be schematically represented as follows:
| (3a) |
| (3b) |
where R is ethyl, i-propyl, n-butyl, etc.184 The size, stability, and morphology of the sol produced from alkoxide route is strongly affected by the water-to-Ti molar ratio (r = [H2O]/[Ti]). At r ≤ 10, spherical, relatively monodisperse particles with diameters of 0.5–1 mm are obtained. On the other hand, at higher r values, the particles formed are unstable and precipitate in the form of large aggregates, which can subsequently be chemically peptized to final sizes that are usually <100 nm in diameter. Because of the small size of particles that are formed under these conditions, formation of colloidal TiO2 at high r values is of great interest. In addition, acidity of the solution has a strong influence on the size distribution of nanoparticles.183 Li and colleagues185 used a solvothermal method to synthesize nanoparticles by controlling the hydrolyzation reaction of titanium butoxide using NH4HCO3 and linoleic acid. They have also described the reaction mechanism and formation process of the TiO2 nanoparticles and nanorods by controlling the solvothermal reaction parameters, such as reaction temperatures, concentrations, reaction durations, and so on. Seo et al186 demonstrated the synthesis of TiO2 nanoparticles by a high-temperature nonhydrolytic method using TiCl4, oleic acid, and oleyl amine mixture in a reaction flask at 270°C. After 10 min, the reaction mixture was quenched, and TiO2 nanoparticles were separated by a size-selective precipitation process. Teleki and colleagues187 used a flame-based spray pyrolysis method to synthesize TiO2 nanoparticles. TTIP, diluted in a mixture of xylene and acetonitrile, was fed into a combustible spray to obtain the powdered nanoparticles. It is to be noted that for the physical- or gas-phase processes of TiO2 nanoparticle syntheses, unlike the liquid-phase methods, process parameters can be adjusted easily to produce nanoparticles with varied crystallinity and surface area without the necessity of posttreatments.
One-dimensional TiO2 nanostructures
One-dimensional nanostructures in the form of nanowires, nanorods, nanotubes, nanopillars, nanocylinders, nano-needles, and nanowhiskers have received considerable attention because of their potential applications in catalysts as carrier materials, in pharmacy as drug-delivery agents, in nanoelectronics for the isolation of ultrasmall wires, or in basic research to study host–guest chemistry in mesoscopic materials.188–190 TiO2-based nanotubes have also attracted wide attention owing to their potential applications in highly efficient photocatalysis,191 lithium ion batteries,192 photovoltaic cells,193–195 and environmental applications.196
Solution-based chemical techniques
Hoyer was the first to attempt to prepare TiO2 nanotubes.197 Starting from a porous alumina membrane (PAM), a polymer mold suitable for the formation of TiO2 nanotubes was obtained. The tubular structure was formed by electrochemical deposition in the mold. After dissolution of the polymer, TiO2 nanotubes were obtained, which were amorphous, and transformed into polycrystalline anatase structure after heat treatment. The typical fabrication steps consist of anodic alumina membrane fabrication and gold evaporation on top of the membrane (step 1), followed by casting of poly(methyl methacrylate) (PMMA) within the pores of alumina membrane (step 2). After that the alumina membrane is removed to obtain negative mold (upside down) with evaporated gold inside the replicated pores of PMMA (step 3). Thereafter, electroless gold deposition on the sidewalls of the PMMA mold is performed (step 4) followed by the electrodeposition of TiO2 on the walls of the mold. Finally, the PMMA mold is removed to obtain free-standing TiO2 nanorods covered with gold layers (step 5).
Kasuga and colleagues198,199 synthesized TiO2 nanoneedles (anatase phase) using sol–gel-derived fne TiO2-based powders under hydrothermal NaOH treatement at 110°C for 20 h. Following their pioneering works, several research groups200–219 have also synthesized 1-dimensional TiO2 nanostructures by similar chemical processes with some variations in experimental conditions and/or reagents. For example, Feng and colleagues210 used tetrabutyl titanate, titanium tetrachloride in HCl with toluene as the nonpolar solvent to form TiO2 nanowires. Liu and Aydil215 used titanium butoxide, TIPO, and titanium tetrachloride as Ti precursors in HCl solution to form nanowires. They also studied the effects of surfactants or salts such as ethylenediamine, ethylenediaminetetraacetic acid, sodium dodecyl sulfate, cetyltrimethylammoniumbromide, polyvinylpyrrolidone, and sodium chloride on the nanowire properties. This group has also used a three-step (hydrothermal + ion exchange + annealing) synthesis of TiO2 nanowires on titanium foil.215 In the first step, sodium titanate nanotubes were hydrothermally formed on Ti foil using a NaOH and H2O2 solution. In the second ion-exchange step, the titanium foil with nanotubes was immersed in HCl to exchange the Na+ with H+ and thus transform the sodium titanate nanotubes to hydrogen titanate nanotubes. In the third step, the hydrogen titanate nanotube-coated titanium foil was annealed at 500°C to convert the nanotubes to anatase TiO2 nanowires. Chang and colleagues216 used a similar hydrothermal method to the Kasuga group198,199 with a microwave oven as the external power source for the reaction. Zhu and colleagues200 used sonication-assisted hydrothermal growth of TiO2 nanotubes and nanowhiskers. Wang and colleagues217 also used a similar hydrothermal growth of TiO2 nanowires on a spiral-shaped Ti wire. Tian and colleagues219 seeded the substrate with TiO2 nanoparticles followed by a similar hydrothermal method.198,199 With respect to the formation mechanism of TiO2 nanotubes, Kasuga et al199 tentatively proposed that TiO2 nanotubes were grown by the connection between the two ends of Ti–OH forming sheets during the process of washing the alkali-treated TiO2 raw materials. Later Yao and colleagues205 explained the nanotube formation mechanism using transmission electron microscopy (TEM) studies. They observed that the TiO2 nanotube walls were not seamless, unlike multiwall carbon nanotubes (CNTs). They argued that crystalline TiO2 raw material underwent delamination in the alkali solution to produce single-layer TiO2 sheets during alkali treatment. These single-layer TiO2 sheets were later rolled to form TiO2 nanotubes.
Encouraged by these methods, various groups reported the syntheses of 1-dimensional TiO2 nanostructures via porous membrane-based sol–gel and electrochemical routes.196,220–261 Use of PAM as the host to grow oriented TiO2 nanowires through the PAM nanopores via sol–gel method has been attempted by several groups.220–237 Ti alkoxides have been used as the source solution for the sol–gel syntheses to fill the PAM pores followed by oxygen/air annealing to obtain the TiO2 nanowires. However, this sol–gel template method has some shortcomings. As the only driving force of this technique is capillary action, for the sol with higher concentration, filling of pores is difficult, whereas for low-concentration sol, the as-synthesized nanomaterials lead to shrinkage and cracking.262 To overcome these difficulties, Miao and colleagues226 reported an electrochemically induced sol-gel method to prepare TiO2 single-crystalline nanowire arrays. For that, one end of the pores are coated with metallic cathode, and an external magnetic field is applied to force the Ti-containing ions to enter into the pores. First, the hydroxyl ion was generated due to the cathodic reduction, and then the generation of OH ions increases the local pH at the electrode surface, resulting in the titanium oxyhydroxide gel formation in the pores of the template. Finally, subsequent heat treatment and the removal of the PAM results in the formation of TiO2 single-crystalline nanowire arrays.226 Similar methods have been adopted by various others groups.227–230 For example, Lin et al227 and Zhang et al228 used a similar electrochemically induced method to form single-crystalline anatase TiO2 nanowires with diameters about 15 nm and lengths about 6 μm within hexagonally packed nanochannels of porous alumina. They have used acidic TiCl3 as the precursor solution, and using the potentiostatic method with a three-electrode arrangement with a saturated calomel reference electrode (SCE) and a Pt counter electrode, anodic oxidative hydrolysis was done followed by oxygen annealing at 500°C to obtain the desired oxide nanowire arrays. Liu and Huang229 used pulsed electrodeposition with acidic TiCl3 as electrolyte solution to grow TiO2 nanowire with PAM.
Caruso et al231 used electrospun polymer fibers as host to coat with amorphous TiO2 using a sol-gel technique. On removal of the thermally degradable polymer, hollow titania fibers are produced. The sol-gel coating was able to mimic the finer details of the fiber, thereby forming nodules on the inner walls of the tubes. Similar methods have been adopted by Formo et al232 and Archana et al233 Jung et al234 and Kobayashi et al235 demonstrated a new methodology to prepare the TiO2 hollow fibers, double-layered tubular structures, and helical ribbons using a crown-appended cholesterol-based organic gelator in the sol-gel polymerization process of Ti[OCH(CH3)2]4 followed by oxygen annealing at elevated temperature to remove organic components and obtain TiO2 nanostructures. Similarly, Zhang and Qi236 used bacterial cellulose membranes as host and followed similar methods stated above to obtain TiO2 nanostructures. Chen and colleagues237 synthesized a TiO2 nanowire network on electrospun polymer template using a H2O2, TiOSO4, and KNO3 sol-gel bath.
Anodization of Ti foils and films is one of the most widely used methods for template-free electrochemical deposition of TiO2 nanowire/nanotube. After the first report of anodic oxidation of Ti film by Zwilling and colleagues,238,239 several groups started working on the fabrication of TiO2 nanowires using the anodization method.196,240–261 HF/NH4F/KF/NaF/(NH4)2SO4/NaHSO4 diluted in organic solvent or water were taken as the electrolyte in standard two-electrode or three-electrode electrodeposition system with Pt as counter electrode and sample (Ti foil or Ti-coated substrate) as anode (along with a reference electrode for three-electrode system). Highly ordered, oriented hollow nanotubes with very high aspect ratio can be obtained by this method. It should be noted that for aqueous HF/NH4F used as electrolyte, the length of the nanorods is rather small, which is due to the dissolution of formed TiO2 under hydrogen ions according to the following reaction process:
| (4) |
This has been overcome by using nonaqueous organic polar electrolytes252 to decrease the formation of hydrogen ions that are derived from the electrolyte solution, thus reducing the chemical dissolution of formed TiO2. Using this method TiO2 nanotube arrays of ~1000 μm in length and approximate 10,000 aspect ratio have been achieved.252
It is also noteworthy that for most of the anodization processes, commercially available Ti foil was used as the source material. Similarly, for PAM-directed growth of the TiO2 nanowires, the host PAM is fabricated from either Al foil or anodiscs purchased from commercially available sources. Since the as-synthesized nanowires/nanotubes are not supported by any rigid substrate (Si, glass, quartz, and so on), they are fragile and unsuitable for practical device applications. For solid-state device compatibility as well as for some specific device applications in solar cells, field emission studies and sensor applications using vertically standing nanorods supported by rigid substrate are very important.263–265 Very few groups reported the syntheses of TiO2 nanowires/rods on rigid substrates.210,237,247,253 These groups have used indium tin oxide (ITO)/fluorine-doped tin oxide-coated glass/Si substrate with sputter-deposited/evaporated Ti thin films on them as the source materials.
For other chemical-based TiO2 nanowire syntheses, Venkataramanan and colleagues266 used an environmentally benign approach for the synthesis of titania nanowire using natural fibers (cellulose) as templates and ionic liquid (1-butyl-3-methylimidazolium chloride) as solvent to obtain a TiO2-nanowire/cellulose composite. Li and Wang267 synthesized rutile TiO2 nanowhiskers by direct annealing of a precursor powder containing homogeneously mixed NaCl and Ti(OH)4 particles. Daothong and colleagues268 fabricated single-crystalline TiO2 nanowires by oxidation of titanium substrates (wire mesh, ϕ = 0.25 mm) in the presence of ethanol vapor at a low pressure (10 Torr) and high temperature (450°C). Kim and colleagues269 used a peptide organogel template to fabricate TiO2 nanonetwork via atomic layer deposition (ALD) process with TTIP and NH3/O2 mixed gas as Ti precursor and reactant gas, respectively. Sander and colleagues270 also used ALD to fabricate TiO2 nanowires within PAM followed by wet etching of the membrane to obtain oriented nanowires. Park and colleagues271 used a simple Cu catalyst-assisted thermal annealing process of Ti foil to obtain TiO2 nanowire and other nanostructures. Wu and Xue272 also used a thermal annealing technique of Ti foil treated with an organic solution containing H2O2.
Vacuum-based physical techniques
Among physical techniques for the syntheses of TiO2 nanowires, various methods have been adopted which include thermal/e-beam evaporation, deposition of Ti and/or TiO2 in a vacuum evaporator, or RF heating in a controlled atmosphere.273–280 Xiang et al273 thermally evaporated Ti powder in a controlled furnace to deposit TiO2 nanowire on a Si substrate. By controlling the growth conditions such as the reaction time and the position of the substrate, this group has reported the synthesis of SiO2/TiO2 shell-core nanostructure.274 Another group275–278 used a two-step thermal evaporation technique, where Ti powder was heated by a radio frequency coil inside a quartz reactor under Ar-diluted O atmosphere to grow TiO2 nanowires on a Si substrate. On the other hand Wolcott and colleagues279 used electron-beam evaporation of TiO2 powder to synthesize nanowires on glass substrates. Other vacuum-based techniques include CVD of TiO2 nanowhiskers from a system of TiF4−H2O at elevated temperatures.280 Francioso and colleagues281 used photolithography techniques to fabricate TiO2 nanowires from thermally annealed TiO2 thin-film deposited on Si substrate. The fabrication steps consist of TiO2 thin-film deposition on Si substrate (in fact on a native oxide layer on the Si substrate) followed by spin coating of photoresist and the pattern transfer via masking and UV exposure. After the development, the TiO2 surface becomes structured with covered photoresist consisting of patterned holes that partially expose the TiO2 surface underneath via the holes on the photoresist. Finally, a plasma-etching treatment was performed to etch the exposed parts of the TiO2 layer vertically downward to obtain 1-dimensional nanostructures of TiO2. After the removal of the photoresist, an array of vertically standing TiO2 nanowires on the Si substrate is obtained. Details of the fabrication procedure can be found in Francioso et al.281
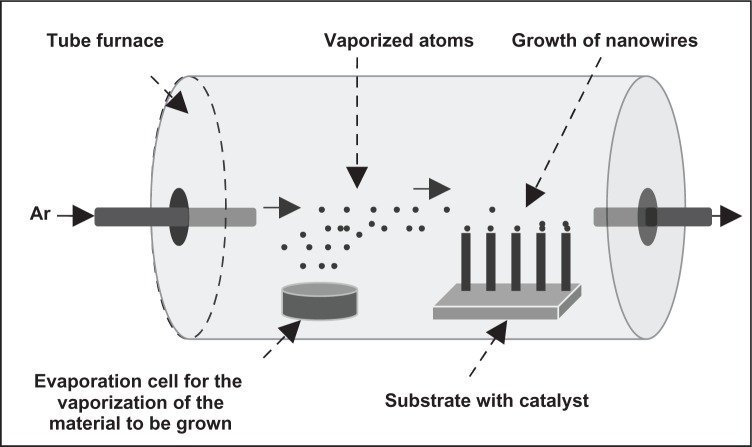
Lee and colleagues282 used vapor–liquid–solid (VLS) growth of TiO2 nanowires on catalyzed (Au) sapphire and quartz substrates using a thermally evaporated Ti powder as source under Ar-diluted O2 as carrier and reactive gases. It is to be noted that the VLS growth technique is a relatively new process, which is mainly used to grow nanowires/rods/whiskers of various elemental and compound materials.283 Generally, nanowires of semiconducting materials are conveniently grown via the VLS process, where material from the vapor is incorporated via a liquid catalyst, commonly a low-melting eutectic alloy. Semiconductor nanowires with diameters ranging from a few to several hundred nanometers can be grown on a solid substrate by this method. By modifying the growth conditions, lateral and longitudinal control over the nanowire size, composition, and doping can be achieved. The central idea of the VLS growth technique is the participation of the catalyst during growth process, which is one of the important key factors for the synthesis of nanowires. Another important factor is to keep the catalytic particles in a liquid state during the VLS growth at high temperatures. In general, the VLS growth process can be divided into two stages: the nucleation and the growth of the liquid droplets, and growth of the nanowires from the droplets due to supersaturation by the VLS mechanism. The temperature should be kept high, but the exact temperature will depend on the catalyst used and can be chosen from the phase diagram of the catalytic material, considering the fact that the melting point of nanosized catalytic particles is less than that of bulk material. For example, in VLS growth of TiO2 nanowires, Au catalyst is used at 1050°C.282 A VLS apparatus, in general, consists of a tube vacuum furnace with Ar flow through it, an effusion cell to provide the vaporized material (to be grown as nanowires), and the substrate with metallic catalyst on which the required nanowire will be grown. A schematic representation of the system is shown in Figure 5. The diameter of the wires is set by the catalyst dimension (typically 1–100 nm), and the nanowire length (typically 1–100 μm) is proportional to the growth time. During growth, the material to be deposited (here Ti powder) is provided by thermal evaporation of a powder target. The resulting vapor is transported to the substrate in an argon (and oxygen for reactive deposition) flow. The vapor dissolves in the metal particles (Au catalyst) and forms a eutectic mixture. When the liquid particle becomes oversaturated, the crystalline nanowire (TiO2) starts to grow. Because new vapor is supplied continuously, the nanowire will keep on growing and can reach lengths exceeding 100 μm. The growth mechanism is schematically described in Figure 6.
Figure 5.
Schematic design of a VLS system.
Figure 6.
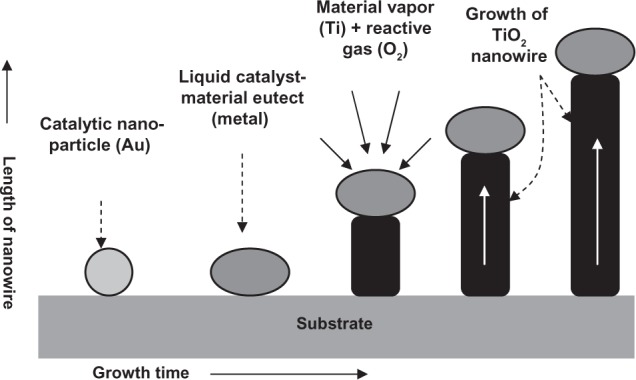
Mechanism of vapor–liquid–solid growth of TiO2 nanowires.
Other TiO2 nanostructures and nanocomposites
Formation of various other nanostructures of TiO2 includes nanoplatelet via hydrothermal/anodization route,255,284 porous nanofoam via direct decomposition and reaction of hydrogen peroxide inside a TiO2/hexadecylamine slurry dispersion,285 and nanocrystalline thin film via spray deposition of TiO2 nanoparticles178. As far as nanocomposites of TiO2 with other organic/inorganic materials are concerned, TiO2 at narrowgap semiconductor materials garnered much interest in dye-sensitized TiO2-based solar cells.144,286–296 Narrow band-gap semiconductors such as CdS, CdSe, CdTe, PbS, Bi2S3, and CuInS2, are used with TiO2 nanomaterials, because these narrow-gap materials absorb light in the visible region and transfer electrons to large band-gap TiO2, and thus serve as sensitizers. The fabrication of core-shell or similar composite structures generally follows chemical/hydrothermal syntheses of TiO2 core followed by the narrow-gap nanoparticles deposition via chemical bath deposition/spray pyrolysis/CVD/ALD/solvothermal methods. Cheng and colleagues297 reported the fabrication of highly ordered WO3/TiO2 composite nanotubes via a combinatorial PAM-based sol–gel method. Brinley et al298 reported SiO2−TiO2 hybrid antireflective coating via a sol–gel process. For enhancement of photocatalytic activity of TiO2, various groups reported the syntheses of TiO2-activated carbon or TiO2–CNT mixture or nanocomposite, which limit electron-hole recombination299–306 and thus improve reactivity. The syntheses of TiO2/CNT nanocomposites include sol–gel, CVD, and PVD techniques.305,307–309 In addition, TiO2-polymer nanocomposite gained renewed interest to create novel organic/inorganic hybrid materials for improved photocatalytic activity, water/air purification, or bactericidal antifouling. Several different methods have been adopted to integrate TiO2 with target materials, which include self-assembly monolayer adsorption on functionalized surfaces, sol–gel synthesis, vacuum vaporization, sputtering, CVD/MOCVD, Langmuir–Blodgett method, ultrasonic irradiation, enzymatic synthesis, or surface-initiated polymerization.310–319
Applications of nanostructured TiO2
Fundamentals of TiO2 photoinduced phenomena
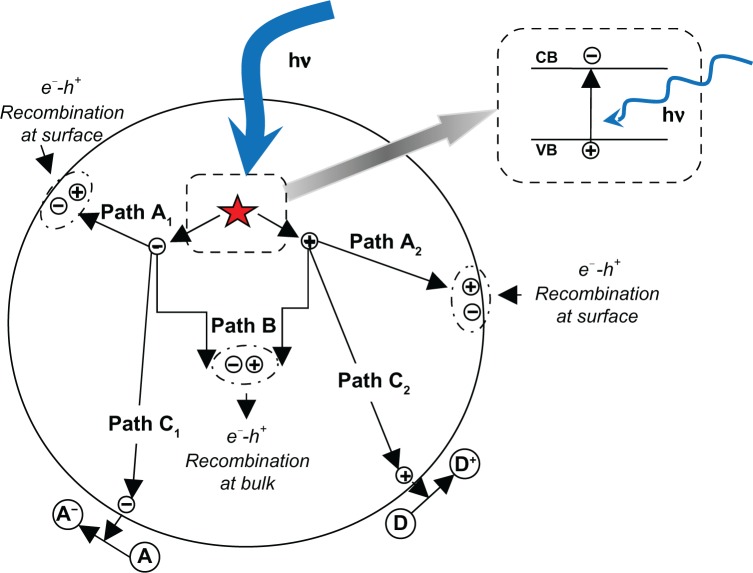
After the pioneering report of Fujishima and Honda on the photocatalytic splitting of water on TiO2 electrodes,64 a new era in the heterogeneous photocatalysis has begun and tremendous research efforts in understanding the fundamental processes and in enhancing the photocatalytic efficiency of TiO2 have been performed by chemists, physicists, materials scientists, chemical engineers, and others. The large oxidizing power of photogenerated holes in titania coupled with the low cost and relative physical and chemical stability of TiO2 render it the semiconductor material of choice for many applications that exploit solar energy including DSSCs where sunlight is converted into electricity, water photoelectrolysis where solar energy is converted into a chemical fuel (hydrogen), photocatalytic conversion of CO2 to hydrocarbon fuels, and as a photocatalyst where organic pollutants are degraded into more environmentally friendly chemical species.320–326 All of these applications require TiO2 to be in contact with a solid, liquid, or gaseous electrolyte; consequently, TiO2 becomes a prime candidate for the development of nanoscale architectures. Titania is a wide band-gap semiconductor having an Eg-value ranging from 3.0 to 3.2 eV, which depends on the crystalline phase,327 capable of converting energy from light into chemical redox energy. A photon with energy equal to or higher than that of the band gap transferred an electron from the VB to the CB leaving a hole to VB (cf, enlarged portion of Figure 7). The number of photogenerated electron-hole pairs (e−−h+) depends on the semiconductor band structure and effective intensity and energy of the incident light. The initial process for heterogeneous photocatalysis of organic and inorganic compounds by semiconductors is this photogeneration of e−−h+ pairs in the semiconductor particles. Upon excitation, the fate of the separated electron and hole can follow different pathways. Figure 7 illustrates these de-excitation pathways for the electrons and holes.
Figure 7.
Schematic representation of various de-excitation pathways for photogenerated electron and holes in a TiO2 particle (adapted and redrawn). Kamat PV. Meeting the clean energy demand: nanostructure architectures for solar energy conversion. J Phys Chem C. 2007;111(7): 2834–2860.345
Abbreviations: CB, conduction band; VB, valance band; hν, photon energy of frequency ν.
In the absence of suitable electron or hole scavenger (adsorbed species as charge carrier trapping site), the charge carriers recombine at the surface (cf, path A1/A2 of Figure 7) and/or bulk of the semiconductor (cf, path B of Figure 7) to dissipate (heat) energy. On the other hand, when a suitable scavenger is available, the charges migrate to the surface of the TiO2 particle and initiate interfacial redox reaction with the adsorbed species. Thus, TiO2 photosensitizes the reduction of an electron acceptor (A) and the oxidation of an electron donor (D) forming anionic (A−) (cf, path C1 of Figure 7) and cationic (D+) (cf, path C2 of Figure 7) species. In nanostructured semiconductors, the surface-to-volume ratio is very high, and hence the reactive surface is also higher in nanomaterial regimes. Therefore, the surface adsorption and interfacial redox reaction can be enhanced by using nanostructured semiconductors.328 This semiconductor-assisted redox reaction is at the core of the heterogeneous photocatalysis. Photocatalysis is generally divided into two classes of processes: 1) when the initial photoexcitation occurs in an adsorbate molecule which then interacts with the ground state catalyst substrate, the process is referred to as a catalyzed photoreaction, and 2) when the initial photoexcitation takes place in the catalyst substrate and the photoexcited catalyst then transfers an electron or energy into a ground state molecule, the process is referred to as a sensitized photoreaction.61 In the subsequent de-excitation processes, which leads to chemical reactions in the heterogeneous photocatalysis process (as mentioned earlier), the electronic population change in the molecular orbitals leads to the different interactions between one reactive center (a reactive center is a molecule or a surface reactive site) in the excited state and another reactive center in the ground state. Generally, this de-excitation process can take place in two forms: 1) electron transfer and 2) energy transfer. Figure 8 schematically illustrates the different interactions between one reactive center in the excited state and another reactive center in the ground state.
Figure 8.
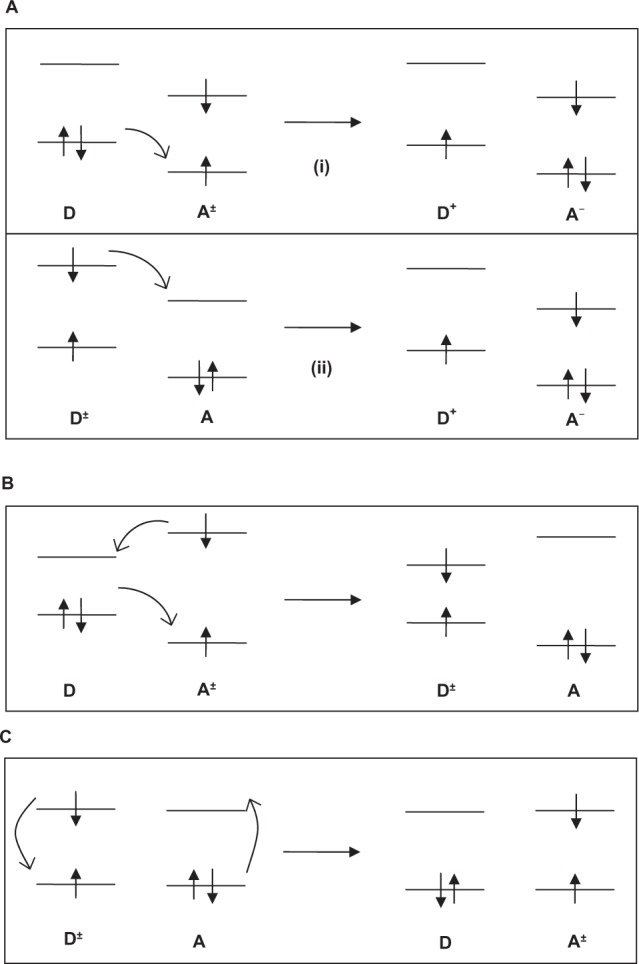
Various electron transfer and energy transfer processes of de-excitation of photogenerated e−−h+ pairs (adapted and redrawn). Kamat PV. Meeting the clean energy demand: nanostructure architectures for solar energy conversion. J Phys Chem C. 2007;111(7): 2834–2860.345
Initially, the donor or acceptor molecules are excited as D → D* or A → A*, where the asterisk represents the excited state. Next, in the electron transfer process, an electron jumps from an occupied orbital of the donor reactant at ground state (D) to the empty orbital of the acceptor reactant at excited state (A*). The electron transfer process requires the overlap between the occupied donor orbital and the empty or half-filled acceptor orbital. The electron transfer results in an ion pair of the donor cation (D+) and the acceptor anion (A−) according to the following equation:
| (5) |
Figure 8A describes the electron transfer from filled ground state donor orbital to half-filled excited acceptor orbital i) or from half-filled excited donor to empty ground state acceptor ii) to form ion pairs (A− + D+). On the other hand in the energy transfer process, either electron exchange between ground D and excited A* (cf, Figure 8B) or dipole-dipole resonant coupling between ground D and excited A* and vice versa (cf, Figure 8C) are considered to be the responsible mechanisms for de-excitation of photogenerated e−−h+ pairs. In the electron exchange process, one electron transfers from the ground state-filled D to half-filled A* coupled with simultaneous transfer of another electron from the upper level of half-filled A* to the empty upper level of D. These two processes occur independently in opposite directions and require simultaneous orbital overlap between the interacting centers. On the other hand, dipole–dipole coupling occurs by a Coulombic resonance interaction in which the oscillating dipole of the excited state molecule, D*, is coupled with the induced dipole in a ground state quencher molecule, A. This coupling process does not require effective orbital overlap between the two interacting centers and can operate over a distance of 0.1–10 nm. A detailed discussion of various electron and energy transfer processes can be found in the literature.329–339
The ability of TiO2 to undergo photoinduced electron transfer to adsorbed species on its surface is governed by the band energy positions of the semiconductor and the redox potentials of the adsorbate. Mills and Hunte340 discuss band-gap values and the redox (reduction) potential of some oxide and other semiconductor materials with respect to the normal hydrogen electrode (NHE). The CB electron of TiO2 is a moderate reducing agent (EO ~ 0.0 V vs NHE), whereas the oxidizing power of VB hole is very strong (EO ~ 3.0 V vs NHE).341 Thermodynamically the redox potentials associated with the CB and VB of TiO2 require the relevant potential level of the acceptor species to be below the CB potential of the TiO2 (ie, more positive), whereas in order to donate an electron to the vacant hole, the potential level of the donor needs to be above the VB position of TiO2 (ie, more negative). Therefore, assuming no kinetic limitations, TiO2-assisted photoinduced interfacial redox reaction will take place with acceptors and donors whose respective reduction potentials are comprised between CB and VB band positions of TiO261,341 It is noteworthy that when the TiO2 surface will be under contact with any fluid (gas/liquid) or metal, there will be band bending at the interface due to the redistribution of the charges (and double layer and Schottky barrier formation). For an n-type semiconductor like TiO2, the band bending will be in the upward direction. Due to this redistributed band structure at the surface, e−−h+ recombination process will be affected and hence, modify the photocatalysis activity of TiO2.
Among anatase and rutile crystal structures of TiO2, anatase shows a higher photocatalytic activity.342 The structure of rutile and anatase are shown in Figures 3 and 4 and described in Table 1. The two crystal structures differ by the distortion of each octahedron and by the assembly pattern of the octahedra chains. These differences in lattice structures cause different mass densities and electronic band structures between the two forms of TiO2 that lead to different photo-activities of these two structures.
Photolysis on TiO2-solar hydrogen
Photolysis or photosplitting of water into gaseous O2 and H2 represents one of the most challenging and promising ways of solar energy accumulation, as solar hydrogen can then be used as an alternative fuel. Numerous comprehensive studies343–354 have been performed since the first report of photocatalytic water splitting on TiO2 surface was published in 1972.64 The band energy position of TiO2 relative to the electrochemical potentials of the H2/H2O and O2/H2O redox couples61,355 demonstrates that due to the presence of a large overpotential for the evolution of H2 and O2 on the TiO2 surface, TiO2 alone cannot photodecompose H2O; rather the photoassisted oxidation of the oxygen vacancy sites on a reduced TiO2 surface is responsible for H evolution.61,356
TiO2-assisted photoelectrolysis of water is achieved using a close circuit photoelectrochemical cell that consists of a TiO2 anode and a Pt counter electrode and is exposed to near-UV light.64,357 Generation of the photoelectrochemical e−−h+ pair in TiO2 is followed by the transfer of electron to the Pt electrode and reduction of water molecule to evolve H2 according to the following reaction:
| (6a) |
whereas at the anode, water oxidation takes place according to following reaction:
| (6b) |
Therefore, the overall photosplitting of water will be given as follows:
| (6c) |
Sometimes the system requires some external electrical (>0.25 V) or chemical (alkali in anode half cell and/or acid in cathode half cell) bias to increase efficiency.320–324,340,357
In another variation, solar hydrogen is generated by mixing TiO2 powders with noble metals (Pt, Ag, and Au) and oxide (RuO2) particles. Here, this system behaves as a microphotoelectrochemical cell in which the metal (Pt) acts as the cathode and oxide (RuO2) as the anode. Band-gap excitation in the TiO2 injects electrons into the Pt particles and holes into the RuO2 particles. Trapped electrons in Pt reduce water to hydrogen, and trapped holes in RuO2 oxidize water to oxygen.358 Various ways of modifying TiO2 particles have been reported such as metal ion or anion doping, metal ion implantation, dye sensitization, addition of sacrificial or other components to the electrolyte, and so on. However, all these processes produce a very low rate of water splitting.344,346 Various measures have been taken to increase the rate of hydrogen evolution over TiO2 surfaces such as using artificial high-power UV light sources and/or nanostructured materials.349 However, the energy conversion efficiency on TiO2 was rather low and the reasons for this are 1) fast recombination of photogenerated electrons and holes, 2) fast backward reaction, 3) inability to harvest visible and IR light at longer wavelengths than ~400 nm, and 4) less effective surface area.344
Surface modification of the TiO2 nanostructure
Apart from photosplitting of water, other photochemical activities of TiO2 include adsorption and desorption of O2, CO2, CO, halides, or various organic compounds on nanostructured TiO2 surface359–373 and photo-oxidation/reduction of molecular nitrogen, NO2, NH3, CO2, or halides.374–389 Photocatalysis being a surface phenomenon, surface area is very important in determining the amount of reaction sites, and charge carriers have to be utilized properly to improve their ability to initiate surface reactions. On the other hand TiO2 crystallinity should be high to prevent the recombination of e− and h+. Highly crystalline TiO2 prevents the recombination of charge carriers relative to amorphous and less crystalline TiO2. As mentioned earlier, because the energy conversion efficiency on TiO2 depends on several factors such as surface area, e−−h+ recombination rates, solar energy spectrum, and so on, as a working hypothesis, surface area, and crystallinity of the TiO2 powder have to be improved for efficient photocatalysis.
Modification of the surface area of TiO2 is performed by using nanostructured materials. Nanoparticulate electrodes are commonly used for these purposes, which consist of a several micrometer-thick film consisting of a random 3-dimensional network of interconnected 15–20 nm particles. While these electrodes possess a high surface-to-volume ratio, the structural disorder at the contact between two crystalline particles leads to an enhanced scattering of free electrons, thus reducing the electron mobility390 On the other hand the nanotube array architecture, being ordered and strongly interconnected eliminates randomization of the grain network and increases contact points for good electrical connection. The nanotube arrays have a large internal surface area and can be easily filled with fluids, thus enabling higher contact with electrolytes. The porosity of the ordered structure allows the incident photons to be more effectively absorbed than on a flat electrode.328 Details of the fabrication processes of various nanostructured TiO2 have been discussed previously. Most of these processes used calcination/annealing steps for liquid-phase syntheses or elevated-temperature gas-phase syntheses methods to obtain high crystalline TiO2 nanostructures for improved photocatalytic activities.
Doping in nanostructured TiO2 for improved photoactivity
Doping of TiO2 nanostructures is another method of improving photoactivities of TiO2. Loading of TiO2 surface with noble metals and/or metal oxides (RuO2, ZnO, WO3, SnO2) creates low-energy states within TiO2 that trap photogenerated charge carriers and thus prevent charge recombination which increases the photoactivity of TiO2. On the other hand, substitutional doping is very important for reducing the band gap of TiO2 nanostructures to utilize the wider fraction of solar radiation, especially the visible and near infrared (NIR) parts.391 Much effort has been expended to narrow the TiO2 band gap by compositional doping. According to the crystal structure of TiO2, it appears that replacement of Ti4+ with any cation is relatively easier than to substitute O2− with any other anion due to the difference in the charge states and ionic radii. Cationic doping of TiO2 with transition and rare earth metals (such as Cu, Zr, Zn, Co, Ni, Cr, Mn, Mo, Nb, V, Fe, Ru, W, Sb, Sn, Au, Ag, Pt, La, Ce, Er, Pr, Gd, Nd, or Sm) has been extensively studied.177,392–405 While several authors have reported that transition metal ion doping decreases the photothreshold energy of TiO2, there is also an increase in thermal instability and a decrease in carrier lifetimes,398,399 which limits overall conversion efficiencies.
For nonmetal doping of TiO2 in anionic site (oxygen), wide varieties of anionic species (N, S, C, B, P, I, or F) have been used. This approach consists of substitution of a nonmetal atom for oxygen.152,405–419 The methods used to introduce the dopants include wet-chemical, electrochemical, and physical methods, which have been described in detail in the previous sections and related references.420–425 Theoretical calculations show that band-gap narrowing originates from the electronic perturbations caused by the change of lattice parameters and/or by the presence of the trap states within conduction and valence bands of TiO2.426,427 Although there is wide consensus that anionic doping produces enhancement in the visible activity of TiO2 photocatalysts, it is a matter of debate whether this anionic doping is really achieving the necessary band-gap narrowing in TiO2 to the extent to be really useful in practical applications.328 Recent studies showed that the visible light activity of TiO2 can be further enhanced by codoping of suitable combination of metals and/or nonmetal ions.405,418,419,428–431
Nanocomposites of TiO2 with semiconductors having lower band-gap energy such as CdS, CdSe, CdTe, PbS, Bi2S3, CuInS2, and so on, which absorb light in the visible region of solar spectrum, can serve as sensitizers because they are able to transfer electrons to large band gap of TiO2.144,286,288,291–294,328,432–435 However, fabrication of metal-chalcogenite/metal oxide (TiO2) composite is somewhat challenging as oxygen intercalation to the chalcogenite sites may alter the band gap of the system and deteriorate photoactivity. Alternatively, metal oxides, such as CuO, Cu2O, Fe2O3, WO3, MoO3, ZnO, SnO2, and so on, have been considered for band-gap engineering of TiO2 as these oxides have compatible processing strategies with TiO2.297,328,436–444 Among these oxides, low band-gap CuO or Cu2O are used as sensitizers to use visible radiation, whereas other large band-gap oxides (eg, ZnO, SnO2) are coupled with TiO2 for extrinsic trapping of photogenerated charge carriers to enhance photoactivity. Among these, coupling TiO2 with SnO2 attracts much attention. The band gaps of SnO2 and TiO2 are 3.88 and 3.2 eV, respectively, and the CB edge of SnO2 is ~0.5 V above that of TiO2. When the two semiconductor particles are coupled, the CB of SnO2 acts as a sink for photogenerated electrons. Since the photogenerated holes move in the opposite direction, they accumulate in the VB of the TiO2 particle, which increases the efficiency of charge separation. Recently, a nanocomposite of TiO2 (anatase)/CNT has been reported to show enhanced photoreactivity by reducing charge recombination at TiO2 surface.299,302–306 It has been observed that single-walled (SW) CNTs can more effectively reduce the charge recombination rate than that of multiwalled (MW) CNTs as the contact area for SWCNT/TiO2 is much higher than that of MWCNT/TiO2 (cf, Figure 9A), enhancing the charge transfer.299 A simple mechanism has been proposed by Yao and colleagues299 to qualitatively explain the reduced charge recombination rate in TIO2/CNT nanocomposite (Figure 9B). Physically, the CB edge of TiO2 is −4.21 eV lower than vacuum level with a band gap around 3.2 eV, whereas work function of (SWCNT) is −4.8 eV (with respect to vacuum level), with a narrow band gap ranging from 0.0 to 1.1 eV.445 Due to this relative position of CB edges between TiO2 and CNTs, the probability of transfer of the (photogenerated) electrons to the CNTs becomes much higher, leading to a higher lifetime for holes, which consequently enhances its photocatalytic activities.
Figure 9.
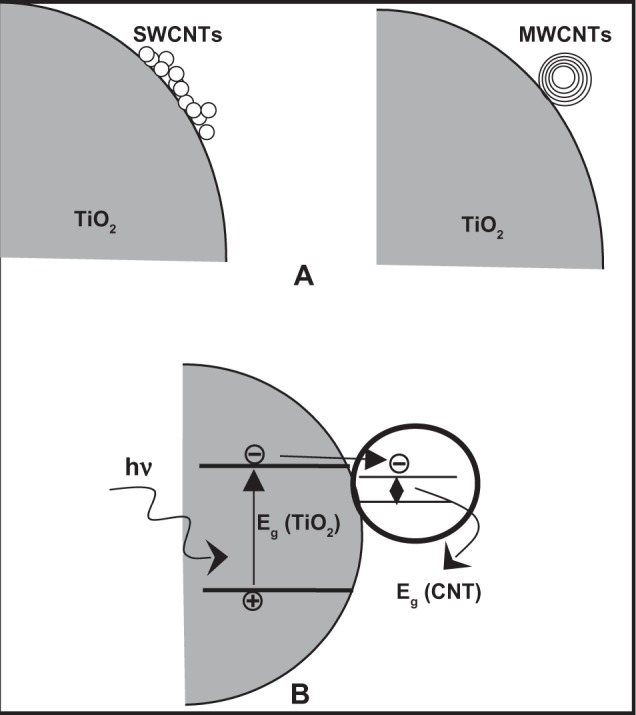
Schematic representation of TiO2/SWCNT and TiO2/MWCNT nanocomposite structures, (b)proposed model for reduction in photogenerated electro-hole recombination in TiO2/CNT nanocomposites (adapted and redrawn). Schnitzler DC, Zarbin AJG. Organic/inorganic hybrid materials formed from TiO2 nanoparticles and polyaniline. J Braz Chem Soc. 2004; 15(3):378–384.315
Abbreviations: CNT, carbon nanotubes; MW, multiwall; SW, single wall.
TiO2-based DSSCs
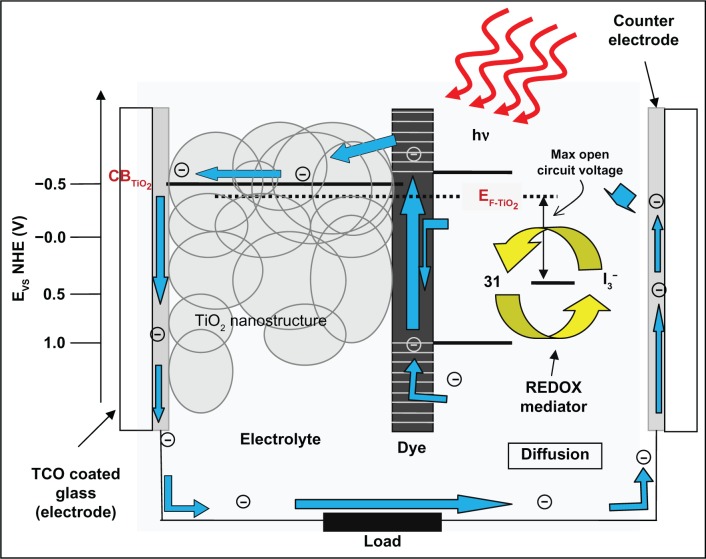
DSSCs have attracted much interest as regenerative low-cost alternatives to conventional solid-state devices due to high energy conversion efficiency and the possibility of large scale production.388,352,446 A DSSC consists of a nanoporous film prepared from nanoparticles of a wide-gap metal oxide (typically TiO2) covered with a monolayer of photosensitizer molecules (a dye, typically ruthenium complexes anchored to the TiO2 surface by a carboxylate bipyridyl ligand) and an electrolyte as a hole transport layer containing redox couples (iodide/tri-iodide). The cell is illuminated through a transparent conducting oxide (TCO) electrode (typically ITO-coated glass) where the semiconductor is deposited. The TiO2 pores are filled with the redox electrolyte which acts as the mediator and establishes electrical contacts with a redox catalyst in the counter electrode.447–451 The dyes on the surface of the films absorb light and inject electrons into the CB of the metal oxide. To collect the electrons at a TCO layer, the electrons should travel the distance in the CB from the point where the electrons were injected to the TCO before recombination. A schematic representation of a typical DSSC is shown in Figure 10. Photoexcitation of the dye (sensitizer) is followed by electron injection into the CB of the TiO2 nanostructure. The dye molecule is regenerated by the redox system, which itself is regenerated at the counter electrode by passing electrons through the load. Potentials are referred to the NHE. The open-circuit voltage of the solar cell corresponds to the difference between the redox potential of the mediator and the Fermi level of the nanocrystalline TiO2 indicated with a dashed line. In the nanoporous TiO2 film, the surface is surrounded by more cations than the electrons, and therefore, it has been assumed that no large electric field gradient is present within the film; hence, the electron transport mechanism is considered to be purely diffusive.452–454
Figure 10.
Schematic representation of principle of operation and energy level scheme of the dye-sensitized nanocrystalline solar cell (adapted and redrawn). Luo H, Takata T, Lee Y, Zhao J, Domen K, Yan Y. Photocatalytic activity enhancing for titanium dioxide by co-doping with bromine and chlorine. Chem Mater. 2004;16(5):846–849.482
Note: The diagram is not drawn to the scale.
DSSCs differ from solid-state p–n junction for the fact that light absorption and the transport occur separately. The process is described through equations 7a through 7d:449
| (7a) |
| (7b) |
| (7c) |
| (7d) |
The light absorption takes place in a monolayer of the photosensitizer (S), which is chemically adsorbed on the surface of TiO2 (Eq. 7a). The photoexcited dye (S*) is then able to transfer electrons into TiO2 (Eq. 7b), which moves toward TCO electrode through the TiO2 porous network. Electrons lost by the sensitizer are recovered using the reduced species present in the electrolyte (I−) (Eq. 7c). Finally, the circuit is closed by the oxidized species of the mediator (I3−) that obtains electrons which flows through the counter electrode through the load (Eq. 7d).
Other photocatalytic applications of nanostructured TiO2
Other photocatalytic activities of TiO2 involve photosensitized oxidation of organic pollutants using TiO2 for environmental remediation, wastewater treatment, recovery of precious metal via TiO2-assisted reduction,325,326,340,341,455–457 organic synthesis,458 photokilling activity,146,356,459,460 or self-cleaning activity.340,356 Photosensitized oxidation of organic pollutants in the aqueous and gaseous phases can be done according to the following reaction:
| (8) |
Similarly, TiO2-assisted organic syntheses comprise 1) oxidation of alkenes substituted with phenyl groups to yield corresponding ketones
| (9) |
2) oxidation of substituted toluene to corresponding acetaldehyde and carboxylic acid, and 3) amine intra N-alkylation.340 On the other hand, the biggest success of TiO2-based photochemistry is probably the self-cleaning and related phenomena. For example, photoactive TiO2 coated on ceramic or glass undergoes a dual action,340 where on one hand TiO2-assisted photo-oxidation takes place to break down the organic compounds (cf, Eq. 8); on the other hand, simultaneous TiO2-assisted, UV-photoinduced superamphiphilicity (forcing detachment of dust and/or organics) takes place followed by a quick drying process.356,461 Photoinduced superamphiphilicity of TiO2 exhibits high quantum yield at low UV radiation intensities and, therefore, can be exploited efficiently within an indoor setup when illuminated with conventional fuorescent light. Major glass and ceramic manufacturers have already commercialized self-actuating materials based on this superamphiphilicity of TiO2 in automobile and/or bathroom mirrors, or self-cleaning windows/panels.462,463
Conclusions
A comprehensive review of the properties, fabrication, and application of nanostructured TiO2 has been presented in detail, especially the syntheses procedures of 0-, 1-, and quasi-2-dimensional nanostructures of TiO2 via various physical and chemical techniques. Synthesis strategies regarding nanocomposites of TiO2 with other inorganic and organic materials and applications of TiO2 in terms of photocatalytic activities has been reviewed. The basic mechanisms of photoactivities of TiO2 and nanostructures were explored. The characteristics of band-structure engineering and surface modification in nanostructured TiO2 in the context of doping, including elemental and compound materials doping as well as nanocomposite formation with various inorganic/organic materials were discussed. The nanostructured TiO2-assisted photocatalytic energy/fuel generations in terms of solar hydrogen and/or DSSCs have also been discussed in detail. Other photochemical activities of nanostructured TIO2 such as wastewater treatment, organic synthesis, photokilling, and self-cleaning activities have also been discussed briefly. TiO2 nanomaterials are of tremendous interest in a wide range of applications such as photocatalysis, DSSCs, gas sensors, photochromic devices, photodegradation of organic compounds, deactivation of microorganisms, organic synthesis, and cell culture. It will not be an exaggeration to say that the next decade will see the renaissance of TiO2-based nanomaterials, and various new, interesting, and novel technological applications are on the verge of exploration.
Future directions
In recent years, TiO2-based photocatalytic oxidation of organic compounds has received the most attention, but there is a rapidly increasing focus on the oxidation of volatile organic or inorganic compounds in the gas phase, including NOx and SOx. Photocatalytic reductions of organic compounds and metal-containing ions and studies on cell killing and disinfection by illuminated TiO2 have also received increasing attention. The current problem with doped TiO2 is the loss of photoactivity during recycling and long-term storage. Future research should be focused on this issue to obtain sustained photoactivity in the long run. Photoactivity of metal-doped TiO2 under visible light strongly depends on preparation methods. A uniform synthetic strategy is the need of the hour to obtain tailored materials for high-efficient UV-Vis photore-sponse. In the case of nonmetal-doped TiO2, the main problem is that its photocatalytic activity under visible light is much lower than that under UV radiation. Therefore, development of new and optimization of the existing photocatalysts exhibiting activity upon visible light with surface characteristics of improved performance and of the high chemical and physical stability are crucial for broader scale utilization of photocatalytic systems in commercial applications. Such materials together with the development of technically applicable self-aligning photocatalytic coating systems adaptable to the major substrates (polymers, glass, ceramics, or metals) will represent a ground-breaking step change in this field, particularly in the economic viability of a range of potential applications. One of the major challenges for the scientific and industrial community involved in photocatalytic research is to increase the spectral sensitivity of TiO2-based photocatalysts to visible and NIR regions. A major area of future research should be the development of new nanostructures of TiO2 with higher surface states, new commercially viable nano-TiO2 fabrication methodology, especially nonlithographic complementary metal–oxide–semiconductor compatible techniques for practical applications, new doping materials, new methods for dopant incorporation into TiO2 nanostructure, as well as new applications for environmental technology. Future research should deal with visible-to-NIR-activated TiO2 functioning in the presence of solar irradiation with predictable photoactivity. Finally, a recent report discusses the photocatalytic cancer-cell treatment using TiO2 nanoparticle,186 which opens up a very important field in nanobiotechnology for defective cell treatment. Very few groups have studied the biocompatibility of nanostructured TiO2.464–469 Since nanostructured TiO2 is extensively used in various applications such as dope, dyes, ceramics, cosmetics, and medicine, where contact with human and/or living cells is necessary, the study of the biocompatibility and biosafety of nano-TiO2 should be considered a very important area of future research.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Efros AlL, Efros AL. Interband absorption of light in a semiconductor sphere. Sov Phys Semicond. 1982;16:772–775. [Google Scholar]
- 2.Brus LE. Electron–electron and electron–hole interactions in small semiconductor crystallites: the size dependence of the lowest excited electronic state. J Chem Phys. 1984;80:4403–4409. [Google Scholar]
- 3.Cox AJ, Louderback JG, Bloomfield LA. Experimental observation of magnetism in rhodium clusters. Phys Rev Lett. 1993;71(6):923–926. doi: 10.1103/PhysRevLett.71.923. [DOI] [PubMed] [Google Scholar]
- 4.Alivisatos AP. Semiconductor clusters, nanocrystals, and quantum dots. Science. 1996;271(5251):933–937. [Google Scholar]
- 5.Heath JR. The chemistry of size and order on a nanometer scale. Science. 1995;270(5240):1315–1316. [Google Scholar]
- 6.Feynman RP.There’s Plenty of Room at the Bottom Available from: http://www.zyvex.com/nanotech/feynman.htmlAccessed 2010 Jun 1
- 7.Demel T, Heitmann D, Grambow P, et al. Coupling of quantum dots on GaAs. Phys Rev Lett. 1990;64(21):2559–2562. doi: 10.1103/PhysRevLett.64.2559. [DOI] [PubMed] [Google Scholar]
- 8.Li Q, Wang C. Fabrication of wurtzite ZnS nanobelts via simple thermal evaporation. Appl Phys Lett. 2003;83(2):359–362. [Google Scholar]
- 9.Demel T, Heitmann D, Grambow P, Ploog K. One-dimensional plasmons in AlGaAs/GaAs quantum wires. Phys Rev Lett. 1991;66(20):2657–2660. doi: 10.1103/PhysRevLett.66.2657. [DOI] [PubMed] [Google Scholar]
- 10.Banerjee AN, Chattopadhyay KK. Recent developments in the emerging field of crystalline p-type transparent conducting oxide thin films. Prog Cryst Growth Charac Mater. 2005;50(1–3):52–105. [Google Scholar]
- 11.Klein DL, Roth R, Lim AKL, Alivisatos AP, McEuen PL. A single-electron transistor made from a cadmium selenide nanocrystal. Nature. 1997;389(6652):699–701. [Google Scholar]
- 12.Feldheim DL, Keating CD. Self-assembly of single electron transistors and related devices. Chem Soc Rev. 1998;27(1):1–12. [Google Scholar]
- 13.Tsuda N, Nasu K, Yanase A, Siratori K. Electronic Conduction in Oxides. Berlin, Germany: Springer-Verlag; 1991. [Google Scholar]
- 14.Rao CNR, Raveau B. Transition Metal Oxides. 2nd ed. New York, NY: Wiley/VCH; 1998. [Google Scholar]
- 15.Moulson AJ, Herbert JM. Electroceramics. 2nd ed. London, UK: Chapman and Hall; 1990. [Google Scholar]
- 16.Cox PA. Transition Metal Oxides. Oxford, UK: Oxford University Press; 1992. [Google Scholar]
- 17.Henrich VE, Cox PA. The Surface Science of Metal Oxides. Cambridge, UK: Cambridge University Press; 1994. [Google Scholar]
- 18.Noguera C. Physics and Chemistry at Oxide Surfaces. Cambridge, UK: Cambridge University Press; 1996. [Google Scholar]
- 19.Fukuyama H, Nagaosa N, editors. Physics and Chemistry of Transition Metal Oxides. Berlin, Germany: Springer; 1999. [Google Scholar]
- 20.Vegard L. Results of crystal analysis. Philos Mag. 1916;32:505–515. [Google Scholar]
- 21.Cronemeyer DC. Electrical and optical properties of rutile single crystals. Phys Rev. 1952;87(5):876–886. [Google Scholar]
- 22.Breckenridge RG, Hosler WR. Electrical properties of titanium dioxide semiconductors. Phys Rev. 1953;91(4):793–802. [Google Scholar]
- 23.Johnson GH. Influence of impurities on electrical conductivity of rutile. J Am Ceram Soc. 1953;36(3):97–101. [Google Scholar]
- 24.Cronemeyer DC. Infrared absorption of reduced rutile TiO2 single crystals. Phys Rev. 1959;113(5):1222–1226. [Google Scholar]
- 25.Grant FA. Properties of rutile (titanium dioxide) Rev Mod Phys. 1959;31(3):646–674. [Google Scholar]
- 26.Frederikse HPR. Recent studies on rutile (TiO2) J Appl Phys. 1961;32(10):2211–2215. [Google Scholar]
- 27.Barbanel VI, Bogomolov VN. Diffusion and defects in rutile during its partial reduction in a vacuum. Sov Phys Solid State. 1970;11:2160–2161. [Google Scholar]
- 28.Kofstad P. Note on the defect structure of rutile (TiO2) J Less Common Met. 1967;13(6):635–638. [Google Scholar]
- 29.Henzler M, Göpel W. Oberflächenphysik des Festkörpers. 2nd ed. Stuttgard (Germany): Teubner; 1994. [Google Scholar]
- 30.Ardakani HK. Electrical and optical properties of in situ “hydrogen-reduced” titanium dioxide thin films deposited by pulsed excimer laser ablation. Thin Solid Films. 1994;248(2):234–239. [Google Scholar]
- 31.Bogomolov VN, Kudinov EK, Mirlin DN, Firsov YA. Sov Phys Solid State. 1968;9:1630–1633. [Google Scholar]
- 32.Bogomolov VN, Firsov YA, Kudinov EK, Mirlin DN. On the experimental observation of small polarons in rutile (TiO2) Phys Stat Sol B. 1969;35(2):555–558. [Google Scholar]
- 33.Agekyan VT, Stepanov YA. Sov Phys Solid State. 1976;17:2390–2393. [Google Scholar]
- 34.Klinger MI. On the experimental observation of small polarons in semiconductors. Phys Stat Sol B. 1968;27(2):479–488. [Google Scholar]
- 35.Tang H. Electronic Properties of Anatase TiO2 Investigated by Electrical and Optical Measurements on Single Crystals and Thin Films. Lausanne, France: Département de physique, Ecole polytechnique fédérale de Lausanne EPFL; 1994. [Google Scholar]
- 36.O’Regan B, Grätzel M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature. 1991;353(6346):737–740. [Google Scholar]
- 37.Berger H, Tang H, Lévy F. Growth and Raman spectroscopic characterization of TiO2 anatase single crystals. J Cryst Growth. 1993;130(1–2):108–112. [Google Scholar]
- 38.Tang H, Prasad K, Sanjinés R, Schmid PE, Lévy F. Electrical and optical properties of TiO2 anatase thin films. J Appl Phys. 1994;75(4):2042–2047. [Google Scholar]
- 39.Sanjinés R, Tang H, Berger H, Gozzo F, Margaritondo G, Lévy F. Electronic structure of anatase TiO2 oxide. J Appl Phys. 1994;75(6):2945–2951. [Google Scholar]
- 40.Tang H, Lévy F, Berger H, Schmid PE. Urbach tail of anatase TiO2. Phys Rev B Condens Matter. 1995;52(11):7771–7774. doi: 10.1103/physrevb.52.7771. [DOI] [PubMed] [Google Scholar]
- 41.Hosaka N, Sekiya T, Satoko C, Kurita S. Optical properties of single-crystal anatase TiO2. J Phys Soc Jpn. 1997;66:877–880. [Google Scholar]
- 42.Sekiya T, Igarashi M, Kurita S, Takekawa S, Fujisawa M. Structure dependence of reflection spectra of TiO2 single crystals. J Electron Spectrosc Relat Phenom. 1998;92(1–3):247–250. [Google Scholar]
- 43.Sekiya T, Ichimura K, Igarashi M, Kurita S. Absorption spectra of anatase TiO2 single crystals heat-treated under oxygen atmosphere. J Phys Chem Solids. 2000;61(8):1237–1242. [Google Scholar]
- 44.Sittig CE. Charakterisierung der Oxidschichten auf Titan und Titanlegierungen sowie deren Reaktionen in Kontakt mit biologisch relevanten Modellösungen. Zürich, Switzerland: Eidgenössische Technische Hochschule ETHZ; 1998. [Google Scholar]
- 45.Clark W, Broadhead P. Optical absorption and photochromism in iron-doped rutile. J Phys C Solid State Phys. 1970;3(5):1047–1050. [Google Scholar]
- 46.Layman P. Outlook brightens for titanium dioxide following recent business pickup. Chem Eng News. 1996;74(4):13–14. [Google Scholar]
- 47.Radecka M, Zahrzewska K, Czternastek H, Stapiński T, Debrus S. The influence of thermal annealing on the structural, electrical and optical properties of TiO2−x thin films. Appl Surf Sci. 1993;(2):65–66. 227–234. [Google Scholar]
- 48.Lee S, Park BH, Oh SG. In-situ ellipsometric investigation of TiO2 thin-film initial growth. J Korean Phys Soc. 1997;31(2):352–356. [Google Scholar]
- 49.Thelen AJ. Design of Optical Interference Coatings. New York, NY: McGraw Hill; 1989. [Google Scholar]
- 50.MacLeod HA. Thin-Film Optical Filters. Bristol, UK: Adam Hiliger Ltd; 2001. [Google Scholar]
- 51.Siefering KL, Griffin GL. Growth kinetics of CVD TiO2: influence of carrier gas. J Electrochem Soc. 1990;137(4):1206–1208. [Google Scholar]
- 52.Harris AW, Ludden BP, Blaikley DCW. The production of titania thin film waveguides by dc reactive magnetron sputtering. Vacuum. 1992;43(1–2):143–147. [Google Scholar]
- 53.Orignac X, Vasconcelos HC, Du XM, Almeida RM. Influence of solvent concentration on the microstructure of SiO2−TiO2 sol–gel films. J Sol Gel Sci Technol. 1997;8(1–3):243–248. [Google Scholar]
- 54.Satake K, Katayama A, Ohkoshi H, Nakahara T, Takeuchi T. Titania NOx sensors for exhaust monitoring. Sens Actuators B Chem. 1994;20(2–3):111–117. [Google Scholar]
- 55.Tang H, Prasad K, Sanjinés R, Lévy F. TiO2 anatase thin films as gas sensors. Sens Actuators B Chem. 1995;26(1–3):71–75. [Google Scholar]
- 56.Baraton MI, Merhari L, Wang J, Gonsalves KE. Investigation of the TiO2/PPV nanocomposite for gas sensing applications. Nanotechnology. 1998;9(4):356–359. [Google Scholar]
- 57.Dai G. A study of the sensing properties of thin film sensor to trimethylamine. Sens Actuators B Chem. 1998;53(1–2):8–12. [Google Scholar]
- 58.Gao L, Li Q, Song Z, Wang J. Preparation of nano-scale titania thick film and its oxygen sensitivity. Sens Actuators B Chem. 2000;71(3):179–183. [Google Scholar]
- 59.Frank SN, Bard AJ. Heterogeneous photocatalytic oxidation of cyanide ion in aqueous solutions at titanium dioxide powder. J Am Chem Soc. 1977;99:303–304. [Google Scholar]
- 60.Sakata T, Kawai T. Heterogeneous photocatalytic production of hydrogen and methane from ethanol and water. Chem Phys Lett. 1981;80(2):341–344. [Google Scholar]
- 61.Linsebigler AL, Lu G, Yates JT., Jr Photocatalysis on TiO2 surfaces: principles, mechanisms, and selected results. Chem Rev. 1995;95(3):735–758. [Google Scholar]
- 62.Ying JY, Sun T. Research needs assessment on nanostructured catalysts. J Electroceram. 1997;1(3):219–238. [Google Scholar]
- 63.Dumitriu D, Bally AR, Ballif C, et al. Photocatalytic degradation of phenol by TiO2 thin films prepared by sputtering. Appl Catal B. 2000;25(2–3):83–92. [Google Scholar]
- 64.Fujishima A, Honda K. Electrochemical photolysis of water at a semiconductor electrode. Nature. 1972;238(5358):37–38. doi: 10.1038/238037a0. [DOI] [PubMed] [Google Scholar]
- 65.Ohko Y, Hashimoto K, Fujishima A. Kinetics of photocatalytic reactions under extremely low-intensity UV illumination on titanium dioxide thin films. J Phys Chem A. 1997;101(43):8057–8062. [Google Scholar]
- 66.Grätzel M, Kalyanasundaram K. Artificial photosynthesis: efficient dye-sensitized photoelectrochemical cells for direct conversion of visible light to electricity. Curr Sci. 1994;66:706–714. [Google Scholar]
- 67.Bonhôte P, Moser JE, Vlachopoulos N, et al. Photoinduced electron transfer and redox-type photochromism of a TiO2-anchored molecular diad. Chem Commun. 1996;(10):1163–1164. [Google Scholar]
- 68.Siebentritt S, Ernst K, Fischer CH, et al. CdTe and CdS as extremely thin absorber materials in an eta solar cell (ETA); 14th European Photovoltaic Solar Energy Conference; Barcelona, Spain. 1997. [Google Scholar]
- 69.Tennakone K, Kumara GRRA, Kottegoda IRM, Perera VPS, Aponsu GMLP. Nanoporous n-TiO2/selenium/p-CuCNS photovoltaic cell. J Phys D Appl Phys. 1998;31(18):2326–2330. [Google Scholar]
- 70.Möller J, Fischer CH, Siebentritt S, et al. CuInS2 as an extremely thin absorber in an eta solar cell; Proceedings of the 2nd World Conference and Exhibition on Photovoltaic Solar Energy Conversion; Vienna. 1998. [Google Scholar]
- 71.Tatsuma T, Saitoh S, Ohko Y, et al. TiO2−WO3 photoelectrochemical anticorrosion system with an energy storage ability. Chem Mater. 2001;13:2838–2842. [Google Scholar]
- 72.Wang R, Hashimoto K, Fujishima A, et al. Light-induced amphiphilic surfaces. Nature. 1997;388:431–432. [Google Scholar]
- 73.Bally AR, Prasad K, Sanjinés R, et al. TiO2: Ce/CeO2 high performance insulators for thin film electroluminescent devices. Mater Res Soc Symp. 1996;424:471–475. [Google Scholar]
- 74.Vullers RJM, Ahlskog M, van Haesendonck C. Titanium nanostructures made by local oxidation with the atomic force microscope. Appl Surf Sci. 1999;144–145:584–588. [Google Scholar]
- 75.Matsumoto K, Takahashi S, Ishii M, et al. Application of STM nanometer-size oxidation process to planar-type mim diode. Jpn J Appl Phys. 1995;34(2B):1387–1390. [Google Scholar]
- 76.Ohko Y, Tatsuma T, Fuji T, et al. Multicolor photochromism of TiO2 films loaded with silver nanoparticles. Nat Mater. 2003;2(1):29–31. doi: 10.1038/nmat796. [DOI] [PubMed] [Google Scholar]
- 77.Ohko Y, Tatsuma T, Fuji T, et al. Multicolor photochromism of TiO2 films loaded with Ag nanoparticles; ECS, Electrochemical Society, 203rd Meeting; Paris. 2003. [Google Scholar]
- 78.Bunshah RF. Deposition Technologies for Films and Coatings: Developments and Applications. 1st ed. Park Ridge: Noyes Publications; 1982. [Google Scholar]
- 79.Jarzebski ZM. Oxide Semiconductors. 1st ed. New York, NY: Pergamon Press; 1973. [Google Scholar]
- 80.Kofstad P. Nonstoichiometry, Diffusion, and Electrical Conductivity in Binary Metal Oxides. New York, NY: John Wiley and Sons, Inc; 1983. [Google Scholar]
- 81.Chen X, Mao SS. Titanium dioxide nanomaterials: synthesis, properties, modifications, and applications. Chem Rev. 2007;107(7):2891–2959. doi: 10.1021/cr0500535. [DOI] [PubMed] [Google Scholar]
- 82.Springer S. Free carriers in nanocrystalline titanium dioxide thin films. Ecole polytechnique fédérale de Lausanne EPFL; Lausanne: 2004. Thèse no 2934. [Google Scholar]
- 83.Diebold U. The surface science of titanium dioxide. Surf Sci Rep. 2003;48(5–8):53–229. [Google Scholar]
- 84.JCPDS, International Centre for Diffraction Data (ICDD) PDF-database Available from: http://www.icdd.comAccessed 2010 Nov 8
- 85.Banfield JF, Bischoff BL, Anderson MA. TiO2 accessory minerals: coarsening, and transformation kinetics in pure and doped synthetic nanocrystalline materials. Chem Geol. 1993;110(1–3):211–231. [Google Scholar]
- 86.Simons PY, Dachille F. The structure of TiO2II, a high-pressure phase of TiO2. Acta Crystallogr. 1967;23(2):334–336. [Google Scholar]
- 87.Tang J, Endo J. P–T boundary of α-PbO2 type and baddeleyite type high-pressure phases of titanium dioxide. J Am Ceram Soc. 1993;76(3):796–798. [Google Scholar]
- 88.Olsen JS, Gerward L, Jiang JZ. On the rutile/α-PbO2-type phase boundary of TiO2. J Phys Chem Solids. 1999;60(2):229–233. [Google Scholar]
- 89.Latroche M, Brohan R, Marchand R, Tournoux M. New hollandite oxides: TiO2(H) and K0.06TiO2. J Solid State Chem. 1989;81(1):78–82. [Google Scholar]
- 90.Akimoto J, Gotoh Y, Oosawa Y, et al. Topotactic oxidation of ramsdellite-type Li0.5TiO2, a new polymorph of titanium dioxide: TiO2(R) J Solid State Chem. 1994;113(1):27–36. [Google Scholar]
- 91.Marchand R, Brohan R, Tournoux M. TiO2(B) a new form of titanium dioxide and the potassium octatitanate K2Ti8O17. Mater Res Bull. 1980;15(8):1129–1133. [Google Scholar]
- 92.Haines J, Leger JM. X-ray diffraction study of TiO2 up to 49 GPa. Physica B. 1993;192(3):233–237. [Google Scholar]
- 93.Horn M, Schwerdtfeger CF, Meagher EP. Refinement of the structure of anatase at several temperatures. Z Kristallogr. 1972;136:273–281. [Google Scholar]
- 94.Henrich VE. The surfaces of metal oxides. Rep Prog Phys. 1985;48(11):1481. [Google Scholar]
- 95.Thien-Nga L, Paxton AT. Electronic structure of 5d transition metals adsorbed on the stoichiometric (110) rutile surface. Phys Rev B. 1998;58(19):13233–13241. [Google Scholar]
- 96.Wyckoff RWG. Crystal Structures Handbook. 2nd ed. New York, NY: Interscience Publishers; 1963. [Google Scholar]
- 97.Wahlbeck PG, Gilles PW. Reinvestigation of the phase diagram for the system titanium–oxygen. J Am Ceram Soc. 1966;49(4):180–183. [Google Scholar]
- 98.Kihlborg L, Olovsson I. Arne Magnéli 1914–1996. Acta Crystallogr A. 1997;53:103–104. [Google Scholar]
- 99.Straumanis ME, Ejima T, James WJ. The TiO2 phase explored by the lattice constant and density method. Acta Crystallogr. 1961;14:493–497. [Google Scholar]
- 100.Carnahan RD, Brittain JO. Optical absorption study of the reduction kinetics of rutile crystals. J Am Ceram Soc. 1965;48(7):365–369. [Google Scholar]
- 101.Cronemeyer DC, Gilleo MA. The optical absorption and photoconductivity of rutile. Phys Rev. 1951;82(6):975–976. [Google Scholar]
- 102.Hosaka N, Sekiya T, Fujisawa M, Satoko C, Kurita S. UV reflection spectra of anatase TiO2. J Electron Spectros Relat Phenom. 1996;78:75–78. [Google Scholar]
- 103.Jellison GA, Jr, Modine FA, Boatner LA. Measurement of the optical functions of uniaxial materials by two-modulator generalized ellipsometry: rutile (TiO2) Opt Lett. 1997;22(23):1808–1810. doi: 10.1364/ol.22.001808. [DOI] [PubMed] [Google Scholar]
- 104.Palik ED, editor. Handbook of Optical Constants of Solids. Orlando, FL: Academic Press; 1985. [Google Scholar]
- 105.Washburn EW. International Critical Tables of Numerical Data, Physics, Chemistry and Technology. New York, NY: McGraw-Hill; 1930. [Google Scholar]
- 106.Tanemura S, Miao L, Jin P, Kaneko K, Terai A, Nabatova-Gabain N. Optical properties of polycrystalline and epitaxial anatase and rutile TiO2 thin films by rf magnetron sputtering. Appl Surf Sci. 2003;212–213:654–660. [Google Scholar]
- 107.Cangiani G. Ab Initio Study of the Properties of TiO2 Rutile and Anatase Polytypes. Lausanne, France: Faculté des sciences de base, Ecole polytechnique fédérale de Lausanne EPFL; 2003. [Google Scholar]
- 108.Paxton AT, Thien-Nga L. Electronic structure of reduced titanium dioxide. Phys Rev B. 1998;57(3):1579–1584. [Google Scholar]
- 109.Bally A. Electronic Properties of Nanocrystalline Titanium Dioxide Thin Films. Lausanne, France: Département de physique, Ecole polytechnique fédérale de Lausanne EPFL; 1999. [Google Scholar]
- 110.Knauth P, Tuller HL. Electrical and defect thermodynamic properties of nanocrystalline titanium dioxide. J Appl Phys. 1999;85(2):897–902. [Google Scholar]
- 111.Mizushima K, Tanaka M, Asai A, Iida S, Goodenough GB. Impurity levels of iron-group ions in TiO2(II) J Phys Chem Solids. 1979;40(12):1129–1140. [Google Scholar]
- 112.Forro L, Chauvet O, Emin D, Zuppiroli L, Berger H, Lévy F. High mobility n-type charge carriers in large single crystals of anatase (TiO2) J Appl Phys. 1994;75(1):633–635. [Google Scholar]
- 113.Yu N, Halley JW. Electronic structure of point defects in rutile TiO2. Phys Rev B. 1995;51(8):4768–4776. doi: 10.1103/physrevb.51.4768. [DOI] [PubMed] [Google Scholar]
- 114.Blumenthal RN, Coburn J, Baukus J, Hirthe WM. Electrical conductivity of nonstoichiometric rutile single crystals from 1000° to 1500°C. J Phys Chem Solids. 1966;27(4):643–654. [Google Scholar]
- 115.Martin N, Santo AME, Sanjinés R, Lévy F. Energy distribution of ions bombarding TiO2 thin films during sputter deposition. Surf Coat Technol. 2001;138(1):77–83. [Google Scholar]
- 116.Bogomolov VN, Kudinov EK, Mirlin DN, et al. Polaron mechanism of light absorption in rutile crystals. Sov Phys Solid State. 1963;9:1630–1639. [Google Scholar]
- 117.Becker JH, Hosler WR. Multiple-band conduction in n-type rutile (TiO2) Phys Rev A. 1965;137(6A):1872–1877. [Google Scholar]
- 118.Hasiguti RR, Yagi E. Electrical conductivity below 3 K of slightly reduced oxygen-deficient rutile TiO2−x. Phys Rev B. 1994;49(11):7251–7256. doi: 10.1103/physrevb.49.7251. [DOI] [PubMed] [Google Scholar]
- 119.Mott NF. Electrons in disordered structures. Adv Phys. 1967;16(61):49–144. [Google Scholar]
- 120.Enright B, Fitzmaurice D. Spectroscopic determination of electron and hole effective masses in a nanocrystalline semiconductor film. J Phys Chem. 1996;100(3):1027–1035. [Google Scholar]
- 121.Nozik AJ, Memming R. Physical chemistry of semiconductor–liquid interfaces. J Phys Chem. 1996;100(31):13061–13078. [Google Scholar]
- 122.Grela MA, Brusa Ma, Colussi AJ. Harnessing excess photon energy in photoinduced surface electron transfer between salicylate and illuminated titanium dioxide nanoparticles. J Phys Chem B. 1997;101(51):10986–10989. [Google Scholar]
- 123.Okuyama K, Kousaka Y, Tohge N, et al. Production of ultrafine metal oxide aerosol particles by thermal decomposition of metal alkoxide vapors. AIChE J. 1986;32(12):2010–2019. [Google Scholar]
- 124.Okuyama K, Jeung JT, Kousaka Y. Experimental control of ultrafine TiO2 particle generation from thermal decomposition of titanium tetraisopropoxide vapor. Chem Eng Sci. 1989;44(6):1369–1375. [Google Scholar]
- 125.Okuyama K, Ushio R, Kousaka Y, Flagan RC. Seinfeld JH Particle generation in a chemical vapor deposition process with seed particles. AIChE J. 1990;36(3):409–419. [Google Scholar]
- 126.Ding Z, Hu X, Lu GQ, Yue P-L, Greenfield PF. Novel silica gel supported TiO2 photocatalyst synthesized by CVD method. Langmuir. 2000;16(15):6216–6222. [Google Scholar]
- 127.Li W, Shah SI, Huang C-P, Jung O, Ni C. Metalorganic chemical vapor deposition and characterization of TiO2 nanoparticles. Mater Sci Eng B. 2002;96(3):247–253. [Google Scholar]
- 128.Li W, Shah SI, Sung M, et al. Structure and size distribution of TiO2 nanoparticles deposited on stainless steel mesh. J Vac Sci Technol B. 2002;20(6):2303–2308. [Google Scholar]
- 129.Li W, Wang Y, Lin H, et al. Band gap tailoring of Nd3+-doped TiO2 nanoparticles. Appl Phys Lett. 2003;83(20):4143–4415. [Google Scholar]
- 130.Leboda R, Gun’ko VM, Marciniak M, et al. Structure of chemical vapor deposition titania/silica gel. J Colloid Interface Sci. 1999;218(1):23–39. doi: 10.1006/jcis.1999.6411. [DOI] [PubMed] [Google Scholar]
- 131.Gun’ko VM, Zarko VI, Turov VV, et al. CVD-titania on fumed silica substrate. J Colloid Interface Sci. 1998;198(1):141–156. [Google Scholar]
- 132.Schrijnemakers K, Impens NREN, Vansant EF. Deposition of a titania coating on silica by means of the chemical surface coating. Langmuir. 1999;15(18):5807–5813. [Google Scholar]
- 133.Stakheev AY, Lee CW, Chong PJ. Preparation and characterization of titanium dioxide embedded onto ZSM-5 zeolite. Bull Korean Chem Soc. 1998;19(5):530–533. [Google Scholar]
- 134.Xia B, Li W, Zhang B, Xie Y. Low temperature vapor-phase preparation of TiO2 nanopowders. J Mater Sci. 1999;34(14):3505–3511. [Google Scholar]
- 135.Mezey EJ. Pigments and reinforcing agents. In: Powell CF, Oxley JH, Blocher JM Jr, editors. Vapor Deposition. New York, NY: John Wiley and Sons; 1966. p. 423. [Google Scholar]
- 136.Akhtar MK, Pratsinis SE, Mastrangelo SVR. Dopants in vapor-phase synthesis of titania powders. J Am Ceram Soc. 1992;75(12):3408–3416. [Google Scholar]
- 137.Suyama Y, Kato A. TiO2 produced by vapor-phase oxygenolysis of TiCI4. J Am Ceram Soc. 1976;59(3–4):146–149. [Google Scholar]
- 138.Vemury S, Pratsinis SE, Kibbey L. Electrically controlled flame synthesis of nanophase TiO2, SiO2, and SnO2 powders. J Mater Res. 1997;12(4):1031–1042. [Google Scholar]
- 139.Vemury S, Pratsinis SE. Corona-assisted flame synthesis of ultrafine titania particles. Appl Phys Lett. 1995;66:3275–3278. [Google Scholar]
- 140.Casey JD, Haggerty JS. Laser-induced vapour-phase synthesis of titanium dioxide. J Mater Sci. 1987;22(12):4307–4312. [Google Scholar]
- 141.Epperson JE, Siegel RW, White JW, et al. Sintering of nanophase TiO2 at 550°C. Mater Res Soc Symp Proc. 1988;132:3–7. [Google Scholar]
- 142.Kato A, Takeshima Y, Katatae Y. Preparation of spherical titania particles from inorganic precursor by homogeneous precipitation. Mater Res Soc Symp Proc. 1989;155:13–15. [Google Scholar]
- 143.Choi W, Termin A, Hoffmann MR. The role of metal ion dopants in quantum-sized TiO2: correlation between photoreactivity and charge carrier recombination dynamics. J Phys Chem. 1994;98(51):13669–13679. [Google Scholar]
- 144.Yu JC, Wu L, Lin J, Li P, Li Q. Microemulsion-mediated solvothermal synthesis of nanosized CdS-sensitized TiO2 crystalline photocatalyst. Chem Commun. 2003;13:1552–1553. [Google Scholar]
- 145.Schneider M, Baiker A. High-surface-area titania aerogels: preparation and structural properties. J Mater Chem. 1992;2:587–589. [Google Scholar]
- 146.Zhang AP, Sun YP. Photocatalytic killing effect of TiO2 nanoparticles on Ls-174-t human colon carcinoma cells. World J Gastroenterol. 2004;10(21):3191–3193. doi: 10.3748/wjg.v10.i21.3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chen LX, Rajh T, Wang Z, Thurnauer MC. XAFS studies of surface structures of TiO2 nanoparticles and photocatalytic reduction of metal ions. J Phys Chem B. 1997;101(50):10688–10697. [Google Scholar]
- 148.Wang CC, Zhang Z, Ying JY. Photocatalytic decomposition of halogenated organics over nanocrystalline titania. Nanostruct Mater. 1997;9(1–8):583–586. [Google Scholar]
- 149.Xue Q, Liu W, Zhang Z. Friction and wear properties of a surface-modified TiO2 nanoparticle as an additive in liquid paraffin. Wear. 1997;213(1–2):29–32. [Google Scholar]
- 150.Jeon S, Braun PV. Hydrothermal synthesis of Er-doped luminescent TiO2 nanoparticles. Chem Mater. 2003;15(6):1256–1263. [Google Scholar]
- 151.Liqiang J, Xiaojun S, Baifu X, Baiqi W, Weimin C, Honggang F. The preparation and characterization of La doped TiO2 nanoparticles and their photocatalytic activity. J Solid State Chem. 2004;177(10):3375–3382. [Google Scholar]
- 152.Burda C, Lou YB, Chen XB, Samia ACS, Stout J, Gole JL. Enhanced nitrogen doping in TiO2 nanoparticles. Nano Lett. 2003;3(8):1049–1051. [Google Scholar]
- 153.Zhang Z, Wang CC, Zakaria R, Ying JY. Role of particle size in nanocrystalline TiO2-based photocatalysts. J Phys Chem B. 1998;102(52):10871–10878. [Google Scholar]
- 154.Sugimoto T, Zhou X, Muramatsu A. Synthesis of uniform anatase TiO2 nanoparticles by gel–sol method. 3. Formation process and size control. J Colloid Interface Sci. 2003;259(1):43–52. doi: 10.1016/s0021-9797(03)00036-5. [DOI] [PubMed] [Google Scholar]
- 155.Stathatos E, Lianos P, Del-Monte F, Levy D, Tsiourvas D. Formation of TiO2 nanoparticles in reverse micelles and their deposition as thin films on glass substrates. Langmuir. 1997;13(16):4295–4300. [Google Scholar]
- 156.Sakai H, Kawahara H, Shimazaki M, Abe M. Preparation of ultrafine titanium dioxide particles using hydrolysis and condensation reactions in the inner aqueous phase of reversed micelles: effect of alcohol addition. Langmuir. 1998;14(8):2208–2212. [Google Scholar]
- 157.Haga Y, An H, Yosomiya R. Photoconductive properties of TiO2 films prepared by the sol–gel method and its application. J Mater Sci. 1997;32(12):3183–3188. [Google Scholar]
- 158.Li K, Wang Y, Sun Y, Yuan C. Preparation of nanocrystalline TiO2 electrode by layer-by-layer screen printing and its application in dye-sensitized solar cell. Mater Sci Eng B. 2010;175(1):44–47. [Google Scholar]
- 159.Xu C, Killmeyer R, Gray ML, Khan SUM. Photocatalytic effect of carbon-modified n-TiO2 nanoparticles under visible light illumination. Appl Catal B. 2006;64(3–2):312–317. [Google Scholar]
- 160.Jagadale TC, Takale SP, Sonawane RS, et al. N-doped TiO2 nanoparticle based visible light photocatalyst by modified peroxide sol-gel method. J Phys Chem C. 2008;112(37):14595–14602. [Google Scholar]
- 161.Shen GX, Chen YC, Lin CJ. Corrosion protection of 316 L stainless steel by a TiO2 nanoparticle coating prepared by sol–gel method. Thin Solid Films. 2005;489(1–2):130–136. [Google Scholar]
- 162.Zaban A, Aruna ST, Tirosh S, Gregg BA, Mastai Y. The effect of the preparation condition of TiO2 colloids on their surface structures. J Phys Chem B. 2000;104(17):4130–4133. [Google Scholar]
- 163.Oskam G, Nellore A, Penn RL, Searson PC. The growth kinetics of TiO2 nanoparticles from titanium(IV) alkoxide at high water/titanium ratio. J Phys Chem B. 2003;107(8):1734–1738. [Google Scholar]
- 164.Wu M, Lin G, Chen D, et al. Sol-hydrothermal synthesis and hydrothermally structural evolution of nanocrystal titanium dioxide. Chem Mater. 2002;14(5):1974–1980. [Google Scholar]
- 165.Pottier A, Chaneac C, Tronc E, Mazerolles L, Jolivet JP. Synthesis of brookite TiO2 nanoparticles by thermolysis of TiCl4 in strongly acidic aqueous media. J Mater Chem. 2001;11:1116–1121. [Google Scholar]
- 160.Aruna ST, Tirosh S, Zaban A. Nanosize rutile titania particle synthesis via a hydrothermal method without mineralizers. J Mater Chem. 2000;10(10):2388–2391. [Google Scholar]
- 167.Wang CC, Ying JY. Sol-gel synthesis and hydrothermal processing of anatase and rutile titania nanocrystals. Chem Mater. 1999;11(11):3113–3120. [Google Scholar]
- 168.Yin H, Wada Y, Kitamura T, et al. Hydrothermal synthesis of nanosized anatase and rutile TiO2 using amorphous phase TiO2. J Mater Chem. 2001;11(6):1694–1703. [Google Scholar]
- 169.Park NG, van de Lagemaat J, Frank AJ. Comparison of dye-sensitized rutile- and anatase-based TiO2 solar cells. J Phys Chem B. 2000;104(38):8989–8994. [Google Scholar]
- 170.Wang W, Gu B, Liang L, Hamilton WA, Wesolowski DJ. Synthesis of rutile (α-TiO2) nanocrystals with controlled size and shape by low-temperature hydrolysis: effects of solvent composition. J Phys Chem B. 2004;108(39):14789–14792. [Google Scholar]
- 171.Li JG, Ishigaki T, Sun X. Anatase, brookite, and rutile nanocrystals via redox reactions under mild hydrothermal conditions: phase-selective synthesis and physicochemical properties. J Phys Chem C. 2007;111(13):4969–4976. [Google Scholar]
- 172.Koelsch M, Cassaignon S, Thanh Minh CT, Guillemoles JF, Jolivet JP. Electrochemical comparative study of titania (anatase, brookite and rutile) nanoparticles synthesized in aqueous medium. Thin Solid Films. 2004;451–452:86–92. [Google Scholar]
- 173.Tomita K, Petrykin V, Kobayashi M, Shiro M, Yoshimura M, Kakihana M. A water-soluble titanium complex for the selective synthesis of nanocrystalline brookite, rutile, and anatase by a hydrothermal method. Angew Chem Int Ed Engl. 2006;45(15):2378–2381. doi: 10.1002/anie.200503565. [DOI] [PubMed] [Google Scholar]
- 174.Reyes-Coronado D, Rodríguez-Gattorno G, Espinosa-Pesqueira ME, Cab C, de Coss R, Oskam G. Phase-pure TiO2 nanoparticles: anatase, brookite and rutile. Nanotechnology. 2008;19:145605. doi: 10.1088/0957-4484/19/14/145605. (10pp) [DOI] [PubMed] [Google Scholar]
- 175.Wang Y, Cheng H, Hao Y, Ma J, Li W, Cai S. Preparation, characterization and photoelectrochemical behaviors of Fe(III)-doped TiO2 nanoparticles. J Mater Sci. 1999;34(15):3721–3729. [Google Scholar]
- 176.Cheng H, Ma J, Zhao Z, Qi L. Hydrothermal preparation of uniform nanosize rutile and anatase particles. Chem Mater. 1995;7(4):663–671. [Google Scholar]
- 177.Wang Y, Hao Y, Cheng H, et al. The photoelectrochemistry of transition metal-ion-doped TiO2 nanocrystalline electrodes and higher solar cell conversion efficiency based on Zn2+-doped TiO2 electrode. J Mater Sci. 1999;34(12):2773–2779. [Google Scholar]
- 178.Rao AR, Dutta V. Low-temperature synthesis of TiO2 nanoparticles and preparation of TiO2 thin films by spray deposition. Solar Energy Mater Solar Cells. 2007;91(12):1075–1080. [Google Scholar]
- 179.Palmisano L, Augugliaro V, Sclafani A, Schiavello M. Activity of chromium-ion-doped titania for the dinitrogen photoreduction to ammonia and for the phenol photodegradation. J Phys Chem. 1988;92(23):6710–6713. [Google Scholar]
- 180.Akhtar MK, Xiong Y. Pratsinis SE Vapor synthesis of titania powder by titanium tetrachloride oxidation. AIChE J. 1991;37(10):1561–1570. [Google Scholar]
- 181.Shi L, Li C, Chen A, Zhu Y, Fang D. Morphology and structure of nanosized TiO2 particles synthesized by gas-phase reaction. Mater Chem Phys. 2000;66(1):51–57. [Google Scholar]
- 182.Gao Y, Masuda Y, Seo WS, Ohta H, Koumoto K. TiO2 nanoparticles prepared using an aqueous peroxotitanate solution. Ceram Int. 2004;30(7):1365–1368. [Google Scholar]
- 183.Mahshid S, Ghamsari MS, Askari M, Afshar N, Lahuti S. Synthesis of TiO2 nanoparticles by hydrolysis and peptization of titanium isopropoxide solution. Semiconductor Phys Quantum Electron Optoelectron. 2006;9(2):65–68. [Google Scholar]
- 184.Vorkapic D, Matsoukas T. Effect of temperature and alcohols in the preparation of titania nanoparticles from alkoxides. J Am Ceram Soc. 1998;81(11):2815–2820. [Google Scholar]
- 185.Li XL, Peng Q, Yi JX, Wang X, Li Y. Near monodisperse TiO2 nanoparticles and nanorods. Chem Eur J. 2006;12(8):2383–2391. doi: 10.1002/chem.200500893. [DOI] [PubMed] [Google Scholar]
- 186.Seo JW, Chung H, Kim MY, Lee J, Choi IH, Cheon J. Development of water-soluble single-crystalline TiO2 nanoparticles for photocatalytic cancer-cell treatment. Small. 2007;3(5):850–853. doi: 10.1002/smll.200600488. [DOI] [PubMed] [Google Scholar]
- 187.Teleki A, Pratsinis SE, Kalyanasundaram K, Gouma PI. Sensing of organic vapors by flame-made TiO2 nanoparticles. Sens Actuators B Chem. 2006;119(2):683–690. [Google Scholar]
- 188.Yager P, Schoen PE. Formation of tubules by a polymerizable surfactant. P Mol Cryst Liq Cryst. 1984;106:371–381. [Google Scholar]
- 189.Iijima S. Helical microtubules of graphitic carbon. Nature. 1991;354:56–58. [Google Scholar]
- 190.Martin CR. Nanomaterials: a membrane-based synthetic approach. Science. 1994;266(5193):1961–1966. doi: 10.1126/science.266.5193.1961. [DOI] [PubMed] [Google Scholar]
- 191.Adachi M, Murata Y, Harada M, Yoshikawa S. Formation of titania nanotubes with high photo-catalytic activity. Chem Lett. 2000;29(8):942–944. [Google Scholar]
- 192.Zhou YK, Cao L, Zhang FB, He BL, Li HL. Lithium insertion into TiO2 nanotube prepared by the hydrothermal process. J Electrochem Soc. 2003;150(9):A1246–A1249. [Google Scholar]
- 193.Poulios I, Kositzi M, Kouras A. Photocatalytic decomposition of triclopyr over aqueous semiconductor suspensions. J Photochem Photobiol A Chem. 1998;115(2):175–183. [Google Scholar]
- 194.Adachi M, Okada I, Ngamsinlapasathian S, Murata Y, Yoshikawa S. Dye-sensitized solar cells using semiconductor thin film composed of titania nanotubes. Electrochemistry. 2002;70(6):449–452. [Google Scholar]
- 195.Uchida S, Chiba R, Tomiha M, Masaki N, Shirai M. Application of titania nanotubes to a dye-sensitized solar cell. Electrochemistry. 2002;70(6):418–420. [Google Scholar]
- 196.Quan X, Yang S, Ruan X, Zhao H. Preparation of titania nanotubes and their environmental applications as electrode. Environ Sci Technol. 2005;39(10):3770–3775. doi: 10.1021/es048684o. [DOI] [PubMed] [Google Scholar]
- 197.Hoyer P. Formation of a titanium dioxide nanotube array. Langmuir. 1996;12(6):1411–1413. [Google Scholar]
- 198.Kasuga T, Hiramatsu M, Hoson A, Sekino T, Niihara K. Formation of titanium oxide nanotube. Langmuir. 1998;14(12):3160–3163. [Google Scholar]
- 199.Kasuga T, Hiramatsu M, Hoson A, Sekino T, Niihara K. Titania nanotubes prepared by chemical processing. Adv Mater. 1999;11(15):1307–1311. [Google Scholar]
- 200.Zhu Y, Li H, Koltypin Y, Hacohen YR, Gedanken A. Sonochemical synthesis of titania whiskers and nanotubes. Chem Commun (Camb) 2001;24:2616–2617. [Google Scholar]
- 201.Du GH, Chen Q, Che RC, Yuan ZY, Peng LM. Preparation and structure analysis of titanium oxide nanotubes. Appl Phys Lett. 2001;79(22):3702–3704. [Google Scholar]
- 202.Seo DS, Lee JK, Kim H. Preparation of nanotube-shaped TiO2 powder. J Cryst Growth. 2001;229(1–4):428–432. [Google Scholar]
- 203.Zhang QH, Gao L, Sun J, Zheng S. Preparation of long TiO2 nanotubes from ultrafine rutile nanocrystals. Chem Lett. 2002;31(2):226–227. [Google Scholar]
- 204.Armstrong AR, Armstrong G, Canales J, Garcia R, Bruce PG. Lithiumion intercalation into TiO2-B nanowires. Adv Mater. 2005;17(7):862–865. [Google Scholar]
- 205.Yao BD, Chan YF, Zhang XY, Zhang WF, Yang ZY, Wang N. Formation mechanism of TiO2 nanotubes. Appl Phys Lett. 2003;82(2):281–283. [Google Scholar]
- 206.Wu Z, Dong F, Zhao W, Wang H, Liu Y, Guan B. The fabrication and characterization of novel carbon doped TiO2 nanotubes, nanowires and nanorods with high visible light photocatalytic activity. Nanotechnology. 2009;20(23):235701. doi: 10.1088/0957-4484/20/23/235701. [DOI] [PubMed] [Google Scholar]
- 207.Zhang YX, Li GH, Jin YX, Zhang Y, Zhang J, Zhang LD. Hydrothermal synthesis and photoluminescence of TiO2 nanowires. Chem Phys Lett. 2002;365(3–4):300–304. [Google Scholar]
- 208.Jitputti J, Suzuki Y, Yoshikawa S. Synthesis of TiO2 nanowires and their photocatalytic activity for hydrogen evolution. Catal Commun. 2008;9(6):1265–1271. [Google Scholar]
- 209.Dong F, Zhao W, Wu Z. Characterization and photocatalytic activities of C, N and S co-doped TiO2 with 1D nanostructure prepared by the nano-confnement effect. Nanotechnology. 2008;19(36):365607. doi: 10.1088/0957-4484/19/36/365607. [DOI] [PubMed] [Google Scholar]
- 210.Feng X, Shankar K, Varghese OK, Paulose M, Latempa TJ, Grimes CA. Vertically aligned single crystal TiO2 nanowire arrays grown directly on transparent conducting oxide coated glass: synthesis details and applications. Nano Lett. 2008;8(11):3781–3786. doi: 10.1021/nl802096a. [DOI] [PubMed] [Google Scholar]
- 211.Kitano M, Mitsui R, Eddy DR, et al. Synthesis of nanowire TiO2 thin films by hydrothermal treatment and their photoelectrochemical properties. Catal Lett. 2007;119(3–4):217–221. [Google Scholar]
- 212.Wang D, Zhou F, Wang C, Liu W. Synthesis and characterization of silver nanoparticle loaded mesoporous TiO2 nanobelts. Microporous Mesoporous Mater. 2008;116(1–3):658–664. [Google Scholar]
- 213.Ye Q, Wang X, Hu H, Wang D, Li S, Zhou F. Polyelectrolyte brush templated multiple loading of Pd nanoparticles onto TiO2 nanowires via regenerative counterion exchange-reduction. J Phys Chem C. 2009;113(18):7677–7683. [Google Scholar]
- 214.Zhang X, Zhang T, Ng J, Sun DD. High-performance multifunctional TiO2 nanowire ultrafiltration membrane with a hierarchical layer structure for water treatment. Adv Funct Mater. 2009;19(23):3731–3736. [Google Scholar]
- 215.Liu B, Aydil ES. Growth of oriented single-crystalline rutile TiO2 nanorods on transparent conducting substrates for dye-sensitized solar cells. J Am Cher Soc. 2009;131(11):3985–3990. doi: 10.1021/ja8078972. [DOI] [PubMed] [Google Scholar]
- 216.Chang M, Lin CH, Deka JR, Chang FC, Chung CC. Nanomechanical characterization of microwave hydrothermally synthesized titania nanowires. J Phys D Appl Phys. 2009;42(14):145105. [Google Scholar]
- 217.Wang H, Liu Y, Li M, Huang H, Zhong M, Shen H. Hydrothermal growth of large-scale macroporous TiO2 nanowires and its application in 3D dye-sensitized solar cells. Appl Phys A Mater Sci Process. 2009;97(1):25–29. [Google Scholar]
- 218.Wen B, Liu C, Liu Y. Bamboo-shaped Ag-doped TiO2 nanowires with heterojunctions. Inorg Chem. 2005;44(19):6503–6505. doi: 10.1021/ic0505551. [DOI] [PubMed] [Google Scholar]
- 219.Tian ZR, Voigt JA, Liu J, McKenzie B, Xu H. Large oriented arrays and continuous films of TiO2-based nanotubes. J Am Chem Soc. 2003;125(41):12384–12385. doi: 10.1021/ja0369461. [DOI] [PubMed] [Google Scholar]
- 220.Maiyalagan T, Viswanathan B, Varadaraju UV. Fabrication and characterization of uniform TiO2 nanotube arrays by sol–gel template method. Bull Mater Sci. 2006;29(7):705–708. [Google Scholar]
- 221.Lei Y, Zhang LD, Meng GW, et al. Preparation and photoluminescence of highly ordered TiO2 nanowire arrays. Appl Phys Lett. 2001;78(8):1125–1127. [Google Scholar]
- 222.Lakshmi BB, Patrissi CJ, Martin CR. Sol-gel template synthesis of semiconductor oxide micro- and nanostructures. Chem Mater. 1997;9(11):2544–2550. [Google Scholar]
- 223.Lakshmi BB, Dorhout PK, Martin CR. Sol-gel template synthesis of semiconductor nanostructures. Chem Mater. 1997;9(3):857–862. [Google Scholar]
- 224.Imai H, Takei Y, Shimizu K, Matsuda M, Hirashima H. Direct preparation of anatase TiO2 nanotubes in porous alumina membranes. J Mater Chem. 1999;9(12):2971–2972. [Google Scholar]
- 225.Michailowski A, AlMawlawi D, Cheng G, Moskovits M. Highly regular anatase nanotubule arrays fabricated in porous anodic templates. Chem Phys Lett. 2001;349(1–2):1–5. [Google Scholar]
- 226.Miao Z, Xu D, Ouyang J, Guo G, Zhao X, Tang Y. Electrochemically induced sol-gel preparation of single-crystalline TiO2 nanowires. Nano Lett. 2002;2(7):717–720. [Google Scholar]
- 227.Lin Y, Wu GS, Yuan XY, Xie T, Zhang LD. Fabrication and optical properties of TiO2 nanowire arrays made by sol–gel electrophoresis deposition into anodic alumina membranes. J Phys Condens Matter. 2003;15(17):2917–2922. [Google Scholar]
- 228.Zhang XY, Zhang LD, Chen W, Meng GW, Zheng MJ, Zhao LX. Electrochemical fabrication of highly ordered semiconductor and metallic nanowire arrays. Chem Mater. 2001;13(8):2511–2515. [Google Scholar]
- 229.Liu S, Huang K. Straightforward fabrication of highly ordered TiO2 nanowire arrays in AAM on aluminum substrate. Solar Energy Mater Solar Cells. 2005;85(1):125–131. [Google Scholar]
- 230.Herderick ED, Tresback JS, Vasiliev AL, Padture NP. Template-directed synthesis, characterization and electrical properties of Au-TiO2–Au heterojunction nanowires. Nanotechnology. 2007;18(15):155204. [Google Scholar]
- 231.Caruso RA, Schattka JH, Greiner A. Titanium dioxide tubes from solgel coating of electrospun polymer fibers. Adv Mater. 2001;13(20):1577–1579. [Google Scholar]
- 232.Formo E, Lee E, Campbell D, Xia Y. Functionalization of electrospun TiO2 nanofibers with Pt nanoparticles and nanowires for catalytic applications. Nano Lett. 2008;8(2):668–672. doi: 10.1021/nl073163v. [DOI] [PubMed] [Google Scholar]
- 233.Archana PS, Jose R, Vijila C, Ramakrishna S. Improved electron diffusion coefficient in electrospun TiO2 nanowires. J Phys Chem C. 2009;113(52):21538–21542. [Google Scholar]
- 234.Jung JH, Kobayashi H, van Bommel KJC, Shinkai S, Shimizu T. Creation of novel helical ribbon and double-layered nanotube TiO2 structures using an organogel template. Chem Mater. 2002;14(4):1445–1447. [Google Scholar]
- 235.Kobayashi S, Hanabusa K, Hamasaki N, Kimura M, Shirai H. Preparation of TiO2 hollow-fibers using supramolecular assemblies. Chem Mater. 2000;12(6):1523–1525. [Google Scholar]
- 236.Zhang D, Qi L. Synthesis of mesoporous titania networks consisting of anatase nanowires by templating of bacterial cellulose membranes. Chem Commun (Camb) 2005;21:2735–2737. doi: 10.1039/b501933h. [DOI] [PubMed] [Google Scholar]
- 237.Chen Y, Kim HC, McVittie J, Ting C, Nishi Y. Synthesis of TiO2 nanoframe and the prototype of a nanoframe solar cell. Nanotechnology. 2010;21(18):185303. doi: 10.1088/0957-4484/21/18/185303. [DOI] [PubMed] [Google Scholar]
- 238.Zwilling V, Aucouturier M, Darque-Ceretti E. Anodic oxidation of titanium and TA6V alloy in chromic media. An electrochemical approach. Electrochim Acta. 1999;45(6):921–929. [Google Scholar]
- 239.Zwilling V, Darque-Ceretti E, Boutry-Forveille A, David D, Perrin MY, Aucouturier M. Structure and physicochemistry of anodic oxide films on titanium and TA6V alloy. Surf Interface Anal. 1999;27(7):629–637. [Google Scholar]
- 240.Jennings JR, Ghicov A, Peter LM, Schmuki P, Walker AB. Dye-sensitized solar cells based on oriented TiO2 nanotube arrays: transport, trapping, and transfer of electrons. J Am Chem Soc. 2008;130(40):13364–13372. doi: 10.1021/ja804852z. [DOI] [PubMed] [Google Scholar]
- 241.Varghese OK, Gong D, Paulose M, Grimes CA, Dickey EC. Crystallization and high-temperature structural stability of titanium oxide nanotube arrays. J Mater Res. 2003;18(1):156–165. [Google Scholar]
- 242.Macák JM, Tsuchiya H, Taveira L, Aldabergerova S, Schmuki P. Smooth anodic TiO2 nanotubes. Angew Chem Int Ed Engl. 2005;44(45):7463–7465. doi: 10.1002/anie.200502781. [DOI] [PubMed] [Google Scholar]
- 243.Gong D, Grimes CA, Varghese OK, Chen Z, Hu W, Dickey EC. Titanium oxide nanotube arrays prepared by anodic oxidation. J Mater Res. 2001;16(12):3331–3334. [Google Scholar]
- 244.Macák JM, Tsuchiya H, Ghicov A, Schmuki P. Dye-sensitized anodic TiO2 nanotubes. Electrochem Commun. 2005;7(11):1133–1137. [Google Scholar]
- 245.Mor GK, Shankar K, Paulose M, Varghese OK, Grimes CA. Use of highly-ordered TiO2 nanotube arrays in dye-sensitized solar cells. Nano Lett. 2006;6(2):215–218. doi: 10.1021/nl052099j. [DOI] [PubMed] [Google Scholar]
- 246.Paulose M, Shankar K, Varghese OK, Mor GK, Hardin B, Grimes CA. Backside illuminated dye-sensitized solar cells based on titania nanotube array electrodes. Nanotechnology. 2006;17(5):1446–1448. [Google Scholar]
- 247.Stergiopoulos T, Valota A, Likodimos V, et al. Dye-sensitization of self-assembled titania nanotubes prepared by galvanostatic anodization of Ti sputtered on conductive glass. Nanotechnology. 2009;20(36):365601. doi: 10.1088/0957-4484/20/36/365601. [DOI] [PubMed] [Google Scholar]
- 248.Stergiopoulos T, Ghicov A, Likodimos V, et al. Dye-sensitized solar cells based on thick highly ordered TiO2 nanotubes produced by controlled anodic oxidation in non-aqueous electrolytic media. Nanotechnology. 2008;19(23):235602. doi: 10.1088/0957-4484/19/23/235602. [DOI] [PubMed] [Google Scholar]
- 249.Zhu K, Neale NR, Miedaner A, Frank AJ. Enhanced charge-collection efficiencies and light scattering in dye-sensitized solar cells using oriented TiO2 nanotubes arrays. Nano Lett. 2007;7(1):69–74. doi: 10.1021/nl062000o. [DOI] [PubMed] [Google Scholar]
- 250.Shankar K, Mor GK, Prakasam HE, Varghese OK, Grimes CA. Self-assembled hybrid polymer–TiO2 nanotube array heterojunction solar cells. Langmuir. 2007;23(24):12445–12449. doi: 10.1021/la7020403. [DOI] [PubMed] [Google Scholar]
- 251.Oh S, Daraio S, Chen LH, Pisanic TR, Fiñones RR, Jin S. Significantly accelerated osteoblast cell growth on aligned TiO2 nanotubes. J Biomed Mater Res A. 2006;78(1):97–103. doi: 10.1002/jbm.a.30722. [DOI] [PubMed] [Google Scholar]
- 252.Paulose M, Prakasam HE, Varghese OK, et al. TiO2 nanotube arrays of 1000 μm length by anodization of titanium foil: phenol red diffusion. J Phys Chem C. 2007;111(41):14992–14997. [Google Scholar]
- 253.Yu BY, Tsai A, Tsai SP, et al. Efficient inverted solar cells using TiO2 nanotube arrays. Nanotechnology. 2008;19(25):255202. doi: 10.1088/0957-4484/19/25/255202. [DOI] [PubMed] [Google Scholar]
- 254.Tsuchiya H, Macák JM, Taveira L, et al. Self-organized TiO2 nanotubes prepared in ammonium fluoride containing acetic acid electrolytes. Electrochem Commun. 2005;7(6):576–580. [Google Scholar]
- 255.Wang J, Zhao L, Lin VSY, Lin Z. Formation of various TiO2 nanostructures from electrochemically anodized titanium. J Mater Chem. 2009;19(22):3682–3687. [Google Scholar]
- 256.Kuang D, Brillet J, Chen P, et al. Application of highly ordered TiO2 nanotube arrays in flexible dye-sensitized solar cells. ACS Nano. 2008;2(6):1113–1116. doi: 10.1021/nn800174y. [DOI] [PubMed] [Google Scholar]
- 257.Xia M, Zhang Q, Li H, et al. The large-scale synthesis of one-dimensional TiO2 nanostructures using palladium as catalyst at low temperature. Nanotechnology. 2009;20(5):055605. doi: 10.1088/0957-4484/20/5/055605. [DOI] [PubMed] [Google Scholar]
- 258.Wu Z, Guo S, Wang H, Liu Y. Synthesis of immobilized TiO2 nanowires by anodic oxidation and their gas phase photocatalytic properties. Electrochem Commun. 2009;11(8):1692–1695. [Google Scholar]
- 259.Kang SH, Kim HS, Kim JY, Sung YE. An investigation on electron behavior employing vertically-aligned TiO2 nanotube electrodes for dye-sensitized solar cells. Nanotechnology. 2009;20(35):355307. doi: 10.1088/0957-4484/20/35/355307. [DOI] [PubMed] [Google Scholar]
- 260.Chen Q, Xu D, Wu Z, Liu Z. Free-standing TiO2 nanotube arrays made by anodic oxidation and ultrasonic splitting. Nanotechnology. 2008;19(36):365708. doi: 10.1088/0957-4484/19/36/365708. [DOI] [PubMed] [Google Scholar]
- 261.Yang Y, Wang X, Sun C, Li L. Electron field emission and photoluminescence of anatase nanotube arrays. J Am Ceram Soc. 2008;91(12):4109–4111. [Google Scholar]
- 262.Limmer SJ, Seraji S, Forbess MJ, et al. Electrophoretic growth of lead zirconate titanate nanorods. Adv Mater. 2001;13(16):1269–1272. [Google Scholar]
- 263.Joo SW, Banerjee AN. Field emission characterization of vertically oriented uniformly grown nickel nanorod arrays on metal-coated silicon substrate. J Appl Phys. 2010;107(11):114317. [Google Scholar]
- 264.Joo SW, Banerjee AN. FESEM studies of densely-packed aligned nickel nanopillars on silicon substrate by electrochemical deposition through porous alumina membrane. Mater Sci Eng B. 2010;175(1):36–40. [Google Scholar]
- 265.Banerjee A, Halder N. Electrochemical growth of ordered nickel nanorods within a composite structure of anodic-alumina-membrane/metal/silicon substrate. J Nanosci Nanotechnol. 2010;10(7):4252–4258. doi: 10.1166/jnn.2010.2675. [DOI] [PubMed] [Google Scholar]
- 266.Venkataramanan NS, Matsui K, Kawanami H, Ikushima Y. Green synthesis of titania nanowire composites on natural cellulose fibers. Green Chem. 2007;9(1):18–19. [Google Scholar]
- 267.Li GL, Wang GH, Hong JM. Synthesis and characterization of rutile TiO2 nanowhiskers. J Mater Res. 1999;14(8):3346–3354. [Google Scholar]
- 268.Daothong S, Songmee N, Thongtem S, Singjai P. Size-controlled growth of TiO2 nanowires by oxidation of titanium substrates in the presence of ethanol vapor. Scripta Mater. 2007;57(7):567–570. [Google Scholar]
- 269.Kim SW, Han TH, Kim J, et al. Fabrication and electrochemical characterization of TiO2 three-dimensional nanonetwork based on peptide assembly. ACS Nano. 2009;3(5):1085–1090. doi: 10.1021/nn900062q. [DOI] [PubMed] [Google Scholar]
- 270.Sander MS, Cote MJ, Gu W, Kile BM, Tripp CP. Template-assisted fabrication of dense, aligned arrays of titania nanotubes with well-controlled dimensions on substrates. Adv Mater. 2004;16(22):2052–2057. [Google Scholar]
- 271.Park J, Ryu Y, Kim H, Yu C. Simple and fast annealing synthesis of titanium dioxide nanostructures and morphology transformation during annealing processes. Nanotechnology. 2009;20(10):105608. doi: 10.1088/0957-4484/20/10/105608. [DOI] [PubMed] [Google Scholar]
- 272.Wu JM, Xue HX. Photocatalytic active titania nanowire arrays on Ti substrates. J Am Ceram Soc. 2009;92(9):2139–2143. [Google Scholar]
- 273.Xiang B, Zhang Y, Wang Z, et al. Field-emission properties of TiO2 nanowire arrays. J Phys D Appl Phys. 2005;38(8):1152–1155. [Google Scholar]
- 274.Zhang H, Luo X, Xu J, Xiang B, Yu D. Synthesis of TiO2/SiO2 core/shell nanocable arrays. J Phys Chem B. 2004;108(39):14866–14869. [Google Scholar]
- 275.Wu JM, Shih HC, Wu WT, Tseng YK, Chen IC. Thermal evaporation growth and the luminescence property of TiO2 nanowires. J Cryst Growth. 2005;281(2–4):384–390. [Google Scholar]
- 276.Wu JM, Shih HC, Wu WT. Growth of TiO2 nanorods by two-step thermal evaporation. J Vac Sci Technol B Microelectron Nanometer Struct Process Meas Phenom. 2005;23(5):2122–2126. [Google Scholar]
- 277.Wu JM, Shih HC, Wu WT. Electron field emission from single crystalline TiO2 nanowires prepared by thermal evaporation. Chem Phys Lett. 2005;413(4–6):490–494. [Google Scholar]
- 278.Wu JM, Shih HC, Wu WT. Formation and photoluminescence of single-crystalline rutile TiO2 nanowires synthesized by thermal evaporation. Nanotechnology. 2006;17(1):105–109. [Google Scholar]
- 279.Wolcott A, Smith WA, Kuykendall TR, Zhao Y, Zhang JZ. Photoelectrochemical water splitting using dense and aligned TiO2 nanorod arrays. Small. 2009;5(1):104–111. doi: 10.1002/smll.200800902. [DOI] [PubMed] [Google Scholar]
- 280.Oota T, Yamai I. Vapor phase growth of titania whiskers by hydrolysis of titanium fouride. J Cryst Growth. 1984;66(2):262–268. [Google Scholar]
- 281.Francioso L, Taurino AM, Forleo A, Siciliano P. TiO2 nanowires array fabrication and gas sensing properties. Sens Actuators B Chem. 2008;130(1):70–76. [Google Scholar]
- 282.Lee JC, Park KS, Kim TG, Choi HJ, Sung YM. Controlled growth of high-quality TiO2 nanowires on sapphire and silica. Nanotechnology. 2006;17(17):4317–4321. [Google Scholar]
- 283.Wang ZL, Liu Y, Zhang Z, editors. Handbook of Nanophase and Nanostructured Materials. New York, NY: Kluwer Academic/Plenum; 2003. [Google Scholar]
- 284.Shan GB, Demopoulos GP. The synthesis of aqueous-dispersible anatase TiO2 nanoplatelets. Nanotechnology. 2010;21(2):025604. doi: 10.1088/0957-4484/21/2/025604. [DOI] [PubMed] [Google Scholar]
- 285.Arabatzis IM, Falaras P. Synthesis of porous nanocrystalline TiO2 foam. Nano Lett. 2003;3(2):249–251. [Google Scholar]
- 286.Gerischer H, Lubke M. A particle size effect in the sensitization of TiO2 electrodes by a CdS deposit. J Electroanal Chem. 1986;204:225–227. [Google Scholar]
- 287.Hickey SG, Riley DJ. Photoelectrochemical studies of CdS nanoparticle-modified electrodes. J Phys Chem B. 1999;103(22):4599–4602. [Google Scholar]
- 288.Toyoda T, Sato J, Shen Q. Effect of sensitization by quantum-sized CdS on photoacoustic and photoelectrochemical current spectra of porous TiO2 electrodes. Rev Sci Instrum. 2003;74(1):297–299. [Google Scholar]
- 289.Vogel R, Pohl K, Weller H. Sensitization of highly porous, polycrystalline TiO2 electrodes by quantum sized CdS. Chem Phys Lett. 1990;174(3–4):241–246. [Google Scholar]
- 290.Kohtani S, Kudo A, Sakata T. Spectral sensitization of a TiO2 semiconductor electrode by CdS microcrystals and its photoelectrochemical properties. Chem Phys Lett. 1993;206(1–4):166–170. [Google Scholar]
- 291.Jia H, Xu H, Hu Y, Tang Y, Zhang L. TiO2@CdS core–shell nanorods films: fabrication and dramatically enhanced photoelectrochemical properties. Electrochem Commun. 2007;9(3):354–360. [Google Scholar]
- 292.O’Hayre R, Nanu M, Schoonman J, Goossens A. A parametric study of TiO2/CuInS2 nanocomposite solar cells: how cell thickness, buffer layer thickness, and TiO2 particle size affect performance. Nanotechnology. 2007;18(5):055702. [Google Scholar]
- 293.Nanu M, Schoonman J, Goossens A. Inorganic nanocomposites of n- and p-type semiconductors: a new type of three-dimensional solar cells. Adv Mater. 2004;16(5):453–456. [Google Scholar]
- 294.Lenzmann F, Nanu M, Kijatkina O, Belaidi A. Substantial improvement of the photovoltaic characteristics of TiO2/CuInS2 interfaces by the use of recombination barrier coatings. Thin Solid Films. 2004;451–452:639–643. [Google Scholar]
- 295.Nanu M, Schoonman J, Goossens A. Nanocomposite three-dimensional solar cells obtained by chemical spray deposition. Nano Lett. 2005;5(9):1716–1719. doi: 10.1021/nl0509632. [DOI] [PubMed] [Google Scholar]
- 296.O’Hayre R, Nanu M, Schoonman J, Goossens A, Wang Q, Gratzel M. The influence of TiO2 particle size in TiO2/CuInS2 nanocomposite solar cells. Adv Funct Mater. 2006;16(12):1566–1576. [Google Scholar]
- 297.Cheng L, Zhang X, Liu B, et al. Template synthesis and characterization of WO3/TiO2 composite nanotubes. Nanotechnology. 2005;16(8):1341–1345. [Google Scholar]
- 298.Brinley E, Seal S, Folks R, Braunstein E, Kramer L, Seal S. High efficiency SiO2−TiO2 hybrid sol-gel antireflective coating for infrared applications. J Vac Sci Technol A. 2006;24(4):1141–1146. [Google Scholar]
- 299.Yao Y, Li G, Ciston S, Lueptow RM, Gray KA. Photoreactive TiO2/carbon nanotube composites: synthesis and reactivity. Environ Sci Technol. 2008;42(13):4952–4957. doi: 10.1021/es800191n. [DOI] [PubMed] [Google Scholar]
- 300.Arana J, Dona-Rodriguez JM, Tello Rendon E, et al. TiO2 activation by using activated carbon as a support. Part I. Surface characterization and decantability study. Appl Catal B. 2003;44(2):161–172. [Google Scholar]
- 301.Arana J, Dona-Rodriguez JM, Tello Rendon E, et al. TiO2 activation by using activated carbon as a support. Part II. Photoreactivity and FTIR study. Appl Catal B. 2003;44(2):153–160. [Google Scholar]
- 302.Wang W, Serp P, Kalck P, Faria JL. Photocatalytic degradation of phenol on MWNT and titania composite catalysts prepared by a modified sol-gel method. Appl Catal B. 2005;56(4):305–312. [Google Scholar]
- 303.Lee SH, Pumprueg S, Moudgil B, Sigmund W. Inactivation of bacterial endospores by photocatalytic nanocomposites. Colloids Surf B. 2005;40(2):93–98. doi: 10.1016/j.colsurfb.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 304.Yu Y, Yu JC, Chan CY, et al. Enhancement of adsorption and photocatalytic activity of TiO2 by using carbon nanotubes for the treatment of azo dye. Appl Catal B. 2005;61(1–2):1–11. [Google Scholar]
- 305.Feng W, Feng Y, Wu Z, Fujii A, Ozaki M, Yoshino K. Optical and electrical characterizations of nanocomposite film of titania adsorbed onto oxidized multiwalled carbon nanotubes. J Phys Condens Matter. 2005;17(27):4361–4368. [Google Scholar]
- 306.Kang SZ, Cui Z, Mu J. Composite of carboxyl-modified multi-walled carbon nanotubes and TiO2 nanoparticles: preparation and photocata-lytic activity. Fullerenes Nanotubes Carbon Nanostruct. 2007;15(2):81–88. [Google Scholar]
- 307.Fan W, Gao L, Sun J. Anatase TiO2-coated multi-wall carbon nanotubes with the vapor phase method. J Am Ceram Soc. 2006;89(2):731–733. [Google Scholar]
- 308.Jitianu A, Cacciaguerra T, Benoit R, Delpeux S, Beguin F, Bonnamy S. Synthesis and characterization of carbon nanotubes-TiO2 nanocomposites. Carbon. 2004;42(5–6):1147–1151. [Google Scholar]
- 309.Yu H, Zhao H, Quan X, Chen S. Preparation and characterization of aligned carbon nanotubes coated with titania nanoparticles. Chin Sci Bull. 2006;51(18):2294–2296. [Google Scholar]
- 310.Kwak SY, Kim SH, Kim SS. Hybrid organic/inorganic reverse osmosis (RO) membrane for bactericidal anti-fouling. 1. Preparation and characterization of TiO2 nanoparticle self-assembled aromatic polyamide thin-film-composite (TFC) membrane. Environ Sci Technol. 2001;35(11):2388–2394. doi: 10.1021/es0017099. [DOI] [PubMed] [Google Scholar]
- 311.Nabid MR, Golbabaee M, Moghaddam AB, Dinarvand R, Sedghi R. Polyaniline/TiO2 nanocomposite: enzymatic synthesis and electrochemical properties. Int J Electrochem Sci. 2008;3:1117–1126. [Google Scholar]
- 312.Zang L, Wan M. Polyaniline/TiO2 composite nanotubes. J Phys Chem B. 2003;107(28):6748–6753. [Google Scholar]
- 313.Gangopadhyay R, De A. Conducting polymer nanocomposites: a brief overview. Chem Mater. 2000;12(3):608–622. [Google Scholar]
- 314.Xia H, Wang Q. Ultrasonic irradiation: a novel approach to prepare conductive polyaniline/nanocrystalline titanium oxide composites. Chem Mater. 2002;14(5):2158–2165. [Google Scholar]
- 315.Schnitzler DC, Zarbin AJG. Organic/inorganic hybrid materials formed from TiO2 nanoparticles and polyaniline. J Braz Chem Soc. 2004;15(3):378–384. [Google Scholar]
- 316.Fan X, Lin L, Messersmith PB. Surface-initiated polymerization from TiO2 nanoparticle surfaces through a biomimetic initiator: a new route toward polymer–matrix nanocomposites. Compos Sci Technol. 2006;66(9):1198–1204. [Google Scholar]
- 317.Liu Y, Wang A, Claus R. Molecular self-assembly of TiO2/polymer nanocomposite films. J Phys Chem B. 1997;101(8):1385–1388. [Google Scholar]
- 318.Rizza R, Fitzmaurice D, Hearne S, et al. Self-assembly of monolayers of semiconductor nanocrystallites. Chem Mater. 1997;9(12):2969–2982. [Google Scholar]
- 319.Kovtyukhova N, Ollivier PJ, Chizhik S, et al. Self-assembly of ultra-thin composite TiO2/polymer films. Thin Solid Films. 1999;337(1–2):166–170. [Google Scholar]
- 320.Bard AJ. Design of semiconductor photoelectrochemical systems for solar energy conversion. J Phys Chem. 1982;86(2):172–177. [Google Scholar]
- 321.Gratzel M, editor. Energy Resources Through Photochemistry and Catalysis. New York, NY: Academic Press; 1983. [Google Scholar]
- 322.Kalyanasundaram K, Gratzel M, Pelizzetti E. Interfacial electron transfer in colloidal metal and semiconductor dispersions and photodecomposition of water. Coord Chem Rev. 1986;69:57–125. [Google Scholar]
- 323.Parmon VN, Zamareav KI. Photocatalysis in energy production. In: Serpone N, Pelizzetti E, editors. Photocatalysis – Fundamentals and Applications. New York, NY: Wiley Interscience; 1989. p. 565. [Google Scholar]
- 324.Pelizzetti E, Schiavello M, editors. Photochemical Conversion and Storage of Solar Energy. Dordrecht, Germany: Kluwer Academic Publishers; 1991. [Google Scholar]
- 325.Schiavello M, editor. Photocatalysis and Environment. Dordrecht, Germany: Kluwer Academic Publishers; 1988. [Google Scholar]
- 326.Ollis DF, Al-Ekabi H, editors. Photocatalytic Purification and Treatment of Water and Air. Amsterdam, The Netherlands: Elsevier; 1993. [Google Scholar]
- 327.Hurum DC, Agrios AG, Gray KA, Rajh T, Thurnauer MC. Explaining the enhanced photocatalytic activity of degussa p25 mixed-phase TiO2 using EPR. J Phys Chem B. 2003;107(19):4545–4549. [Google Scholar]
- 328.Shankar K, Basham JI, Allam NK, et al. Recent advances in the use of TiO2 nanotube and nanowire arrays for oxidative photoelectrochemistry. J Phys Chem C. 2009;113(16):6327–6359. [Google Scholar]
- 329.Mauzerall D, Ballard SG. Ionization in solution by photoactivated electron transfer. Annu Rev Phys Chem. 1982;33:377–407. [Google Scholar]
- 330.Forster T. Transfer mechanisms of electronic excitation. Faraday Discuss Chem Soc. 1959;27:7–17. [Google Scholar]
- 331.Okabe H. Photochemistry of Small Molecules. New York, NY: Wiley-Interscience; 1978. [Google Scholar]
- 332.Gilbert A, Baggott J. Essentials of Molecular Photochemistry. Boca Raton, FL: CRC Press; 1991. [Google Scholar]
- 333.Fox MA, Chanon M, editors. Photoinduced Electron Transfer, Part A: Conceptual Basis. Amsterdam, The Netherlands: Elsevier; 1988. [Google Scholar]
- 334.Serpone N, Pelizzetti E, editors. Photocatalysis, Fundamentals and Applications. New York, NY: Wiley-Interscience; 1989. [Google Scholar]
- 335.Gratzel M. Heterogeneous Photochemical Electron Transfer. Boca Raton, FL: CRC Press; 1989. [Google Scholar]
- 336.Julliard M, Chanon M. Photoelectron-transfer catalysis: its connections with thermal and electrochemical analogs. Chem Rev. 1983;83(4):425–506. [Google Scholar]
- 337.Kavarnos GJ, Turro NJ. Photosensitization by reversible electron transfer: theories, experimental evidence, and examples. Chem Rev. 1986;86(2):401–449. [Google Scholar]
- 338.Fox MA, Dulay MT. Heterogeneous photocatalysis. Chem Rev. 1993;93(1):341–357. [Google Scholar]
- 339.Kamat PV. Photochemistry on nonreactive and reactive (semiconductor) surfaces. Chem Rev. 1993;93(1):267–300. [Google Scholar]
- 340.Mills A, Hunte SL. An overview of semiconductor photocatalysis. J Photochem Photobiol A Chem. 1997;108(1):1–35. [Google Scholar]
- 341.Hoffmann MR, Martin ST, Choi W, Bahnemannt DW. Environmental applications of semiconductor photocatalysis. Chem Rev. 1995;95:69–96. [Google Scholar]
- 342.Augustynski J. The role of the surface intermediates in the photoelectrochemical behavior of anatase and rutile TiO2. Electrochim Acta. 1993;38(1):43–46. [Google Scholar]
- 343.Fujishima A, Zhang X, Tryk DA. TiO2 photocatalysis and related surface phenomena. Surf Sci Rep. 2008;63(12):515–582. [Google Scholar]
- 344.Ni M, Leung MKH, Leung DY, Sumathy K. A review and recent developments in photocatalytic water splitting using TiO2 for hydrogen production. Renew Sustain Energy Rev. 2007;11(3):401–425. [Google Scholar]
- 345.Kamat PV. Meeting the clean energy demand: nanostructure architectures for solar energy conversion. J Phys Chem C. 2007;111(7):2834–2860. [Google Scholar]
- 346.Nowotny J, Bak T, Nowotny MK, Sheppard LR. Titanium dioxide for solar-hydrogen. I. Functional properties. Int J Hydrogen Energy. 2007;32(14):2609–2629. [Google Scholar]
- 347.Nowotny J, Bak T, Nowotny MK, Sheppard LR. Titanium dioxide for solar-hydrogen. III. Kinetic effects at elevated temperatures. Int J Hydrogen Energy. 2007;32(14):2644–2650. [Google Scholar]
- 348.Wold A. Photocatalytic properties of TiO2. Chem Mater. 1993;5(3):280–283. [Google Scholar]
- 349.Mor GK, Varghese OK, Paulose M, Shankar K, Grimes CA. A review on highly ordered, vertically oriented TiO2 nanotube-arrays: fabrication, material properties, and solar energy applications. Solar Energy Mater Solar Cells. 2006;90(14):2011–2075. [Google Scholar]
- 350.Tachikawa T, Fujitsuka M, Majima T. Mechanistic insight into the TiO2 photocatalytic reactions: design of new photocatalysts. J Phys Chem C. 2007;111(14):5259–5275. [Google Scholar]
- 351.Aroutiounian VM, Arakelyan VM, Shahnazaryan GE. Metal oxide photoelectrodes for hydrogen generation using solar radiation-driven water splitting. Solar Energy. 2005;78(5):581–592. [Google Scholar]
- 352.Gratzel M. Photoelectrochemical cells. Nature. 2001;414:338–344. doi: 10.1038/35104607. [DOI] [PubMed] [Google Scholar]
- 353.Murphy TW., Jr Home photovoltaic systems for physicists. Phys Today. 2008;61(7):42–47. [Google Scholar]
- 354.Juodkazis K, Juodkazyte J, Jelmakas E, et al. Photoelectrolysis of water: solar hydrogen – achievements and perspectives. Opt Express. 2010;18(Suppl 2):A147–A160. doi: 10.1364/OE.18.00A147. [DOI] [PubMed] [Google Scholar]
- 355.Sato S, White JM. Photoassisted hydrogen production from titania and water. J Phys Chem. 1981;85(5):592–594. [Google Scholar]
- 356.Fujishima A, Rao TN, Tryk DA. Titanium dioxide photocatalysis. J Photochem Photobiol C Photochem Rev. 2000;1(1):1–21. [Google Scholar]
- 357.Wrighton MS, Ginley DS, Wolczanski PT, Ellis AB, Morse DL, Linz A. Photoassisted electrolysis of water by irradiation of a titanium dioxide electrode. Proc Natl Acad Sci U S A. 1975;72(4):1518–1522. doi: 10.1073/pnas.72.4.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Duonghong D, Borgarello E, Graetzel M. Dynamics of light-induced water cleavage in colloidal systems. J Am Chem Soc. 1981;103(16):4685–4690. [Google Scholar]
- 359.Van Hieu N, Lichtman D. Band gap radiation-induced photodesorption from polycrystalline powder surfaces of SrTiO3. J Catal. 1982;73(2):329–336. [Google Scholar]
- 360.Courbon H, Formenti M, Pichat P. Study of oxygen isotopic exchange over ultraviolet irradiated anatase samples and comparison with the photooxidation of isobutane into acetone. J Phys Chem. 1977;81(6):550–554. [Google Scholar]
- 361.Bickley RI, Munuera G, Stone FS. Photoadsorption and photocatalysis at rutile surfaces: II. Photocatalytic oxidation of isopropanol. J Catal. 1973;31(3):398–407. [Google Scholar]
- 362.Munuera G, Rives-Amau V, Saucedo A. Photo-adsorption and photodesorption of oxygen on highly hydroxylated TiO2 surfaces. Part 1. Role of hydroxyl groups in photo-adsorption. J Chem Soc Faraday Trans 1. 1979;75:736–747. [Google Scholar]
- 363.Anpo M, Kubokawa Y, Fujii T, Suzuki S. Quantum chemical and molecular oxygen-18 tracer studies of the activation of oxygen in photocatalytic oxidation reactions. J Phys Chem. 1984;88(12):2572–2575. [Google Scholar]
- 364.Lu G, Linsebigler A, Yates JT., Jr The photochemical identification of two chemisorption states for molecular oxygen on TiO2(110) J Chem Phys. 1995;102(7):3005–3008. [Google Scholar]
- 365.Yanagisawa Y, Ota Y. Thermal and photo-stimulated desorption of chemisorbed oxygen molecules from titanium dioxide surfaces. Surf Sci Lett. 1991;254(1–3):L433–L436. [Google Scholar]
- 366.Funk S, Hokkanen B, Burghaus U, Ghicov A, Schmuki P. Unexpected adsorption of oxygen on TiO2 nanotube arrays: influence of crystal structure. Nano Lett. 2007;7(4):1091–1094. doi: 10.1021/nl062797j. [DOI] [PubMed] [Google Scholar]
- 367.Inderwildi OR, Kraft M. Adsorption, diffusion and desorption of chlorine on and from rutile TiO2{110}: a theoretical investigation. Chemphyschem. 2007;8(3):444–451. doi: 10.1002/cphc.200600653. [DOI] [PubMed] [Google Scholar]
- 368.Uner D, Tapan NA, Özen Ý, Üner M. Oxygen adsorption on Pt/TiO2 catalysts. Appl Catal A. 2003;251(2):225–234. [Google Scholar]
- 369.Kimmel GA, Petrik NG. Tetraoxygen on reduced TiO2(110): oxygen adsorption and reactions with bridging oxygen vacancies. Phys Rev Lett. 2008;100(19):196102. doi: 10.1103/PhysRevLett.100.196102. [DOI] [PubMed] [Google Scholar]
- 370.Petrik NG, Kimmel GA. Off-normal CO2 desorption from the photooxidation of CO on reduced TiO2(110) J Phys Chem Lett. 2010;1(17):2508–2513. [Google Scholar]
- 371.Ajo HM, Bondzie VA, Campbell CT. Propene adsorption on gold particles on TiO2(110) Catal Lett. 2002;78(1–4):359–368. [Google Scholar]
- 372.Chen MT, Lin YF, Liao LF, Lien CF, Lin JL. Adsorption and reactions of CH2Br2 on TiO2: effects of H2O and O2. Int J Photoenergy. 2004;6(1):35–41. [Google Scholar]
- 373.Lee J, Zhang Z, Yates JT., Jr Electron-stimulated positive-ion desorption caused by charge transfer from adsorbate to substrate: oxygen adsorbed on TiO2(110) Phys Rev B. 2009;79(8):081408.1–081408.4. [Google Scholar]
- 374.Schrauzer GN, Guth TD. Photocatalytic reactions. 1. Photolysis of water and photoreduction of nitrogen on titanium dioxide. J Am Chem Soc. 1977;99(22):7189–7193. [Google Scholar]
- 375.Bickley RI, Jayanty RKM, Navio JA, Real C, Macias M. Photo-oxidative fixation of molecular nitrogen on TiO2 (rutile) surfaces: the nature of the adsorbed nitrogen-containing species. Surf Sci. 1991;251–252:1052–1056. [Google Scholar]
- 376.Seiji Y, Taro O, Tsunehiro T. Ammonia photo-oxidation over TiO2 photo-catalyst. Catalysts Catal. 2006;48(2):110–112. [Google Scholar]
- 377.Inoue T, Fujishima A, Konishi S, et al. Photoelectrocatalytic reduction of carbon dioxide in aqueous suspensions of semiconductor powders. Nature. 1979;277(22):637–638. [Google Scholar]
- 378.Hirano K, Inoue K, Yatsu T. Photocatalysed reduction of CO2 in aqueous TiO2 suspension mixed with copper powder. J Photochem Photobiol A: Chem. 1992;64:255–258. [Google Scholar]
- 379.Tennakone K. Photoreduction of carbonic acid by mercury coated n-titanium dioxide. Sol Energy Mater. 1984;10(2):235–238. [Google Scholar]
- 380.Rophael WM, Malati MA. The photocatalysed reduction of aqueous sodium carbonate to carbon using platinised titania. J Chem Soc Chem Commun. 1987:1418–1420. [Google Scholar]
- 381.Ishitani O, Inoue C, Suzuki Y, Ibusuki T. Photocatalytic reduction of carbon dioxide to methane and acetic acid by an aqueous suspension of metal-deposited TiO2. J Photochem Photobiol A Chem. 1993;72(3):269–271. [Google Scholar]
- 382.Woolerton TW, Sheard S, Reisner E, Pierce E, Ragsdale SW, Armstrong FA. Efficient and clean photoreduction of CO2 to CO by enzyme-modified TiO2 nanoparticles using visible light. J Am Chem Soc. 2010;132(7):2132–2133. doi: 10.1021/ja910091z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Wang C, Thompson RL, Baltrus J, Matranga C. Visible light photoreduction of CO2 using CdSe/Pt/TiO2 heterostructured catalysts. J Phys Chem Lett. 2010;1(1):48–53. [Google Scholar]
- 384.Chen L, Graham ME, Li G, Gentner DR, Dimitrijevic NM, Gray KA. Photoreduction of CO2 by TiO2 nanocomposites synthesized through reactive direct current magnetron sputter deposition. Thin Solid Films. 2009;517(19):5641–5645. [Google Scholar]
- 385.Cueto LF, Martínez GT, Zavala G, Sánchez EM. Surface characterization and CO2 reduction using electrodeposited silver particles over TiO2 thin film. J Nano Res. 2010;9:89–100. [Google Scholar]
- 386.Draper RB, Fox MA. Titanium dioxide photosensitized reactions studied by diffuse reflectance flash photolysis in aqueous suspensions of TiO2 powder. Langmuir. 1990;6(8):1396–1402. [Google Scholar]
- 387.Fitzmaurice DJ, Eschle M, Frei H, Moser J. Time-resolved rise of iodine molecule (I−) upon oxidation of iodide at aqueous titania colloid. J Phys Chem. 1993;97(15):3806–3812. [Google Scholar]
- 388.O’Regan B, Gratzel M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature. 1991;353:737–740. [Google Scholar]
- 389.Henderson MA. Photooxidation of acetone on TiO2(110): conversion to acetate via methyl radical ejection. J Phys Chem B. 2005;109(24):12062–12070. doi: 10.1021/jp0507546. [DOI] [PubMed] [Google Scholar]
- 390.De Jongh PE, Vanmaekelbergh D. Trap-limited electronic transport in assemblies of nanometer-size TiO2 particles. Phys Rev Lett. 1996;77(16):3427–3430. doi: 10.1103/PhysRevLett.77.3427. [DOI] [PubMed] [Google Scholar]
- 391.Zaleska A. Doped-TiO2: a review. Recent Patents Eng. 2008;2:157–164. [Google Scholar]
- 392.Dvoranová D, Brezová V, Mazúr M, Malati MA. Investigations of metal-doped titanium dioxide photocatalysts. Appl Catal B Environ. 2002;37(2):91–105. [Google Scholar]
- 393.Umebayashi T, Yamaki T, Itoh H, Asak K. Analysis of electronic structures of 3d transition metal-doped TiO2 based on band calculations. J Phys Chem Solids. 2002;63(10):1909–1920. [Google Scholar]
- 394.Davydov L, Reddy EP, France P, Smirniotis PG. Transition metal-substituted titania-loaded MCM-41 as photocatalysts for the degradation of aqueous organics in visible light. J Catal. 2001;203(1):157–167. [Google Scholar]
- 395.Anpo M. Use of visible light. Second-generation titanium dioxide photocatalysts prepared by the application of an advanced metal ion-implantation method. Pure Appl Chem. 2000;72(9):1787–1792. [Google Scholar]
- 396.Fuerte A, Hernández-Alonso MD, Maira AJ, et al. Visible light-activated nanosized doped-TiO2 photocatalysts. Chem Commun. 2001;24:2718–2719. [Google Scholar]
- 397.Yamashita H, Harada M, Misaka J, et al. Application of ion beam techniques for preparation of metal ion-implanted TiO2 thin film photocatalyst available under visible light irradiation: metal ion-implantation and ionized cluster beam method. J Synchrotron Radiat. 2001;8(Pt 2):569–571. doi: 10.1107/s090904950001712x. [DOI] [PubMed] [Google Scholar]
- 398.Wilke K, Breuer HD. The influence of transition metal doping on the physical and photocatalytic properties of titania. J Photochem Photobiol A Chem. 1999;121(1):49–53. [Google Scholar]
- 399.Wilke K, Breuer HD. Transition metal doped titania: physical properties and photocatalytic behaviour. Z Phys Chem. 1999;213:135–140. [Google Scholar]
- 400.Hong NH, Sakai J, Prellier W, Ruyter A. Room temperature ferromagnetism in laser ablated transition-metal-doped TiO2 thin films on various types of substrates. J Phys D Appl Phys. 2005;38(6):816–821. [Google Scholar]
- 401.Narayanan BN, Koodathil R, Gangadharan T, Yaakob Z, Saidu FK, Chandralayam S. Preparation and characterization of exfoliated polyaniline/montmorillonite nanocomposites. Mater Sci Eng B. 2010;168(1–3):242–244. [Google Scholar]
- 402.Xu AW, Gao Y, Liu HQ. The preparation, characterization, and their photocatalytic activities of rare-earth-doped TiO2 nanoparticles. J Catal. 2002;207(2):151–157. [Google Scholar]
- 403.Moon J, Takagi H, Fujishiro Y, Awano M. Preparation and characterization of the Sb-doped TiO2 photocatalysts. J Mater Sci. 2001;36(4):949–955. [Google Scholar]
- 404.Li J, Zeng HC. Hollowing Sn-doped TiO2 nanospheres via Ostwald ripening. J Am Chem Soc. 2007;129(51):15839–15847. doi: 10.1021/ja073521w. [DOI] [PubMed] [Google Scholar]
- 405.Shen Y, Xiong T, Li T, Yang K. Tungsten and nitrogen co-doped TiO2 nano-powders with strong visible light response. Appl Catal B. 2008;83(3–4):177–185. [Google Scholar]
- 406.Asahi R, Morikawa T, Ohwaki T, Aoki K, Taga Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science. 2001;293(5528):269–271. doi: 10.1126/science.1061051. [DOI] [PubMed] [Google Scholar]
- 407.Ihara T, Miyoshi M, Iriyama Y, Matsumoto O, Sugihara S. Visible-light-active titanium oxide photocatalyst realized by an oxygen deficient structure and by nitrogen doping. Appl Catal B Environ. 2003;42(4):403–409. [Google Scholar]
- 408.Sakthivel S, Kisch H. Photocatalytic and photoelectrochemical properties of nitrogen-doped titanium dioxide. Chemphyschem. 2003;4(5):487–490. doi: 10.1002/cphc.200200554. [DOI] [PubMed] [Google Scholar]
- 409.Irie H, Watanabe Y, Hashimoto K. Carbon-doped anatase TiO2 powders as a visible-light sensitive photocatalyst. Chem Lett. 2003;32(8):772–773. [Google Scholar]
- 410.Park JH, Kim S, Bard AJ. Novel carbon-doped TiO2 nanotube arrays with high aspect ratios for efficient solar water splitting. Nano Lett. 2006;6(1):24–28. doi: 10.1021/nl051807y. [DOI] [PubMed] [Google Scholar]
- 411.Yamaki T, Umebayashi T, Sumita T, et al. Fluorine-doping in titanium dioxide by ion implantation technique. Nucl Instrum Methods Phys Res Sec B. 2003;206:254–258. [Google Scholar]
- 412.Ohno T, Mitsui T, Matsumura M. Photocatalytic activity of S-doped TiO2 photocatalyst under visible light. Chem Lett. 2003;32(4):364–365. [Google Scholar]
- 413.Liu Y, Chen X, Li J, Burda C. Photocatalytic degradation of azo dyes by nitrogen-doped TiO2 nanocatalysts. Chemosphere. 2005;61(1):11–18. doi: 10.1016/j.chemosphere.2005.03.069. [DOI] [PubMed] [Google Scholar]
- 414.Yu JC, Zhang L, Zheng Z, Zhao J. Synthesis and characterization of phosphated mesoporous titanium dioxide with high photocatalytic activity. Chem Mater. 2003;15(11):2280–2286. [Google Scholar]
- 415.Lei L, Su Y, Zhou M, Zhang X, Chen X. Fabrication of multi-nonmetal-doped TiO2 nanotubes by anodization in mixed acid electrolyte. Mater Res Bull. 2007;42(12):2230–2236. [Google Scholar]
- 416.Kang IC, Zhang Q, Yin S, Stao T, Saito F. Preparation of a visible sensitive carbon doped TiO2 photo-catalyst by grinding TiO2 with ethanol and heating treatment. Appl Catal B. 2008;80(1–2):81–87. [Google Scholar]
- 417.Bettinelli M, Dallacasa V, Falcomer D, et al. Photocatalytic activity of TiO2 doped with boron and vanadium. J Hazard Mater. 2007;146(3):529–534. doi: 10.1016/j.jhazmat.2007.04.053. [DOI] [PubMed] [Google Scholar]
- 418.Liu C, Tang X, Mo C, Qiang Z. Characterization and activity of visible-light-driven TiO2 photocatalyst codoped with nitrogen and cerium. J Solid State Chem. 2008;181(4):913–919. [Google Scholar]
- 419.Kim SW, Khan R, Kim TJ, Kim WJ. Synthesis, characterization, and application of Zr, S co-doped TiO2 as visible-light active photocatalyst. Bull Korean Chem Soc. 2008;29(6):1217–1223. [Google Scholar]
- 420.Sakthivel S, Janczarek M, Kisch H. Visible light activity and photoelectrochemical properties of nitrogen-doped TiO2. J Phys Chem B. 2004;108(50):19384–19387. [Google Scholar]
- 421.Shankar K, Tep KC, Mor GK, et al. An electrochemical strategy to incorporate nitrogen in nanostructured TiO2 thin films: modification of bandgap and photoelectrochemical properties. J Phys D Appl Phys. 2006;39(11):2361–2366. [Google Scholar]
- 422.Wu ZB, Dong F, Zhao WR, Guo S. Visible light induced electron transfer process over nitrogen doped TiO2 nanocrystals prepared by oxidation of titanium nitride. J Hazard Mater. 2008;157(1):57–63. doi: 10.1016/j.jhazmat.2007.12.079. [DOI] [PubMed] [Google Scholar]
- 423.Lindgren T, Lu J, Hoel A, Granqvist CG, Torres GR, Lindquist SE. Photo electrochemical study of sputtered nitrogen-doped titanium dioxide thin films in aqueous electrolyte. Solar Energy Mater Solar Cells. 2004;84(1–4):145–157. [Google Scholar]
- 424.Lindgren T, Mwabora JM, Avendano E, et al. Photoelectrochemical and optical properties of nitrogen doped titanium dioxide films prepared by reactive DC magnetron sputtering. J Phys Chem B. 2003;107(24):5709–5716. [Google Scholar]
- 425.Diwald O, Thompson TL, Goralski EG, Walck SD, Yates JT., Jr The effect of nitrogen ion implantation on the photoactivity of TiO2 rutile single crystals. J Phys Chem B. 2004;108(1):52–57. [Google Scholar]
- 426.Serpone N. Is the band gap of pristine TiO2 narrowed by anion- and cation-doping of titanium dioxide in second-generation photocatalysts? J Phys Chem B. 2006;110(48):24287–24293. doi: 10.1021/jp065659r. [DOI] [PubMed] [Google Scholar]
- 427.Wang H, Lewis JP. Second-generation photocatalytic materials: anion-doped TiO2. J Phys Condens Matter. 2006;18(2):421–434. [Google Scholar]
- 428.Zhao W, Ma W, Chen C, Zhao J, Shuai Z. Efficient degradation of toxic organic pollutants with Ni2O3/TiO2−x Bx under visible irradiation. J Am Chem Soc. 2004;126(15):4782–4783. doi: 10.1021/ja0396753. [DOI] [PubMed] [Google Scholar]
- 429.Gao B, Ma Y, Cao Y, Yang W, Yao J. Great enhancement of photocatalytic activity of nitrogen-doped titania by coupling with tungsten oxide. J Phys Chem B. 2006;110(29):14391–14397. doi: 10.1021/jp0624606. [DOI] [PubMed] [Google Scholar]
- 430.Ohno T, Miyamoto Z, Nishijima K, et al. Sensitization of photocatalytic activity of S- or N-doped TiO2 particles by adsorbing Fe3+cations. Appl Catal A. 2006;302(1):62–68. [Google Scholar]
- 431.Morikawa T, Irokawa Y, Ohwaki T. Enhanced photocatalytic activity of TiO2−x Nx loaded with copper ions under visible light irradiation. Appl Catal A. 2006;314(1):123–127. [Google Scholar]
- 432.Hirai T, Suzuki K, Komasawa I. Preparation and photocatalytic properties of composite CdS nanoparticles–titanium dioxide particles. J Colloid Interface Sci. 2001;244(2):262–265. [Google Scholar]
- 433.Lettmann C, Hinrichs H, Maier WF. Combinatorial discovery of new photocatalysts for water purification with visible light. Angew Chem Int Ed Engl. 2001;40(17):3160–3164. doi: 10.1002/1521-3773(20010903)40:17<3160::AID-ANIE3160>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 434.Liu B, Zhao X, Zhang N, Zhao Q, He X, Feng J. Photocatalytic mechanism of TiO2−CeO2 films prepared by magnetron sputtering under UV and visible light. Surf Sci. 2005;595(1–3):203–211. [Google Scholar]
- 435.Grasso C, Burgelman M. Theoretical study on the effect of an intermediate layer in CIS-based ETA-solar cells. Thin Solid Films. 2004;451–452:156–159. [Google Scholar]
- 436.Papp J, Soled S, Dwight K, Wold A. Surface acidity and photocatalytic activity of TiO2, WO3/TiO2, and MoO3/TiO2 photocatalysts. Chem Mater. 1994;6(4):496–500. [Google Scholar]
- 437.Mane RS, Lee WJ, Pathan HM, Han SH. Nanocrystalline TiO2/ZnO thin films: fabrication and application to dye-sensitized solar cells. J Phys Chem B. 2005;109(51):24254–24259. doi: 10.1021/jp0531560. [DOI] [PubMed] [Google Scholar]
- 438.Liao DL, Badour CA, Liao BQ. Preparation of nanosized TiO2/ZnO composite catalyst and its photocatalytic activity for degradation of methyl orange. J Photochem Photobiol A: Chem. 2008;194(1):11–19. [Google Scholar]
- 439.Kansal SK, Singh M, Sud D. Studies on TiO2/ZnO photocatalysed degradation of lignin. J Hazard Mater. 2008;153(1–2):412–417. doi: 10.1016/j.jhazmat.2007.08.091. [DOI] [PubMed] [Google Scholar]
- 440.Jiang Y, Sun Y, Liu H, Zhu F, Yin H. Solar photocatalytic decolorization of C.I. Basic Blue 41 in an aqueous suspension of TiO2−ZnO. Dyes Pigments. 2008;78(1):77–83. [Google Scholar]
- 441.Tada H, Hattori A, Tokihisa Y, Imai K, Tohge N, Ito S. A patterned-TiO2/SnO2 bilayer type photocatalyst. J Phys Chem B. 2000;104(19):4585–4587. [Google Scholar]
- 442.Liu Z, Sun DD, Guo P, Leckie JO. An efficient bicomponent TiO2/SnO2 nanofiber photocatalyst fabricated by electrospinning with a side-by-side dual spinneret method. Nano Lett. 2007;7(4):1081–1085. doi: 10.1021/nl061898e. [DOI] [PubMed] [Google Scholar]
- 443.Wang C, Xu BQ, Wang X, Zhao J. Preparation and photocatalytic activity of ZnO/TiO2/SnO2 mixture. J Solid State Chem. 2005;178(11):3500–3506. [Google Scholar]
- 444.Lin CF, Wu CH, Onn ZN. Degradation of 4-chlorophenol in TiO2, WO3, SnO2, TiO2/WO3 and TiO2/SnO2 systems. J Hazard Mater. 2008;154(1–3):1033–1039. doi: 10.1016/j.jhazmat.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 445.Robel I, Bunker BA, Kamat PV. Single-walled carbon nanotube-CdS nanocomposites as light-harvesting assemblies: photoinduced chargetransfer interactions. Adv Mater. 2005;17(20):2458–2463. [Google Scholar]
- 446.Grätzel M. Dye-sensitized solar cells. J Photochem Photobiol C Photochem Rev. 2003;4:145–153. [Google Scholar]
- 447.Kubo W, Murakoshi K, Kitamura T, et al. Quasi-solid-state dye-sensitized tio2 solar cells: effective charge transport in mesoporous space filled with gel electrolytes containing iodide and iodine. J Phys Chem B. 2001;105(51):12809–12815. [Google Scholar]
- 448.Bisquert J, Cahen D, Hodes G, Rühle S, Zaban R. Physical chemical principles of photovoltaic conversion with nanoparticulate, mesoporous dye-sensitized solar cells. J Phys Chem B. 2004;108(24):8106–8118. [Google Scholar]
- 449.Smestad GP. Education and solar conversion: demonstrating electron transfer. Sol Energy Mater Solar Cells. 1998;55(1–2):157–178. [Google Scholar]
- 450.Nakade S, Saito Y, Kubo W, Kitamura T, Wada Y, Yanagida S. Influence of TiO2 nanoparticle size on electron diffusion and recombination in dye-sensitized TiO2 solar cells. J Phys Chem B. 2003;107(33):8607–8611. [Google Scholar]
- 451.Nakade S, Matsuda M, Kambe S, et al. Dependence of TiO2 nanoparticle preparation methods and annealing temperature on the efficiency of dye-sensitized solar cells. J Phys Chem B. 2002;106(39):10004–10010. [Google Scholar]
- 452.Sodergren S, Hagfeldt A, Olsson J, Lindquist SE. Theoretical models for the action spectrum and the current–voltage characteristics of microporous semiconductor films in photoelectrochemical cells. J Phys Chem. 1994;98(21):5552–5556. [Google Scholar]
- 453.Cao F, Oskam G, Meyer GJ, Searson PC. Electron transport in porous nanocrystalline TiO2. J Phys Chem. 1996;100(42):17021–17027. [Google Scholar]
- 454.Solbrand A, Lindstrom H, Rensmo H, Hagfelddt A, Lindquist SE. Electron transport in the nanostructured TiO2–electrolyte system studied with time-resolved photocurrents. J Phys Chem B. 1997;101(14):2514–2518. [Google Scholar]
- 455.Kabra K, Chaudhury R, Sawney RL. Treatment of hazardous organic and inorganic compounds through aqueous-phase photocatalysis: a review. Ind Eng Chem Res. 2004;43(24):7683–7696. [Google Scholar]
- 456.Hermann JM. Heterogeneous photocatalysis: state of the art and present applications. Top Catal. 2005;34(1–4):49–65. [Google Scholar]
- 457.Melemeni M, Stamatakis D, Xekoukoulotakis NP, Mantzavinos D, Kalogerakis N. Disinfection of municipal wastewater by TiO2 photocatalysis with UV-A, visible and solar irradiation and BDD electrolysis. Global NEST J. 2009;11(3):357–363. [Google Scholar]
- 458.Palmisano G, Augugliaro V, Pagliaro M, Palmisano L. Photocatalysis: a promising route for 21st century organic chemistry. Chem Commun (Camb) 2007;(33):3425–3437. doi: 10.1039/b700395c. [DOI] [PubMed] [Google Scholar]
- 459.Ollis DF. Photocatalytic purification and remediation of contaminated air and water. C R Acad Sci Paris Chem. 2000;3(6):405–411. [Google Scholar]
- 460.McCullagh C, Robertson JMC, Bahnemann DW, Robertson PKJ. The application of TiO2 photocatalysis for disinfection of water contaminated with pathogenic micro-organisms: a review. Res Chem Intermed. 2007;33(3–5):359–375. [Google Scholar]
- 461.Carp O, Huisman CL, Reller A. Photoinduced reactivity of titanium dioxide. Prog Solid State Chem. 2004;32(1–2):33–177. [Google Scholar]
- 462.Saint Gobain Glass Co. Ltd Available from: www.selfcleaningglass.comAccessed 2010 Jun 1
- 463.Nippon Sheet Glass Co. Ltd Website Available from: http://www.nsg.co.jp/en/Accessed 2010 Jun 1
- 464.Han W, Wang YD, Zheng YF. In vitro biocompatibility study of nano TiO2 materials. Adv Mater Res. 2008;47–50:1438–1441. [Google Scholar]
- 465.Carbone R, Marangi I, Zanardi A, et al. Biocompatibility of cluster-assembled nanostructured TiO2 with primary and cancer cells. Biomaterials. 2006;27(17):3221–3229. doi: 10.1016/j.biomaterials.2006.01.056. [DOI] [PubMed] [Google Scholar]
- 466.Karpagavalli R, Zhou A, Chellamuthu P, Nguyen K. Corrosion behavior and biocompatibility of nanostructured TiO2 film on Ti6Al4V. J Biomed Mater Res A. 2007;83(4):1087–1095. doi: 10.1002/jbm.a.31447. [DOI] [PubMed] [Google Scholar]
- 467.Cui C, Liu H, Li Y, et al. Fabrication and biocompatibility of nano-TiO2/titanium alloys biomaterials. Mater Lett. 2005;59(24–25):3144–3148. [Google Scholar]
- 468.Wei J, Chen QZ, Stevens MM, Roether JA, Boccaccini AR. Biocompatibility and bioactivity of PDLLA/TiO2 and PDLLA/TiO2/Bioglass® nanocomposites. Mater Sci Eng C. 2008;28(1):1–10. [Google Scholar]
- 469.Yuan Q, He G, Tan Z, et al. Biocompatibility of nano-TiO2/HA bio-ceramic coating for nerve regeneration around dental implants. Key Eng Mater. 2007;330–332:1393–1396. [Google Scholar]
- 470.Abrahams SC, Bernstein JL. Rutile: normal probability plot analysis and accurate measurement of crystal structure. J Chem Phys. 1971;55:3206–3211. [Google Scholar]
- 471.Pascual J, Camassel J, Mathieu H. Fine structure in the intrinsic absorption edge of TiO2. Phys Rev B. 1978;18(10):5606–5614. [Google Scholar]
- 472.Mathieu H, Pascual J, Camassel J. Uniaxial stress dependence of the direct-forbidden and indirect-allowed transitions of TiO2. Phys Rev B. 1978;18:6920–6929. [Google Scholar]
- 473.Eucken A, Dannöhl W. Die thermisce Ausdehnung einiger Alkalihalogenide and Metalle bei hohen Temperaturen. Z Elektrochem. 1934;40:814–821. [Google Scholar]
- 474.Eucken A, Büchner A. Die Dielecrizitatskonstante schwach polare Kristalle and ihre Temperaturabhangigkeit. Z Phys Chem B. 1935;27:321–349. [Google Scholar]
- 475.Roberts S. Dielectric constants and polarizabilities of ions in simple crystals and barium titanate. Phys Rev. 1949;76(8):1215–1220. [Google Scholar]
- 476.Poumellec B, Marucco JF, Lagnel F. Electron transport in TiO2−x at intermediate temperatures 300 K < T< 1500 K. Phys Stat Sol A. 1985;89(1):375–382. [Google Scholar]
- 477.Acket GA, Volger J. On the electron mobility and the donor centers in reduced and lithium-doped rutile (TiO2) Physica. 1966;32(10):1680–1692. [Google Scholar]
- 478.Jellison GE, Jr, Boatner LA, Budai JD, Jeong B-S, Norton DP. Spectroscopic ellipsometry of thin film and bulk anatase (TiO2) J Appl Phys. 2003;93(12):9537–9541. [Google Scholar]
- 479.Jaeger CD, Bard AJ. Spin trapping and electron spin resonance detection of radical intermediates in the photodecomposition of water at titanium dioxide particulate systems. J Phys Chem. 1979;83:3146–3152. [Google Scholar]
- 480.Yuan ZH, Jia JH, Zhang LD. Influence of co-doping of Zn(II) + Fe(III) on the photocatalytic activity of TiO2 for phenol degradation. Mater Chem Phys. 2002;73(2–3):323–326. [Google Scholar]
- 481.Kim DH, Woo SI, Moon SH, et al. Effect of Co/Fe co-doping in TiO2 rutile prepared by solid state reaction. Solid State Commun. 2005;136(9–10):554–558. [Google Scholar]
- 482.Luo H, Takata T, Lee Y, Zhao J, Domen K, Yan Y. Photocatalytic activity enhancing for titanium dioxide by co-doping with bromine and chlorine. Chem Mater. 2004;16(5):846–849. [Google Scholar]
- 483.Cong Y, Zhang J, Chen F, Anpo M, He D. Preparation, photocatalytic activity, and mechanism of nano-TiO2 co-doped with nitrogen and iron (III) J Phys Chem C. 2007;111(28):10618–10623. [Google Scholar]
- 484.Chen D, Jiang Z, Geng J, Wang Q, Yang D. Carbon and nitrogen co-doped TiO2 with enhanced visible-light photocatalytic activity. Ind Eng Chem Res. 2007;46(9):2741–2746. [Google Scholar]
- 485.Shi JW, Zheng JT, Hu Y, Zhao YC. Influence of Fe3+ and Ho3+ co-doping on the photocatalytic activity of TiO2. Mater Chem Phys. 2007;106(2–3):247–249. [Google Scholar]
- 486.Gu DE, Yang BC, Hu YD. V and N co-doped nanocrystal anatase TiO2 photocatalysts with enhanced photocatalytic activity under visible light irradiation. Catal Commun. 2008;9(6):1472–1476. [Google Scholar]
- 487.Obata K, Irie H, Hashimoto K. Enhanced photocatalytic activities of Ta, N co-doped TiO2 thin films under visible light. Chem Phys. 2007;339(1–3):124–132. [Google Scholar]