Abstract
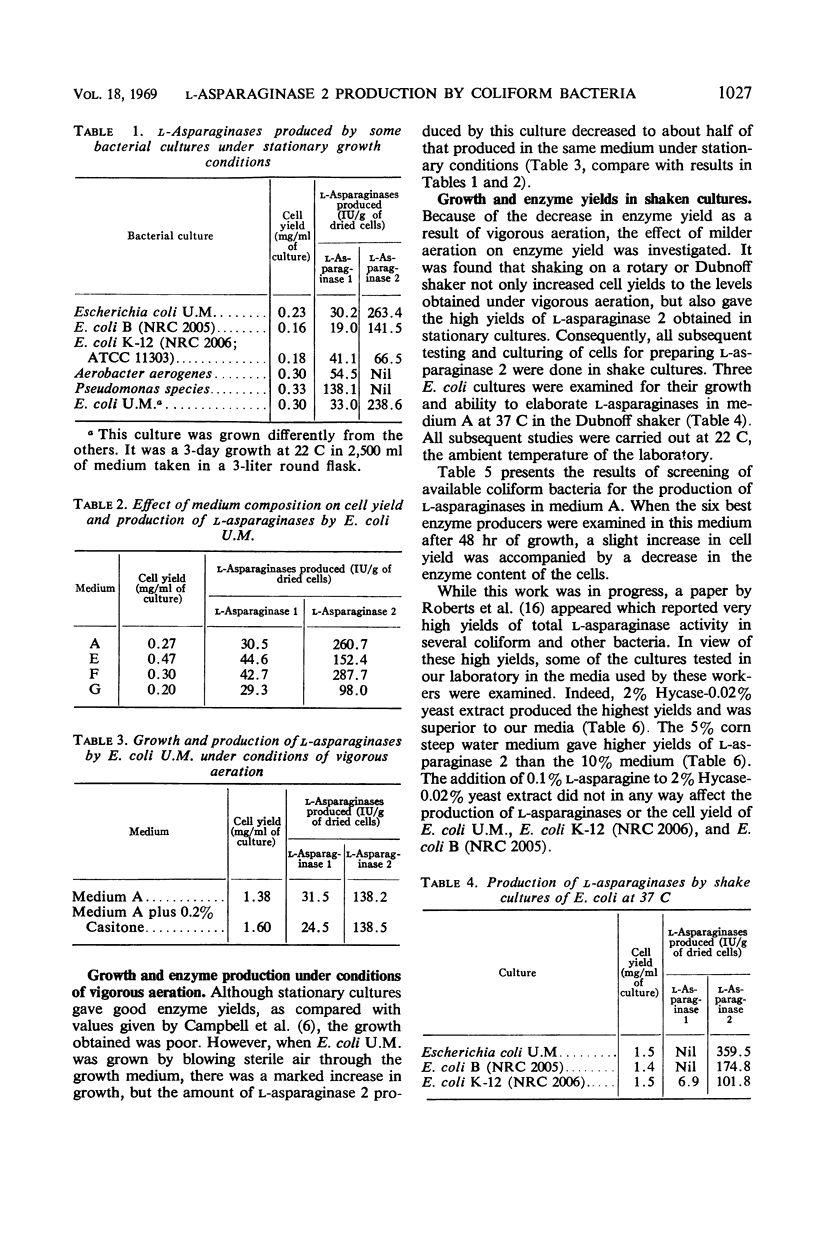
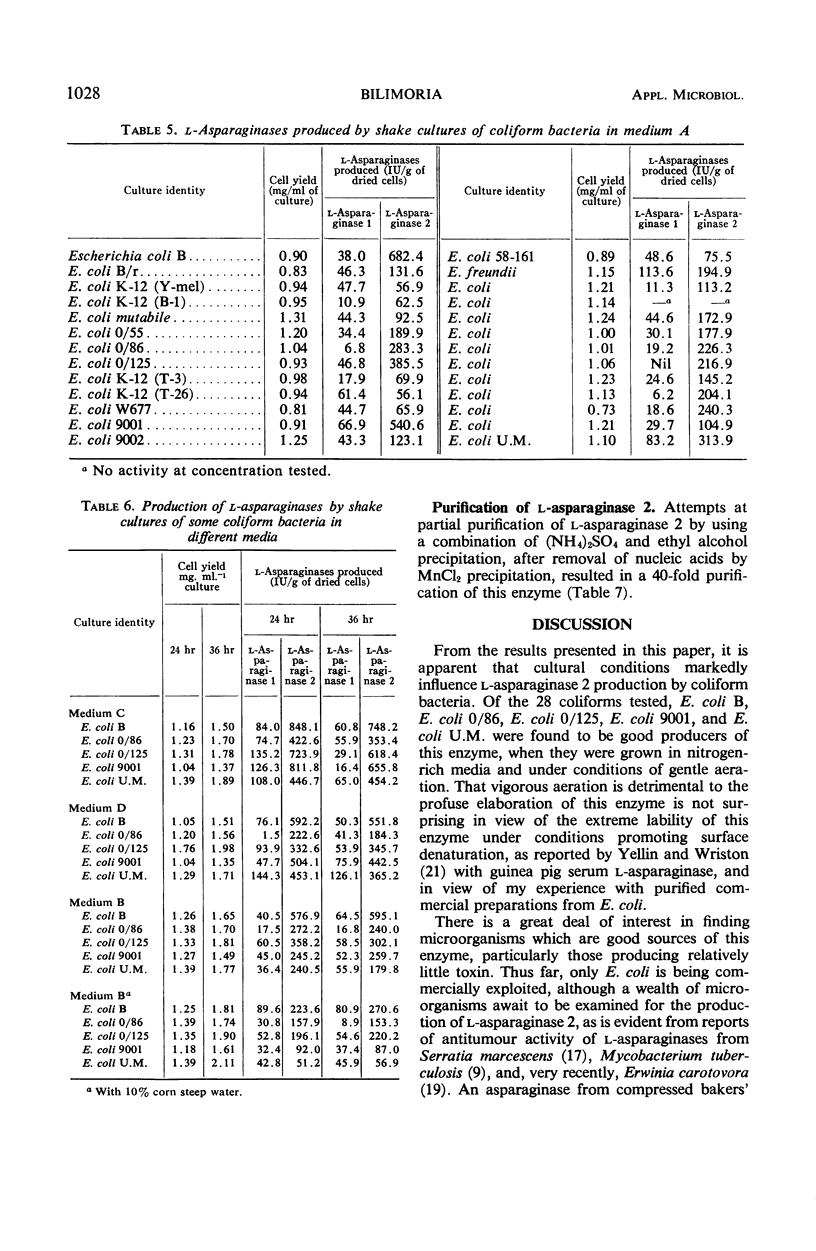
Of 28 coliforms, five strains of Escherichia coli were particularly active in elaborating L-asparaginase 2, the form of the enzyme useful in the treatment of some forms of cancer. Since it is advantageous to start purification of the enzyme from highly active cells, cultural conditions necessary for good growth and high enzyme yield have been studied. Gentle aeration proved suitable for good growth as well as high enzyme content. Stationary cultures gave poor growth, whereas vigorous aeration gave good growth but resulted in a marked decrease in the enzyme content of the cells. L-Asparaginase 2 has been purified about 40-fold by a combination of ammonium sulfate and ethyl alcohol precipitations.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROOME J. D. Evidence that the L-asparaginase of guinea pig serum is responsible for its antilymphoma effects. I. Properties of the L-asparaginase of guinea pig serum in relation to those of the antilymphoma substance. J Exp Med. 1963 Jul;118:99–120. doi: 10.1084/jem.118.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROOME J. D. Evidence that the L-asparaginase of guinea pig serum is responsible for its antilymphoma effects. II. Lymphoma 6C3HED cells cultured in a medium devoid of L-asparagine lose their susceptibility to the effects of guinea pig serum in vivo. J Exp Med. 1963 Jul;118:121–148. doi: 10.1084/jem.118.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broome J. D. Antilymphoma activity of L-asparaginase in vivo: clearance rates of enzyme preparations from guinea pig serum and yeast in relation to their effect on tumor growth. J Natl Cancer Inst. 1965 Dec;35(6):967–974. [PubMed] [Google Scholar]
- Broome J. D. L-asparaginase: the evolution of a new tumor inhibitory agent. Trans N Y Acad Sci. 1968 Mar;30(5):690–704. doi: 10.1111/j.2164-0947.1968.tb02511.x. [DOI] [PubMed] [Google Scholar]
- Campbell H. A., Mashburn L. T., Boyse E. A., Old L. J. Two L-asparaginases from Escherichia coli B. Their separation, purification, and antitumor activity. Biochemistry. 1967 Mar;6(3):721–730. doi: 10.1021/bi00855a011. [DOI] [PubMed] [Google Scholar]
- Cedar H., Schwartz J. H. Localization of the two-L-asparaginases in anaerobically grown Escherichia coli. J Biol Chem. 1967 Aug 25;242(16):3753–3755. [PubMed] [Google Scholar]
- Groves W. E., Davis F. C., Jr, Sells B. H. Spectrophotometric determination of microgram quantities of protein without nucleic acid interference. Anal Biochem. 1968 Feb;22(2):195–210. doi: 10.1016/0003-2697(68)90307-2. [DOI] [PubMed] [Google Scholar]
- Jayaram H. N., Ramakrishnan R., Vaidyanathan C. S. L-asparaginases from Mycobacterium tuberculosis strains H37Rv and H37Ra. Arch Biochem Biophys. 1968 Jul;126(1):165–174. doi: 10.1016/0003-9861(68)90570-5. [DOI] [PubMed] [Google Scholar]
- KIDD J. G. Regression of transplanted lymphomas induced in vivo by means of normal guinea pig serum. I. Course of transplanted cancers of various kinds in mice and rats given guinea pig serum, horse serum, or rabbit serum. J Exp Med. 1953 Dec;98(6):565–582. doi: 10.1084/jem.98.6.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIDD J. G. Regression of transplanted lymphomas induced in vivo by means of normal guinea pig serum. II. Studies on the nature of the active serum constituent: histological mechanism of the regression: tests for effects of guinea pig serum on lymphoma cells in vitro: discussion. J Exp Med. 1953 Dec;98(6):583–606. doi: 10.1084/jem.98.6.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASHBURN L. T., WRISTON J. C., Jr TUMOR INHIBITORY EFFECT OF L-ASPARAGINASE FROM ESCHERICHIA COLI. Arch Biochem Biophys. 1964 May;105:450–452. doi: 10.1016/0003-9861(64)90032-3. [DOI] [PubMed] [Google Scholar]
- Mashburn L. T., Wriston J. C., Jr Change in ribonuclease concentrations in L-asparaginase-treated lymphosarcomata. Nature. 1966 Sep 24;211(5056):1403–1404. doi: 10.1038/2111403a0. [DOI] [PubMed] [Google Scholar]
- Oettgen H. F., Old L. J., Boyse E. A., Campbell H. A., Philips F. S., Clarkson B. D., Tallal L., Leeper R. D., Schwartz M. K., Kim J. H. Inhibition of leukemias in man by L-asparaginase. Cancer Res. 1967 Dec;27(12):2619–2631. [PubMed] [Google Scholar]
- Old L. J., Boyse E. A., Campbell H. A. L-asparagine and leukemia. Sci Am. 1968 Aug;219(2):34–40. doi: 10.1038/scientificamerican0868-34. [DOI] [PubMed] [Google Scholar]
- Roberts J., Burson G., Hill J. M. New procedures for purification of L-asparaginase with high yield from Escherichia coli. J Bacteriol. 1968 Jun;95(6):2117–2123. doi: 10.1128/jb.95.6.2117-2123.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley B., Wriston J. C., Jr Partial purification and antilymphoma activity of Serratia marcescens L-asparaginase. Biochem Biophys Res Commun. 1967 Jul 21;28(2):160–165. doi: 10.1016/0006-291x(67)90423-8. [DOI] [PubMed] [Google Scholar]
- Schwartz J. H., Reeves J. Y., Broome J. D. Two L-asparaginases from E. coli and their action against tumors. Proc Natl Acad Sci U S A. 1966 Nov;56(5):1516–1519. doi: 10.1073/pnas.56.5.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade H. E., Elsworth R., Herbert D., Keppie J., Sargeant K. A new L-asparaginase with antitumour activity? Lancet. 1968 Oct 5;2(7571):776–777. doi: 10.1016/s0140-6736(68)90977-x. [DOI] [PubMed] [Google Scholar]
- Yellin T. O., Wriston J. C., Jr Antagonism of purified asparaginase from guinea pig serum toward lymphoma. Science. 1966 Feb 25;151(3713):998–999. doi: 10.1126/science.151.3713.998. [DOI] [PubMed] [Google Scholar]
- Yellin T. O., Wriston J. C., Jr Purification and properties of guinea pig serum asparaginase. Biochemistry. 1966 May;5(5):1605–1612. doi: 10.1021/bi00869a022. [DOI] [PubMed] [Google Scholar]


