Abstract
Approved therapeutic antineoplastic antibodies have targeted extracellular or cell-surface molecules. ESK1 is the first fully human T-cell receptor-like antibody targeting an intracellular tumor-associated antigen, Wilm’s tumor 1 (WT1). In murine xenograft models, ESK1 exhibits a high specificity and exert robust antineoplastic effects against human cancers that express WT1 epitopes on HLA-A201 molecules.
Keywords: WT1, TCR-like, monoclonal antibodies, cancer, MHC
Monoclonal antibody (mAb)-based antineoplastic regimens have emerged as a highly effective therapeutic option for the treatment of several cancers. mAbs represent advantageous immunotherapeutics as they exhibit a high target specificity and efficacy, are associated with limited side effects and have a prolonged half-life in vivo, being compatible with infrequent dosing schedules. Naked antibodies can exert robust antineoplastic functions by directly conveying a pro-apoptotic stimulus or through the activation of immune effector mechanisms. These include antibody-dependent cellular cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC) and antibody-dependent cellular phagocytosis (ADCP). In addition, mAbs can serve as antigen-specific vehicles that specifically deliver potent cytotoxic agents such as toxins, drugs, or radionuclides to cancer cells. Finally, mAbs can be engineered to generate chimeric antigen receptors or bi-specific antibodies, further enhancing the specificity and the potency of T cell-based anticancer therapy. However, so far commercial therapeutic mAbs have only targeted extracellular or cell-surface proteins, including differentiation-associated antigens, secreted growth factors and their receptors.1-3 Obviously, several among the most interesting and truly specific tumor-associated antigens (TAAs) are cytoplasmic or nuclear proteins and hence are inaccessible to conventional mAbs. The generation of T cell-based responses against these TAAs, often through vaccines, has been a major goal of cancer immunotherapy during the past 3 decades.
Most TAAs arise from mutated proteins, differentiation antigens that are poorly expressed by normal tissues, or overexpressed gene products. These proteins are generally degraded by proteasomes and presented on the cell surface in the context of MHC Class I molecules, often as 8–10 mer antigenic peptides, to be recognized by the T-cell receptor (TCR) of cytotoxic T lymphocytes. Initially, vaccines aimed at stimulating T-cell responses or adoptive T cell-based therapies were the only approaches to target intracellular TAAs. The active immunizations of cancer patients with peptide- or DNA-based vaccines targeting specific TAAs, dendritic cells loaded with TAAs, or the adoptive transfer of TAA-specific T cells is currently being investigated in several clinical trials. A number of issues has hampered the success of these T cell-based immunotherapies. Typically, TAA-specific CTLs arise at a low frequency only in a small portion of patients, exhibit a low affinity for their targets and are characterized by a short lifespan. Therefore, this approach generally fails to mediate therapeutic effects in the presence of large tumor burdens.4
Combining TCR recognition with the potency and versatility of mAbs may represent the ideal next step for immunotherapeutic approaches targeting intracellular TAAs (Fig. 1). It has taken more than a decade to generate the first fully human therapeutic TCR-like mAb, ESK1, since immunologists initially used TCR-like mAbs to illustrate and study antigen processing and presentation to T cells.5 In addition, technical problems hampered the generation of highly specific TCR-like mAbs by traditional hybridoma techniques. The introduction of phage display technology allowed for the selection of rare/unique mAbs targeting very defined epitopes, such as peptide/MHC complexes, among a large number of candidates.
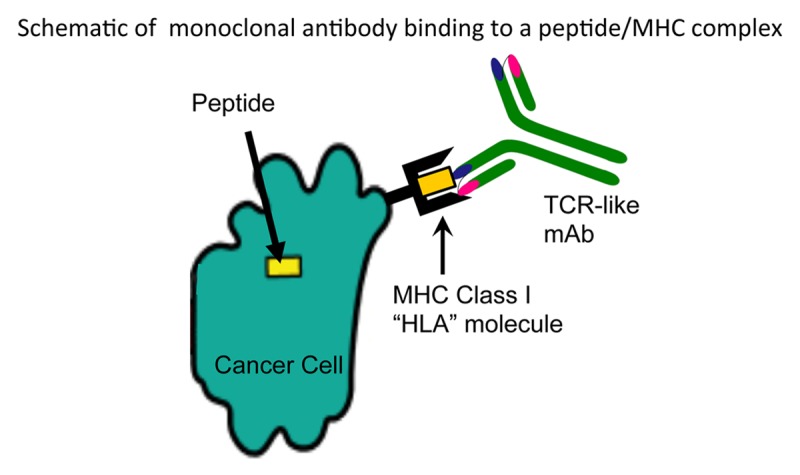
Figure 1. TCR-like monoclonal antibody binding to a peptide/MHC complex on a cancer cell. Intracellular tumor-associated antigens (TAAs) are generally processed and presented on the surface of malignant cells in the context of MHC Class I molecules. Highly specific, T-cell receptor (TCR)-like monoclonal antibodies (mAbs) can now be isolated and used to specifically target malignant cells exhibiting specific TAA/MHC complexes on their surface.
The Reiter group pioneered the isolation of a number of TAA-specific monovalent antibody fragments (Fab or ScFV) from phage-display libraries. These mAbs constituted excellent tools for elucidating the intracellular generation and trafficking of peptide/MHC complexes. The same authors also demonstrated that a TCR-like Fab specific for the melanoma-associated epitope MART-126–35 complexed with HLA-A2 can be used to deliver a toxin to human melanoma cells and inhibits tumor growth in murine melanoma models.6 More recently, two murine TCR-like mAbs, namely, 3.2G1 min, which is specific for human chronic chorionic β,7 and 8F4, which is specific for PR1 presented by HLA-A0201,8 have been generated. 3.2G1 min kills human breast carcinoma cells by triggering apoptosis, while 8F4 mediates CDC against human acute myeloid leukemia (AML) blasts and stem cells. These studies have spurred the concept that TCR-like mAbs might be used as therapeutic agents.
The Wilms’ tumor 1 (WT1) protein represented an attractive target for TCR-like mAbs, since it is poorly expressed by normal tissues but is abundant in a wide range of human neoplasms. Importantly, a fragment of WT1 protein, RMFPNAPYL, presented by the HLA-A0201 molecule has been intensively studied and validated as a CD8+ TCR epitope.9
Using the phage-display technology, our group had successfully isolated a high-affinity human IgG1 that is highly specific for the RMFPNAPYL/HLA-A0201 complex, ESK1. ESK1 appears to bind to a wide range of human leukemic and solid tumor cell lines as well as to primary leukemia cells, in a WT1- and HLA-A0201-restricted manner. ADCC seems to be the primary mechanism accounting for the cytotoxic effects of ESK1, both in vitro and in vivo.10
Through these studies, we learned the following:
(1) The general assumption that the expression level of T-cell epitope/MHC complexes on surface of tumors cells is insufficient to elicit an immune response may not always be true. We used ESK1 to detect the RMFPNAPYL/HLA-A2 complex in levels that ranged from a few hundred epitopes to more than 6,000 epitopes per cell.
(2) The affinity of TCR-like mAbs is crucial for the binding of peptide/MHC complex. We have shown that mAbs with low affinity have problems in detecting TAA/MHC complexes.
(3) We have demonstrated that ADCC provides a relevant contribution to the antineoplastic effects of ESK1. In vivo, however, ESK1 may mediate antitumor effect via other mechanisms, which remain to be elucidated.
If upcoming clinical trials based this approach will be successful, TCR-like mAbs could mark the emergence of new era for mAb-based immunotherapy, bringing about a new universe of critical targets for the treatment of cancer as well as inflammatory and infectious diseases.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/24678
References
- 1.Weiner LM, Murray JC, Shuptrine CW. Antibody-based immunotherapy of cancer. Cell. 2012;148:1081–4. doi: 10.1016/j.cell.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer. 2012;12:278–87. doi: 10.1038/nrc3236. [DOI] [PubMed] [Google Scholar]
- 3.Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5:77ra38. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, et al. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15:5323–37. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Porgador A, Yewdell JW, Deng Y, Bennink JR, Germain RN. Localization, quantitation, and in situ detection of specific peptide-MHC class I complexes using a monoclonal antibody. Immunity. 1997;6:715–26. doi: 10.1016/S1074-7613(00)80447-1. [DOI] [PubMed] [Google Scholar]
- 6.Dahan R, Reiter Y. T-cell-receptor-like antibodies - generation, function and applications. Expert Rev Mol Med. 2012;14:e6. doi: 10.1017/erm.2012.2. [DOI] [PubMed] [Google Scholar]
- 7.Verma B, Neethling FA, Caseltine S, Fabrizio G, Largo S, Duty JA, et al. TCR mimic monoclonal antibody targets a specific peptide/HLA class I complex and significantly impedes tumor growth in vivo using breast cancer models. J Immunol. 2010;184:2156–65. doi: 10.4049/jimmunol.0902414. [DOI] [PubMed] [Google Scholar]
- 8.Sergeeva A, Alatrash G, He H, Ruisaard K, Lu S, Wygant J, et al. An anti-PR1/HLA-A2 T-cell receptor-like antibody mediates complement-dependent cytotoxicity against acute myeloid leukemia progenitor cells. Blood. 2011;117:4262–72. doi: 10.1182/blood-2010-07-299248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maslak PG, Dao T, Krug LM, Chanel S, Korontsvit T, Zakhaleva V, et al. Vaccination with synthetic analog peptides derived from WT1 oncoprotein induces T-cell responses in patients with complete remission from acute myeloid leukemia. Blood. 2010;116:171–9. doi: 10.1182/blood-2009-10-250993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dao T, Yan S, Veomett N, Pankov D, Zhou L, Korontsvit T, et al. Targeting the intracellular WT1 oncogene product with a therapeutic human antibody. Sci Transl Med. 2013;5:76ra33. doi: 10.1126/scitranslmed.3005661. [DOI] [PMC free article] [PubMed] [Google Scholar]


