Abstract
Immunotherapeutic strategies including the blockade of programmed death 1 (PD-1) receptors hold promise for the treatment of various cancers including non-small cell lung carcinoma (NSCLC). Preclinical data suggest that pre-existing tumor immunity is important for disease regression upon checkpoint blockade-based therapies. However, the nature of antigen-specific T-cell responses that correlate with the clinical response to immunotherapy in NSCLC patients is not known. The embryonic stem cell gene SRY (sex determining region Y)-box 2 (SOX2) has recently emerged as a major oncogenic driver in NSCLC. Here, we show that nearly 50% of a cohort of NSCLC patients mounted both CD4+ and CD8+ T-cell responses against SOX2, which could be readily detected among peripheral blood mononuclear cells. T-cell responses against SOX2 were associated with NSCLC regression upon immunotherapy with anti-PD-1 monoclonal antibodies, whereas none of the patients lacking SOX2-specific T cells experienced disease regression following immune checkpoint blockade. Conversely, cellular and humoral responses against viral antigens or another tumor-associated antigen (NY-ESO-1) failed to correlate with the clinical response of NSCLC patients to immunotherapy. Of note, the administration of PD-1-blocking antibodies was associated with intramolecular epitope spread as well as with the amplification of SOX2-specific immune responses in vivo. These findings identify SOX2 as an important tumor-associated antigen in NSCLC and link the presence of SOX2-specific T cells with the clinical response of lung cancer patients to immunotherapy.
Keywords: sox2, embryonal stem cells, lung cancer, programmed death 1, immunotherapy, checkpoint blockade
Introduction
Non-small cell lung carcinoma (NSCLC) is the leading cause of cancer-related mortality in the United States. Although NSCLC was initially considered to be non-immunogenic, several studies now support a beneficial effect of immunotherapy for NSCLC patients. Endogenous tumor-specific T-cell responses indeed delay tumor progression in mouse models of lung adenocarcinoma,1 and the presence of tumor-infiltrating T cells has been linked to improved survival among NSCLC patients.2-4 Along similar lines, recent studies have demonstrated that the blockade of immune checkpoints mediates promising clinical effects in patients affected by this tumor.5-7 In particular, the antibody-mediated blockade of programmed death 1 (PD-1) has been reported to lead to objective tumor regression in 18% of patients bearing advanced NSCLC.6
SRY (sex determining region Y)-box 2 (SOX2) is a transcription factor critically involved in the pluripotency and stemness of human embryonic stem cells.8 SOX2 has recently been identified as a common target of genomic amplifications and as a lineage survival oncogene in lung cancer.9-11 In addition, SOX2 has been implicated in the function of lung stem cells and putative cancer stem cells.12,13 We and others have previously shown the capacity of the immune system to target SOX2,14,15 and several groups have studied the presence of humoral responses against SOX2, especially in patients with small-cell lung cancer (SCLC).16 Moreover, we have previously demonstrated that the presence of naturally occurring SOX2-targeting T cells is associated with an improved survival and reduced risk of malignant progression among patients with monoclonal gammopathies.14 However, the nature of SOX2-specific T-cell responses in lung cancer patients has not yet been characterized.
Results and Discussion
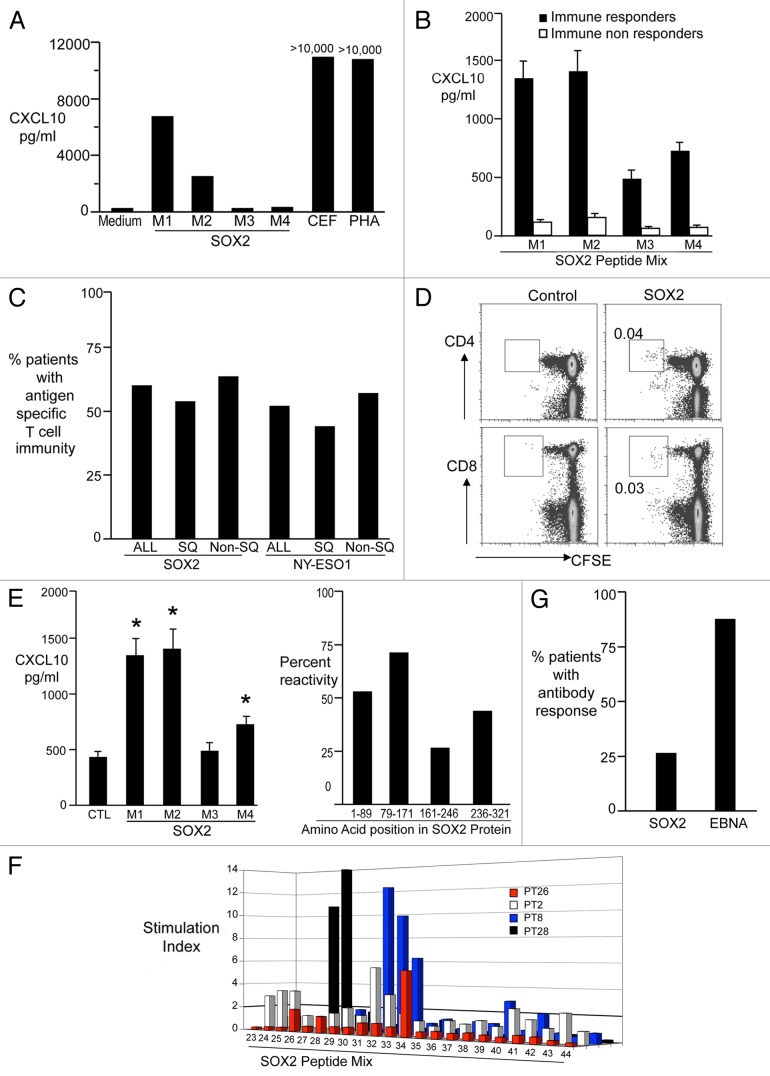
In view of the emerging interest in SOX2 as a potential therapeutic target in lung cancer, we analyzed the expression of SOX2 in samples from a cohort of NSCLC patients (Table S1) by immunohistochemistry. SOX2 expression was detected in the neoplastic lesions of patients affected by both most common NSCLC subtypes, although, consistent with other studies,17,18 expression levels were greater in squamous as compared with non-squamous NSCLC (Fig. 1). These findings prompted us to study T-cell immune responses targeting SOX2 in NSCLC patients. In order to detect the presence of SOX2-specific T cells, freshly isolated peripheral blood mononuclear cells (PBMCs) from patients with advanced NSCLC were stimulated using a library of overlapping peptides that spans the entire SOX2 protein, as previously described.14 Phytohemagglutinin (PHA) or a cocktail of viral antigens (derived from cytomegalovirus, Epstein–Barr virus and influenza virus; CEF) were employed as positive control conditions (Fig. 2A and B). Immune responses to NY-ESO-1 were also monitored, as NY-ESO-1 is an established tumor-associated antigen in this setting.19 Overall, T-cell immunity against SOX2 or NY-ESO-1 was detected in 21/35 (60%) and 12/23 (52%) of patients tested, respectively. SOX2- and NY-ESO-1-specific T-cell responses were equally common among patients with squamous and non-squamous NSCLC (Fig. 2C). The presence of SOX2-reactive T cells was confirmed using a CFSE dilution assay, and this cell population was found to consist of both CD4+ and CD8+ components (Fig. 2D). We detected T cells reacting against epitopes derived from the entire sequence of SOX2 (Fig. 2E), with some preference for peptides corresponding to the N-terminus of the protein. In order to confirm the nature of the epitopes responsible for SOX2-specific T-cell responses in NSCLC patients, peptides from the reactive cocktails were tested individually (Fig. 2F). We also examined the presence of SOX-specific antibodies in the plasma of NSCLC patients by ELISA. Consistent with other studies,16 anti-SOX2 antibodies were detected in only 7 of 25 (28%) patients tested (Fig. 2G). As a control, the presence of EBV nuclear antigen 1 (EBNA1)-specific antibodies was detected in a majority of patients (22 of 25, 88%). Together, these data demonstrate that SOX2 is a common target of endogenous T-cell immunity in patients with NSCLC.
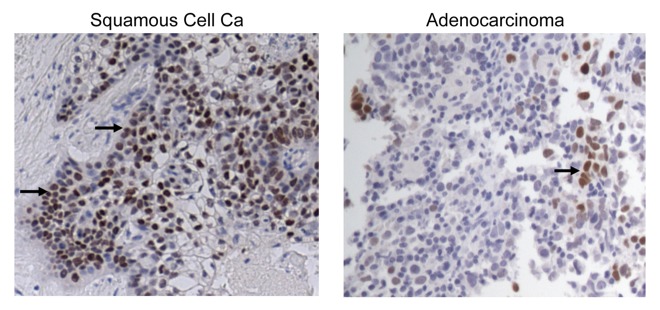
Figure 1. Expression of SOX2 in human lung cancer. The expression of SOX2 was analyzed by immunohistochemistry on malignant tissues from paraffin-embedded tumor sections. SOX2 expression in samples from patients bearing squamous and non-squamous non-small cell lung carcinoma is illustrated.
Figure 2. Detection of antigen-specific cellular and humoral responses in non-small cell lung carcinoma patients. Peripheral blood mononuclear cells (PBMCs) were co cultured with medium alone, overlapping peptides from the SOX2 library (3 μg/ml, M1 M2 M3 M4), CEF peptides or phytohemagglutinin (PHA). After 48 h, supernatants were harvested and examined for the abundance of CXCL10. M1 peptides cover SOX2 residues 1–89, M2 residues 79–171, M3 residues 161–246 and M4 residues 236–321. See also Table S2. (A) Representative results from a patient exhibiting SOX2-reactive T cells. (B) CXCL10 production (mean ± SEM) in response to SOX2 antigens (M1–4) for patients classified as immune responders (n = 15) vs. non-responders (n = 9). (C) PBMCs obtained from patients with advanced non-small cell lung carcinoma (NSCLC) were tested for the presence of T cells reacting against SOX2 (n = 35) or NY-ESO-1 (n = 23) using overlapping peptides as in Figure 2A and B. The percentage of all NSCLC patients (ALL), squamous NSCLC patients (SQ) and non-squamous (Non-SQ) NSCLC patients exhibiting immunity to SOX2 (ALL = 60%) or NY-ESO-1 (ALL = 52%) is reported. (D) PBMCs from lung cancer patients were labeled with CFSE and co-cultured with peptides from the SOX2 library (5 μg/mL) in the presence of anti-CD28 and anti-CD49d (both at 1 μm/mL) for 7 d. Eventually, T-cell proliferation was determined using flow cytometry. (E) Reactivity of T cells from SOX2-immune patients to the different regions of the SOX2 protein. Both the amount of CXCL10 (mean ± SEM; left panel) and the % samples reacting to individual mixes are reported. *p < 0.05 compared with control. Please note PBMCs from some patients reacted against more than one region of SOX2. (F) Fine mapping of SOX2 reactivity. PBMCs were incubated with individual peptides from the SOX2 library for 48 h and tested for the presence of CXCL10 using a luminex assay. Figure shows individual peptide reactivity in PBMCs from 4 different patients. (G) Detection of anti-SOX2 antibodies. Plasma obtained from patients was tested for the presence of antibodies specific for SOX2 and EBV nuclear antigen 1 (EBNA1) by ELISA. The percentage of patients with bearing antibodies against SOX2 (28%) and EBNA1 (88%) detectable at a titer of > 1:100 is reported.
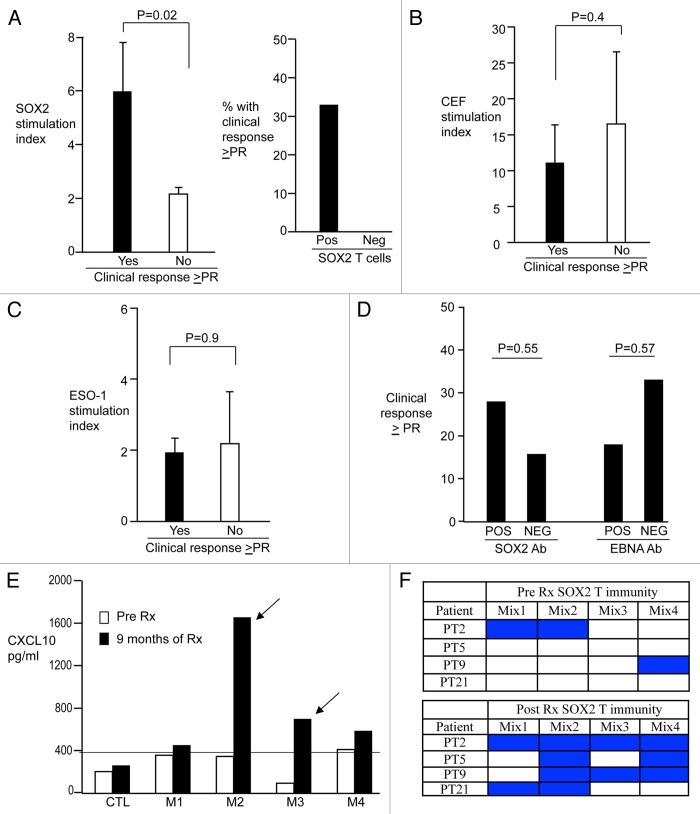
Several patients of our cohort were enrolled in a clinical trial testing the PD-1-blocking antibody BMS-936558.5 Therefore, we analyzed the correlation between SOX2-specific T-cell responses and disease outcome upon the administration of anti-PD-1 antibodies. Of 24 patients evaluable for a clinical response upon PD-1 blockade, those who responded to therapy (at least partial response, PR, as defined by RECIST; n = 5) exhibited significantly greater immune response against SOX2 as compared with those who did not (p = 0.02) (Fig. 3A). This held true when the analysis was restricted to samples studied at baseline or during the first cycle of immunotherapy (n = 22; p = 0.04). Overall, 5 of 15 (33%) patients exhibiting SOX2-specific immune responses at baseline achieved an objective response (≥PR) to therapy. In contrast, delete no objective response could be documented among the 9 patients lacking T-cell immunity against SOX2 (Fig. 3A). The immune reactivity to viral antigens of patients who responded to anti-PD1 therapy and those who did not was comparable, indicating that differences in SOX2-specific responses were not due to issues of immune incompetence (Fig. 3B). NY-ESO-1-specific T-cell reactivity has previously been correlated with clinical responses to anti-CTLA4 antibodies in melanoma patients.20 Nonetheless, NY-ESO-1-targeting T-cell responses did not differ between NSCLC patients who achieved an objective clinical response upon PD-1 blockade and those who did not (Fig. 3C). Along similar lines, the presence of circulating antibodies specific for SOX2 or EBNA1 did not correlate with clinical responses to PD-1 blockade in NSCLC patients (Fig. 3D).
Figure 3. Correlation between endogenous antigen-specific immunity and response to PD-1 blockade in non-small cell lung carcinoma patients. (A–C) Antigen-specific cellular immunity and objective response to immunotherapy. Peripheral blood mononuclear cells (PBMCs) from non-small cell lung carcinoma (NSCLC) patients were stimulated with overlapping peptides derived from SOX2 (A), with CEF peptides (B), or overlapping peptides derived from SOX2 (B), cellular immunity was quantified as stimulation index and correlated with objective clinical responses (partial response, PR, or better) as achieved upon the administration of anti-PD1 monoclonal antibodies. In (A), stimulation indexes (mean ± SEM) are illustrated on the left, while the percentage of patients exhibiting (n = 15) or not (n = 9) SOX2-specific T cells who achieved an objective clinical response is depicted on the right. (B) and (C) report stimulation indexes only. (D) Humoral immunity and response to immunotherapy. The percentage of clinical responses (≥ PR) of NSCLC patients exhibiting (POS) or not (NEG) circulating antibodies specific for SOX2 or EBV nuclear antigen 1 (EBNA1) is depicted. (E and F) In vivo epitope spread upon immunotherapy. (E) reports representative data from one patient showing T-cell reactivity against different regions (M1, M2, M3, M4) of the SOX2 protein before and 9 mo after immunotherapy with anti-PD1 monoclonal antibodies. (F) summarizes data on T-cell reactivity against SOX2 peptide mixes before and after immunotherapy with anti-PD1 monoclonal antibodies, in 4 patients exhibiting a detectable epitope spread upon treatment.
The chemotherapy-induced lysis of tumor cells can, at least in principle, amplify immune responses in vivo, in part owing to the phenomenon known as epitope spread. To address whether this was the case also in NSCLC patients receiving PD-1-targeting antibodies, serial samples from 21 patients were analyzed to assess the emergence of T cells reacting against new regions of SOX2 (Fig. 3E and F). Some extent of epitope spread or immune amplification was documented in 4 patients. Two of these patients experienced PRs and remained on therapy for > 1 y. In the other two patients (who exhibited no anti-SOX2 immune responses at baseline), the development of SOX2-specific immunity was associated with disease stabilization as the best objective response. Overall, 5 of 17 patients bearing SOX2-targeting T cells (either at initial analysis, n = 15, or on follow up, n = 2) received anti-PD-1 antibodies for at least one year, indicating the durability of therapeutic responses. In contrast, none of the patients lacking SOX2 immunity remained on therapy for >1 y.
These findings are consistent with preclinical studies suggesting that tumor regression upon checkpoint blockade depend on pre-existing immune responses.21 Endogenous tumor immunity has been proposed to induce the expression of PD-1 ligand 1 (PD-L1) on melanoma cells,22 which in principle can suppress immune responses by engaging PD-1 on T cells. The expression of PD-L1 on the surface of tumor cells has recently been correlated with the clinical response of NSCLC patients to anti-PD-1 therapy.5 However, this analysis only included 10 NSCLC patients, of whom just one responded to therapy. More importantly, the evaluation and monitoring of PD-L1 expression on tumor cells are challenging as they entail invasive delete procedures, as PD-L1 expression by tumor cells is often highly heterogeneous, as the levels of membrane-exposed PD-L1 should specifically be assessed rather than those of the total protein/RNA, as several commercial antibodies lack sufficient specificity, and as substantial difficulties have been encountered in the development of reagents and methods for detection of PD-L1 expression on formalin-fixed, paraffin-embedded (FFPE) tissues.7 It has therefore been suggested that the nature of host immune responses may provide biomarkers that complement or even replace the need to assess PD-L1 expression on tumor cells.7 The finding that SOX2-specific T cells can be detected in the peripheral blood of NSCLC patients may provide a simple and straightforward strategy to monitor these responses.
Our findings provide evidence in support of a direct link between antigen-specific T-cell immunity and clinical responses to PD-1 blockade in NSCLC patients, and suggest that individuals with pre-existing antitumor immune responses may be those who obtain clinical benefits from immune checkpoint blocking-agents. In addition, our data point to SOX2 as an attractive tumor-associated antigen in the context of NSCLC and suggest that the detection of SOX2-specific T cells may be a simple strategy to identify patients who are more likely to benefit from anti-PD-1 monoclonal antibodies. In this scenario, patients lacking T-cell responses against SOX2 may perhaps constitute ideal candidates for SOX2-targeting vaccines.
As this stage, a confirmation of our preliminary data in large clinical cohorts is needed. Moreover, it would be interesting to dissect the role of other checkpoint regulators (e.g., CTLA4) in patients exhibiting SOX2-specific immune responses who do not obtain clinical benefits from anti-PD-1 antibodies. SOX2 and other embryonic stem cell factors have now been implicated in the development and progression of several human neoplasm.15 Harnessing natural immune responses against these antigens may therefore provide an opportunity for the development of novel anticancer immunotherapeutics.
Materials and Methods
Patients
Blood samples were obtained from NSCLC patients (n = 35) after obtaining informed consent and upon the approval or the Yale University Institutional Review Board. Some of these patients were enrolled in a clinical trial testing the anti-PD1 antibody BMS-936558.5 Clinical responses in patients undergoing immunotherapy was evaluated using Response Evaluation Criteria In Solid Tumors (RECIST) v1.0. Clinical data were confirmed with the central study database at Bristol Myers Squibb.
Peptide libraries
Libraries of overlapping peptides spanning the entire length of SOX2 and NY-ESO-1 were synthesized as previously described.14,23 The SOX2 library consisted of 86 peptides divided into 4 sub-mixes, while the NY-ESO-1 library consisted of 30 peptides subdivided in 3 sub-mixes (Table S2). A pool of peptides derived from cytomegalovirus, Epstein–Barr virus and influenza virus (CEF; Anaspec Inc.) was used as a positive control.
Detection of antigen-specific T cells
The presence of SOX2-, NY-ESO-1- and CEF-reactive T cells was detected based on antigen-dependent cytokine production and proliferation, as previously described.14,23 Briefly, PBMCs were isolated using Ficoll Hypaque and cultured either alone (control) or together with CEF peptides or peptide pools from the SOX2 and NY-ESO-1 libraries, employed at 3 μg/mL in 5% PHS, in 96-well round bottom plates (2.5 × 105 cells/well). PHA was used as a positive control. After 48 h, culture supernatants were harvested and examined for the presence of chemokine (C-X-C motif) ligand 10 (CXCL10, also known as IP10) using a luminex assay, as per manufacturer’s instructions. Values ≥2-fold over the negative control were deemed positive, based on the analysis of inter- and intra-assays variation. Of note, in this assay the peptide-induced secretion of CXCL10 serves as a marker of T-cell reactivity and depends on the presence of CD3+ T cells as well as on the induction of interferon γ, as previously described.14,23 Stimulation indexes were calculated as fold increases in CXCL10 secretion over that recorded control wells, with values of at least 100 pg/mL. In some cases, the reactivity of T cells against a specific peptide of SOX2 sub-mixes was confirmed by stimulating PBMCs with single peptides.
Antigen-dependent proliferation assays
The presence of antigen-dependent proliferation was monitored using a CFSE dilution assay, as previously described.23 Freshly isolated PBMCs were labeled with 0.5 μM CFSE (Molecular Probes) and cultured with 1 μg/mL anti-CD28 and anti-CD49d antibodies (BD Biosciences), alone or in the presence of 5 μg/mL SOX2 peptide mixes, 3 μg/mL CEF peptides or 2 μg/mL PHA. Seven days later, PBMCs were stained with anti-CD3, anti-CD4 and anti-CD8 antibodies and T-cell proliferation was analyzed on a FACSCalibur cytofluorometer (Becton Dickinson). Flow cytometry data were analyzed using the FlowJo software.
Detection of SOX2- and EBNA1-specific antibodies against
The presence of antibodies specific for SOX2 or EBNA1 was analyzed by ELISA, as previously described.14 Full-length SOX2 (Abcam) was used to coat ELISA plates (25 μl/well, at 1 mg/mL) for 2 h at room temperature. Plates were then washed twice with PBS with Tween and blocked overnight with 4% non-fat milk at 4 °C. Patient plasma was diluted 1:100 and 1:400 in 4% non-fat milk and 200 μL of diluted plasma were added to the wells of ELISA plates and incubated for 2 h at room temperature. Plates were finally washed and probed with horseradish peroxidase (HRP)-coupled goat anti-human IgG (1:6000 in 5% non-fat milk; Southern Biotech) for 1 h at room temperature. Finally, 30 μL of HRP substrate was added to each well for 15 min, followed by the addition of a commercial stop solution (both from Biosource). Absorbance was read at 450 nm on an ELISA plate reader. Antibodies against EBNA1 were detected using a commercial ELISA kit (Scimedx), as per manufacturer’s instructions.
Immunohistochemistry
Paraffin sections from lung cancer specimens were subjected to antigen retrieval at low pH with citrate buffer. Slides were then stained with monoclonal mouse anti-human SOX2 antibodies (1:100 dilution, clone 245610, R&D) and Envision anti-mouse antibodies (Dako).
Statistical analyses
Data from different groups was compared using a non-parametric Mann–Whitney U test. The threshold for the statistical significance of p values was set to 0.05.
Supplementary Material
Acknowledgments
The authors thank Yale-New Haven Hospital nursing and support staff for their help with the collection of samples and patients for their participation in these studies. The authors thank R. Sipples, E. Rowen, E. Duffield, K. Drosier, for their help with the collection of samples, and S. Soni and R. Sundaram for technical assistance with assays, and patients for their participation in these studies.
Glossary
Abbreviations:
- CEF
cytomegalovirus, Epstein–Barr virus and influenza virus
- EBNA1
EBV nuclear antigen 1
- FFPE
formalin-fixed, paraffin-embedded
- NSCLC
non-small cell lung carcinoma
- PBMC
Peripheral blood mononuclear cell
- PD-1
programmed death 1
- PHA
phytohemagglutinin
- PR
partial response
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplementary Material
Supplementary materials may be found here:
http://www.landesbioscience.com/journals/oncoimmunology/article/25205
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/25205
References
- 1.DuPage M, Cheung AF, Mazumdar C, Winslow MM, Bronson R, Schmidt LM, et al. Endogenous T cell responses to antigens expressed in lung adenocarcinomas delay malignant tumor progression. Cancer Cell. 2011;19:72–85. doi: 10.1016/j.ccr.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruffini E, Asioli S, Filosso PL, Lyberis P, Bruna MC, Macrì L, et al. Clinical significance of tumor-infiltrating lymphocytes in lung neoplasms. Ann Thorac Surg. 2009;87:365–71, discussion 371-2. doi: 10.1016/j.athoracsur.2008.10.067. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki K, Kadota K, Sima CS, Nitadori J, Rusch VW, Travis WD, et al. Clinical impact of immune microenvironment in stage I lung adenocarcinoma: tumor interleukin-12 receptor β2 (IL-12Rβ2), IL-7R, and stromal FoxP3/CD3 ratio are independent predictors of recurrence. J Clin Oncol. 2013;31:490–8. doi: 10.1200/JCO.2012.45.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawai O, Ishii G, Kubota K, Murata Y, Naito Y, Mizuno T, et al. Predominant infiltration of macrophages and CD8(+) T Cells in cancer nests is a significant predictor of survival in stage IV nonsmall cell lung cancer. Cancer. 2008;113:1387–95. doi: 10.1002/cncr.23712. [DOI] [PubMed] [Google Scholar]
- 5.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sznol M, Chen L. Antagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer. Clin Cancer Res. 2013;19:1021–34. doi: 10.1158/1078-0432.CCR-12-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–56. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bass AJ, Watanabe H, Mermel CH, Yu S, Perner S, Verhaak RG, et al. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nat Genet. 2009;41:1238–42. doi: 10.1038/ng.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hussenet T, du Manoir S. SOX2 in squamous cell carcinoma: amplifying a pleiotropic oncogene along carcinogenesis. Cell Cycle. 2010;9:1480–6. doi: 10.4161/cc.9.8.11203. [DOI] [PubMed] [Google Scholar]
- 11.McCaughan F, Pole JC, Bankier AT, Konfortov BA, Carroll B, Falzon M, et al. Progressive 3q amplification consistently targets SOX2 in preinvasive squamous lung cancer. Am J Respir Crit Care Med. 2010;182:83–91. doi: 10.1164/rccm.201001-0005OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakatsugawa M, Takahashi A, Hirohashi Y, Torigoe T, Inoda S, Murase M, et al. SOX2 is overexpressed in stem-like cells of human lung adenocarcinoma and augments the tumorigenicity. Lab Invest. 2011;91:1796–804. doi: 10.1038/labinvest.2011.140. [DOI] [PubMed] [Google Scholar]
- 13.Xiang R, Liao D, Cheng T, Zhou H, Shi Q, Chuang TS, et al. Downregulation of transcription factor SOX2 in cancer stem cells suppresses growth and metastasis of lung cancer. Br J Cancer. 2011;104:1410–7. doi: 10.1038/bjc.2011.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spisek R, Kukreja A, Chen LC, Matthews P, Mazumder A, Vesole D, et al. Frequent and specific immunity to the embryonal stem cell-associated antigen SOX2 in patients with monoclonal gammopathy. J Exp Med. 2007;204:831–40. doi: 10.1084/jem.20062387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dhodapkar MV, Dhodapkar KM. Vaccines targeting cancer stem cells: are they within reach? Cancer J. 2011;17:397–402. doi: 10.1097/PPO.0b013e318233e730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Güre AO, Stockert E, Scanlan MJ, Keresztes RS, Jäger D, Altorki NK, et al. Serological identification of embryonic neural proteins as highly immunogenic tumor antigens in small cell lung cancer. Proc Natl Acad Sci U S A. 2000;97:4198–203. doi: 10.1073/pnas.97.8.4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen S, Xu Y, Chen Y, Li X, Mou W, Wang L, et al. SOX2 gene regulates the transcriptional network of oncogenes and affects tumorigenesis of human lung cancer cells. PLoS One. 2012;7:e36326. doi: 10.1371/journal.pone.0036326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Velcheti V, Schalper K, Yao X, Cheng H, Kocoglu M, Dhodapkar K, et al. High SOX2 Levels Predict Better Outcome in Non-Small Cell Lung Carcinomas. PLoS One. 2013;8:e61427. doi: 10.1371/journal.pone.0061427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jungbluth AA, Chen YT, Stockert E, Busam KJ, Kolb D, Iversen K, et al. Immunohistochemical analysis of NY-ESO-1 antigen expression in normal and malignant human tissues. Int J Cancer. 2001;92:856–60. doi: 10.1002/ijc.1282. [DOI] [PubMed] [Google Scholar]
- 20.Yuan J, Adamow M, Ginsberg BA, Rasalan TS, Ritter E, Gallardo HF, et al. Integrated NY-ESO-1 antibody and CD8+ T-cell responses correlate with clinical benefit in advanced melanoma patients treated with ipilimumab. Proc Natl Acad Sci U S A. 2011;108:16723–8. doi: 10.1073/pnas.1110814108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Elsas A, Sutmuller RP, Hurwitz AA, Ziskin J, Villasenor J, Medema JP, et al. Elucidating the autoimmune and antitumor effector mechanisms of a treatment based on cytotoxic T lymphocyte antigen-4 blockade in combination with a B16 melanoma vaccine: comparison of prophylaxis and therapy. J Exp Med. 2001;194:481–9. doi: 10.1084/jem.194.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:27ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dhodapkar KM, Feldman D, Matthews P, Radfar S, Pickering R, Turkula S, et al. Natural immunity to pluripotency antigen OCT4 in humans. Proc Natl Acad Sci U S A. 2010;107:8718–23. doi: 10.1073/pnas.0915086107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.