Abstract
Little is known about the relative contributions of self-specific regulatory T cells (Tregs) of thymic origin and induced Tregs generated extrathymically to the pool of tumor-infiltrating Tregs. We have recently demonstrated that thymic-derived Tregs reactive to a prostate-associated self antigen are highly and recurrently enriched within oncogene-driven murine prostate cancers.
Keywords: AIRE, cancer, development, FOXP3, induced regulatory T cells, thymus, tumor
Regulatory T cells (Tregs), which are characterized by the expression of the transcription factor FOXP3, are essential regulators of immune responses to foreign, self and tumor-associated antigens. In several human cancers, the density of Tregs negatively correlates with disease outcome,1 suggesting that Tregs may play a functional role in modulating tumor progression. Moreover, Treg-depleting agents have been shown to enhance the efficacy of various immunotherapeutic regimens aimed at inducing antitumor immune responses, sparking considerable interest in the manipulation of Tregs in cancer patients.2 However, efforts to selectively target tumor-associated Tregs have been hampered by a limited understanding of the basic biology of these cells. In particular, it is largely unknown whether the Tregs enriched within tumor lesions are pre-existing, thymic-derived Tregs (tTregs) specific for self antigens, induced Tregs (iTregs) generated de novo within the tumor environment, or a mixture of both. It has been hypothesized that the tumor environment can drive the differentiation of conventional CD4+ T (Tconv) cells reactive to tumor-associated antigens into iTregs,3 thereby promoting disease progression.
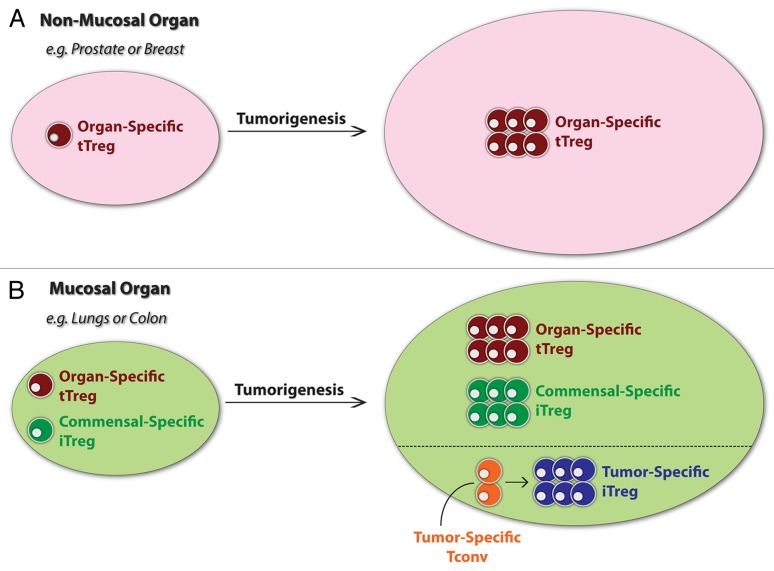
In a recent study aimed at addressing these issues, we identified a monospecific Treg population, which we termed “MJ23” Tregs, that was recurrently enriched in oncogene-driven murine prostate tumors.4 When seeded at low clonal frequency, MJ23 Tregs developed in the thymus, prior to exposure to the tumor environment, implying a thymic origin of these cells. Furthermore, MJ23 Tregs reacted to a prostate-associated antigen that was present in both tumor-bearing and tumor-free male mice. Thus, the antigen recognized by MJ23 Tregs is not a unique “tumor-specific” antigen generated by cancer-associated mutations or post-translational modifications. These findings are consistent with a model in which developing neoplasms do not drive the de novo conversion of Tconv cells into iTregs in the tumor microenvironment, but instead recruit pre-existing tTregs specific for self antigens associated with the organs from which the neoplastic lesions originated (Fig. 1).
Figure 1. Origin of tumor-associated regulatory T cells. The relative contribution of thymic-derived regulatory T cells (tTregs) and induced Tregs (iTregs) of extrathymic origin to the immune infiltrate of neoplastic lesions is unknown. Here, a conceptual model is presented in which the contribution of tTregs and iTregs is strongly influenced by the organ of cancer origin. (A) In tumors originating within organs that lack an extensive mucosal interface with the external environment (such as prostate and breast carcinomas), tTregs reactive to (organ-specific or less restricted) self antigens may predominate. (B) In tumors developing within organs with extensive mucosal surfaces that are constitutively exposed to microbes or non-microbial foreign substances (such as colon and lung carcinomas), iTregs may play a more prevalent role. At these sites, the tumor microenvironment may promote the expansion of pre-existing iTregs specific for commensal or environmental antigens, or may be permissive for the differentiation of tumor-specific conventional T (Tconv) cells into iTregs.
Unexpectedly, despite their reactivity against a prostate-associated antigen, MJ23 Tregs developed in the thymus of both male and female mice.4 Why would female mice generate Tregs specific for a prostate-associated antigen? To understand the mechanisms driving this process, we hypothesized that the development of MJ23 Tregs would depend on autoimmune regulator (AIRE). AIRE is a transcriptional regulator that has been shown to induce the ectopic expression of peripheral tissue antigens by epithelial cells in the thymic medulla.5 Extensive evidence demonstrates that the AIRE-driven display of peripheral tissue antigens within the thymus promotes the deletion of thymocytes reacting to these antigens, thereby enforcing immune tolerance.5 However, a role for AIRE in the development of Tregs has not been previously established. The study of Treg development in Aire+/+ and Aire−/− mice demonstrated that AIRE is critical for the generation of both MJ23 Tregs and a second specificity of tumor-associated Tregs analyzed in our study. These results provide direct evidence that AIRE is required for the thymic development of some tTreg populations. Thus, AIRE appears to serve a dual role in immune tolerance, driving the deletion of autoreactive thymocytes and promoting the development of some Treg specificities.
In the same study, we surveyed the T-cell receptor (TCR) repertoire expressed by polyclonal tumor-infiltrating Tregs and Tconv cells, obtaining 2 interesting results.4 First, several TCRs expressed by tumor-infiltrating Tregs were reproducibly observed in many neoplastic lesions, demonstrating that prostate tumors drive the recurrent enrichment of Tregs of selected specificities. Second, within a given prostate tumor, the TCRs expressed by Tregs were largely distinct from those expressed by Tconv cells. Similar findings have also been reported in mouse models of transplantable and carcinogen-induced tumors.6,7 These data suggest that the antigens recognized by Tregs within prostate tumors are different from those recognized by Tconv cells. It is currently unknown whether this reflects the recognition of different antigens presented by the same antigen-presenting cells or the interaction of Tconv cells and Tregs with distinct antigen-presenting cell types. In addition, the finding that the TCRs expressed by Tregs and Tconv cells within the tumor microenvironment are distinct has implications for our understanding of the developmental origin of tumor-infiltrating Tregs. Indeed, if Tconv cell clones specific for tumor-associated antigens differentiate into iTregs within the tumor microenvironment, then it is likely that these TCRs would be found in both Tconv and Treg subsets, reflecting a precursor-product relationship between these cell types. However, the TCR repertoire data from the studies discussed above4,6,7 reveal a minimal overlap between the TCR repertoires of tumor-infiltrating Tconv cells and Tregs, suggesting that the generation of iTregs is negligible within the tumors examined.
The results of our study suggest that tTregs reactive to organ-specific self antigens are preferentially enriched in murine prostate tumors. However, these findings do not exclude a potential role for iTregs within neoplastic lesions (Fig. 1). Recent work has demonstrated that iTregs play a critical function at mucosal sites that interface with the external environment, such as the lung and the colon.8 For cancers originating at these sites, the inflammatory environment may be permissive for the generation of iTregs specific for tumor-associated antigens as well as for the local recruitment of pre-existing iTregs reactive to antigens derived from commensal microorganisms.9 Further work is required to determine the relative contribution of tTregs and iTregs to the microenvironment of cancers originating at internal and mucosal sites (Fig. 1).
Glossary
Abbreviations:
- AIRE
autoimmune regulator
- iTregs
induced Tregs
- Tconv
conventional CD4+ T cell
- TCR
T-cell receptor
- Tregs
regulatory T cells
- tTregs
thymic-derived Tregs
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/24898
References
- 1.deLeeuw RJ, Kost SE, Kakal JA, Nelson BH. The prognostic value of FoxP3+ tumor-infiltrating lymphocytes in cancer: a critical review of the literature. Clin Cancer Res. 2012;18:3022–9. doi: 10.1158/1078-0432.CCR-11-3216. [DOI] [PubMed] [Google Scholar]
- 2.Gajewski TF, Chesney J, Curriel TJ. Emerging strategies in regulatory T-cell immunotherapies. Clin Adv Hematol Oncol. 2009;7:1–10, quiz 11-2. [PubMed] [Google Scholar]
- 3.Bilate AM, Lafaille JJ. Induced CD4+Foxp3+ regulatory T cells in immune tolerance. Annu Rev Immunol. 2012;30:733–58. doi: 10.1146/annurev-immunol-020711-075043. [DOI] [PubMed] [Google Scholar]
- 4.Malchow S, Leventhal DS, Nishi S, Fischer BI, Shen L, Paner GP, et al. Aire-dependent thymic development of tumor-associated regulatory T cells. Science. 2013;339:1219–24. doi: 10.1126/science.1233913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathis D, Benoist C. Aire. Annu Rev Immunol. 2009;27:287–312. doi: 10.1146/annurev.immunol.25.022106.141532. [DOI] [PubMed] [Google Scholar]
- 6.Sainz-Perez A, Lim A, Lemercier B, Leclerc C. The T-cell receptor repertoire of tumor-infiltrating regulatory T lymphocytes is skewed toward public sequences. Cancer Res. 2012;72:3557–69. doi: 10.1158/0008-5472.CAN-12-0277. [DOI] [PubMed] [Google Scholar]
- 7.Hindley JP, Ferreira C, Jones E, Lauder SN, Ladell K, Wynn KK, et al. Analysis of the T-cell receptor repertoires of tumor-infiltrating conventional and regulatory T cells reveals no evidence for conversion in carcinogen-induced tumors. Cancer Res. 2011;71:736–46. doi: 10.1158/0008-5472.CAN-10-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Josefowicz SZ, Niec RE, Kim HY, Treuting P, Chinen T, Zheng Y, et al. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature. 2012;482:395–9. doi: 10.1038/nature10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lathrop SK, Bloom SM, Rao SM, Nutsch K, Lio CW, Santacruz N, et al. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011;478:250–4. doi: 10.1038/nature10434. [DOI] [PMC free article] [PubMed] [Google Scholar]