Abstract
The accumulation of immunosuppressive cells and exhausted effector T cells highlight an important immune dysfunction in advanced stage hepatocellular carcinoma (HCC) patients. These cells significantly hamper the efficacy immunotherapies and facilitate HCC progression. We have recently demonstrated that the multipronged depletion of immunosuppressive cells potentially restores effector T-cell function in HCC.
Keywords: HCC, GARP, granzyme B, MDSCs, Tregs
The immune dysfunction elicited by developing neoplasms occurs at several checkpoints during tumor progression and overlapping immunosuppressive mechanisms act in concert for malignant cells to evade antitumor immunity. Several studies have focused on the role that regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs) play in the abrogation of antitumor effector T-cell function. However, there is a paucity of published data on the overall composition of immunosuppressive networks that are established in cancer patients, as most studies have simply described one single dysfunctional aspect of immunity. To fix this critical gap in knowledge, we completed a comprehensive evaluation of immunosuppressive networks in advanced stage hepatocellular carcinoma (HCC) patients.1 Our study represents the first global analysis of immune dysfunction in this cancer patient population.
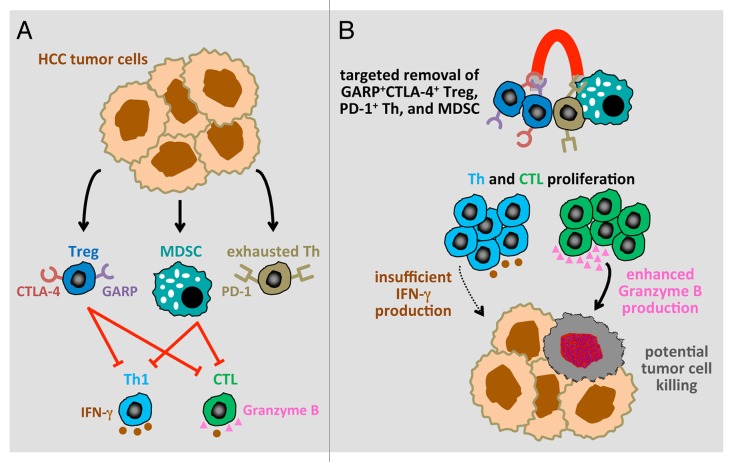
The identification of Tregs based on CD25 only has been highly debated, as this marker fails to accurately discriminate between activated effector T cells and immunosuppressive Tregs.2 Relying on FOXP3 alone is also problematic as this protein is also expressed by non-immunosuppressive Tregs.3 To circumvent both these issues, we utilized FOXP3 and CD127 as Treg markers and observed an elevated frequency of CD4+FOXP3+CD127− Tregs in HCC patients. The intracellular localization of FOXP3 precludes its use for the depletion of immunosuppressive Tregs with specific antibodies. Thus, in order to identify immunosuppressive Tregs exhibiting markers that can be targeted therapeutically, we evaluated the expression of cytotoxic T lymphocyte antigen 4 (CTLA4) and glycoprotein A repetitions predominant (GARP)4 on the surface of FOXP3+ Tregs. HCC patients exhibited elevated levels of CTLA4+GARP+FOXP3+ Tregs (Fig. 1A). The accumulation of this Treg population highlights the severe immune dysfunction of these patients and provides a rational target for eliminating truly immunosuppressive Tregs rather than effector T cells.
Figure 1. Targeted depletion of regulatory T cells, exhausted helper T cells and myeloid-derived suppressor cells may restore effete antitumor T-cell function. (A) The accumulation of exhausted effector helper T cells, immunosuppressive GARP+CTLA4+ regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs) reduces the antitumor effects of TH1 cells and cytotoxic T lymphocytes (CTLs) by inhibiting granzyme B production and interferon γ (IFNγ) secretion. (B) The restoration of effector T-cell proliferation and granzyme B production (but not IFNγ secretion) by the combined removal of all these immunosuppressive cells may restore antitumor TH1 and CTL responses.
MDSCs had not been previously evaluated in HCC patients despite their importance as an immunosuppressive cell population in many cancers. Due to the interconnectedness of MDSC and Treg generation during tumor progression, we measured the frequency of CD14−HLA-DR−CD11b+CD33+ MDSCs in each HCC patient in which Tregs has also been quantified. Correlating with elevated Tregs levels, the abundance of MDSCs was also significantly increased in HCC patients. The accumulation of both Tregs and MDSCs is likely to contribute to the inability of effector CD4+ T cells and CD8+ T cells from HCC patients to proliferate and produce granzyme B and antitumor cytokines such as interferon γ (IFNγ) (Fig. 1A). Effete TH1 and cytotoxic T lymphocyte (CTL) responses further promote the evasion of antitumor immunity by HCC cells and create a milieu that facilitate the accumulation of exhausted PD-1+ effector cells. All these cell subsets limit the efficacy of immunotherapeutic interventions. Thus, the restoration of HCC-targeting immune responses in spite of the (hyper)additive structure of this immunosuppressive network calls for approaches that target all cell subsets promoting immune dysfunction.
We reasoned that the combined depletion of GARP+CTLA4+ Tregs, MDSCs and PD-1+ exhausted T cells in vitro might be able to restore—at least in part—effector T-cell functions in HCC patients. Indeed, our study represents the first formal demonstration that the targeted depletion of multiple immunosuppressive cells is capable of restoring effector T-cell function in advanced HCC (Fig. 1B). The combined depletion of these cells restored the proliferation of both CD4+ helper T cells and CD8+ CTLs, but not to levels equivalent to those observed with T cells from healthy individuals. Importantly, however, the combined depletion of GARP+CTLA4+ Tregs, MDSCs and PD-1+ exhausted T cells was able to restore granzyme B production by CD8+ cells to levels equivalent to those observed with T cells from healthy donors. The secretion of IFNγ by CD4+ T cells was also improved, yet to a lesser extent. Thus, elevated levels of immunosuppressive cells in HCC patients compromise CTL functions in part by inhibiting granzyme B expression, in turn resulting in attenuated tumor-cell killing. In patients with follicular lymphoma, CD8+ T cells expressing high levels of granzyme B have been correlated with prolonged progression-free survival in response to rituximab plus chemotherapy.5 Our findings demonstrate that the depletion of suppressor cells does not completely resolve the immune deregulation of HCC patients.
Some of the biggest successes in immunotherapy have been obtained with monoclonal antibodies that target CTLA4 (ipilimumab),6 PD-1 (BMS-936558)7 and CD25 (daclizumab).8 Ipilimumab improved the survival of advanced refractory melanoma patients by 4 mo and has been the first immunostimulatory monotherapy to be approved for the treatment of an advanced solid tumor. Although the use of ipilimumab has been associated with serious adverse events in 10–15% of patients, the success of several ipilimumab-based clinical trials has demonstrated the therapeutic potential of targeting immune cells. Similarly, PD-1 blockade has shown promise in melanoma, renal cell carcinoma (RCC), and lung cancer patients, even though 14% of patients developed grade 3/4 toxicities. A clinical trial is underway to test escalating doses of BMS-936558 (an anti-PD-1 monoclonal antibody) in HCC patients (NCT01658878). In addition, several studies are now examining the therapeutic of combinatorial approaches and preliminary data are encouraging. Although daclizumab is still in the early phases of development for the treatment of solid tumors, it has already been shown to decrease circulating Tregs in metastatic breast carcinoma patients and to improve the efficacy of a peptide-based anticancer vaccine.
Our studies suggest that removal of all immunosuppressive cell subsets may be required to enhance therapeutic outcomes in HCC patients by eliciting endogenous antitumor effector T cells. The administration of multi-kinase inhibitors (e.g., sunitinib, sorafenib) that target conserved signaling pathways in angiogenesis and immune function may result in immunomodulatory effects similar to those achieved with blocking monoclonal antibodies. RCC patients have been shown to respond to sunitinib with a decrease in MDSCs9 and Tregs,10 as well as with an increase in IFNγ production.10 Mitigating immunosuppressive networks to enable TH1 and CTL responses appears to be critical for improving the survival of advanced HCC patients.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
These authors contributed equally to this article.
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/24679
References
- 1.Kalathil S, Lugade AA, Miller A, Iyer R, Thanavala Y. Higher frequencies of GARP+ CTLA-4+ Foxp3+ T regulatory cells and myeloid-derived suppressor cells in hepatocellular carcinoma patients are associated with impaired T cell functionality. Cancer Res. 2013;73:2435–44. doi: 10.1158/0008-5472.CAN-12-3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ormandy LA, Hillemann T, Wedemeyer H, Manns MP, Greten TF, Korangy F. Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Cancer Res. 2005;65:2457–64. doi: 10.1158/0008-5472.CAN-04-3232. [DOI] [PubMed] [Google Scholar]
- 3.Kryczek I, Liu R, Wang G, Wu K, Shu X, Szeliga W, et al. FOXP3 defines regulatory T cells in human tumor and autoimmune disease. Cancer Res. 2009;69:3995–4000. doi: 10.1158/0008-5472.CAN-08-3804. [DOI] [PubMed] [Google Scholar]
- 4.Wang R, Kozhaya L, Mercer F, Khaitan A, Fujii H, Unutmaz D. Expression of GARP selectively identifies activated human FOXP3+ regulatory T cells. Proc Natl Acad Sci U S A. 2009;106:13439–44. doi: 10.1073/pnas.0901965106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laurent C, Müller S, Do C, Al-Saati T, Allart S, Larocca LM, et al. Distribution, function, and prognostic value of cytotoxic T lymphocytes in follicular lymphoma: a 3-D tissue-imaging study. Blood. 2011;118:5371–9. doi: 10.1182/blood-2011-04-345777. [DOI] [PubMed] [Google Scholar]
- 6.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rech AJ, Mick R, Martin S, Recio A, Aqui NA, Powell DJ, Jr., et al. CD25 blockade depletes and selectively reprograms regulatory T cells in concert with immunotherapy in cancer patients. Sci Transl Med. 2012;4:34ra62. doi: 10.1126/scitranslmed.3003330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15:2148–57. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- 10.Finke JH, Rini B, Ireland J, Rayman P, Richmond A, Golshayan A, et al. Sunitinib reverses type-1 immune suppression and decreases T-regulatory cells in renal cell carcinoma patients. Clin Cancer Res. 2008;14:6674–82. doi: 10.1158/1078-0432.CCR-07-5212. [DOI] [PubMed] [Google Scholar]