Abstract
The kynurenine pathway (KP) is the main catabolic pathway of the essential amino acid tryptophan. The KP has been identified to play a critical role in regulating immune responses in a variety of experimental settings. It is also known to be involved in several neuroinflammatory diseases including Huntington’s disease, amyotrophic lateral sclerosis, and Alzheimer’s disease. This review considers the current understanding of the role of the KP in stem cell biology. Both of these fundamental areas of cell biology have independently been the focus of a burgeoning research interest in recent years. A systematic review of how the two interact has not yet been conducted. Several inflammatory and infectious diseases in which the KP has been implicated include those for which stem cell therapies are being actively explored at a clinical level. Therefore, it is highly relevant to consider the evidence showing that the KP influences stem cell biology and impacts the functional behavior of progenitor cells.
Keywords: kynurenine pathway, tryptophan, indoleamine 2, 3-dioxygenase, embryonic stem cell, haematopoietic stem cell, mesenchymal stem cell, neural stem cell
Introduction
Stem cells
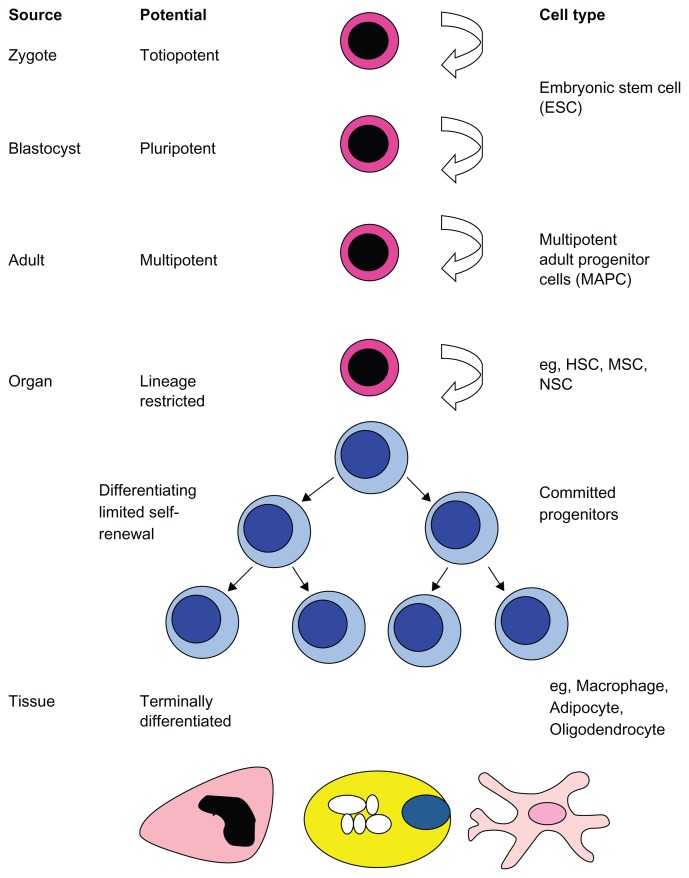
A stem cell is defined by two fundamental properties: the capacity to self-renew and the ability to differentiate into mature cells. A hierarchy of stem cell potential exists, with pluripotent embryonic stem cells (ESCs) at the apex (Fig. 1). ESCs are derived from the inner cell mass of the developing blastocyst and can give rise to mature cells of all three germ layers. Due to a range of moral, bioethical, and technical issues, there are numerous hurdles to the clinical application of ESCs. Consequently, the last three to four decades have witnessed an increased interest in the use of adult stem cells. These cells can be isolated postnatally from a host of different organs and tissues. They typically have a more limited differentiation potential, often restricted to mature cells of one germ layer. Of the diverse range of adult stem cells the hematopoietic stem cell (HSC) represents perhaps the best-studied multipotent cell and can give rise to all cells of the blood. Another bone marrow resident stem cell is the mesenchymal stem cell (MSC). MSCs can differentiate into cells of mesodermal origin, typically osteoblasts, chondrocytes, and adipocytes. Neural stem cells (NSCs) represent a relatively recently identified organ-specific adult stem cell that can differentiate into neurons, astrocytes, and oligodendrocytes (Fig. 1).
Figure 1.
Hierarchy of stem cells.
The kynurenine pathway
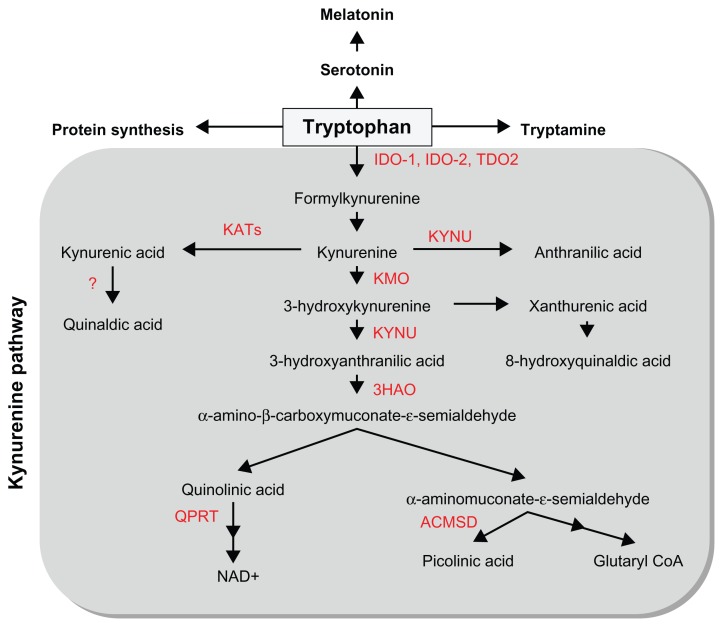
Tryptophan is one of the 9 essential amino acids that the human body is unable to synthesize and thus must be provided through diet. Once absorbed by the body, tryptophan travels through the peripheral circulation. Tryptophan is the only amino acid that binds to albumin in the plasma with approximately 10%–15% of the total plasma tryptophan in the free form and 85%–90% transported bound to albumin, with these two states existing in equilibrium.1 However, tryptophan can only be transported across the blood-brain barrier in its free form by the competitive and non-specific L-type amino acid transporter. Once in the central nervous system (CNS), tryptophan acts as a precursor to several metabolic pathways including general protein synthesis, serotonin/melatonin synthesis, and kynurenine production (Fig. 2).1
Figure 2.
Overview of the kynurenine pathway of tryptophan metabolism.
Note: Key enzymes are indicated in red.
Abbreviations: IDO, Indoleamine 2,3-dioxygenase; TDO2, Tryptophan 2,3-dioxygenase; KYNU, Kynureninase; KATs, Kynurenine aminotransferases; KMO, Kynurenine 3-monooxygenase; 3HAO, 3-hydroxyanthranilic acid oxygenase; ACMSD, Aminocarboxymuconate-semialdehyde decarboxylase; QPRT, quinolinic acid phosphoribosyltransferase.
In both the peripheral and central nervous systems, the kynurenine pathway (KP) represents the major route for the catabolism of L-tryptophan, resulting in the production of the essential co-factor pyridine nucleotide nicotinamide adenine dinucleotide (NAD+) and other neuroactive intermediates (Fig. 2). Tryptophan is oxidized by cleavage of the indole ring, initiated either by tryptophan 2,3-dioxygenase (TDO2), indoleamine 2,3-dioxygenase 1 (IDO-1) or IDO-2.2–5 TDO2 is primarily expressed in the liver,6,7 but is also present in the CNS.8 TDO2 is induced by its substrate tryptophan and by corticosteroids.7 TDO2 can be inhibited by indoleamines and nicotinamide analogs as well as by some antidepressant drugs.9 Extra-hepatically, IDO-1 is the predominant enzyme and can be found in most cell types, including macrophages, microglia, neurons, and astrocytes, but not in oligodendrocytes.10–12 IDO-1 is up-regulated by several inflammatory molecules including lipopolysaccharides, amyloid peptides, and HIV proteins,13–15 but its most potent activator is interferon gamma (IFN-γ).5,16 IFN-γ induces both the gene expression and enzymatic activity of IDO-1.17,18 While IDO-2 possesses similar structural and enzymatic activities to IDO-1, IDO-2 displays a different expression pattern and signaling pathway.4,19 In the CNS, KP enzymes are variably expressed in most cell types,11 including astrocytes,20 neurons,21 infiltrating macrophages and microglia,22 oligodendrocytes,12 and endothelial cells.23 Infiltrating macrophages, activated microglia, and neurons express the full range of KP enzymes, whereas astrocytes and likely oligodendrocytes lack the crucial enzymes kynurenine 3-monooxygenase (KMO) and IDO-1, respectively.12,20
In the production of KP metabolites, kynurenine (KYN) is the first stable intermediate formed. Subsequently, several other neuroactive intermediates are generated, including the free-radical generator, 3-hydroxyanthranilic acid (3HAA),24 the excitotoxin and N-methyl-D-aspartate (NMDA) receptor agonist, quinolinic acid (QUIN),25 the NMDA antagonist, kynurenic acid (KYNA),26 and the neuroprotectant, picolinic acid (PIC).27 Among KP metabolites, QUIN appears to be one of the most important in terms of biological activity.28
IDO-1 has recently been the focus of attention because of its potent immunosuppressive effects on T lymphocytes, resulting in part from tryptophan depletion and from direct effects of tryptophan catabolites.29–32 Some kynurenines, such as QUIN and 3HAA, can selectively target immune cells undergoing activation, consequently suppressing T cell proliferation.33,34 More recently, KYN is also involved in immuno-regulation through its ligand function for the Aryl hydrocarbon receptor (AhR).35 KP metabolites can also act in concert to produce an additive effect.36 IDO-1 up-regulation as well as accelerated and sustained degradation of tryptophan represent key indicators of inflammation. Indeed, inflammation and resulting immune activation lead to KP activation and the concomitant increased production of the excitotoxin QUIN.37 To date, QUIN has been shown to be associated with the pathogenesis of a wide range of inflammatory diseases and disorders.38–41
Evidence of KP Involvement in Stem Cell Biology
Embryonic stem cells
Currently, very little is known regarding the role of the KP in ESC biology. As expected for such a critical metabolic pathway, the rate-limiting and key enzymes of the KP are expressed in human ESCs.42 In a quest for biomarkers of developmental toxicity Cezar et al42 identified that KP molecules, particularly TDO2, were up-regulated in ESCs when cells were treated with the anti-epileptic drug valproate. The authors proposed that the KP plays a role in the pathogenesis of neurodevelopmental disorders. They hypothesised that activation of the KP reduces the bioavailability of tryptophan, leading to reduced serotonin synthesis and subsequent neurodevelopmental defects. Aside from this study, there has been limited exploration of the KP in ESC biology.
Hematopoietic stem cells
HSCs represent a population of progenitor cells that, relative to other adult stem cells, have been well-characterized as precursors of cells of their respective tissue systems. The role of HSCs and their niche in hemopoiesis, transplantation biology, and treatment of malignancies has been a strong research focus in recent years. Cells of the hematopoietic lineage were among the first studied to show a link between the KP and immune regulation. IDO expression has been well-characterized in professional antigen-presenting cells.43,44 In these studies, the activation of the KP in dendritic cells and macrophages was a potent mechanism for the regulation of T cell proliferation. In fact, IDO production from donor monocytes following hematopoietic stem cell transplantation is thought to be responsible for the depressed T cell function often observed in these patients.45 Work on tryptophan catabolism in lineage committed hematopoietic cells has recently been extended to investigate activation of the KP in their progenitor precursor: the HSC.46 In this study, the authors found that both acute and chronic stimulation with IFN-γ caused an increase in the production of kynurenine in CD34+ HSC cultures. They hypothesized that IFN-γ mediated activation of the KP inhibited hematopoiesis. However, they did not observe any functional suppressive effect of IDO on erythropoiesis in their experiments. This may have occurred because tryptophan concentrations were not sufficiently depleted to starve HSCs. Kurz et al suggested that other bone marrow resident progenitor cells could therefore account for the inhibition of erythropoiesis observed in conditions such as anemia. These mesenchymal progenitors will be the focus of the next section.
Mesenchymal stem cells
As a purported population of adult stem cells, links between tryptophan metabolism and MSC biology have been widely investigated. MSCs are multipotent progenitors that were initially identified in the bone marrow,47 where they are thought to play a physiological role in maintaining the hematopoietic stem cell niche.48 Recently MSCs have also been isolated and expanded from a wide range of post-natal and fetal tissues,49–51 with some investigators suggesting that these cells are also present in nearly all adult tissues.52 The defining feature of MSCs is their ability to differentiate in various tissues of mesodermal origin, typically osteoblasts, chondrocytes and adipocytes.53 Interestingly, it seems MSCs are not restricted to a mesodermal fate. They have also been shown to differentiate into endothelial cells54 and neural cells.55 This plasticity of MSCs, along with their ability to migrate to sites of inflammation, has attracted significant attention in the last decade for their potential use in transplant and regeneration studies.56 Another aspect of MSC biology that makes them of particular interest is that they display potent immunomodulatory functions. MSCs from a variety of species inhibit the response of T cells to antigenic, mitogenic, and polyclonal stimuli.57–59 This effect is targeted mainly at the level of T cell proliferation and has been further characterized as arrest of the cell cycle in the G1 phase.60 This has been shown to be a pro-survival property, with MSCs rescuing T cells from apoptosis.61 The immunosuppressive effects of MSCs are not limited to T cells. B cell proliferation and differentiation,62 dendritic cell maturation and antigen-presenting function,63 and NK cell proliferation and cytotoxicity64 are all targets of MSC-mediated suppression. There is also growing evidence that MSCs exert similar effects on the innate immune system (for a review of this subject65).
The mechanisms by which MSCs produce these compelling immunomodulatory functions remain unresolved. While cell-to-cell contact appears to be required for ‘licensing’ MSCs to become suppressive, the inhibitory effect is ultimately mediated by a soluble factor(s).61 Several candidate molecules have been proposed, including transforming growth factor-β1,58 hemeoxygenase-1,66 prostaglandin E2 (PGE2),67 and nitric oxide (NO).68 Interestingly, activation of the KP has also been investigated as a potential mechanism for this immunosuppressive effect. Meisel et al showed that IDO could be induced in MSCs by exposure to IFN-γ in a dose-dependent manner. This finding was confirmed in co-cultures of MSCs and mixed lymphocyte reactions (MLR), in which significant IDO activity was detected compared to MSC or MLR cultures alone, suggesting that MSCs were the primary source of IDO activity. Importantly, in the MSC/MLR co-cultures, addition of tryptophan significantly restored T cell proliferation. The authors did not investigate the effect of IDO inhibitors in this system.69 In support of these findings a more comprehensive study of the MSC immunomodulatory function found that the separate addition of two different competitive inhibitors of IDO, 1-methyl-tryptophan (1-MT) and norharmane, reduced the suppressive effect of MSCs on T cell proliferation.70 A third study also confirmed a role for IDO in MSC-mediated suppression of allogeneic T cell proliferation. Treatment of MSC/MLR co-cultures with 1-MT significantly, but not completely, restored allo-driven proliferation of lymphocytes.71 Interestingly, it has been documented that ESCs share similar immunosuppressive functions with MSCs.72 However, it remains unclear whether the KP is involved in this inhibitory effect. In a recent study using murine ESCs and MSCs, the authors found that IDO production was not involved in stem cell-mediated immunosuppression in their system.73
Involvement of the KP in the immunosuppressive effects of MSCs has been supported by in vivo studies. Using experimental autoimmune encephalomyelitis (EAE), a murine model of multiple sclerosis, Matysiak et al showed that mice transplanted with MSCs showed significantly lower clinical scores and greater improvement than control mice with EAE.74 The authors reported a higher than two-fold increase in the expression of IDO in the spleens of EAE mice treated with MSCs; upon closer analysis, it was found that CD11c+ dendritic cells were the population predominantly expressing IDO. The use of 1-MT to treat MSC-transplanted EAE mice restored clinical scores to similar levels as those of control mice with EAE and no transplanted MSCs. Therefore, blocking IDO activity led to a loss of the immunosuppressive function of MSCs in vivo and exacerbation of inflammatory disease.74
In addition to their effects on T cells, MSCs have been shown to inhibit NK cell proliferation and cytotoxicity, which is at least partially mediated by IDO.75 Furthermore, in this study, addition of the PGE2 inhibitor NS-398 to MSC and NK cell co-cultures along with 1-MT (inhibitor of IDO) nearly fully recovered IL-2-stimulated NK cell proliferation. This suggests that mechanistic synergy exists between IDO and PGE2 in the immunosuppressive effect of MSCs. The authors speculate that the synthesis of IDO by MSCs is induced both directly by exposure to pro-inflammatory cytokines such as IFN-γ and indirectly by autocrine stimulation of cells by PGE2. This hypothesis is supported by earlier work using dendritic cells in which PGE2 exposure induced de novo expression of IDO mRNA.76
Despite the evidence outlined above, the hypothesis that the KP regulates MSC-mediated suppression of immune cells remains controversial. Gieseke and co-workers used MSCs cultured from the bone marrow of a boy with a frameshift mutation in the IFN-γ receptor 1 (R1). These MSCIFNγR1− cells failed to respond to IFN-γ in vitro but displayed the same ability to inhibit PBMC proliferation as wild-type MSCs. Additionally, IDO expression was completely absent and non-inducible in MSCIFNγR1− cells, yet they were still able to inhibit the proliferation of allogeneic PBMC.77 These findings clearly contest the notion that IFN-γ-stimulated IDO expression is required for MSCs to exert their inhibitory effects on immune cells. However, this does not rule out the fact that other factors can upregulate IDO. More recently, Lanz et al demonstrated that murine MSCs suppress the activation of myelin specific T cells independently of IDO.78 The pharmacological inhibition of IDO, using 1-MT, failed to rescue immunosuppression of MOG activated splenocytes. This can be explained by the observation that 1-MT is not a potent inhibitor of IDO.79 Work from our laboratory and others has shown that 1-MT, along with other pharmacological inhibitors, at best only offers partial inhibition of IDO activity. Moreover, the authors showed that in an EAE model, IDO gene ablation (IDO1−/− MSC) did not attenuate the therapeutic effects of MSCs. Both wild-type MSCs and IDO1−/− MSCs reduced EAE clinical scores by a similar magnitude, potentially by inhibiting proinflammatory cytokines, notably IL-17.78
Interestingly, there appears to be more species differences between human and murine KP expression than what might be expected for an evolutionarily conserved pathway. Our group has found that in contrast to human MSCs, mouse MSCs do not experience significant IFN-γ inducible changes in the expression of several KP enzymes at the RNA level.79 Such findings may explain the conflicting evidence outlined above. In the same paper, Croitoru-Lamoury et al showed that the inhibitory effects of KP activation are not limited to immune cells. In fact, IFN-γ inhibits the proliferation of both mouse and human MSCs through activation of the KP. The addition of excess tryptophan to cultures of MSCs stimulated with IFN-γ blocked the anti-proliferative activity of the cytokine on MSCs. Moreover, blocking IDO action by addition of 1-MT rescued cell viability of cytokine-stimulated MSCs. However, in long-term cultures of 50 days, addition of the IDO inhibitors norharmane and 1-MT did not recover the significant decrease in proliferation of IFN-γ-treated MSCs. The effect of IFN-γ-mediated activation of the KP on MSC proliferation was extended to investigate its effects on MSC differentiation. Based on the gene expression of recognized osteogenic and adipogenic differentiation markers (osteopontin, integrin-binding sialoprotein II, and adipsin, adipoQ, Fabp4 respectively), it was demonstrated that treatment with IFN-γ inhibited the gene expression of all these markers. Importantly, addition of norharmane to differentiation cultures partially recovered the gene expression of osteopontin, adipsin, and adipoQ. Similar findings were extended to the neural differentiation of MSCs.79 Taken together, this evidence points to a role for the KP in modulating essential stem cell functions: proliferation and differentiation potential.
Neural stem cells
NSCs have been shown to express high levels of IDO mRNA and protein when activated by IFN-γ. This feature of NSCs was utilized in a cell-based assay to examine the effects of a number of naturally occurring anti-inflammatory phytochemicals on IDO expression. The authors suggested that the suppressive effect of some of these compounds on IDO may account for their observed anti-tumor and neuroprotective properties.80 Our group previously established that much of the KP “machinery” is expressed in NSCs.79 Using murine NSCs, transcripts encoding all the major KP enzymes, were detected under basal conditions. On-going experiments in our laboratory are examining the expression and function of the KP in neural progenitor cells. We hope to ascertain whether modulation of the KP can affect the survival, proliferation, and differentiation of neural progenitor cells and ultimately their functional ability to remyelinate damaged neurons. This has huge potential to offer novel therapeutic opportunities for the treatment of inflammatory and neurodegenerative disorders.
The KP has been implicated in the pathophysiology of depression and its associated deficits in neurogenesis. Zunsdain et al found that human hippocampal progenitor cells constitutively express IDO and TDO and that treatment with IL-1β significantly up-regulated IDO. IL-1β also induced a decrease in expression of the 3 major KAT enzyme isoforms, which catalyze the conversion of kynurenine (KYN) into the neuroprotectant kynurenic acid (KYNA). In contrast, transcripts for two enzymes that act along the neurotoxic branches of the KP, kynurenine 3-monooxygenase (KMO) and kynureninase (KYNU), were increased. Thus, the authors postulated that IL-1β inhibits neurogenesis in human hippocampal progenitor cells by increasing neurotoxicity and supressing neuroprotection, although they provided no direct evidence. Treatment with the KMO inhibitor Ro 61-8048 partially reversed the detrimental effects of IL-1β on neurogenesis, confirming that the observed effects were mediated via the KP.81 Interestingly, the authors showed that IL-1β had contrasting effects on the differentiation and proliferation of neural progenitor cells; IL-1β inhibits neural differentiation while promoting proliferation of undifferentiated cells. It was postulated that this difference was facilitated through activation of the neurotoxic branch and inhibition of the neuroprotective branch of the KP during differentiation. In contrast, IL-1β activated both the neuroprotective and neurotoxic branches in proliferating cells. A major limitation of this study was the use of a progenitor cell line under in vitro experimental conditions.
However, in vivo support for a role of the KP in NSC differentiation comes from a report exploring the function of the KP in neurogenesis and anxiety-related behavior using TDO−/− mice.82 TDO−/− mice had significantly elevated plasma levels of tryptophan; however, concentrations of KYN and KYNA remained at physiological levels. IDO was suggested as the most likely candidate to compensate for the lack of TDO in maintaining downstream KP metabolite concentrations. In terms of the phenotype of neural progenitor cells, this study reported that there was a marked increase in the proliferation of NSCs in the subventricular zone of TDO−/− mice brains. This suggests that the KP is involved in regulating adult neurogenesis and possibly higher brain functions. Evidence to support the latter came from observations that TDO−/− mice displayed anxiety-related behavior in two classical behavior tests.
An investigation into the effect of alcohol exposure on neural development in the foetus, treated human ESCs, neural progenitors and neurons with ethanol in vitro and analysed the metabolome of human ESCs during these stages of neurogenesis.83 Interestingly ethanol induced the significant alteration of tryptophan metabolism in human ESCs and NSCs. A number of KP metabolites were elevated upon alcohol exposure including 3-hydroxy-L-kynurenine, 5-hydroxy-L-kynurenine, L-kynurenine and indole-3-acetaldehyde. This may provide potential biomarkers to help with the diagnosis of foetal alcohol spectrum disorders (FASD) and also suggests aberrant tryptophan metabolism plays a mechanistic role in FASD.
Therapeutic Applications
This review has discussed the considerable body of evidence suggsting that the KP plays a significant role in the development and function of stem cells. The clinical application of this knowledge is in its infancy, but there is promising evidence that targeting stem cells through the manipulation of the KP is clinically beneficial. Several synthetic tryptophan catabolites (Tranilast, Teriflunomide, and Laquinimod) are in phase II and phase III clinical trials for the prevention of a number of autoimmune disorders, including multiple sclerosis.84 Although it is thought the mechanistic action of these compounds is mediated largely through their immunosuppressive effect on T cells and NK cells, their influence on endogenous progenitor cells has not been investigated. Notably, in a recent double-blind, placebo controlled, phase III study investigating the efficacy of Teriflunomide in MS patients, treatment groups showed a significantly reduced risk of disability progression.85 This suggests a regenerative action above and beyond a solely anti-inflammatory effect.
Two studies outlined in this review identify KP metabolites in stem cells as potential biomarkers for neurodevelopmental disorders.42,83 In addition to their putative role as markers of disease, it is highly likely that the KP plays a mechanistic role in a number of cognitive and motor deficits associated with neurodegeneration. There is recent compelling evidence in both Drosophila86 and mice87 that the genetic and pharmacological inhibition of KMO reverses neurodegeneration in models of Alzheimer’s disease and Huntington’s disease. This effect was mediated by concomitant decrease in the neurotoxin 3-HK and increase in KYNA. Thus, use of KMO inhibitors should be explored at the clinical level. Interestingly, the mode of action of some antidepressant drugs has been linked to reducing KMO levels and stimulating KATs activity, which leads to the production of KYNA from KYN.88 Ensuring the correct balance of neurotoxic and neuroprotective KP metabolites in future stem cell therapies must be of prime importance. This hypothesis is consistent with clinical data demonstrating a decrease in KYNA levels in depressed patients.89
Conclusion
In this review, we outlined the known links between the KP and stem cell biology. In ESCs and HSCs, there is relatively little known about the role of tryptophan metabolism in their function. However, in the case of MSCs and NSCs, there exists a rapidly growing body of evidence demonstrating a crucial link between the KP and the role of these progenitor cells in healthy and diseased tissue. The KP has significant potential for use in treating a wide range of inflammatory and degenerative diseases. The precise mechanistic role that progenitor cells play in this remains to be further elucidated. A better understanding of how KP metabolites impact stem cell populations may help to overcome some of the current barriers preventing their use in animal models and enhance future therapeutic transplantation. This review heralds an exciting time for the exploration of tryptophan metabolism as a modulator of stem cell behavior.
Footnotes
Author Contributions
Wrote the first draft of the manuscript: SPJ. Contributed to the writing of the manuscript: GJG, BJB. Agree with manuscript results and conclusions: SPJ, GJG, BJB. Jointly developed the structure and arguments for the paper: SPJ, GJG, BJB. Made critical revisions and approved final version: SPJ, GJG, BJB. All authors reviewed and approved of the final manuscript.
Competing Interests
Author(s) disclose no potential conflicts of interest.
Disclosures and Ethics
As a requirement of publication the authors have provided signed confirmation of their compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published elsewhere, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests.
Funding
This work was supported by the Multiple Sclerosis Research Association (MSRA) and The University of New South Wales (UNSW).
References
- 1.Ruddick JP, Evans AK, Nutt DJ, Lightman SL, Rook GA, Lowry CA. Tryptophan metabolism in the central nervous system: medical implications. Expert Rev Mol Med. 2006;8(20):1–27. doi: 10.1017/S1462399406000068. [DOI] [PubMed] [Google Scholar]
- 2.Tan L, Yu JT, Tan L. The kynurenine pathway in neurodegenerative diseases: Mechanistic and therapeutic considerations. J Neurol Sci. 2012;323(1–2):1–8. doi: 10.1016/j.jns.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Indoleamine 2,3-dioxygenase. A new vista in tryptophan metabolism. Acta Vitaminol Enzymol. 1975;29(1–6):17–20. [PubMed] [Google Scholar]
- 4.Ball HJ, Sanchez-Perez A, Weiser S, et al. Characterization of an indoleamine 2,3-dioxygenase-like protein found in humans and mice. Gene. 2007;396(1):203–13. doi: 10.1016/j.gene.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 5.Shimizu T, Nomiyama S, Hirata F, Hayaishi O. Indoleamine 2,3-dioxygenase. purification and some properties. J Biol Chem. 1978;253(13):4700–6. [PubMed] [Google Scholar]
- 6.Salter M, Pogson CI. The role of tryptophan 2,3-dioxygenase in the hormonal control of tryptophan metabolism in isolated rat liver cells. Effects of glucocorticoids and experimental diabetes. Biochem J. 1985;229(2):499–504. doi: 10.1042/bj2290499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller CL, Llenos IC, Dulay JR, Barillo MM, Yolken RH, Weis S. Expression of the kynurenine pathway enzyme tryptophan 2,3-dioxygenase is increased in the frontal cortex of individuals with schizophrenia. Neurobiol Dis. 2004;15(3):618–29. doi: 10.1016/j.nbd.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 8.Ohira K, Hagihara H, Toyama K, et al. Expression of tryptophan 2,3-dioxygenase in mature granule cells of the adult mouse dentate gyrus. Mol Brain. 2010;3(1):26. doi: 10.1186/1756-6606-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies NWS, Guillemin GJ, Brew BJ, Tryptophan Neurodegeneration. HIV-Associated Neurocognitive Disorder. Int J Tryptophan Res. 2010;3:1–20. doi: 10.4137/ijtr.s4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guillemin GJ, Kerr SJ, Smythe GA, et al. Kynurenine pathway metabolism in human astrocytes: a paradox for neuronal protection. J Neurochem. 2001;78:1–13. doi: 10.1046/j.1471-4159.2001.00498.x. [DOI] [PubMed] [Google Scholar]
- 11.Guillemin GJ, Smythe G, Takikawa O, Brew BJ. Expression of indoleamine 2,3-dioxygenase and production of quinolinic acid by human microglia, astrocytes, and neurons. Glia. 2005;49(1):15–23. doi: 10.1002/glia.20090. [DOI] [PubMed] [Google Scholar]
- 12.Lim CK, Smythe GA, Stocker R, Brew BJ, Guillemin GJ. Characterization of the kynurenine pathway in human oligodendrocytes. International Congress Series. 2007;1304(1):213–7. [Google Scholar]
- 13.Fujigaki S, Saito K, Takemura M, et al. Species differences in L-tryptophan-kynurenine pathway metabolism: quantification of anthranilic acid and its related enzymes. Arch Biochem Biophys. 1998;358(2):329–35. doi: 10.1006/abbi.1998.0861. [DOI] [PubMed] [Google Scholar]
- 14.Guillemin GJ, Smythe GA, Veas LA, Takikawa O, Brew BJ. A beta 1–42 induces production of quinolinic acid by human macrophages and microglia. Neuroreport. 2003;14(18):2311–5. doi: 10.1097/00001756-200312190-00005. [DOI] [PubMed] [Google Scholar]
- 15.Takikawa O. Biochemical and medical aspects of the indoleamine 2,3-dioxygenase-initiated L-tryptophan metabolism. Biochem Biophys Res Commun. 2005;338(1):12–9. doi: 10.1016/j.bbrc.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 16.Werner-Felmayer G, Werner ER, Fuchs D, Hausen A, Reibnegger G, Wachter H. Characteristics of interferon induced tryptophan metabolism in human cells in vitro. Biochim Biophys Acta. 1989;1012(2):140–7. doi: 10.1016/0167-4889(89)90087-6. [DOI] [PubMed] [Google Scholar]
- 17.Yasui H, Takai K, Yoshida R, Hayaishi O. Interferon enhances tryptophan metabolism by inducing pulmonary indoleamine 2,3-dioxygenase: its possible occurrence in cancer patients. Proc Natl Acad Sci U S A. 1986;83(17):6622–6. doi: 10.1073/pnas.83.17.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dai W, Gupta SL. Regulation of indoleamine 2,3-dioxygenase gene expression in human fibroblasts by interferon-gamma. Upstream control region discriminates between interferon-gamma and interferon-alpha. J Biol Chem. 1990;265(32):19871–7. [PubMed] [Google Scholar]
- 19.Metz R, Duhadaway JB, Kamasani U, Laury-Kleintop L, Muller AJ, Prendergast GC. Novel tryptophan catabolic enzyme IDO2 is the preferred biochemical target of the antitumor indoleamine 2,3-dioxygenase inhibitory compound D-1-methyl-tryptophan. Cancer Res. 2007;67(15):7082–7. doi: 10.1158/0008-5472.CAN-07-1872. [DOI] [PubMed] [Google Scholar]
- 20.Guillemin GJ, Smith DG, Kerr SJ, et al. Characterisation of kynurenine pathway metabolism in human astrocytes and implications in neuropathogenesis. Redox Rep. 2000;5(2–3):108–11. doi: 10.1179/135100000101535375. [DOI] [PubMed] [Google Scholar]
- 21.Guillemin GJ, Cullen KM, Lim CK, et al. Characterization of the kynurenine pathway in human neurons. J Neurosci. 2007;27(47):12884–92. doi: 10.1523/JNEUROSCI.4101-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guillemin GJ, Smith DG, Smythe GA, Armati PJ, Brew BJ. Expression of the kynurenine pathway enzymes in human microglia and macrophages. Adv Exp Med Biol. 2003;527:105–12. doi: 10.1007/978-1-4615-0135-0_12. [DOI] [PubMed] [Google Scholar]
- 23.Owe-Young R, Webster NL, Mukhtar M, et al. Kynurenine pathway metabolism in human blood-brain-barrier cells: implications for immune tolerance and neurotoxicity. J Neurochem. 2008;105(4):1346–57. doi: 10.1111/j.1471-4159.2008.05241.x. [DOI] [PubMed] [Google Scholar]
- 24.Goldstein LE, Leopold MC, Huang X, et al. 3-Hydroxykynurenine and 3-hydroxyanthranilic acid generate hydrogen peroxide and promote alpha-crystallin cross-linking by metal ion reduction. Biochemistry. 2000;39(24):7266–75. doi: 10.1021/bi992997s. [DOI] [PubMed] [Google Scholar]
- 25.Stone TW, Perkins MN. Quinolinic acid: a potent endogenous excitant at amino acid receptors in CNS. Eur J Pharmacol. 1981;72(4):411–2. doi: 10.1016/0014-2999(81)90587-2. [DOI] [PubMed] [Google Scholar]
- 26.Perkins MN, Stone TW. An iontophoretic investigation of the actions of convulsant kynurenines and their interaction with the endogenous excitant quinolinic acid. Brain Res. 1982;247(1):184–7. doi: 10.1016/0006-8993(82)91048-4. [DOI] [PubMed] [Google Scholar]
- 27.Jhamandas K, Boegman RJ, Beninger RJ, Bialik M. Quinolinate-induced cortical cholinergic damage: modulation by tryptophan metabolites. Brain Res. 1990;529(1–2):185–91. doi: 10.1016/0006-8993(90)90826-w. [DOI] [PubMed] [Google Scholar]
- 28.Guillemin GJ. Quinolinic acid, the inescapable neurotoxin. FEBS J. 2012;279(8):1356–65. doi: 10.1111/j.1742-4658.2012.08485.x. [DOI] [PubMed] [Google Scholar]
- 29.Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999;189(9):1363–72. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munn DH, Zhou M, Attwood JT, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281(5380):1191–3. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 31.Terness P, Bauer TM, Rose L, et al. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J Exp Med. 2002;196(4):447–57. doi: 10.1084/jem.20020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pilotte L, Larrieu P, Stroobant V, et al. Reversal of tumoral immune resistance by inhibition of tryptophan 2,3-dioxygenase. Proc Natl Acad Sci U S A. 2012;109(7):2497–502. doi: 10.1073/pnas.1113873109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. The J Exp Med. 2002;196(4):459–68. doi: 10.1084/jem.20020121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fallarino F, Grohmann U, Hwang KW, et al. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4(12):1206–12. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 35.Opitz CA, Litzenburger UM, Sahm F, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478(7368):197–203. doi: 10.1038/nature10491. [DOI] [PubMed] [Google Scholar]
- 36.Terness P, Bauer TM, Röse L, et al. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J Exp Med. 2002;196(4):447–57. doi: 10.1084/jem.20020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moffett JR, Els T, Espey MG, Walter SA, Streit WJ, Namboodiri MA. Quinolinate immunoreactivity in experimental rat brain tumors is present in macrophages but not in astrocytes. Exp Neurol. 1997;144(2):287–301. doi: 10.1006/exnr.1996.6365. [DOI] [PubMed] [Google Scholar]
- 38.Vécsei L, Szalárdy L, Fülöp F, Toldi J. Kynurenines in the CNS: recent advances and new questions. Nat Rev Drug Discov. 2012;12(1):64–82. doi: 10.1038/nrd3793. [DOI] [PubMed] [Google Scholar]
- 39.Zádori D, Klivényi P, Vámos E, Fülöp F, Toldi J, Vécsei L. Kynurenines in chronic neurodegenerative disorders: future therapeutic strategies. J Neural Transm. 2009;116(11):1403–9. doi: 10.1007/s00702-009-0263-4. [DOI] [PubMed] [Google Scholar]
- 40.Stone TW, Stoy N, Darlington LG. An expanding range of targets for kynurenine metabolites of tryptophan. Trends Pharmacol Sci. 2013;34(2):136–43. doi: 10.1016/j.tips.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 41.Stone TW, Mackay GM, Forrest CM, Clark CJ, Darlington LG. Tryptophan metabolites and brain disorders. Clin Chem Lab Med. 2003;41(7):852–9. doi: 10.1515/CCLM.2003.129. [DOI] [PubMed] [Google Scholar]
- 42.Cezar GG, Quam JA, Smith AM, et al. Identification of small molecules from human embryonic stem cells using metabolomics. Stem Cells Dev. 2007;16(6):869–82. doi: 10.1089/scd.2007.0022. [DOI] [PubMed] [Google Scholar]
- 43.Hwu P, Du MX, Lapointe R, Do M, Taylor MW, Young HA. Indoleamine 2,3-dioxygenase production by human dendritic cells results in the inhibition of T cell proliferation. J Immunol. 2000;164(7):3596–9. doi: 10.4049/jimmunol.164.7.3596. [DOI] [PubMed] [Google Scholar]
- 44.Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999;189(9):1363–72. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hainz U, Obexer P, Winkler C, et al. Monocyte-mediated T-cell suppression and augmented monocyte tryptophan catabolism after human hematopoietic stem-cell transplantation. Blood. 2005;105(10):4127–34. doi: 10.1182/blood-2004-05-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kurz K, Gluhcheva Y, Zvetkova E, Konwalinka G, Fuchs D. Interferon-gamma-mediated pathways are induced in human CD34(+) haematopoietic stem cells. Immunobiology. 2010;215(6):452–7. doi: 10.1016/j.imbio.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 47.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 48.Dazzi F, Ramasamy R, Glennie S, Jones SP, Roberts I. The role of mesenchymal stem cells in haemopoiesis. Blood Rev. 2006;20(3):161–71. doi: 10.1016/j.blre.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 49.Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I, Fisk NM. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001;98(8):2396–402. doi: 10.1182/blood.v98.8.2396. [DOI] [PubMed] [Google Scholar]
- 50.Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005;52(8):2521–9. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- 51.Zvaifler NJ, Marinova-Mutafchieva L, Adams G, et al. Mesenchymal precursor cells in the blood of normal individuals. Arthritis Res. 2000;2(6):477–88. doi: 10.1186/ar130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119(Pt 11):2204–13. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 53.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 54.Reyes M, Lund T, Lenvik T, Aguiar D, Koodie L, Verfaillie CM. Purification and ex vivo expansion of postnatal human marrow mesodermal progenitor cells. Blood. 2001;98(9):2615–25. doi: 10.1182/blood.v98.9.2615. [DOI] [PubMed] [Google Scholar]
- 55.Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61(4):364–70. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 56.Ren G, Chen X, Dong F, et al. Concise review: mesenchymal stem cells and translational medicine: emerging issues. Stem Cells Transl Med. 2012;1(1):51–8. doi: 10.5966/sctm.2011-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bartholomew A, Sturgeon C, Siatskas M, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30(1):42–8. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 58.Di Nicola M, Carlo-Stella C, Magni M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99(10):3838–43. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 59.Krampera M, Glennie S, Dyson J, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101(9):3722–9. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- 60.Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105(7):2821–7. doi: 10.1182/blood-2004-09-3696. [DOI] [PubMed] [Google Scholar]
- 61.Jones S, Horwood N, Cope A, Dazzi F. The antiproliferative effect of mesenchymal stem cells is a fundamental property shared by all stromal cells. J Immunol. 2007;179(5):2824–31. doi: 10.4049/jimmunol.179.5.2824. [DOI] [PubMed] [Google Scholar]
- 62.Corcione A, Benvenuto F, Ferretti E, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107(1):367–72. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 63.Ramasamy R, Fazekasova H, Lam EW, Soeiro I, Lombardi G, Dazzi F. Mesenchymal stem cells inhibit dendritic cell differentiation and function by preventing entry into the cell cycle. Transplantation. 2007;83(1):71–6. doi: 10.1097/01.tp.0000244572.24780.54. [DOI] [PubMed] [Google Scholar]
- 64.Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006;24(1):74–85. doi: 10.1634/stemcells.2004-0359. [DOI] [PubMed] [Google Scholar]
- 65.Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12(5):383–96. doi: 10.1038/nri3209. [DOI] [PubMed] [Google Scholar]
- 66.Chabannes D, Hill M, Merieau E, et al. A role for heme oxygenase-1 in the immunosuppressive effect of adult rat and human mesenchymal stem cells. Blood. 2007;110(10):3691–4. doi: 10.1182/blood-2007-02-075481. [DOI] [PubMed] [Google Scholar]
- 67.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815–22. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 68.Sato K, Ozaki K, Oh I, et al. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood. 2007;109(1):228–34. doi: 10.1182/blood-2006-02-002246. [DOI] [PubMed] [Google Scholar]
- 69.Meisel R, Zibert A, Laryea M, Göbel U, Däubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103(12):4619–21. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- 70.Krampera M, Cosmi L, Angeli R, et al. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24(2):386–98. doi: 10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]
- 71.Ryan JM, Barry F, Murphy JM, Mahon BP. Interferon-gamma does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin Exp Immunol. 2007;149(2):353–63. doi: 10.1111/j.1365-2249.2007.03422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Koch CA, Geraldes P, Platt JL. Immunosuppression by embryonic stem cells. Stem Cells. 2008;26(1):89–98. doi: 10.1634/stemcells.2007-0151. [DOI] [PubMed] [Google Scholar]
- 73.Han KH, Ro H, Hong JH, et al. Immunosuppressive mechanisms of embryonic stem cells and mesenchymal stem cells in alloimmune response. Transpl Immunol. 2011;25(1):7–15. doi: 10.1016/j.trim.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 74.Matysiak M, Stasiolek M, Orlowski W, et al. Stem cells ameliorate EAE via an indoleamine 2,3-dioxygenase (IDO) mechanism. J Neuroimmunol. 2008;193(1–2):12–23. doi: 10.1016/j.jneuroim.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008;111(3):1327–33. doi: 10.1182/blood-2007-02-074997. [DOI] [PubMed] [Google Scholar]
- 76.Braun D, Longman RS, Albert ML. A two-step induction of indoleamine 2,3 dioxygenase (IDO) activity during dendritic-cell maturation. Blood. 2005;106(7):2375–81. doi: 10.1182/blood-2005-03-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gieseke F, Schutt B, Viebahn S, et al. Human multipotent mesenchymal stromal cells inhibit proliferation of PBMCs independently of IFN R1 signaling and IDO expression. Blood. 2007;110(6):2197–200. doi: 10.1182/blood-2007-04-083162. [DOI] [PubMed] [Google Scholar]
- 78.Lanz TV, Opitz CA, Ho PP, et al. Mouse mesenchymal stem cells suppress antigen-specific TH cell immunity independent of indoleamine 2,3-dioxygenase 1 (IDO1) Stem Cells Dev. 2010;19(5):657–68. doi: 10.1089/scd.2009.0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Croitoru-Lamoury J, Lamoury FM, Caristo M, et al. Interferon-gamma regulates the proliferation and differentiation of mesenchymal stem cells via activation of indoleamine 2,3 dioxygenase (IDO) PloS ONE. 2011;6(2):e14698. doi: 10.1371/journal.pone.0014698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen S, Corteling R, Stevanato L, Sinden J. Natural inhibitors of indoleamine 3,5-dioxygenase induced by interferon-gamma in human neural stem cells. Biochem Biophys Res Commun. 2012;429(1–2):117–23. doi: 10.1016/j.bbrc.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 81.Zunszain PA, Anacker C, Cattaneo A, et al. Interleukin-1beta: a new regulator of the kynurenine pathway affecting human hippocampal neurogenesis. Neuro Psycho Pharmacology. 2012;37(4):939–49. doi: 10.1038/npp.2011.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kanai M, Funakoshi H, Takahashi H, et al. Tryptophan 2,3-dioxygenase is a key modulator of physiological neurogenesis and anxiety-related behavior in mice. Mol Brain. 2009;2:8. doi: 10.1186/1756-6606-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Palmer JA, Poenitzsch AM, Smith SM, Conard KR, West PR, Cezar GG. Metabolic biomarkers of prenatal alcohol exposure in human embryonic stem cell-derived neural lineages. Alcohol Clin Exp Res. 2012;36(8):1314–24. doi: 10.1111/j.1530-0277.2011.01732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Platten M, Ho PP, Steinman L. Anti-inflammatory strategies for the treatment of multiple sclerosis—tryptophan catabolites may hold the key. Drug Discovery Today: Therapeutic Strategies. 2006;3(3):401–8. [Google Scholar]
- 85.O’Connor P, Wolinsky JS, Confavreux C, et al. Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med. 2011;365(14):1293–303. doi: 10.1056/NEJMoa1014656. [DOI] [PubMed] [Google Scholar]
- 86.Campesan S, Green EW, Breda C, et al. The kynurenine pathway modulates neurodegeneration in a Drosophila model of Huntington’s disease. Curr Biol. 2011;21(11):961–6. doi: 10.1016/j.cub.2011.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zwilling D, Huang SY, Sathyasaikumar KV, et al. Kynurenine 3-monooxygenase inhibition in blood ameliorates neurodegeneration. Cell. 2011;145(6):863–74. doi: 10.1016/j.cell.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kocki T, Wnuk S, Kloc R, Kocki J, Owe-Larsson B, Urbanska EM. New insight into the antidepressants action: modulation of kynurenine pathway by increasing the kynurenic acid/3-hydroxykynurenine ratio. J Neural Transm. 2012;119(2):235–43. doi: 10.1007/s00702-011-0668-8. [DOI] [PubMed] [Google Scholar]
- 89.Myint AM, Kim YK, Verkerk R, Scharpe S, Steinbusch H, Leonard B. Kynurenine pathway in major depression: evidence of impaired neuroprotection. J Affect Disord. 2007;98(1–2):143–51. doi: 10.1016/j.jad.2006.07.013. [DOI] [PubMed] [Google Scholar]