Abstract
In the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (n=6632), eplerenone-associated reduction in all-cause mortality was significantly greater in those with a history of hypertension (Hx-HTN). There were 4007 patients with Hx-HTN (eplerenone: n=1983) and 2625 patients without Hx-HTN (eplerenone: n=1336). Propensity scores for eplerenone use, separately calculated for patients with and without Hx-HTN, were used to assemble matched cohorts of 1838 and 1176 pairs of patients. In patients with Hx-HTN, all-cause mortality occurred in 18% of patients treated with placebo (rate, 1430/10 000 person-years) and 14% of patients treated with eplerenone (rate, 1058/10 000 person-years) during 2350 and 2457 years of follow-up, respectively (hazard ratio [HR]: 0.71; 95% CI: 0.59 to 0.85; P<0.0001). Composite end point of cardiovascular hospitalization or cardiovascular mortality occurred in 33% of placebo-treated patients (3029/10 000 person-years) and 28% of eplerenone-treated patients (2438/10 000 person-years) with Hx-HTN (HR: 0.82; 95% CI: 0.72 to 0.94; P=0.003). In patients without Hx-HTN, eplerenone reduced heart failure hospitalization (HR: 0.73; 95% CI: 0.55 to 0.97; P=0.028) but had no effect on mortality (HR: 0.91; 95% CI: 0.72 to 1.15; P=0.435) or on the composite end point (HR: 0.91; 95% CI: 0.76 to 1.10; P=0.331). Eplerenone should, therefore, be prescribed to all of the post–acute myocardial infarction patients with reduced left ventricular ejection fraction and heart failure regardless of Hx-HTN.
Keywords: Eplerenone, hypertension, myocardial infarction, heart failure, morbidity, mortality
Introduction
In the Eplerenone Post-acute myocardial infarction Heart failure Efficacy and SUrvival Study (EPHESUS), eplerenone, a selective aldosterone blocker, significantly reduced all-cause mortality and the coprimary combined end points of cardiovascular (CV) hospitalization or CV mortality.1 A subgroup analysis of the EPHESUS suggested that the effect of eplerenone on all-cause mortality was greater in patients with a history of hypertension (Hx-HTN) than in those without Hx-HTN (P for interaction=0.05).1 However, there was no significant difference between these groups for eplerenone on the coprimary combined end points of CV hospitalization or CV mortality. To gain further insight into this relationship, we examined the effects of eplerenone on mortality and morbidity in a propensity score–matched cohort of patients with and without Hx-HTN.
Methods
Study Design and Patients
EPHESUS was a multicenter, international, randomized, double-blind, placebo-controlled clinical trial of eplerenone.1 Briefly, 6632 patients with acute myocardial infarction (AMI) complicated by low (≤40%) left ventricular ejection fraction (LVEF) and symptomatic heart failure (HF) were randomly assigned within 3 to 14 days of their AMI to receive eplerenone 25 mg/d titrated to 50 mg/d (n=3319) or matching placebo (n=3313). Patients were receiving standard medical therapy, including an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker (87%) and a beta-blocker. Patients were followed for up to 2.5 years, with a mean follow-up of 16 months. Patients in the placebo and eplerenone groups were receiving a mean dose of 43.5 mg and 42.6 mg per day, respectively. Exclusion criteria included the use of potassium-sparing diuretics, a serum creatinine concentration >2.5 mg/dL (220 μmol/L), and a serum potassium concentration >5.0 mEq/L (mmol/L). Of the EPHESUS participants, 4007 patients had a history of hypertension (Hx-HTN), and 2625 had no Hx-HTN at the time of enrollment. Of the 4007 patients with Hx-HTN, 2024 (50.5%) were in the placebo group, and 1983 (49.5%) were in the eplerenone group. Of the 2625 patients without Hx-HTN, 1289 (49.1%) were in the placebo group, and 1336 (50.9%) were in the eplerenone group.
The two primary end points of the EPHESUS, all-cause mortality and the combined end point of CV hospitalization or CV mortality, were also the primary end points for this analysis. Major secondary end points from EPHESUS, such as CV mortality, which included mortality because of AMI, HF, stroke, and sudden cardiac death (SCD), as well as hospitalization because of AMI and HF, were also studied. The cause of death or the primary diagnosis leading to hospitalization was adjudicated by a blinded EPHESUS critical events committee.
Statistical Analysis
Because the balance achieved by randomization in the main trial may have been lost in the groups with and without Hx-HTN, propensity scores for the receipt of eplerenone were used to assemble a balanced cohort. The propensity score for the receipt of eplerenone for a patient is defined as the conditional probability of receiving eplerenone given that patient’s measured covariates.2–6 Propensity scores were calculated separately for each of the 4007 and 2625 patients with and without Hx-HTN, respectively, using a nonparsimonious multivariable logistic regression model, incorporating the 36 baseline covariates (Table 1). Patients receiving eplerenone and placebo were matched based on their propensity to receive eplerenone. In all, 1838 pairs of patients with Hx-HTN and 1176 pairs of patients without Hx-HTN were matched. Absolute standardized differences were estimated to assess residual balance after matching.6,7
TABLE 1.
Baseline patient characteristics of propensity score matched patients
| Characteristics n (%) or mean (±SD) |
History of hypertension | No history of hypertension | |||||
|---|---|---|---|---|---|---|---|
| Placebo (n=1838) |
Eplerenone (n=1838) |
P value |
Placebo (n=1176) |
Eplerenone (n=1176) |
P value | ||
| Age, y* | 65.5 (±11) | 65.4 (±11) | 0.654 | 61.7 (±13) | 61.8 (±12) | 0.859 | |
| Age ≥65 y* | 1032 (56) | 1010 (55) | 0.486 | 500 (43) | 502 (42) | 0.934 | |
| Women* | 634 (35) | 623 (24) | 0.702 | 248 (21) | 244 (21) | 0.879 | |
| Nonwhites | 177 (10) | 185 (10) | 0.698 | 115 (10) | 119 (9) | 0.674 | |
| Smoking status* | |||||||
| Current | 453 (25) | 461 (25) | 0.895 | 471 (40) | 464 (40) | 0.957 | |
| Never | 836 (46) | 822 (45) | 354 (30) | 357 (30) | |||
| Former | 549 (30) | 555 (30) | 351 (30) | 355 (30 | |||
| Medical history | |||||||
| AMI* | 545 (30) | 543 (30) | 0.971 | 277 (24) | 278 (23) | 1.000 | |
| Angina* | 881 (48) | 893 (49) | 0.717 | 357 (30) | 363 (21) | 0.823 | |
| HF* | 322 (18) | 319 (17) | 0.896 | 117 (10) | 119 (10) | 0.891 | |
| Prior HF hospitalization* | 161 (9) | 159(9) | 0.953 | 68 (6) | 66 (6) | 0.859 | |
| Diabetes* | 685 (37) | 689 (38) | 0.919 | 288 (25) | 288 (25) | 1.000 | |
| Killip status* | |||||||
| I | 294 (16) | 308 (17) | 0.928 | 179 (15) | 174 (15) | 0.964 | |
| II | 1157 (63) | 1152 (63) | 790 (67) | 799 (68) | |||
| III | 332 (18) | 323 (18) | 167 (14) | 161 (14) | |||
| IV | 55 (3) | 55 (3) | 40 (3) | 42 (4) | |||
| Medications | |||||||
| ACEIs* | 1584 (86) | 1585 (86) | 1.000 | 966 (82) | 964 (82) | 0.957 | |
| ARBs | 60 (3) | 63 (3) | 0.855 | 32 (3) | 32 (3) | 1.000 | |
| Beta-blockers | 1364 (74) | 1370 (75) | 0.850 | 886 (75) | 886 (75) | 1.000 | |
| Alpha-blockers* | 49 (3) | 46 (3) | 0.757 | 8 (1) | 7 (1) | 0.803 | |
| Calcium channel blockers* | 356 (19) | 356 (19) | 1.000 | 127 (11) | 128 (11) | 1.000 | |
| Glycoprotein IIb/IIIa blockers | 14 (1) | 18 (1) | 0.595 | 10 (1) | 10 (1) | 1.000 | |
| Antiarrhythmic drugs | 230 (13) | 221 (12) | 0.651 | 126 (11) | 129 (11) | 0.843 | |
| Antiplatelet drugs* | 464 (25) | 469 (26) | 0.880 | 402 (34) | 394 (34) | 0.760 | |
| Anticoagulants* | 339 (18) | 327 (18) | 0.638 | 173 (15) | 171 (15) | 0.953 | |
| Aspirin | 1640 (89) | 1640 (89) | 1.000 | 1037 (88) | 1039 (88) | 0.949 | |
| Statins* | 798 (43) | 809 (44) | 0.740 | 595 (51) | 596 (51) | 0.997 | |
| Other lipid-lowering agents | 31 (2) | 34 (2) | 0.803 | 16 (1) | 16 (1) | 1.000 | |
| Digoxin† | 296 (16) | 290 (16) | 0.787 | 159 (14) | 160 (14) | 0.952 | |
| Nitrates* | 1232 (67) | 1223 (67) | 0.753 | 651 (55) | 652 (55) | 1.000 | |
| Loop diuretics* | 1069 (58) | 1068 (58) | 1.000 | 593 (50) | 595 (51) | 0.967 | |
| Other diuretics* | 176 (10) | 168 (9) | 0.692 | 66 (6) | 65 (6) | 1.000 | |
| Potassium supplements | 322 (18) | 309 (17) | 0.570 | 180 (15) | 180 (15) | 1.000 | |
| Magnesium supplements | 77 (4) | 76 (4) | 1.000 | 40 (3) | 36 (3) | 0.643 | |
| Body mass index, kg/m2* | 28 (±5) | 28 (±4) | 0.812 | 27 (±4) | 26 (±4) | 0.738 | |
| Blood pressure, mm Hg | |||||||
| Systolic* | 123 (±17) | 123 (±17) | 0.894 | 114 (±14) | 113 (±15) | 0.795 | |
| Diastolic* | 74 (±11) | 74 (±11) | 0.997 | 70 (±10) | 70 (±10) | 0.934 | |
| Heart rate per minute | 74 (±11) | 74 (±11) | 0.762 | 75 (±12) | 75 (±12) | 0.822 | |
| LVEF, %† | 33 (±6) | 33 (±6) | 0.767 | 33 (±6) | 33 (±6) | 0.735 | |
| Serum concentrations, mean | |||||||
| Sodium, mmol/L* | 140 (±4) | 140 (±5) | 0.850 | 139 (±4) | 139 (±4) | 0.610 | |
| Potassium, mmol/L* | 4.3 (±0.5) | 4.3 (±0.5) | 0.949 | 4.3 (±0.4) | 4.3 (±0.4) | 0.567 | |
| Creatinine, mg/dL* | 1.16 (±0.4) | 1.15 (±0.3) | 0.784 | 1.09 (±0.4) | 1.09 (±0.3) | 0.949 | |
indicates P<0.0001;
P<0.05 for significant difference between patients with and without a history of hypertension.
ACEI indicates angiotensin-converting enzyme inhibitor; AMI, acute myocardial infarction; ARB, angiotensin receptor blocker; HF, heart failure, LVEF, left ventricular ejection fraction.
We used Kaplan–Meier plots and matched Cox regression analysis to estimate the effect of eplerenone in patients with and without Hx-HTN. We used multivariable Cox regression analyses in the prematch cohort, separately adjusting for the raw propensity scores. All of the analyses were based on intent to treat. All of the statistical tests were evaluated using 2-tailed 95% CIs.
Because the sample size of matched patients with Hx-HTN (n=3676) was larger than matched patients without Hx-HTN (n=2352), we conducted a sensitivity analysis by repeating our analysis in a smaller group of patients with Hx-HTN. In addition, we conducted a sensitivity analysis to determine the potential effects of an unmeasured covariate that may potentially invalidate our main conclusions.6,8,9
Results
Study Patients
Baseline characteristics of patients with and without Hx-HTN are presented in Table 1. In both groups with and without Hx-HTN, the distribution of all of the measured baseline covariates was balanced, and there were no statistically significant differences between treatment groups.
Eplerenone and All-Cause Mortality
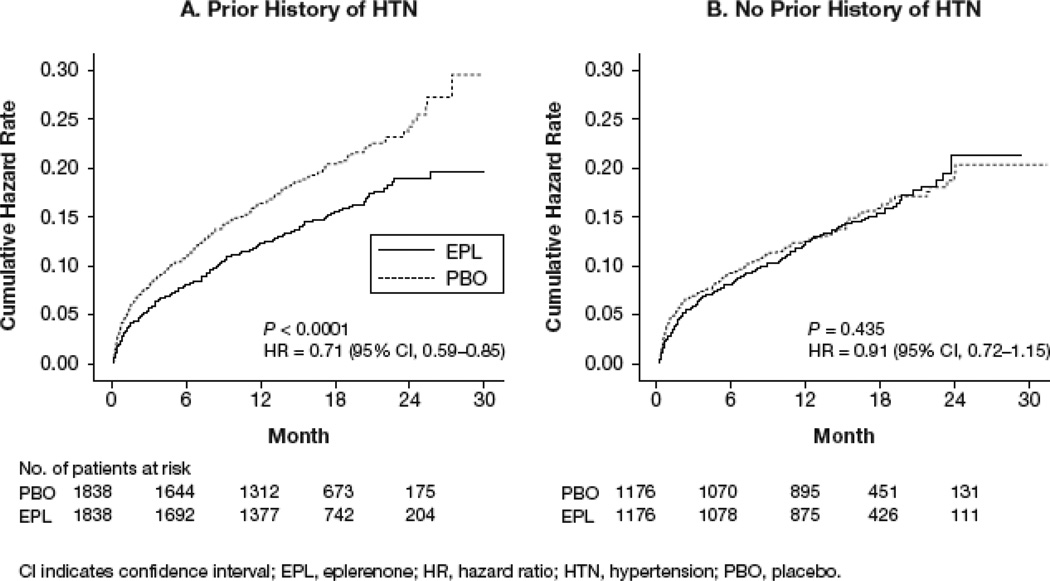
During a median follow-up of 16 months, 596 matched patients with Hx-HTN (16.2%) and 336 matched patients without Hx-HTN (14.3%) died of all causes. Among patients with Hx-HTN, all-cause mortality occurred in 18% of the placebo group and 14% of the eplerenone group (hazard ratio [HR]: 0.71; 95% CI: 0.59 to 0.85; P<0.0001; Figure 1A and Table 2). Among patients without Hx-HTN, all-cause mortality occurred in 14.4% of the placebo group and 14.2% of the eplerenone group (HR: 0.91; 95% CI: 0.72 to 1.15; P=0.435; Figure 1B and Table 2).
Figure 1.
Kaplan-Meier plots for all-cause mortality in patients (a) with and (b) without Hx-HTN.
TABLE 2.
Effects of Eplerenone on Primary and Secondary End Points
| History of hypertension | |||||
|---|---|---|---|---|---|
| Primary end points | Rate, per 10,000 person- years follow-up (events/follow-up in years) |
Rate difference* per 10,000 person-years) |
Matched hazard ratio (95% CI) |
P value | |
| Placebo (n=1838) |
Eplerenone (n=1838) |
||||
| Death from any cause | 1430 (336/2350) | 1058 (260/2457) | −372 | 0.71 (0.59–0.85) | <0.0001 |
| CV hospitalization or CV death | 3029 (603/1991) | 2438 (518/2125) | −591 | 0.82 (0.72–0.94) | 0.003 |
| Secondary end points | |||||
| Death from CV causes | 1255 (295/2350) | 916 (225/2457) | −339 | 0.72 (0.59–0.87) | 0.001 |
| Sudden death from cardiac causes | 511 (120/2350) | 374 (92/2457) | −137 | 0.71 (0.53–0.96) | 0.028 |
| Death from AMI | 213 (50/2350) | 155 (38/2457) | −58 | 0.72 (0.47–1.13) | 0.150 |
| Death from HF | 328 (77/2350) | 240 (59/2457) | −88 | 0.70 (0.47–1.04) | 0.076 |
| Hospitalization for AMI | 723 (161/2227) | 588 (138/2348) | −135 | 0.84 (0.66–1.07) | 0.157 |
| Hospitalization for HF | 1258 (268/2130) | 1116 (251/2250) | −142 | 0.87 (0.72–1.05) | 0.135 |
| No history of hypertension | |||||
| Primary end points | Placebo (n=1176) | Eplerenone (n=1176) | |||
| Death from any cause | 1091 (169/1548) | 1086 (167/1537) | −5 | 0.91 (0.72–1.15) | 0.435 |
| CV hospitalization or CV death | 2188 (298/1362) | 2019 (277/1372) | −169 | 0.91 (0.76–1.10) | 0.331 |
| Secondary end points | |||||
| Death from CV causes | 950 (147/1548) | 898 (138/1537) | −52 | 0.89 (0.69–1.15) | 0.360 |
| Sudden death from cardiac causes | 394 (61/1548) | 338 (52/1537) | −137 | 0.75 (0.49–1.13) | 0.170 |
| Death AMI | 233 (36/1548) | 202 (31/1537) | −31 | 0.85 (0.51–1.40) | 0.523 |
| Death from HF | 258 (40/1548) | 241 (37/1537) | −17 | 1.00 (0.60–1.66) | 1.000 |
| Hospitalization for AMI | 578 (86/1489) | 629 (92/1463) | +51 | 1.05 (0.77–1.45) | 0.744 |
| Hospitalization for HF | 902 (130/1442) | 681 (100/1468) | –221 | 0.73 (0.55–0.97) | 0.028 |
AMI indicates acute myocardial infarction; CI, confidence interval; CV, cardiovascular; HF, heart failure.
Eplerenone and CV Hospitalization or CV Mortality
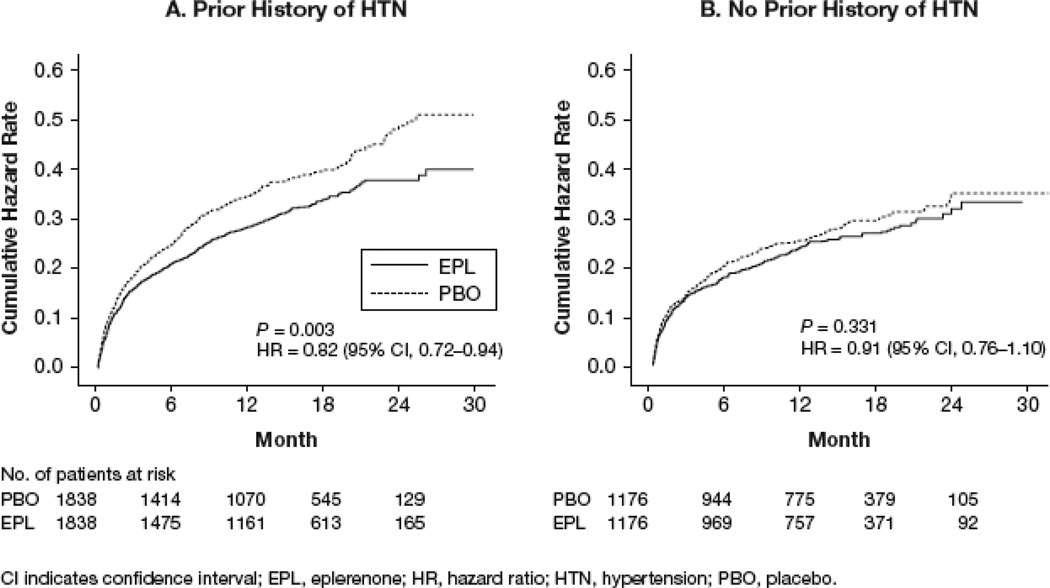
Among patients with Hx-HTN, the coprimary combined end points of CV hospitalization or CV mortality occurred in 32.8% patients in the placebo group and 28.2% of patients in the eplerenone group (HR: 0.82; 95% CI: 0.72 to 0.94; P=0.003; Figure 2A and Table 2). Among patients without Hx-HTN, the coprimary combined end points of CV hospitalization or CV mortality occurred in 25.3% of patients in the placebo group and 23.6% of patients in the eplerenone group (HR: 0.91; 95% CI: 0.76 to 1.10; P=0.331; Figure 2B and Table 2).
Figure 2.
Kaplan-Meier plots for co-primary combined end point of cardiovascular (CV) hospitalization or CV mortality in patients (a) with and (b) without Hx-HTN.
Eplerenone, SCD, and Other Secondary Study End Points
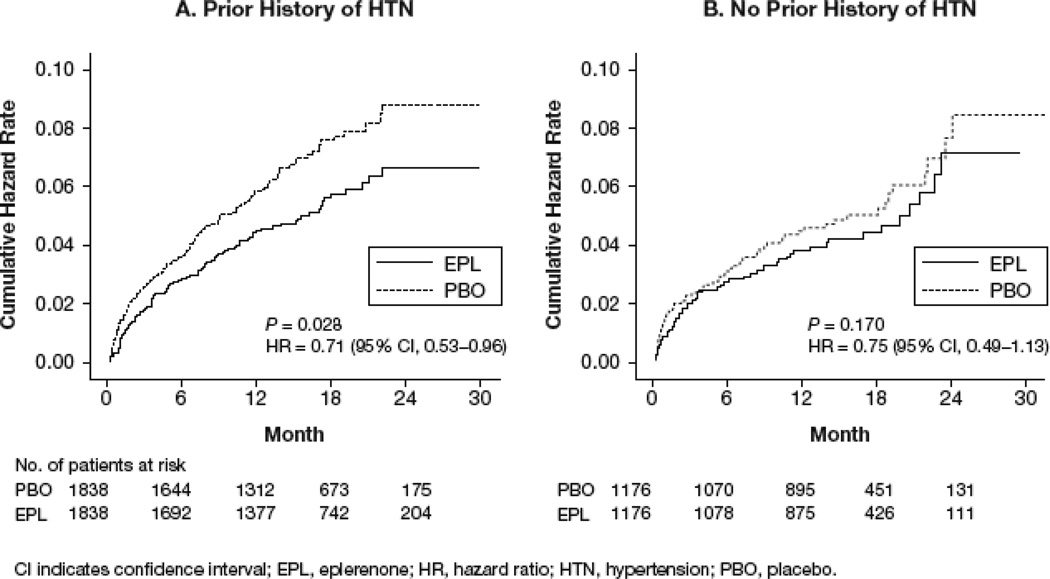
SCD occurred in 6.5% of patients with Hx-HTN in the placebo group and 5.0% of patients with Hx-HTN in the eplerenone group (Figure 3A and Table 2). Among patients without Hx-HTN, SCD occurred in 5.2% patients in the placebo group and 4.4% patients in the eplerenone group (Figure 3B and Table 2). Effects of eplerenone on other secondary end points in patients with and without Hx-HTN are displayed in Table 2.
Figure 3.
Kaplan-Meier plots for sudden cardiac death in patients (a) with and (b) without Hx-HTN.
Eplerenone and Adverse Events
In patients with Hx-HTN, the incidence of severe hyperkalemia was significantly higher in patients receiving eplerenone (5.9% versus 4.2%; odds ratio for patients receiving eplerenone: 1.43; 95% CI: 1.06 to 1.93; P=0.019; Table 3). The incidence of severe hypokalemia was lower in patients receiving eplerenone (14.7% versus 9.6%; odds ratio for patients receiving eplerenone: 0.62; 95% CI: 0.51 to 0.76; P<0.0001). Other adverse events are displayed in Table 3.
TABLE 3.
Adverse events in patients with a history of hypertension
| Adverse events, n (%) | History of Hypertension | No History of Hypertension | ||||
|---|---|---|---|---|---|---|
| Placebo (n = 1838) |
Eplerenone (n = 1838) |
P value |
Placebo (n = 1176) |
Eplerenone (n = 1176) |
P value |
|
| Cardiovascular disorder* | 947 (51.7) | 891 (48.7) | 0.080 | 555 (47.4) | 562 (47.8) | 0.869 |
| Respiratory disorder | 437 (23.8) | 402 (22.0) | 0.182 | 295 (25.2) | 266 (22.6) | 0.147 |
| Cough | 99 (5.4) | 91 (5.0) | 0.602 | 81 (6.9) | 67 (5.7) | 0.235 |
| Dyspnea | 158 (8.6) | 132 (7.2) | 0.126 | 125 (10.7) | 87 (7.4) | 0.006 |
| Pneumonia | 68 (3.7) | 53 (2.9) | 0.195 | 40 (3.4) | 28 (2.4) | 0.141 |
| Metabolic or nutritional disorder | 381 (20.8) | 319 (17.4) | 0.010 | 188 (16.1) | 193 (16.4) | 0.823 |
| Hyperkalemia† | 41 (2.2) | 60 (3.3) | 0.056 | 16 (1.4) | 38 (3.2) | 0.003 |
| Hypoglycemia | 22 (1.2) | 12 (0.7) | 0.120 | 8 (0.7) | 5 (0.4) | 0.422 |
| Hypokalemia† | 29 (1.6) | 9 (0.5) | 0.002 | 15 (1.3) | 6 (0.5) | 0.051 |
| Hyperuricemia‡ | 70 (3.8) | 57 (3.1) | 0.278 | 31 (2.6) | 21 (1.8) | 0.163 |
| Neoplasm | 35 (1.9) | 31 (1.7) | 0.710 | 18 (1.5) | 23 (2.0) | 0.529 |
| Urinary tract disorder | 270 (14.7) | 292 (16.0) | 0.313 | 112 (9.6) | 146 (12.4) | 0.029 |
| Disorder of skin or appendages | 113 (6.2) | 127 (6.9) | 0.350 | 85 (7.3) | 81 (6.9) | 0.748 |
| Musculoskeletal disorder | 107 (5.8) | 111 (6.1) | 0.780 | 76 (6.5) | 80 (6.8) | 0.804 |
| Nervous system disorder‡ | 220 (12.0) | 263 (14.4) | 0.036 | 187 (16.0) | 180 (15.3) | 0.691 |
| Psychiatric disorder | 145 (7.9) | 139 (7.6) | 0.757 | 100 (8.5) | 97 (8.3) | 0.823 |
| Gastrointestinal disorder‡ | 327 (17.8) | 363 (19.8) | 0.128 | 195 (16.7) | 243 (20.7) | 0.013 |
| Endocrine disorder | 14 (0.8) | 20 (1.1) | 0.308 | 5 (0.4) | 12 (1.0) | 0.142 |
| Disorder in men§ | ||||||
| Gynecomastia | 9 (0.8) | 5 (0.4) | 0.299 | 3 (0.3) | 5 (0.5) | 0.726 |
| Impotence | 9 (0.8) | 9 (0.7) | 1.000 | 10 (1.1) | 10 (1.1) | 1.000 |
| Disorder in women‖ | ||||||
| Breast pain | 1 (0.2) | 1 (0.2) | 1.000 | 2 (0.8) | 0 (0.0) | 0.499 |
| Serum potassium ≥6 mmol/L‡¶ | 77 (4.2) | 108 (5.9) | 0.019 | 37 (3.2) | 53 (4.5) | 0.106 |
| Serum potassium <3.5 mmol/Lঠ| 269 (14.7) | 176 (9.6) | <0.001 | 116 (9.9) | 73 (6.2) | 0.001 |
Data are for all cardiovascular adverse events reported, whether or not they were related to a study end point.
Data are based on investigators’ reports.
P<0.05 indicates significant difference between patients with and without Hx-HTN.
Among patients with Hx-HTN, 1200 men were in the placebo group and 1208 men were in the eplerenone group. Among those without Hx-HTN, 925 men were in the placebo group and 931 men were in the eplerenone group.
Among patients with Hx-HTN, 633 women were in the placebo group and 621 women were in the eplerenone group. Among patients without Hx-HTN, 245 women were in the placebo group and 244 women were in the eplerenone group.
Data are based on laboratory measurements. Data were available for 3622 patients with Hx-HTN (1833 patients, placebo group; 1829 patients, eplerenone group) and 2345 patients without Hx-HTN (1170 patients, placebo group; 1175 patients, eplerenone group).
Eplerenone, History of Hypertension and Baseline Blood Pressure
Eplerenone had a favorable effect on outcomes in patients with Hx-HTN regardless of their baseline systolic blood pressure (SBP). In the subgroups of patients with Hx-HTN and a baseline SBP of ≤120 mm Hg, all-cause mortality occurred in 191 placebo patients (rate: 1465/10 000 person-years) and 157 eplerenone patients (rate: 1176/10,000 person-years; HR: 0.80; 95% CI: 0.65 to 0.99; P=0.043). Eplerenone also reduced CV hospitalization or CV mortality (HR: 0.86; 95% CI: 0.73 to 0.997; P=0.046) in these patients. In Hx-HTN patients with a baseline SBP of >120 mm Hg, similarly, eplerenone reduced both all-cause mortality (HR: 0.68; 95% CI: 0.52 to 0.87; P=0.002) and the coprimary composite end point (HR: 0.79; 95% CI: 0.66 to 0.94; P=0.010).
Eplerenone and Changes in Baseline Blood Pressure
Increases in SBP from baseline (Table 1) were similar in both groups of patients with or without Hx-HTN and were slightly greater in the placebo group. The SBP increased by 6 and 3 mm Hg in the placebo and eplerenone patients, respectively, in both groups with and without Hx-HTN.
Results of Sensitivity Analyses
Because the group with Hx-HTN (n=3676) was larger than the group without Hx-HTN (n=2352), we assembled a cohort of patients with Hx-HTN that was similar in size to that of those without Hx-HTN (please see the data supplement, available online at http://hyper.ahajournals.org). In this smaller group of patients with Hx-HTN (n=2334), we observed that eplerenone use was associated with reduction in all-cause mortality (HR: 0.76; 95% CI: 0.61 to 0.95; P=0.014).
Within the Hx-HTN–matched cohort, sign-score tests for matched survival data with censoring provide evidence that the use of eplerenone, compared with placebo, was associated with decreased all-cause mortality (z=3.73; 2-tailed, P=0.0002) and decreased rates of CV hospitalization or CV mortality (z=2.92; P=0.0035). Sensitivity analyses suggest that an unmeasured binary covariate would need to increase the odds of eplerenone use by >17.6% to explain the all-cause mortality association and by >6.6% to explain the association with the coprimary end points.
Discussion
The results of this analysis demonstrate that the use of eplerenone in patients with Hx-HTN within 3 to 14 days of an AMI, complicated by reduced LVEF and symptomatic HF, was associated with significant reductions in all-cause mortality and the coprimary end points of CV hospitalization or CV mortality. In addition, the use of eplerenone was associated with a significant reduction in SCD in these patients. Although the direction of change with eplerenone in the subgroup of patients without Hx-HTN was similar, significant reductions in these end points were not observed in this subgroup; however, in patients without Hx-HTN, eplerenone significantly reduced hospitalization for HF, suggesting a long-term effect on ventricular remodeling. Although approximately two thirds of patients in EPHESUS had Hx-HTN, their mean blood pressure at the time of random assignment post-AMI was within normal limits
There are several plausible explanations for the differential benefit of eplerenone in patients with Hx-HTN. Effects of a treatment on outcomes are known to vary between groups of patients based on differences in pathophysiology, natural history, severity of disease, comorbidity, and absolute risks between groups.10 EPHESUS patients with Hx-HTN were older and had more severe disease and comorbidity burden than those without Hx-HTN. They had 14% greater risk for all-cause mortality and 28% increased risk for CV mortality or CV hospitalizations than those without Hx-HTN. Pathophysiologically, compared with patients without Hx-HTN, those with Hx-HTN are at an increased risk of incident AMI, subsequent ventricular remodeling, and CV mortality.11–14
Hypertension predisposes patients to an increase in reactive oxygen species, vascular remodeling, and myocardial collagen formation.15–18 After AMI, patients with Hx-HTN have been found to have a greater degree of ventricular remodeling than those without Hx-HTN.13,19,20 Ventricular remodeling results in myocardial stretch, which is an important stimulus for activation of various neurohormones, including angiotensin II and aldosterone.21,22 HF is associated with an upregulation of mineralocorticoid receptors and aldosterone with an increase in myocardial calcium channel expression.23–26 Recently, AMI has been shown to cause electric remodeling before mechanical remodeling and LV hypertrophy.27 The increase in intracellular calcium associated with electrical remodeling has been suggested to increase the risk of ventricular arrhythmias and SCD.28,29 Alterations in intracellular calcium and potassium may be greater in patients with AMI and Hx-HTN, many of whom have LV hypertrophy, and an increase in mineralocorticoid receptors and calcium channel expression.25,26 Therefore, the effectiveness of eplerenone in patients with Hx-HTN, most of whom were treated with an angiotensin-converting enzyme inhibitor or an angiotensin receptor blocker and a 13-adrenergic receptor blocker, in reducing total mortality and SCD along with a trend toward a reduction in death because of progressive HF can be explained by the effects of aldosterone blockade in preventing electric remodeling, as well as by improving sympathetic/parasympathetic balance related to a decrease in reactive oxygen species production and an improvement in NO availability.30–33 These effects are in addition to the effects of eplerenone on ventricular mechanical remodeling, LV hypertrophy, and collagen formation, all of which are likely of greater magnitude in those with Hx-HTN.34–38
Results of our subgroup analysis suggest that the effects of eplerenone were observed regardless of baseline SBP, suggesting a long-term effect of HTN on target organs rather than baseline blood pressure levels as the underlying mechanistic explanation for the differential effect of eplerenone in the group with Hx-HTN. Another plausible explanation of a differential benefit of eplerenone in patients with Hx-HTN is the larger sample of these patients; however, sensitivity analysis suggests that eplerenone was beneficial in a smaller subset of patients with Hx-HTN. In addition, statistical analysis showed no significant difference in the treatment effect of eplerenone on SBP between patients with and without Hx-HTN, suggesting that our findings were not driven by higher absolute reductions in SBP among patients with Hx-HTN.
This analysis suggests that patients with AMI complicated by a low LVEF and symptomatic HF should be risk-stratified based on Hx-HTN. Those with Hx-HTN should be prescribed eplerenone to improve outcomes. Although we did not observe a significant effect of eplerenone on mortality in patients without Hx-HTN, we did observe a significant reduction in HF hospitalization, likely mediated by a reduction in ventricular remodeling. There was a trend toward a reduction in mortality, but analysis of this subgroup may have been underpowered to detect a significant difference in mortality and other outcomes, which is not surprising given the low baseline risk in these patients.11 Reduction in HF hospitalization would suggest a long-term effect of eplerenone on mortality in these patients.
Eplerenone was well tolerated in patients with and without Hx-HTN. Although more patients receiving eplerenone experienced hyperkalemia (>6 mEq/L), overall absolute rates were low, and no deaths were attributed to hyperkalemia in patients receiving eplerenone. Patients receiving eplerenone had a lower risk of developing hypokalemia (<3.5 mEq/L). This is important, because the overall absolute rate of hypokalemia was higher than that of hyperkalemia, which has been associated with increased mortality.39–42
Perspectives
This analysis suggests that patients with AMI, reduced LVEF, and symptomatic HF should be risk stratified based on their Hx-HTN; and those with Hx-HTN should be treated early post-AMI with eplerenone to prevent death, especially SCD. Because HF hospitalization is an important predictor of CV death and eplerenone reduced HF hospitalization in patients without Hx-HTN, eplerenone should also be initiated in these patients
Acknowledgements
The authors thank Richard Zhang, University of Alabama at Birmingham, and Gabriel Saltan, COMSYS, Chicago, IL, for their assistance in data preparation and cleanup.
Dr. Ahmed is supported by the National Institutes of Health through grants from the National Heart, Lung, and Blood Institute (5-R01-HL085561-02 and P50-HL077100), and a generous gift from Ms. Jean B. Morris of Birmingham, Alabama.
Sources of Funding:
This study was funded by Pfizer Inc. Editorial support was provided by PAREXEL and was funded by Pfizer Inc.
Footnotes
Author Contributions
Bertram Pitt conceived the study hypothesis for this subanalysis of EPHESUS. Ali Ahmed developed the subanalysis design, and wrote the first draft of the manuscript. Ali Ahmed conducted statistical analyses in consultation with Thomas Love. All authors interpreted the data, participated in critical revision of the paper for important intellectual content, and approved the final version of the article. Ali Ahmed had full access to the data.
References
- 1.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 2.Rosenbaum PR, Rubin DB. The central role of propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 3.Rosenbaum PR, Rubin DB. Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Assoc. 1984;79:516–524. [Google Scholar]
- 4.Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127(8 Pt 2):757–763. doi: 10.7326/0003-4819-127-8_part_2-199710151-00064. [DOI] [PubMed] [Google Scholar]
- 5.Rubin DB. Using propensity score to help design observational studies: application to the tobacco litigation. Health Services Outcomes Res Methodol. 2001;2:169–188. [Google Scholar]
- 6.Ahmed A, Husain A, Love TE, Gambassi G, Dell'Italia LJ, Francis GS, Gheorghiade M, Allman RM, Meleth S, Bourge RC. Heart failure, chronic diuretic use, increase in mortality and hospitalization: an observational study using propensity score methods. Eur Heart J. 2006;27:1431–1439. doi: 10.1093/eurheartj/ehi890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D'Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 8.Rosenbaum PR. Sensitivity to hidden bias. In: Rosenbaum PR, editor. Observational Studies. 2 ed. New York, NY: Springer-Verlag; 2002. pp. 110–124. [Google Scholar]
- 9.Rosenbaum PR. Sensitivity analysis for matching with multiple controls. Biometrika. 1988;75:577–581. [Google Scholar]
- 10.Rothwell PM. Treating individuals. 2. Subgroup analysis in randomised controlled trials: importance, indications, and interpretation. Lancet. 2005;365:176–186. doi: 10.1016/S0140-6736(05)17709-5. [DOI] [PubMed] [Google Scholar]
- 11.Tuomilehto J, Salonen JT, Nissinen A. Isolated systolic hypertension and its relationship to the risk of myocardial infarction, cerebrovascular disease and death in a middle-aged population. Eur Heart J. 1984;5:739–744. doi: 10.1093/oxfordjournals.eurheartj.a061735. [DOI] [PubMed] [Google Scholar]
- 12.Haider AW, Chen L, Larson MG, Evans JC, Chen MH, Levy D. Antecedent hypertension confers increased risk for adverse outcomes after initial myocardial infarction. Hypertension. 1997;30:1020–1024. doi: 10.1161/01.hyp.30.5.1020. [DOI] [PubMed] [Google Scholar]
- 13.Richards AM, Nicholls MG, Troughton RW, Lainchbury JG, Elliott J, Frampton C, Espiner EA, Crozier IG, Yandle TG, Turner J. Antecedent hypertension and heart failure after myocardial infarction. J Am Coll Cardiol. 2002;39:1182–1188. doi: 10.1016/s0735-1097(02)01737-0. [DOI] [PubMed] [Google Scholar]
- 14.Kenchaiah S, Davis BR, Braunwald E, Rouleau JL, Dagenais GR, Sussex B, Steingart RM, Brown EJ, Jr, Lamas GA, Gordon D, Bernstein V, Pfeffer MA. Antecedent hypertension and the effect of captopril on the risk of adverse cardiovascular outcomes after acute myocardial infarction with left ventricular systolic dysfunction: insights from the Survival and Ventricular Enlargement Trial. Am Heart J. 2004;148:356–364. doi: 10.1016/j.ahj.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 15.Peterson JR, Sharma RV, Davisson RL. Reactive oxygen species in the neuropathogenesis of hypertension. Curr Hypertens Rep. 2006;8:232–241. doi: 10.1007/s11906-006-0056-1. [DOI] [PubMed] [Google Scholar]
- 16.Zhou MS, Jaimes EA, Raij L. Vascular but not cardiac remodeling is associated with superoxide production in angiotensin II hypertension. J Hypertens. 2005;23:1737–1743. doi: 10.1097/01.hjh.0000179513.71018.09. [DOI] [PubMed] [Google Scholar]
- 17.Intengan HD, Schiffrin EL. Vascular remodeling in hypertension: roles of apoptosis, inflammation, and fibrosis. Hypertension. 2001;38(3 Pt 2):581–587. doi: 10.1161/hy09t1.096249. [DOI] [PubMed] [Google Scholar]
- 18.Davies MJ. Hypertension and atherosclerotic (ischaemic) heart disease. J Hum Hypertens. 1991;5(suppl 1):23–29. [PubMed] [Google Scholar]
- 19.Parodi G, Carrabba N, Santoro GM, Memisha G, Valenti R, Buonamici P, Dovellini EV, Antoniucci D. Heart failure and left ventricular remodeling after reperfused acute myocardial infarction in patients with hypertension. Hypertension. 2006;47:706–710. doi: 10.1161/01.HYP.0000210549.47167.db. [DOI] [PubMed] [Google Scholar]
- 20.Kenchaiah S, Pfeffer MA, St John Sutton M, Plappert T, Rouleau JL, Lamas GA, Sasson Z, Parker JO, Geltman EM, Solomon SD. Effect of antecedent systemic hypertension on subsequent left ventricular dilation after acute myocardial infarction (from the Survival and Ventricular Enlargement trial) Am J Cardiol. 2004;94:1–8. doi: 10.1016/j.amjcard.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 21.Pitt B. Aldosterone blockade in patients with acute myocardial infarction. Circulation. 2003;107:2525–2527. doi: 10.1161/01.CIR.0000072746.11078.36. [DOI] [PubMed] [Google Scholar]
- 22.Yamazaki T, Komuro I, Kudoh S, Zou Y, Shiojima I, Mizuno T, Takano H, Hiroi Y, Ueki K, Tobe K, Kadowaki T, Nagai R, Yazaki Y. Angiotensin II partly mediates mechanical stress-induced cardiac hypertrophy. Circ Res. 1995;77:258–265. doi: 10.1161/01.res.77.2.258. [DOI] [PubMed] [Google Scholar]
- 23.Ohtani T, Ohta M, Yamamoto K, Mano T, Sakata Y, Nishio M, Takeda Y, Yoshida J, Miwa T, Okamoto M, Masuyama T, Nonaka Y, Hori M. Elevated cardiac tissue level of aldosterone and mineralocorticoid receptor in diastolic heart failure: Beneficial effects of mineralocorticoid receptor blocker. Am J Physiol Regul Integr Comp Physiol. 2007;292:R946–R954. doi: 10.1152/ajpregu.00402.2006. [DOI] [PubMed] [Google Scholar]
- 24.Yoshida M, Ma J, Tomita T, Morikawa N, Tanaka N, Masamura K, Kawai Y, Miyamori I. Mineralocorticoid receptor is overexpressed in cardiomyocytes of patients with congestive heart failure. Congest Heart Fail. 2005;11:12–16. doi: 10.1111/j.1527-5299.2005.03722.x. [DOI] [PubMed] [Google Scholar]
- 25.Hersel J, Jung S, Mohacsi P, Hullin R. Expression of the L-type calcium channel in human heart failure. Basic Res Cardiol. 2002;97(suppl 1):I4–I10. doi: 10.1007/s003950200022. [DOI] [PubMed] [Google Scholar]
- 26.Lalevee N, Rebsamen MC, Barrere-Lemaire S, Perrier E, Nargeot J, Bénitah JP, Rossier MF. Aldosterone increases T-type calcium channel expression and in vitro beating frequency in neonatal rat cardiomyocytes. Cardiovasc Res. 2005;67:216–224. doi: 10.1016/j.cardiores.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 27.Perrier E, Kerfant BG, Lalevee N, Bideaux P, Rossier MF, Richard S, Gómez AM, Benitah JP. Mineralocorticoid receptor antagonism prevents the electrical remodeling that precedes cellular hypertrophy after myocardial infarction. Circulation. 2004;110:776–783. doi: 10.1161/01.CIR.0000138973.55605.38. [DOI] [PubMed] [Google Scholar]
- 28.Zhou S, Cao JM, Ohara T, KenKnight BH, Chen LS, Karagueuzian HS, Chen PS. Torsade de pointes and sudden death induced by thiopental and isoflurane anesthesia in dogs with cardiac electrical remodeling. J Cardiovasc Pharmacol Ther. 2002;7:39–43. doi: 10.1177/107424840200700i106. [DOI] [PubMed] [Google Scholar]
- 29.Chen PS, Chen LS, Cao JM, Sharifi B, Karagueuzian HS, Fishbein MC. Sympathetic nerve sprouting, electrical remodeling and the mechanisms of sudden cardiac death. Cardiovasc Res. 2001;50:409–416. doi: 10.1016/s0008-6363(00)00308-4. [DOI] [PubMed] [Google Scholar]
- 30.Shroff SC, Ryu K, Martovitz NL, Hoit BD, Stambler BS. Selective aldosterone blockade suppresses atrial tachyarrhythmias in heart failure. J Cardiovasc Electrophysiol. 2006;17:534–541. doi: 10.1111/j.1540-8167.2006.00372.x. [DOI] [PubMed] [Google Scholar]
- 31.Boixel C, Gavillet B, Rougier JS, Abriel H. Aldosterone increases voltage-gated sodium current in ventricular myocytes. Am J Physiol Heart Circ Physiol. 2006;290:H2257–H2266. doi: 10.1152/ajpheart.01060.2005. [DOI] [PubMed] [Google Scholar]
- 32.Healey JS, Morillo CA, Connolly SJ. Role of the renin-angiotensin-aldosterone system in atrial fibrillation and cardiac remodeling. Curr Opin Cardiol. 2005;20:31–37. [PubMed] [Google Scholar]
- 33.Benitah JP, Vassort G. Aldosterone upregulates Ca(2+) current in adult rat cardiomyocytes. Circ Res. 1999;85:1139–1145. doi: 10.1161/01.res.85.12.1139. [DOI] [PubMed] [Google Scholar]
- 34.Nishiyama A, Yao L, Nagai Y, Miyata K, Yoshizumi M, Kagami S, Kondo S, Kiyomoto H, Shokoji T, Kimura S, Kohno M, Abe Y. Possible contributions of reactive oxygen species and mitogen-activated protein kinase to renal injury in aldosterone/salt-induced hypertensive rats. Hypertension. 2004;43:841–848. doi: 10.1161/01.HYP.0000118519.66430.22. [DOI] [PubMed] [Google Scholar]
- 35.Rude MK, Duhaney TA, Kuster GM, Judge S, Heo J, Colucci WS, Siwik DA, Sam F. Aldosterone stimulates matrix metalloproteinases and reactive oxygen species in adult rat ventricular cardiomyocytes. Hypertension. 2005;46:555–561. doi: 10.1161/01.HYP.0000176236.55322.18. [DOI] [PubMed] [Google Scholar]
- 36.Liu SL, Schmuck S, Chorazcyzewski JZ, Gros R, Feldman RD. Aldosterone regulates vascular reactivity: short-term effects mediated by phosphatidylinositol 3-kinase-dependent nitric oxide synthase activation. Circulation. 2003;108:2400–2406. doi: 10.1161/01.CIR.0000093188.53554.44. [DOI] [PubMed] [Google Scholar]
- 37.Thai HM, Do BQ, Tran TD, Gaballa MA, Goldman S. Aldosterone antagonism improves endothelial-dependent vasorelaxation in heart failure via upregulation of endothelial nitric oxide synthase production. J Card Fail. 2006;12:240–245. doi: 10.1016/j.cardfail.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 38.Pitt B, Reichek N, Willenbrock R, Zannad F, Phillips RA, Roniker B, Kleiman J, Krause S, Burns D, Williams GH. Effects of eplerenone, enalapril, and eplerenone/enalapril in patients with essential hypertension and left ventricular hypertrophy: the 4E-left ventricular hypertrophy study. Circulation. 2003;108:1831–1838. doi: 10.1161/01.CIR.0000091405.00772.6E. [DOI] [PubMed] [Google Scholar]
- 39.Packer M. Potential role of potassium as a determinant of morbidity and mortality in patients with systemic hypertension and congestive heart failure. Am J Cardiol. 1990;65:45E–51E. doi: 10.1016/0002-9149(90)90251-u. discussion 52E. [DOI] [PubMed] [Google Scholar]
- 40.Macdonald JE, Struthers AD. What is the optimal serum potassium level in cardiovascular patients? J Am Coll Cardiol. 2004;43:155–161. doi: 10.1016/j.jacc.2003.06.021. [DOI] [PubMed] [Google Scholar]
- 41.Srivastava TN, Young DB. Impairment of cardiac function by moderate potassium depletion. J Card Fail. 1995;1:195–200. doi: 10.1016/1071-9164(95)90024-1. [DOI] [PubMed] [Google Scholar]
- 42.Ahmed A, Zannad F, Love TE, Tallaj J, Gheorghiade M, Ekundayo OJ, Pitt B. A propensity matched study of the association of low serum potassium levels and mortality in chronic heart failure. Eur Heart J. 2007;28:1334–1143. doi: 10.1093/eurheartj/ehm091. [DOI] [PMC free article] [PubMed] [Google Scholar]