Abstract
The devil facial tumor disease (DFTD) is a contagious cancer that has recently emerged among Tasmanian devils, rapidly decimating the population. We have recently discovered that DFTD cells lose the expression MHC molecules on the cell surface, explaining how this tumor avoids recognition by host CD8+ T cells.
Keywords: contagious cancer, transmissible tumor, Tasmanian devil, DFTD, CTVT, MHC, epigenetics, interferon, extinction, conservation
On rare occasions, tumors acquire the ability to move among individuals as a contagious disease.1,2 For a tumor to become contagious, malignant cells must have a physical route of transmission between individuals, must be able to grow in a new microenvironment and must evade host immune responses. These challenges are similar to those faced by a metastasizing cancer cell within a single individual, with the key difference that once the tumor leaves its original host it becomes an allograft. The immune system is generally very efficient at detecting and responding to allografts. For this reason, even though endogenous cancers can be difficult for the immune system to deal with, contagious cancers should be easily rejected.
One such contagious tumor, devil facial tumor disease (DFTD), emerged around 1996 among Tasmanian devils, carnivorous marsupials that are confined to the island of Tasmania. Robust evidence suggests that DFTD cells are physically transmitted from animal to animal, rather than arising from a transforming viral infection.2-4 DFTD is characterized by growth of large tumors on the face and neck of infected animals. Together with the propensity of devils to bite and jaw wrestle during feeding, this suggests that DFTD is transmitted by biting.2 No devils have been reported to raise protective immune responses against DFTD and the disease is presumed to be fatal, with metastasis occurring in ~65% of cases.5 DFTD has had an alarming effect on the population of Tasmanian devils, with local declines of up to 83%.5
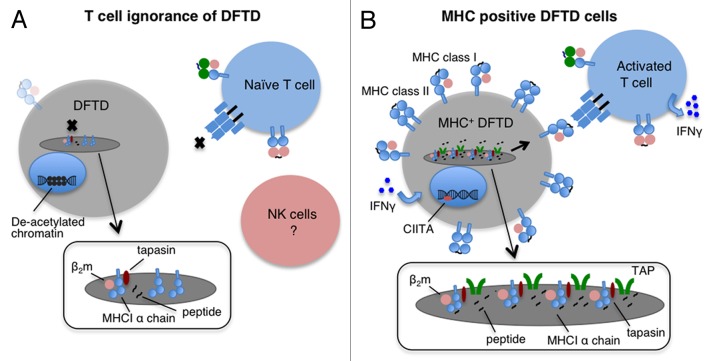
As histocompatibility barriers usually prevent the transplantation of foreign grafts between individuals, we asked whether DFTD cells express MHC molecules. Towards this aim, we developed two antibodies, one against the cytoplasmic tails of devil MHC Class I α chains and one specific for native devil β2-microglobulin (β2m), a protein that associates with MHC Class I α chains. By means of these antibodies, we detected only very low levels of MHC Class I molecules within DFTD cells and at most trace amounts of β2m on the cell surface. Immunohistochemical assays confirmed that primary DFTD tumors also exhibit very low levels of β2m. In order to determine the reasons for such a lack of MHC Class I molecules and β2m, we examined gene expression at the mRNA level. We found that DFTD cells only exhibit trace levels of transcripts for β2m, and transporter associated with antigen presentation (TAP) 1 and TAP2 coding transcripts. These results explain the loss of MHC Class I molecules, because in the absence of β2m and peptides (which are pumped into the endoplasmic reticulum by the TAP heterodimer), MHC Class I molecules are unstable and are retained in the endoplasmic reticulum.6 Thus, DFTD cells lack MHC Class I molecules on their surface due to the downregulation of genes that are essential for antigen presentation, in turn explaining why host devil CD8+ T cells do not recognize allogeneic DFTD cells (Fig. 1).
Figure 1. Mechanisms of immune evasion by DFTD cells. (A) Devil T lymphocytes fail to recognize devil facial tumor disease (DFTD) cells as the latter lack MHC molecules on their surface. This is mainly due to the deacetylation-dependent repression of transcription from β2-microglobulin (β2m), transporter associated with antigen presentation (TAP) 1 and TAP2-coding genes. In this situation, MHC Class I heavy chains are produced but retained in the endoplasmic reticulum (ER). Low levels of MHC Class I molecules may be found on the surface of DFTD cells owing to the synthesis of trace amounts of β2m and to peptides derived from ER-resident proteins. (B) DFTD cells can re-express MHC Class I molecules on their surface. Upon interferon γ (IFNγ) treatment of DFTD cells, β2m, TAP1, TAP2, MHC Class II molecules and the transcription factor Class II transactivator (CIITA) are upregulated and MHC Class I molecules are expressed on the cell surface. Devils vaccinated with MHC Class I-expressing DFTD cells are expected to activate a protective T-cell response. Insets represent magnified view of the ER.
In human tumors, MHC Class I molecules are often lost owing to structural mutations in various important genes, which permanently shut off transcription.7 We found no evidence for haplotype loss or for structural mutations in the coding regions and promoter regions of the β2m, TAP1 and TAP2, genes. Such a lack of structural mutations led us to investigate potential epigenetic changes that would affect the transcription of these genes. While we found no evidence of hypermethylation in the corresponding promoter regions, the inhibition of deacetylases led to the upregulation of β2m and TAPs.6 Thus, changes in the acetylation state of promoters affect transcription of β2m and TAPs in DFTD cells. However, treatment with deacetylase inhibitor did not restore the expression of MHC Class I molecules on the surface of DFTD cells in vitro. Armed with this knowledge, we decided to test the effect of pro-inflammatory cytokines on MHC Class I expression by DFTD cells.
The treatment of DFTD cells in vitro with recombinant devil interferon γ (IFNγ) resulted in significant upregulation of MHC Class I molecules on the cell surface as well as upregulation of Class II transactivator (CIITA) and MHC Class II gene expression.6 Interestingly, we observed rare instances of strong β2-m expression by primary DFTD biopsies in which malignant cells were adjacent to clusters of CD3+ lymphocytes. Thus, the antigen processing and presentation machinery of DFTD cells is functional in vitro and in vivo, indicating that the devil immune system does respond to DFTD. We propose to exploit the fact that DFTD constitutes an allograft and employ DFTD cells induced to express MHC Class I molecules as a whole-cell vaccine against this contagious tumor (Fig. 1).
Our results raise interesting questions about the evolution of DFTD and about how this tumor interacts with the immune system. The only other naturally occurring contagious cancer, canine transmissible venereal tumor (CTVT), is not fatal, due to a modulation of MHC expression that allows the dog immune system to control tumor growth.1 The ability of DFTD cells to regulate the expression of MHC molecules suggests that DFTD may evolve into a less aggressive disease that is controlled by the immune system. In addition, it is likely that DFTD exploits other, hitherto undiscovered, mechanisms to evade immune responses. For example, why host natural killer (NK) cells do not respond to the absence of an inhibitory self-signal (as normally provided by MHC Class I molecules) on the surface of DFTD cells remains unclear. Despite the success of DFTD as an infectious pathogen, the allograft nature should facilitate the stimulation of the immune system by tumor cells if they express MHC Class I molecules. In vivo experiments are urgently needed to determine whether a whole-cell vaccine could efficiently prevent the further spread of DFTD and contribute to saving the Tasmanian devil in the wild. Finally, transmissible cancers have been reported in humans, primarily passing from the mother to the fetus8 or being transmitted with transplants.9 Thus, understanding naturally occurring contagious tumors in animals is important in case such a cancer would emerge among humans.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/25235
References
- 1.Murgia C, Pritchard JK, Kim SY, Fassati A, Weiss RA. Clonal origin and evolution of a transmissible cancer. Cell. 2006;126:477–87. doi: 10.1016/j.cell.2006.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pearse AM, Swift K. Allograft theory: transmission of devil facial-tumour disease. Nature. 2006;439:549. doi: 10.1038/439549a. [DOI] [PubMed] [Google Scholar]
- 3.Murchison EP, Schulz-Trieglaff OB, Ning Z, Alexandrov LB, Bauer MJ, Fu B, et al. Genome sequencing and analysis of the Tasmanian devil and its transmissible cancer. Cell. 2012;148:780–91. doi: 10.1016/j.cell.2011.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deakin JE, Bender HS, Pearse AM, Rens W, O’Brien PC, Ferguson-Smith MA, et al. Genomic restructuring in the Tasmanian devil facial tumour: chromosome painting and gene mapping provide clues to evolution of a transmissible tumour. PLoS Genet. 2012;8:e1002483. doi: 10.1371/journal.pgen.1002483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hawkins C, Baars C, Hesterman H, Hocking G, Jones M, Lazenby B, et al. Emerging disease and population decline of an island endemic, the Tasmanian devil Sarcophilus harrisii. Biol Conserv. 2006;131:307–24. doi: 10.1016/j.biocon.2006.04.010. [DOI] [Google Scholar]
- 6.Siddle HV, Kreiss A, Tovar C, Yuen CK, Cheng Y, Belov K, et al. Reversible epigenetic down-regulation of MHC molecules by devil facial tumour disease illustrates immune escape by a contagious cancer. Proc Natl Acad Sci U S A. 2013;110:5103–8. doi: 10.1073/pnas.1219920110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang CC, Campoli M, Ferrone S. Classical and nonclassical HLA class I antigen and NK Cell-activating ligand changes in malignant cells: current challenges and future directions. Adv Cancer Res. 2005;93:189–234. doi: 10.1016/S0065-230X(05)93006-6. [DOI] [PubMed] [Google Scholar]
- 8.Isoda T, Ford AM, Tomizawa D, van Delft FW, De Castro DG, Mitsuiki N, et al. Immunologically silent cancer clone transmission from mother to offspring. Proc Natl Acad Sci U S A. 2009;106:17882–5. doi: 10.1073/pnas.0904658106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krapp JD, Brauer RB, Matevossian E, Gerauer KE, Thorban S, Becker K, et al. Donor transmitted anaplastic carcinoma in a kidney-transplant recipient. Transpl Int. 2005;18:1109–12. doi: 10.1111/j.1432-2277.2005.00178.x. [DOI] [PubMed] [Google Scholar]