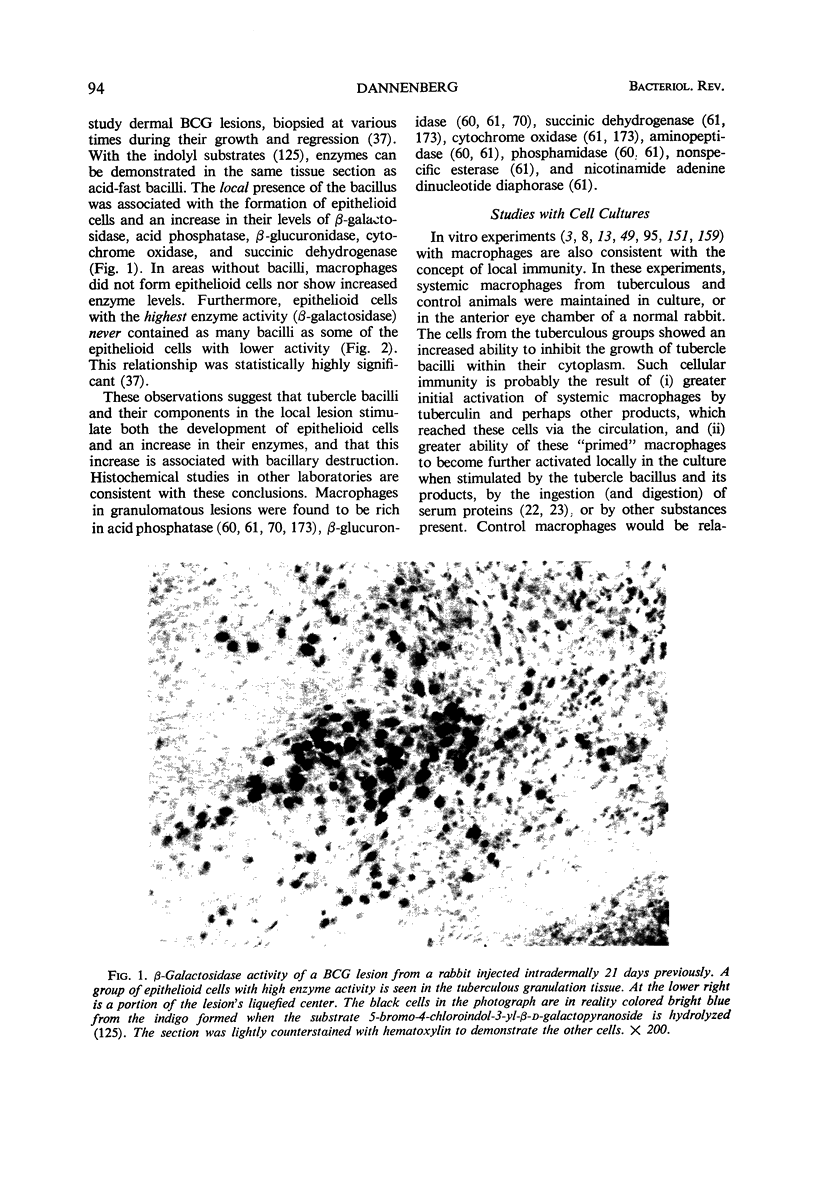
Full text
PDF
















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON M. J., ZAPPASODI P., LURIE M. B. Metabolic studies on mononuclear cells from rabbits of varying genetic resistance to tuberculosis. II. Studies on cells from BCG-vaccinated animals. Am Rev Respir Dis. 1962 Mar;85:364–372. doi: 10.1164/arrd.1962.85.3.364. [DOI] [PubMed] [Google Scholar]
- ARNASON B. G., WAKSMAN B. H. TUBERCULIN SENSITIVITY. IMMUNOLOGIC CONSIDERATIONS. Bibl Tuberc. 1964;19:1–97. [PubMed] [Google Scholar]
- AUZINS I., ROWLEY D. On the question of the specificity of cellular immunity. Aust J Exp Biol Med Sci. 1962 Aug;40:283–291. doi: 10.1038/icb.1962.32. [DOI] [PubMed] [Google Scholar]
- Anacker R. L., Barclay W. R., Brehmer W., Larson C. L., Ribi E. Duration of immunity to tuberculosis in mice vaccinated intravenously with oil-treated cell walls of Mycobacterium bovis strain BCG. J Immunol. 1967 Jun;98(6):1265–1273. [PubMed] [Google Scholar]
- BLOCH H. Studies on the virulence of tubercle bacilli; isolation and biological properties of a constituent of virulent organisms. J Exp Med. 1950 Feb;91(2):197-218, pl. doi: 10.1084/jem.91.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYDEN S. V. The immunological response to antigens of the tubercle bacillus; some experimental aspects. Prog Allergy. 1958;5:149–214. doi: 10.1159/000277644. [DOI] [PubMed] [Google Scholar]
- Baram P., Yuan L., Mosko M. M. Studies on the transfer of human delayed-type hypersensitivity. I. Partial purification and characterization of two active components. J Immunol. 1966 Sep;97(3):407–420. [PubMed] [Google Scholar]
- Barclay W. R., Anacker R., Brehmer W., Ribi E. Effects of oil-treated mycobacterial cell walls on the organs of mice. J Bacteriol. 1967 Nov;94(5):1736–1745. doi: 10.1128/jb.94.5.1736-1745.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benacerraf B. Cytophilic immunoglobulins and delayed hypersensitivity. Fed Proc. 1968 Jan-Feb;27(1):46–48. [PubMed] [Google Scholar]
- Bennett B., Bloom B. R. Reactions in vivo and in vitro produced by a soluble substance associated with delayed-type hypersensitivity. Proc Natl Acad Sci U S A. 1968 Mar;59(3):756–762. doi: 10.1073/pnas.59.3.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett B., Bloom B. R. Studies on the migration inhibitory factor associated with delayed-type hypersensitivity: cytodynamics and specificity. Transplantation. 1967 Jul;5(4 Suppl):996–1000. [PubMed] [Google Scholar]
- Bennett W. E., Cohn Z. A. The isolation and selected properties of blood monocytes. J Exp Med. 1966 Jan 1;123(1):145–160. doi: 10.1084/jem.123.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V., Mackaness G. B., Collins F. M. Mechanisms of acquired resistance in mouse typhoid. J Exp Med. 1966 Oct 1;124(4):585–600. doi: 10.1084/jem.124.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom B. R., Bennett B. Mechanism of a reaction in vitro associated with delayed-type hypersensitivity. Science. 1966 Jul 1;153(3731):80–82. doi: 10.1126/science.153.3731.80. [DOI] [PubMed] [Google Scholar]
- Bloom B. R., Bennett B. Migration inhibitory factor associated with delayed-type hypersensitivity. Fed Proc. 1968 Jan-Feb;27(1):13–15. [PubMed] [Google Scholar]
- Bloom B. R., Chase M. W. Transfer of delayed-type hypersensitivity. A critical review and experimental study in the guinea pig. Prog Allergy. 1967;10:151–255. [PubMed] [Google Scholar]
- Brewer D. B., Heath D. Electron microscopy of anoxic vacuolation in the liver cell and its comparison with sucrose vacuolation. J Pathol Bacteriol. 1965 Oct;90(2):437–441. doi: 10.1002/path.1700900211. [DOI] [PubMed] [Google Scholar]
- CARSON M. E., DANNENBERG A. M., Jr HYDROLYTIC ENZYMES OF RABBIT MONONUCLEAR EXUDATE CELLS. II. LYSOZYME: PROPERTIES AND QUANTITATIVE ASSAY IN TUBERCULOUS AND CONTROL INBRED RABBITS. J Immunol. 1965 Jan;94:99–104. [PubMed] [Google Scholar]
- COHN Z. A., BENSON B. THE DIFFERENTIATION OF MONONUCLEAR PHAGOCYTES. MORPHOLOGY, CYTOCHEMISTRY, AND BIOCHEMISTRY. J Exp Med. 1965 Jan 1;121:153–170. doi: 10.1084/jem.121.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A., BENSON B. THE IN VITRO DIFFERENTIATION OF MONONUCLEAR PHAGOCYTES. II. THE INFLUENCE OF SERUM ON GRANULE FORMATION, HYDROLASE PRODUCTION, AND PINOCYTOSIS. J Exp Med. 1965 May 1;121:835–848. doi: 10.1084/jem.121.5.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A., WIENER E. THE PARTICULATE HYDROLASES OF MACROPHAGES. I. COMPARATIVE ENZYMOLOGY, ISOLATION, AND PROPERTIES. J Exp Med. 1963 Dec 1;118:991–1008. doi: 10.1084/jem.118.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A., WIENER E. THE PARTICULATE HYDROLASES OF MACROPHAGES. II. BIOCHEMICAL AND MORPHOLOGICAL RESPONSE TO PARTICLE INGESTION. J Exp Med. 1963 Dec 1;118:1009–1020. doi: 10.1084/jem.118.6.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLWELL C., HESS A. R., TAVASTSTJERNA M. Mononuclear cells from animals of divergent susceptibility to tuberculosis. I. Enzyme studies. Am Rev Respir Dis. 1963 Jul;88:37–46. doi: 10.1164/arrd.1963.88.1.37. [DOI] [PubMed] [Google Scholar]
- COWLING D. C., QUAGLINO D., DAVIDSON E. CHANGES INDUCED BY TUBERCULIN IN LEUCOCYTE CULTURES. Lancet. 1963 Nov 23;2(7317):1091–1094. doi: 10.1016/s0140-6736(63)92860-5. [DOI] [PubMed] [Google Scholar]
- Carr I. The cellular basis of reticulo-endothelial stimulation. J Pathol Bacteriol. 1967 Oct;94(2):323–330. doi: 10.1002/path.1700940209. [DOI] [PubMed] [Google Scholar]
- Chatterjee B. R. Growth habits of Mycobacterium leprae. Their implications. Int J Lepr. 1965 Jul-Sep;33(3 Suppl):551–562. [PubMed] [Google Scholar]
- Coe J. E., Feldman J. D., Lee S. Immunologic competence of thoracic duct cells. I. Delayed hypersensitivity. J Exp Med. 1966 Feb 1;123(2):267–281. doi: 10.1084/jem.123.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn Z. A., Benson B. The in vitro differentiation of mononuclear phagocytes. 3. The reversibility of granule and hydrolytic enzyme formation and the turnover of granule constituents. J Exp Med. 1965 Sep 1;122(3):455–466. doi: 10.1084/jem.122.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn Z. A., Fedorko M. E., Hirsch J. G. The in vitro differentiation of mononuclear phagocytes. V. The formation of macrophage lysosomes. J Exp Med. 1966 Apr 1;123(4):757–766. doi: 10.1084/jem.123.4.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn Z. A., Hirsch J. G., Fedorko M. E. The in vitro differentiation of mononuclear phagocytes. IV. The ultrastructure of macrophage differentiation in the peritoneal cavity and in culture. J Exp Med. 1966 Apr 1;123(4):747–756. doi: 10.1084/jem.123.4.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn Z. A., Parks E. The regulation of pinocytosis in mouse macrophages. II. Factors inducing vesicle formation. J Exp Med. 1967 Feb 1;125(2):213–232. doi: 10.1084/jem.125.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn Z. A., Parks E. The regulation of pinocytosis in mouse macrophages. IV. The immunological induction of pinocytic vesicles, secondary lysosomes, and hydrolytic enzymes. J Exp Med. 1967 Jun 1;125(6):1091–1104. doi: 10.1084/jem.125.6.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn Z. A. The regulation of pinocytosis in mouse macrophages. I. Metabolic requirements as defined by the use of inhibitors. J Exp Med. 1966 Oct 1;124(4):557–571. doi: 10.1084/jem.124.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M., Mackaness G. B., Blanden R. V. Infection-immunity in experimental salmonellosis. J Exp Med. 1966 Oct 1;124(4):601–619. doi: 10.1084/jem.124.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M. D., Raymond D. A., Peterson R. D., South M. A., Good R. A. The functions of the thymus system and the bursa system in the chicken. J Exp Med. 1966 Jan 1;123(1):75–102. doi: 10.1084/jem.123.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANNENBERG A. M., Jr, BENNETT W. E. HYDROLASES OF MONONUCLEAR EXUDATE CELLS AND TUBERCULOSIS. I. EXUDATE CHARACTERISTICS, ESTERASES, PROTEINASES, AND LIPASE. Arch Pathol. 1963 Nov;76:581–591. [PubMed] [Google Scholar]
- DANNENBERG A. M., Jr, BENNETT W. E. HYDROLYTIC ENZYMES OF RABBIT MONONUCLEAR EXUDATE CELLS. I. QUANTITATIVE ASSAY AND PROPERTIES OF CERTAIN PROTEASES, NON-SPECIFIC ESTERASES, AND LIPASES OF MONONUCLEAR AND POLYMORPHONUCLEAR CELLS AND ERYTHROCYTES. J Cell Biol. 1964 Apr;21:1–13. doi: 10.1083/jcb.21.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANNENBERG A. M., Jr, BURSTONE M. S., WALTER P. C., KINSLEY J. W. A histochemical study of phagocytic and enzymatic functions of rabbit mononuclear and polymorphonuclear exudate cells and alveolar macrophages. I. Survey and quantitation of enzymes, and states of cellular activation. J Cell Biol. 1963 Jun;17:465–486. doi: 10.1083/jcb.17.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVID J. R., AL-ASKARI S., LAWRENCE H. S., THOMAS L. DELAYED HYPERSENSITIVITY IN VITRO. I. THE SPECIFICITY OF INHIBITION OF CELL MIGRATION BY ANTIGENS. J Immunol. 1964 Aug;93:264–273. [PubMed] [Google Scholar]
- DAVID J. R., LAWRENCE H. S., THOMAL L. DELAYED HYPERSENSITIVITY IN VITRO. II. EFFECT OF SENSITIVE CELLS ON NORMAL CELLS IN THE PRESENCE OF ANTIGEN. J Immunol. 1964 Aug;93:274–278. [PubMed] [Google Scholar]
- DAVID J. R., LAWRENCE H. S., THOMAS L. THE IN VITRO DESENSITIZATION OF SENSITIVE CELLS BY TRYPSIN. J Exp Med. 1964 Dec 1;120:1189–1200. doi: 10.1084/jem.120.6.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBOS R. J., SCHAEDLER R. W. Effects of cellular constituents of mycobacteria on the resistance of mice to heterologous infections I. Protective effects. J Exp Med. 1957 Nov 1;106(5):703–717. doi: 10.1084/jem.106.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBOS R. J., SCHAEDLER R. W. Reversible changes in the susceptibility of mice to bacterial infections. I. Changes brought about by injection of pertussis vaccine or of bacterial endotoxins. J Exp Med. 1956 Jul 1;104(1):53–65. doi: 10.1084/jem.104.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannenberg A. M., Jr, Meyer O. T., Esterly J. R., Kambara T. The local nature of immunity in tuberculosis, illustrated histochemically in dermal BCG lesions. J Immunol. 1968 May;100(5):931–941. [PubMed] [Google Scholar]
- David J. R. Delayed hypersensitivity in vitro: its mediation by cell-free substances formed by lymphoid cell-antigen interaction. Proc Natl Acad Sci U S A. 1966 Jul;56(1):72–77. doi: 10.1073/pnas.56.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David J. R. Macrophage migration. Fed Proc. 1968 Jan-Feb;27(1):6–12. [PubMed] [Google Scholar]
- Dumonde D. C. The role of the macrophage in delayed hypersensitivity. Br Med Bull. 1967 Jan;23(1):9–14. doi: 10.1093/oxfordjournals.bmb.a070524. [DOI] [PubMed] [Google Scholar]
- Dunn D. J., Patnode R. A. Passive transfer of tuberculin hypersensitivity with cellular fractions from sensitized guinea pigs. J Immunol. 1967 Sep;99(3):467–475. [PubMed] [Google Scholar]
- ELBERG S. S. Cellular immunity. Bacteriol Rev. 1960 Mar;24(1):67–95. doi: 10.1128/br.24.1.67-95.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELBERG S. S. Factors affecting resistance to infection. Annu Rev Microbiol. 1956;10:1–20. doi: 10.1146/annurev.mi.10.100156.000245. [DOI] [PubMed] [Google Scholar]
- ELBERG S. S., SCHNEIDER P., FONG J. Cross-immunity between Brucella melitensis and Mycobacterium tuberculosis; intracellular behavior of Brucella melitensis in monocytes from vaccinated animals. J Exp Med. 1957 Oct 1;106(4):545–554. doi: 10.1084/jem.106.4.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenreich B. A., Cohn Z. A. The uptake and digestion of iodinated human serum albumin by macrophages in vitro. J Exp Med. 1967 Nov 1;126(5):941–958. doi: 10.1084/jem.126.5.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein W. L. Granulomatous hypersensitivity. Prog Allergy. 1967;11:36–88. [PubMed] [Google Scholar]
- Everett N. B., Tyler R. W. Lymphopoiesis in the thymus and other tissues: functional implications. Int Rev Cytol. 1967;22:205–237. doi: 10.1016/s0074-7696(08)61836-7. [DOI] [PubMed] [Google Scholar]
- FONG J., CHIN D., AKIYAMA H. J., ELBERG S. S. Studies on tubercle bacillus-monocyte relationship. III. Conditions affecting the action of serum and cells; modification of bacilli in an immune system. J Exp Med. 1959 Jun 1;109(6):523–543. doi: 10.1084/jem.109.6.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FONG J., CHIN D., ELBERG S. S. STUDIES OF TUBERCLE BACILLUS-HISTIOCYTE RELATIONSHIPS. VI. INDUCTION OF CELLULAR RESISTANCE BY RIBOSOMES AND RIBOSOMAL RNA. J Exp Med. 1963 Sep 1;118:371–386. doi: 10.1084/jem.118.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FONG J., CHIN D., ELBERG S. S. Studies on tubercle bacillus-histiocyte relationship. V. Passive transfer of cellular resistance. J Exp Med. 1962 Mar 1;115:475–489. doi: 10.1084/jem.115.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORBES I. J., MACKANESS G. B. MITOSIS IN MACROPHAGES. Lancet. 1963 Dec 7;2(7319):1203–1204. doi: 10.1016/s0140-6736(63)92927-1. [DOI] [PubMed] [Google Scholar]
- Fireman P., Boesman M., Haddad Z. H., Gitlin D. Passive transfer of tuberculin reactivity in vitro. Science. 1967 Jan 20;155(3760):337–338. doi: 10.1126/science.155.3760.337. [DOI] [PubMed] [Google Scholar]
- Fireman P., Boesman M., Haddad Z., Gitlin D. In vitro passive transfer of tuberculin reactivity. Fed Proc. 1968 Jan-Feb;27(1):29–30. [PubMed] [Google Scholar]
- GEDIGK P., BONTKE E. Uber die Enzymaktivität im Fremdkörpergranulationsgewebe. Virchows Arch Pathol Anat Physiol Klin Med. 1957;330(5):538–568. doi: 10.1007/BF00956748. [DOI] [PubMed] [Google Scholar]
- GEDIGK P., FISCHER R. [On the adaptation of enzymatic activity of histiocytes to functional performance]. Klin Wochenschr. 1960 Aug 15;38:806–809. doi: 10.1007/BF01487756. [DOI] [PubMed] [Google Scholar]
- GELL P. G. H., HINDE I. T. The histology of the tuberculin reaction and its modification by cortisone. Br J Exp Pathol. 1951 Dec;32(6):516–529. [PMC free article] [PubMed] [Google Scholar]
- GELL P. G. Cellular hypersensitivity. Int Arch Allergy Appl Immunol. 1961;18:39–54. doi: 10.1159/000229149. [DOI] [PubMed] [Google Scholar]
- GELZER J., SUTER E. The effect of antibody on intracellular parasitism of Salmonella typhimurium in mononuclear phagocytes in vitro: prolonged survival of infected monocytes in presence of antibody. J Exp Med. 1959 Nov 1;110:715–730. doi: 10.1084/jem.110.5.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEORGE M., VAUGHAN J. H. In vitro cell migration as a model for delayed hypersensitivity. Proc Soc Exp Biol Med. 1962 Nov;111:514–521. doi: 10.3181/00379727-111-27841. [DOI] [PubMed] [Google Scholar]
- GROGG E., PEARSE A. G. E. The enzymic and lipid histochemistry of experimental tuberculosis. Br J Exp Pathol. 1952 Dec;33(6):567–576. [PMC free article] [PubMed] [Google Scholar]
- Gowans J. L. Life-span, recirculation, and transformation of lymphocytes. Int Rev Exp Pathol. 1966;5:1–24. [PubMed] [Google Scholar]
- HEISE E. R., MYRVIK Q. N., LEAKE E. S. EFFECT OF BACILLUS CALMETTE-GU'ERIN ON THE LEVELS OF ACID PHOSPHATASE, LYSOZYME AND CATHEPSIN IN RABBIT ALVEOLAR MACROPHAGES. J Immunol. 1965 Jul;95:125–130. [PubMed] [Google Scholar]
- Heilman D. H. In vitro studies on polysaccharides of Mycobacterium tuberculosis and delayed hypersensitivity. Am Rev Respir Dis. 1967 Aug;96(2):198–203. doi: 10.1164/arrd.1967.96.2.198. [DOI] [PubMed] [Google Scholar]
- Heilman D. H., McFarland W. Inhibition of tuberculin-induced mitogenesis in cultures of lymphocytes from tuberculous donors. Int Arch Allergy Appl Immunol. 1966;30(1):58–66. doi: 10.1159/000229793. [DOI] [PubMed] [Google Scholar]
- Holm G., Perlmann P. Cytotoxic potential of stimulated human lymphocytes. J Exp Med. 1967 Apr 1;125(4):721–736. doi: 10.1084/jem.125.4.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey J. H. Cell-mediated immunity--general perspectives. Br Med Bull. 1967 Jan;23(1):93–97. doi: 10.1093/oxfordjournals.bmb.a070525. [DOI] [PubMed] [Google Scholar]
- JENKIN C., BENACERRAF B. In vitro studies on the interaction between mouse peritoneal macrophages and strains of Salmonella and Escherichia coli. J Exp Med. 1960 Aug 1;112:403–417. doi: 10.1084/jem.112.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLY L. S., BROWN B. A., DOBSON E. L. Cell division and phagocytic activity in liver reticulo-endothelial cells. Proc Soc Exp Biol Med. 1962 Jul;110:555–559. doi: 10.3181/00379727-110-27578. [DOI] [PubMed] [Google Scholar]
- KHOO K. K., MACKANESS G. B. MACROPHAGE PROLIFERATION IN RELATION TO ACQUIRED CELLULAR RESISTANCE. Aust J Exp Biol Med Sci. 1964 Dec;42:707–716. doi: 10.1038/icb.1964.67. [DOI] [PubMed] [Google Scholar]
- KOSUNEN T. U., WAKSMAN B. H., FLAX M. H., TIHEN W. S. Radioautographic study of cellular mechanisms in delayed hypersensitivity. I. Delayed reactions to tuberculin and purified proteins in the rat and guinea-pig. Immunology. 1963 May;6:276–290. [PMC free article] [PubMed] [Google Scholar]
- Kanai K. Acquired resistance to tuberculous infection in experimental model. Jpn J Med Sci Biol. 1967 Feb;20(1):21–72. doi: 10.7883/yoken1952.20.21. [DOI] [PubMed] [Google Scholar]
- LARSON C. L., BELL J. F., LIST R. H., RIBI E., WICHT W. C. SYMPOSIUM ON RELATIONSHIP OF STRUCTURE OF MICROORGANISMS TO THEIR IMMUNOLOGICAL PROPERTIES. II. HOST-REACTIVE PROPERTIES OF CELL WALLS AND PROTOPLASM FROM MYCOBACTERIA. Bacteriol Rev. 1963 Dec;27:341–351. doi: 10.1128/br.27.4.341-351.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVY F. M., CONGE G. A., PASQUIER J. F., MAUSS H., DUBOS R. J., SCHAEDLER R. W. The effect of BCG vaccination on the fate of virulent tubercle bacilli in mice. Am Rev Respir Dis. 1961 Jul;84:28–36. doi: 10.1164/arrd.1961.84.1.28. [DOI] [PubMed] [Google Scholar]
- LURIE M. B., HEPPLESTON A. G., ABRAMSON S., SWARTZ I. B. Evaluation of the method of quantitative airborne infection and its use in the study of the pathogenesis of tuberculosis. Am Rev Tuberc. 1950 Jun;61(6):765–797. [PubMed] [Google Scholar]
- LURIE M. B. Native and acquired resistance to tuberculosis. Am J Med. 1950 Nov;9(5):591–610. doi: 10.1016/0002-9343(50)90210-5. [DOI] [PubMed] [Google Scholar]
- LURIE M. B., ZAPPASODI P., BLAKER R. G., LEVY R. S. On the role of the thyroid in native resistance to tuberculosis. II. The effect of hypothyroidism; the mode of action of thyroid hormones. Am Rev Tuberc. 1959 Feb;79(2):180–203. doi: 10.1164/artpd.1959.79.2.180. [DOI] [PubMed] [Google Scholar]
- LURIE M. B., ZAPPASODI P., DANNENBERG A. M., Jr, CARDONA-LYNCH E. The effect of cortisone and ACTH on the pathogenesis of tuberculosis. Ann N Y Acad Sci. 1953 Jul 17;56(4):779–792. doi: 10.1111/j.1749-6632.1953.tb27402.x. [DOI] [PubMed] [Google Scholar]
- LURIE M. B., ZAPPASODI P., DANNENBERG A. M., Jr Constitutional factors in resistance to infection; the effect of cortisone on the pathogenesis of tuberculosis. Science. 1951 Mar 2;113(2931):234–237. doi: 10.1126/science.113.2931.234. [DOI] [PubMed] [Google Scholar]
- LURIE M. B., ZAPPASODI P., LEVY R. S., BLAKER R. G. On the role of the thyroid in native resistance to tuberculosis. I. The effect of hyperthyroidism. Am Rev Tuberc. 1959 Feb;79(2):152–179. doi: 10.1164/artpd.1959.79.2.152. [DOI] [PubMed] [Google Scholar]
- LURIE M. B., ZAPPASODI P., TICKNER C. On the nature of genetic resistance to tuberculosis in the light of the host-parasite relationships in natively resistant and susceptible rabbits. Am Rev Tuberc. 1955 Sep;72(3):297–329. doi: 10.1164/artpd.1955.72.3.297. [DOI] [PubMed] [Google Scholar]
- Lurie M. B., Dannenberg A. M. Macrophage Function in Infectious Disease with Inbred Rabbits. Bacteriol Rev. 1965 Dec;29(4):466–476. doi: 10.1128/br.29.4.466-476.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. THE IMMUNOLOGICAL BASIS OF ACQUIRED CELLULAR RESISTANCE. J Exp Med. 1964 Jul 1;120:105–120. doi: 10.1084/jem.120.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIDDLEBROOK G. A hemolytic modification of the hemagglutination test for antibodies against tubercle bacillus antigens. J Clin Invest. 1950 Nov;29(11):1480–1485. doi: 10.1172/JCI102388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLMAN I. Nonspecific resistance to tuberculosis. Am Rev Respir Dis. 1961 May;83:668–675. doi: 10.1164/arrd.1961.83.5.668. [DOI] [PubMed] [Google Scholar]
- MYRVIK Q. N., LEAKE E. S., OSHIMA S. A study of macrophages and epitheloid-like cells from granulomatous (BCG-induced) lungs of rabbits. J Immunol. 1962 Nov;89:745–751. [PubMed] [Google Scholar]
- Mackaness G. B., Blanden R. V. Cellular immunity. Prog Allergy. 1967;11:89–140. [PubMed] [Google Scholar]
- Mackaness G. B. The immunology of antituberculous immunity. Am Rev Respir Dis. 1968 Mar;97(3):337–344. doi: 10.1164/arrd.1968.97.3.337. [DOI] [PubMed] [Google Scholar]
- Mackaness G. B. The relationship of delayed hypersensitivity to acquired cellular resistance. Br Med Bull. 1967 Jan;23(1):52–54. doi: 10.1093/oxfordjournals.bmb.a070516. [DOI] [PubMed] [Google Scholar]
- McCune R. M., Feldmann F. M., Lambert H. P., McDermott W. Microbial persistence. I. The capacity of tubercle bacilli to survive sterilization in mouse tissues. J Exp Med. 1966 Mar 1;123(3):445–468. doi: 10.1084/jem.123.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills J. A. The immunologic significance of antigen induced lymphocyte transformation in vitro. J Immunol. 1966 Aug;97(2):239–247. [PubMed] [Google Scholar]
- Mizunoe K., Dannenberg A. M., Jr Hydrolases of rabbit macrophages. 3. Effect of BCG vaccination, tissue culture, and ingested tubercle bacilli. Proc Soc Exp Biol Med. 1965 Nov;120(2):284–290. doi: 10.3181/00379727-120-30513. [DOI] [PubMed] [Google Scholar]
- NEGRE L. Résistance antituberculeuse sans allergie conférée aux animaux de laboratoire par l'antigène méthylique; étude de sa durée et de l'action préventive de ce produit associée a celle du BCG. Ann Inst Pasteur (Paris) 1952 Oct;83(4):429–436. [PubMed] [Google Scholar]
- Nelson D. S., Boyden S. V. Macrophage cytophilic antibodies and delayed hypersensitivity. Br Med Bull. 1967 Jan;23(1):15–20. doi: 10.1093/oxfordjournals.bmb.a070508. [DOI] [PubMed] [Google Scholar]
- OKADA Y., MORISAWA S., SYOJIMA K., KITAGAWA M., NAKASHIMA S., YAMAMURA Y. IMPROVED METHOD FOR THE ISOLATION AND PROPERTIES OF TUBERCULIN ACTIVE PEPTIDES. J Biochem. 1963 Dec;54:484–490. doi: 10.1093/oxfordjournals.jbchem.a127820. [DOI] [PubMed] [Google Scholar]
- Oppenheim J. J. Relationship of in vitro lymphocyte transformation to delayed hypersensitivity in guinea pigs and man. Fed Proc. 1968 Jan-Feb;27(1):21–28. [PubMed] [Google Scholar]
- PEARMAIN G., LYCETTE R. R., FITZGERALD P. H. Tuberculin-induced mitosis in peripheral blood leucocytes. Lancet. 1963 Mar 23;1(7282):637–638. doi: 10.1016/s0140-6736(63)91275-3. [DOI] [PubMed] [Google Scholar]
- PEARSON B., WOLF P. L., VAZQUEZ J. A COMPARATIVE STUDY OF A SERIES OF NEW INDOLYL COMPOUNDS TO LOCALIZE BETA-GALACTOSIDASE IN TISSUES. Lab Invest. 1963 Dec;12:1249–1259. [PubMed] [Google Scholar]
- Pincus W. B. Cell-free cytotoxic fluids from tuberculin-treated guinea pigs. J Reticuloendothel Soc. 1967 May;4(2):140–150. [PubMed] [Google Scholar]
- Pincus W. B. Formation of cytotoxic factor by macrophages from normal guinea pigs. J Reticuloendothel Soc. 1967 May;4(2):122–139. [PubMed] [Google Scholar]
- RAFFEL S. Chemical factors involved in the induction of infectious allergy. Experientia. 1950 Nov 15;6(11):410–419. doi: 10.1007/BF02150118. [DOI] [PubMed] [Google Scholar]
- RAMSEIER H., SUTER E. AN ANTI-MYCOBACTERIAL PRINCIPLE OF PERITONEAL MONONUCLEAR CELLS. I. THE INHIBITION OF TUBERCLE BACILLI BY DISRUPTED MONONUCLEAR CELLS OBTAINED FROM NORMAL AND BCG-IMMUNIZED GUINEA PIGS. J Immunol. 1964 Sep;93:511–517. [PubMed] [Google Scholar]
- REES R. J., HART P. D. Analysis of the host-parasite equilibrium in chronic murine tuberculosis by total and viable bacillary counts. Br J Exp Pathol. 1961 Feb;42:83–88. [PMC free article] [PubMed] [Google Scholar]
- ROWLEY D., TURNER K. J., JENKIN C. R. THE BASIS FOR IMMUNITY TO MOUSE TYPHOID. 3. CELL-BOUND ANTIBODY. Aust J Exp Biol Med Sci. 1964 Apr;42:237–248. doi: 10.1038/icb.1964.25. [DOI] [PubMed] [Google Scholar]
- Ruddle N. H., Waksman B. H. Cytotoxic effect of lymphocyte-antigen interaction in delayed hypersensitivity. Science. 1967 Sep 1;157(3792):1060–1062. doi: 10.1126/science.157.3792.1060. [DOI] [PubMed] [Google Scholar]
- SAITO K., SUTER E. LYSOSOMAL ACID HYDROLASES IN MICE INFECTED WITH BCG. J Exp Med. 1965 May 1;121:727–738. doi: 10.1084/jem.121.5.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHREK R. Cell transformations and mitoses produced in vitro by tuberculin purified protein derivative in human blood cells. Am Rev Respir Dis. 1963 May;87:734–738. doi: 10.1164/arrd.1963.87.5.734. [DOI] [PubMed] [Google Scholar]
- SEIBERT F. B., FIGUEROA E. S., DUFOUR E. H. Isolation, identification, and classification of proteins of tuberculin and the tubercle bacillus. Am Rev Tuberc. 1955 May;71(5):704–721. doi: 10.1164/artpd.1955.71.5.704. [DOI] [PubMed] [Google Scholar]
- SHEE J. C. Myelosclerosis: an autoimmune disease. Lancet. 1963 Apr 6;1(7284):773–773. doi: 10.1016/s0140-6736(63)91599-x. [DOI] [PubMed] [Google Scholar]
- SUTER E. Interaction between phagocytes and pathogenic microorganisms. Bacteriol Rev. 1956 Jun;20(2):94–132. doi: 10.1128/br.20.2.94-132.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schild H. O., Willoughby D. A. Possible pharmacological mediators of delayed hypersensitivity. Br Med Bull. 1967 Jan;23(1):46–51. doi: 10.1093/oxfordjournals.bmb.a070515. [DOI] [PubMed] [Google Scholar]
- Spector W. G. Histology of allergic inflammation. Br Med Bull. 1967 Jan;23(1):35–38. doi: 10.1093/oxfordjournals.bmb.a070513. [DOI] [PubMed] [Google Scholar]
- Spector W. G., Lykke A. W. The cellular evolution of inflammatory granulomata. J Pathol Bacteriol. 1966 Jul;92(1):163–167. doi: 10.1002/path.1700920117. [DOI] [PubMed] [Google Scholar]
- Spector W. G., Lykke A. W., Willoughby D. A. A quantitative study of leucocyte emigration in chronic inflammatory granulomata. J Pathol Bacteriol. 1967 Jan;93(1):101–107. doi: 10.1002/path.1700930109. [DOI] [PubMed] [Google Scholar]
- Sutton J. S. Ultrastructural aspects of in vitro development of monocytes into macrophages, epithelioid cells, and multinucleated giant cells. Natl Cancer Inst Monogr. 1967 Sep;26:71–141. [PubMed] [Google Scholar]
- Svejcar J., Johanovský J., Pekárek J. Studies on the mechanism of delayed type hypersenitivity in tissue cultures. XI. The influence of the substances released during the cultivation of lymph nodes cells from sensitized organism with antigen on the migration activity of normal splee cells. Z Immunitatsforsch Allerg Klin Immunol. 1967 Aug;133(3):258–274. [PubMed] [Google Scholar]
- TAKEYA K., HISATSUNE K., INOUE Y. Mycobacterial cell walls. II. Chemical composition of the "basal layer". J Bacteriol. 1963 Jan;85:24–30. doi: 10.1128/jb.85.1.24-30.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEYA K., HISATSUNE K. Mycobacterial cell walls. I. Methods of preparation and treatment with various chemicals. J Bacteriol. 1963 Jan;85:16–23. doi: 10.1128/jb.85.1.16-23.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THACORE H., WILLETT H. P. FORMATION OF SPHEROPLASTS OF MYCOBACTERIUM TUBERCULOSIS BY LYSOZYME. Proc Soc Exp Biol Med. 1963 Oct;114:43–47. doi: 10.3181/00379727-114-28580. [DOI] [PubMed] [Google Scholar]
- THORBECKE G. J., BENACERRAF B. The reticulo-endothelial system and immunological phenomena. Prog Allergy. 1962;6:559–598. [PubMed] [Google Scholar]
- THORBECKE G. J., OLD L. J., BENACERRAF B., CLARKE D. A. A histochemical study of acid and alkaline phosphatase in mouse livers during various conditions modifying activity of the reticuloendothelial system. J Histochem Cytochem. 1961 Jul;9:392–399. doi: 10.1177/9.4.392. [DOI] [PubMed] [Google Scholar]
- Thor D. E. Delayed hypersensitivity in man: a correlate in vitro and transfer by an RNA extract. Science. 1967 Sep 29;157(3796):1567–1569. doi: 10.1126/science.157.3796.1567. [DOI] [PubMed] [Google Scholar]
- Thor D. E. Human delayed hypersensitivity: an in vitro correlate and transfer by an RNA extract. Fed Proc. 1968 Jan-Feb;27(1):16–20. [PubMed] [Google Scholar]
- Trotter N. L. Electron-opaque, lipid-containing bodies in mouse liver at early intervals after partial hepatectomy and sham operation. J Cell Biol. 1965 Jun;25(3 Suppl):41–52. doi: 10.1083/jcb.25.3.41. [DOI] [PubMed] [Google Scholar]
- Turk J. L., Polák L. Studies on the origin and reactive ability in vivo of peritoneal exudate cells in delayed hypersensitivity. Int Arch Allergy Appl Immunol. 1967;31(4):403–416. doi: 10.1159/000229886. [DOI] [PubMed] [Google Scholar]
- Uhr J. W. Delayed hypersensitivity. Physiol Rev. 1966 Jul;46(3):359–419. doi: 10.1152/physrev.1966.46.3.359. [DOI] [PubMed] [Google Scholar]
- Uriuhara T., Movat H. Z. Role of PMN-leukocyte lysosomes in tissue injury, inflammation and hypersensitivity. V. Partial suppresssion in leukopenic rabbits of vascular hyper-permeability due to thermal injury. Proc Soc Exp Biol Med. 1967 Jan;124(1):279–284. doi: 10.3181/00379727-124-31723. [DOI] [PubMed] [Google Scholar]
- VOLKMAN A., GOWANS J. L. THE ORIGIN OF MACROPHAGES FROM BONE MARROW IN THE RAT. Br J Exp Pathol. 1965 Feb;46:62–70. [PMC free article] [PubMed] [Google Scholar]
- VOLKMAN A., GOWANS J. L. THE PRODUCTION OF MACROPHAGES IN THE RAT. Br J Exp Pathol. 1965 Feb;46:50–61. [PMC free article] [PubMed] [Google Scholar]
- Volkman A. The origin and turnover of mononuclear cells in peritoneal exudates in rats. J Exp Med. 1966 Aug 1;124(2):241–254. doi: 10.1084/jem.124.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAKSMAN B. H., MATOLTSY M. The effect of tuberculin on peritoneal exudate cells of sensitized guinea pigs in surviving cell culture. J Immunol. 1958 Sep;81(3):220–234. [PubMed] [Google Scholar]
- WEISS D. W., DUBOS R. J. Antituberculous immunity induced in mice by vaccination with killed tubercle bacilli or with a soluble bacillary extract. J Exp Med. 1955 Mar 1;101(3):313–330. doi: 10.1084/jem.101.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WESSLEN T. A histological study of the tuberculin reaction in animals with passively transferred hypersensitivity. Acta Tuberc Scand. 1952;26(3):175–182. [PubMed] [Google Scholar]
- WESSLEN T. Passive transfer of tuberculin hypersensitivity by viable lymphocytes from the thoracic duct. Acta Tuberc Scand. 1952;26(1-2):38–53. [PubMed] [Google Scholar]
- WILLOUGHBY D. A., BOUGHTON B., SCHILD H. O. A FACTOR CAPABLE OF INCREASING VASCULAR PERMEABILITY PRESENT IN LYMPH NODE CELLS. A POSSIBLE MEDIATOR OF THE DELAYED REACTION. Immunology. 1963 Sep;6:484–498. [PMC free article] [PubMed] [Google Scholar]
- WILLOUGHBY D. A., SPECTOR W. G., BOUGHTON B. A LYMPH-NODE PERMEABILITY FACTOR IN THE TUBERCULIN REACTION. J Pathol Bacteriol. 1964 Apr;87:353–363. doi: 10.1002/path.1700870216. [DOI] [PubMed] [Google Scholar]
- Weissmann G., Uhr J. W. Studies on lysosomes. IX. Localization of bacteriophages and thorotrast and their inflammatory properties. Biochem Pharmacol. 1968 Mar;(Suppl):5–17. doi: 10.1016/0006-2952(68)90289-x. [DOI] [PubMed] [Google Scholar]
- White R. G. Role of adjuvants in the production of delayed hypersensitivity. Br Med Bull. 1967 Jan;23(1):39–45. doi: 10.1093/oxfordjournals.bmb.a070514. [DOI] [PubMed] [Google Scholar]
- Wiener E. DNA-synthesis in peritoneal mononuclear leucocytes. Exp Cell Res. 1967 Feb;45(2):450–459. doi: 10.1016/0014-4827(67)90193-0. [DOI] [PubMed] [Google Scholar]
- Willett H. P., Thacore H. Formation of spheroplasts of Mycobacterium tuberculosis by lysozyme in combination with certain enzymes of rabbit peritoneal monocytes. Can J Microbiol. 1967 May;13(5):481–488. doi: 10.1139/m67-063. [DOI] [PubMed] [Google Scholar]
- Willoughby D. A., Coote E., Spector W. G. A monocytogenic humoral factor released after lymph node stimulation. Immunology. 1967 Feb;12(2):165–178. [PMC free article] [PubMed] [Google Scholar]
- YAMAMURA Y. The pathogenesis of tuberculous cavities. Bibl Tuberc. 1958;13:13–37. [PubMed] [Google Scholar]
- YAMORI T. ON PHAGOCYTES: THEIR STRUCTURES AND PARTICIPATION IN INFLAMMATION. Acta Pathol Jpn. 1964 Jan;14:1–43. [PubMed] [Google Scholar]
- YOUMANS G. P., YOUMANS A. S. AN ACUTE PULMONARY GRANULOMATOUS RESPONSE IN MICE PRODUCED BY MYCOBACTERIAL CELLS AND ITS RELATION TO INCREASED RESISTANCE AND INCREASED SUSCEPTIBILITY TO EXPERIMENTAL TUBERCULOUS INFECTION. J Infect Dis. 1964 Apr;114:135–151. doi: 10.1093/infdis/114.2.135. [DOI] [PubMed] [Google Scholar]
- Youmans G. P., Youmans A. S. Nonspecific factors in resistance of mice to experimental tuberculosis. J Bacteriol. 1965 Dec;90(6):1675–1681. doi: 10.1128/jb.90.6.1675-1681.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]