Abstract
Zinc, the most abundant trace metal in the brain, has numerous functions in health and disease. It is released into the synaptic cleft alongside glutamate and this connection between zinc and glutamatergic neurotransmission allows the ion to modulate overall excitability of the brain and influence synaptic plasticity. To maintain healthy synapses, extracellular zinc levels need to be tightly regulated. We recently reported that the cellular prion protein (PrPC) can directly influence neuronal zinc concentrations by promoting zinc uptake via AMPA receptors. The octapeptide repeat region of PrPC is involved in zinc sensing or scavenging and the AMPA receptor provides the channel for transport of the metal across the membrane, facilitated by a direct interaction between the N-terminal polybasic region of PrPC and AMPA receptors. PrPC has been evolutionarily linked to the Zrt/Irt-like protein (ZIP) metal ion transport family with the C-terminus of PrPC sharing sequence similarities with the N-terminal extracellular domains of ZIP 5, 6 and 10. By incorporating the properties of ZIP transporters (both zinc sensing and zinc transport) into two existing neuronal proteins, (PrPC as zinc sensor, AMPA receptor as zinc transporter), neuronal cells are enhancing their biological efficiency and functionality.
Keywords: prion, zinc, AMPA receptor, ZIP transporter
Zinc and the Brain
Zinc is the most abundant trace metal in the brain where it is found as an essential component of a great number of proteins. The significance of the ion is exemplified by the many and varied cellular roles it performs. These include being a cofactor necessary for structure (i.e., zinc-finger proteins), for catalytic activity of some metalloenzymes or acting directly as a signaling molecule. As perturbations of zinc either above or below physiological concentrations are detrimental to cell survival, neurons must maintain tight homeostatic control on their zinc content. Pre-synaptic vesicles of glutamatergic neurons contain a significant proportion of brain zinc in a loosely-bound, chelatable form which is co-released with the glutamate upon excitation.1 The released zinc must then be cleared from the synaptic cleft to prevent damage, although the molecular mechanisms involved are not clear. In non-neuronal cells, the transport of zinc across the plasma membrane is performed by members of the ZIP (Zrt/Irt-like Protein) family which import zinc from the extracellular space or organellar lumen into the cytoplasm. In neuronal cells, a number of possible import channels have been identified, including, activated voltage-gated Ca2+ channels, Ca2+ and zinc-permeable α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) channels, N-methyl-D-aspartate (NMDA) channels1 and most recently, the transient receptor potential melastatin 7 (TRPM7) channel.2 While there is a well-established relationship between the cellular prion protein (PrPC) and copper, which has been discussed elsewhere,3,4 we focus this short, concise review on the interaction between PrPC and zinc transport in the neuron, highlighting a novel aspect of prion biology.
As a protein found in the outer leaflet of the plasma membrane, glycosyl-phosphatidylinositol-anchored PrPC protrudes from the cell into the synaptic cleft at both pre-and post-synaptic sites.5 This means that PrPC is exposed to the extracellular fluid in the synaptic cleft and can react to any changes that occur within it. Under basal conditions, ionic zinc is found in the synaptic cleft at a concentration below the current limits of detection (< 1nM). During neuronal depolarisation, exocytosis of synaptic vesicles containing concentrations of zinc in the low millimolar range results in a transient spike in zinc concentration in the extracellular fluid.6 This dramatically increases the extracellular zinc concentration to one in the micromolar range. Together this implies that there is a supply of rapidly exchangeable zinc (200–600µM) contained within the synaptic bouton.7
Ten years ago, we proposed a role for PrPC in neuronal zinc homeostasis.8 Multiple authors have shown that in addition to binding copper, PrPC could bind zinc in a similar fashion albeit with a lower affinity (low nanomolar to micromolar range for Cu, ~200µM for Zn).9-11 Furthermore, following the binding of zinc to the octapeptide repeat region of the protein, PrPC was rapidly internalised.12 The concentration of zinc required to bring about this response (100 µM) was comparable to the physiological concentration of zinc found within the brain (100–350 µM),13 whereas, the copper concentration required for endocytosis (100 µM) was higher than the reported neuronal copper concentration (70 µM). These data suggested that PrPC could play a role in binding or transporting zinc ions in the brain.
We hypothesized that there were three possible ways that PrPC could contribute to neuronal zinc homeostasis.8 First, acting simply as a zinc sink; PrPC would bind zinc released during synaptic transmission, sequestering it in an inert form before passing it on to high affinity plasma membrane transporters to help return the ion to intracellular stores. Second, PrPC could be directly involved in zinc uptake via endocytosis. The decreasing endosomal pH following internalisation would promote the dissociation of the zinc from PrPC, releasing it for transport through a transporter into the cytosol. Apo-PrPC would then recycle back to the cell surface to pick up more zinc. The third mechanism suggested was that PrPC behaves as a zinc sensor, monitoring levels of metal ion in the extracellular space. If the zinc concentration rises above a certain threshold, a signaling cascade is triggered that results in an appropriate cellular response such as increased transcription of metal transporter genes.8
Prion Protein Facilitates Zinc Uptake
In our recent study,14 we examined the role of PrPC in regulating zinc uptake into neurons. Using zinc specific dyes (Zinpyr-1 and Newport Green), we found that PrPC significantly enhanced the rate of zinc uptake following its addition to primary hippocampal neurons and neuronal (SH-SY5Y and N2a) cell lines. These data were confirmed by siRNA knockdown or overexpression of PrPC. We eliminated the possibility of cross-reactivity of the dyes with other divalent metals, and further verified their specificity for zinc using zinc-specific chelators. By monitoring zinc stores in the presence and absence of PrPC, we established that PrPC was not acting by retaining zinc within the cell.
Both copper and zinc bind to the octapeptide repeat region of PrPC and mediate its endocytosis.12 We showed that, like Cu2+, Zn2+-mediated endocytosis of PrPC was also dependent on the transmembrane low-density lipoprotein receptor-related protein-1 (LRP1).15 The reduction of LRP1 expression by siRNA or blocking the interaction between LRP1 and PrPC using receptor-associated protein (RAP) prevented zinc-mediated endocytosis, but did not reduce the uptake of zinc into cells, thus implicating another transporter or channel protein in the uptake mechanism. Interestingly, we observed a small but significant increase in the uptake of zinc when the endocytosis of PrPC was blocked, suggesting that the increased availability of PrPC at the cell surface could be facilitating the enhanced uptake of zinc.
Following excitation, zinc is released with glutamate from the presynaptic terminal into the synaptic cleft, membrane depolarisation occurs with the opening of zinc-permeable ion channels that clear the ion from the extracellular fluid.1 Using an AMPA/kainate receptor antagonist (CNQX), we demonstrated that the PrPC-enhanced uptake was mediated by AMPA receptors.14 Direct activation of AMPA receptors caused an increase in zinc uptake which was also ablated when PrPC expression was knocked down, thus providing additional support that PrPC can mediate zinc uptake via AMPA receptors. AMPA receptors are composed of four subunits (GluA1 – GluA4) arranged in various combinations and are expressed throughout the central nervous system, although GluA1 and GluA2 are predominately found in the hippocampus and cerebral cortex. GluA2-lacking receptors display high Ca2+ and zinc permeability but low Ca2+ and zinc permeability when GluA2 is present. To investigate the mechanism behind the zinc uptake, we established that PrPC forms immunoprecipitable complexes with GluA1- and GluA2-containing AMPA receptors in mouse brain.14 PrPC also influenced the trafficking of GluA1 and was found to increase its cell surface expression. Blocking AMPA receptors with either IEM-1460 (antagonist of GluA2-lacking AMPA receptors) or pentobarbital (antagonist of GluA2-containing AMPA receptors) diminished the PrPc mediated zinc uptake. This was surprising given that the presence of the GluA2 subunit reduces Ca2+ and Zn2+ permeability, and would therefore not be expected to facilitate zinc uptake. It is possible that non-GluA2 containing subunits still display some sensitivity to pentobarbital.16 Using various constructs of PrPC and antibody protection techniques, we were able to establish the regions of PrPC that were involved in zinc binding and uptake. The binding of PrPC to either GluA1 or GluA2 was still found in a mutant form of PrPC in which the octapeptide repeat region had been deleted, but a significantly reduced uptake of zinc was seen. Deletion of the extreme N-terminal KKRP sequence of PrPC prevented its binding to the GluA2 subunit and significantly reduced zinc uptake. These data identified that the metal-binding octapeptide repeats in PrPc are critical for the presentation of zinc and that the N-terminal polybasic region is necessary for the interaction with AMPA receptors. Thus our recent work identified PrPC as a zinc sensor/scavenger that binds zinc via the octapeptide repeats prior to facilitating the transport of the metal across the membrane through interaction with AMPA receptors (Fig. 1).
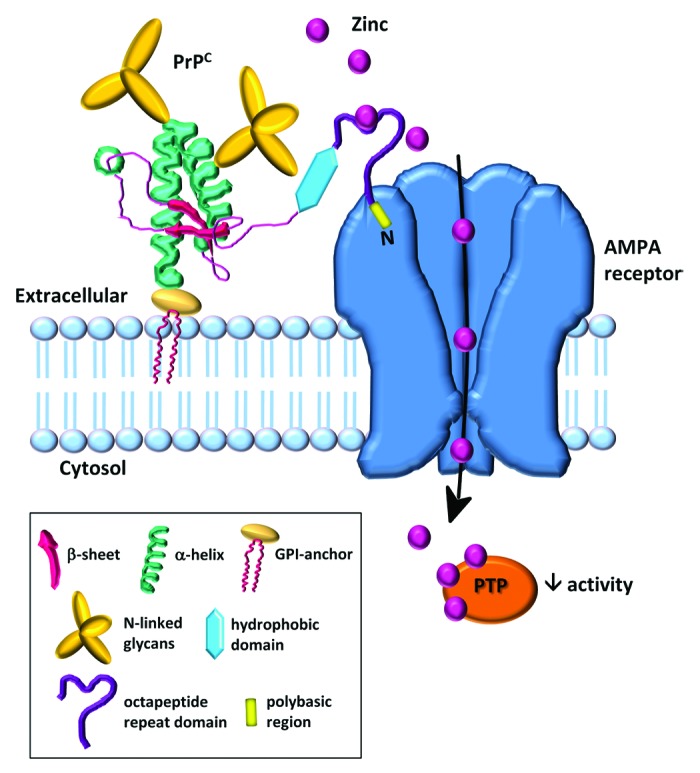
Figure 1. Prion and AMPA receptor zinc transporter. PrPC acts as a sensor for zinc in the extracellular space and coordinates the low affinity binding of zinc to the octapeptide repeats. The N-terminal polybasic region of PrPC interacts with the AMPA receptor subunits; this interaction facilitates the transport of zinc through the channel. The PrPC mediated increase in intracellular zinc leads to downstream inhibition of protein tyrosine phosphatases (PTP).
Zinc and Prion Disease
Zinc concentrations decrease in the brain during prion disease17,18 and there is debate about whether prion diseases involve a loss of a normal function of PrPC in addition to the toxic gain of function upon the conformational conversion to PrPSc.19,20 We reported that the PrPC-mediated zinc uptake was disrupted in scrapie-infected N2a cells.14 This would imply that either zinc binding is altered when PrPC is converted to PrPSc and can no longer be presented to the AMPA receptor for transport across the membrane, or that the extreme N-terminus of PrPSc is unavailable for the interaction with the AMPA receptor. Interestingly, the zinc content in whole brain homogenates from scrapie-infected mice has been shown to be decreased by > 70%.17
In addition, using various disease-associated mutations, we established that none of the mutants of PrPC investigated were able to enhance zinc uptake.14 Neither D177N nor P101L mutant forms interacted with GluA1 or GluA2 subunits, suggesting that their inability to modulate zinc uptake is due to a lack of interaction with the AMPA receptors. Both the PG14 and A116V mutations of PrPC were able to form immunoprecipitatable complexes with GluA2 but GluA1 was undetectable in the cells expressing these mutants. The loss of GluA1 was, therefore, the most likely reason for the disruption of zinc uptake in cells expressing PG14 and A116V.
A recent study by Spevacek et al.21 used magnetic resonance to demonstrate that zinc binding induces a structural change in murine PrPC. Specifically, they found that zinc coordinates with the histidine residues found in the octapeptide repeat region to bring about a partial ordering which then allows the N-terminal region to form a tertiary interaction with the exposed surfaces of helices 2 and 3 in the C-terminal domain. This interaction limits the conformational freedom of PrPC and enhances zinc-mediated inter-domain interactions. It is interesting to speculate that this zinc-mediated conformational change in PrPC may also promote its interaction with the AMPA receptor and other interacting partner proteins. The C-terminal region, identified by Spevacek and colleagues,21 that forms the docking site when zinc binds to the octapeptide repeat region also contains the majority of the PrPC point mutations which lead to familial prion diseases.22 For example, the D177N and P101L mutations of PrPC were found to destabilize the interaction between the N-terminal and C-terminal domains by altering either the charge (D177N) or removing conformational restrictions in the linker region (P101L), resulting in inhibition of the inter-domain interaction.21 These findings could provide further molecular explanation for the reduced uptake of zinc into cells expressing these two mutant constructs of PrPC.14 Another study, using peptides corresponding to the full metal-binding octapeptide repeats anchored to the surface of lipid vesicles, demonstrated that copper and, to a greater extent, zinc could promote PrP-PrP interactions.23 The metal-induced conformational change in PrPC may also promote interactions with other proteins. This would be consistent with the binding of metals to the octapeptide repeat region resulting in the displacement of PrPC from rafts, possibly by disrupting interactions with raft-based components.24
If PrPC does contribute to neuronal zinc uptake, it could be anticipated that mouse models in which the prion gene is disrupted would show altered neuronal zinc levels. Unfortunately, there are limited and conflicting data on the concentration of metal ions in the brain of PrPC knockout mice.25-27 A possible explanation for these conflicting results is that, because of the essential nature of zinc regulation in the brain, other uptake mechanisms compensate for the lack of prion-mediated metal transport during development in the germ-line knockout models. Alternatively, highly localized redistribution of zinc could occur with no loss in measurable concentration in the gross sample. Considering these caveats, we measured intracellular protein tyrosine phosphatase activity, an enzyme that is exquisitely sensitive to zinc28,29 to try and assess the bioavailability of zinc within the brain. Protein tyrosine phosphatase activity, which has been shown to be important for hippocampal synapse formation and learning,30 is inhibited by zinc. In cells expressing PrPC there was a significant reduction in protein tyrosine phosphatase activity as compared with cells lacking PrPC, consistent with an increase in intracellular zinc concentration.14 Similarly, a decrease in protein tyrosine phosphatase activity was measured in the brains of wild-type mice compared with PrP null mice.14 These data support the hypothesis that PrPC-mediated changes in cellular zinc concentration have a physiological consequence for the neuron.
Prion Protein and ZIP Transporters
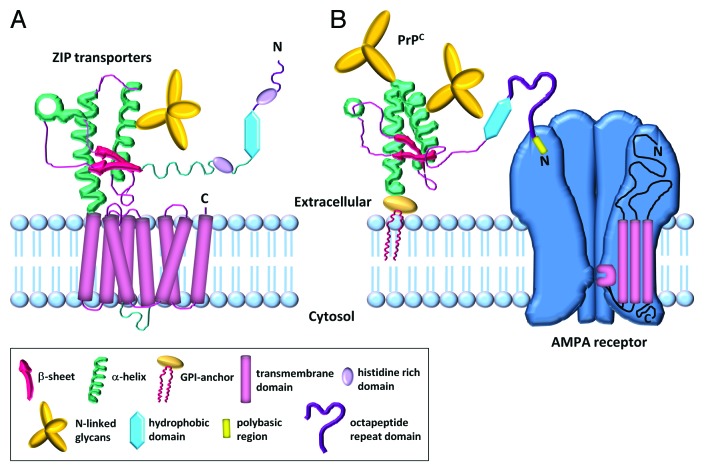
While we were gathering the data on a PrPC-mediated route of zinc uptake into neurons, Schmitt-Ulms et al.31 reported that PrPC was evolutionarily linked to the ZIP family of transmembrane zinc transporters. They initially performed a quantitative interactome study to identify proteins that co-purified with a tagged construct of PrPC. From this analysis, three proteins stood out for further analysis, namely ZIP5, 6 and 10. Following extensive bioinformatic analysis, a region in the ZIP proteins was shown to possess extensive sequence similarity to a region of PrPC. A 111 amino acid fragment in the N-terminal extracellular domain of murine ZIP10 (residues 285−395) has some alignment (16% identity, 42% similarity) to the C-terminal globular domain of murine PrPC. Within this “prion-like” (PL) domain of ZIP10, there was positional agreement of a cysteine-flanked core domain and the first N-linked glycosylation site. These ZIP proteins are also equipped with histidine-rich sequences, N-terminal to their “PL” domain, capable of divalent metal binding, which is reminiscent of the octapeptide repeat domain of PrPC. The sequence similarity between the PL domain of ZIP10 and PrPC is comparable to that seen between PrPC and its paralogue doppel (18% identity, 44% similarity).
While the orientation and distance of the cysteine-flanked core domain to the respective membrane attachment sites in both PrPC and the ZIP proteins are similar, the primary sequence of the first transmembrane domain in the ZIP protein and the glycosylphosphatidylinositol (GPI) anchor attachment sequence of PrPC are also comparable. As minimal changes to an area of hydrophobicity can bring about a shift such that a transmembrane region can be converted to a GPI anchor,32 this could suggest that the signal peptide relating to the attachment of the GPI anchor (in PrPC) may have evolutionarily originated from the first transmembrane domain of the ZIP ancestral molecule.33
Identification of numerous commonalities between ZIP transporters and PrPC led Schmitt-Ulms and colleagues to conclude that PrPC must have descended from a progenitor in the ZIP family and that divergent evolution led to the formation of the two proteins as they are today.31 However, PrPC is a GPI-anchored protein residing in the outer leaflet of the plasma membrane, whereas ZIP transporters are multispanning membrane proteins with an N-terminal domain that is predicted to be extracellular (or luminal) and a C-terminal region that forms a channel to allow zinc influx across the membrane (see Fig. 2A). In order to correlate a function for PrPC in the transport of zinc across the plasma membrane, it is necessary to consider how that may be possible in the absence of a transmembrane domain within the protein. Many proteins require the activity of another in order to perform their biological role and we proposed that PrPC requires the assistance of the channel properties of AMPA receptors in order to achieve zinc transport (Fig. 2B).14 The combination of the octapeptide repeats in the GPI-anchored PrPC act as a zinc sensor/scavenger in the extracellular environment which then presents the metal to the transmembrane AMPA receptor for transport of the zinc across the membrane. This incorporates the two properties (zinc sensing and transport) held by the ZIP transporters into two neuronal proteins (PrPC as zinc sensor, AMPA receptor as zinc transporter) potentially increasing their biological efficiency and functionality.
Figure 2. Structure comparison of ZIP transporters and the prion-AMPA receptor zinc transporter. (A) Schematic model of the LIV-1 subfamily of zinc transporters (LZT) with a prion-like ectodomain. The ectodomain contains two histidine-rich zinc binding domains, two α-helices containing a cysteine-flanked core, two β-sheets and a hydrophobic domain. The ectodomain is coupled to a C-terminal multispanning membrane domain which facilitates the uptake of zinc across the plasma membrane. (B) PrPC is structurally similar to the ectodomain of the ZIP transporter. It contains an octapeptide repeat region which binds zinc, and an N-terminal polybasic region which facilitates its interaction with the GluA1 subunit of the AMPA receptor. The AMPA receptor is composed of four subunits, each with multispanning membrane domains. The receptor forms a channel across the membrane that can facilitate the uptake of zinc in a manner similar to the C-terminal region of the ZIP transporter. The combination of the extracellular PrPC acting as a zinc scavenger/ sensor and AMPA receptor mediating the influx of zinc into the cell links the two proteins, and encapsulates the properties held by the ZIP transporters. (Figs. adapted from Ehsani et al., 2011 and Shepherd JD, Huganir RL., 2007).
In summary, PrPC has been identified as a neuronal zinc sensor, modulating zinc binding, and promoting zinc uptake via AMPA receptors, with a subsequent downstream effect on the activity of intracellular protein tyrosine phosphatases. PrPC and AMPA receptors incorporate the properties of the ZIP transporters (both zinc sensing and zinc transport), which allows them to function together to maintain healthy levels of zinc in the synaptic cleft of neurons. The binding of zinc promotes inter-domain interactions in PrPC, and is also likely to mediate the interaction between the N-terminus of PrPC and the AMPA receptor subunits. Familial disease associated mutations in PrPC prevent the inter-domain interactions, and disrupt the interaction between PrPC and the AMPA receptor, providing a possible explanation for the reduction in zinc in prion disease-affected brains. Together, these findings provide new insight on a functional role of PrPC in neuronal zinc homeostasis.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/prion/article/24503
References
- 1.Paoletti P, Vergnano AM, Barbour B, Casado M. Zinc at glutamatergic synapses. Neuroscience. 2009;158:126–36. doi: 10.1016/j.neuroscience.2008.01.061. [DOI] [PubMed] [Google Scholar]
- 2.Inoue K, Branigan D, Xiong ZG. Zinc-induced neurotoxicity mediated by transient receptor potential melastatin 7 channels. J Biol Chem. 2010;285:7430–9. doi: 10.1074/jbc.M109.040485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Viles JH, Klewpatinond M, Nadal RC. Copper and the structural biology of the prion protein. Biochem Soc Trans. 2008;36:1288–92. doi: 10.1042/BST0361288. [DOI] [PubMed] [Google Scholar]
- 4.Millhauser GL. Copper and the prion protein: methods, structures, function, and disease. Annu Rev Phys Chem. 2007;58:299–320. doi: 10.1146/annurev.physchem.58.032806.104657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salès N, Rodolfo K, Hässig R, Faucheux B, Di Giamberardino L, Moya KL. Cellular prion protein localization in rodent and primate brain. Eur J Neurosci. 1998;10:2464–71. doi: 10.1046/j.1460-9568.1998.00258.x. [DOI] [PubMed] [Google Scholar]
- 6.Frederickson CJ, Bush AI. Synaptically released zinc: physiological functions and pathological effects. Biometals. 2001;14:353–66. doi: 10.1023/A:1012934207456. [DOI] [PubMed] [Google Scholar]
- 7.Frederickson CJ, Giblin LJ, 3rd, Balaji RV, Masalha R, Frederickson CJ, Zeng Y, et al. Synaptic release of zinc from brain slices: factors governing release, imaging, and accurate calculation of concentration. J Neurosci Methods. 2006;154:19–29. doi: 10.1016/j.jneumeth.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 8.Watt NT, Hooper NM. The prion protein and neuronal zinc homeostasis. Trends Biochem Sci. 2003;28:406–10. doi: 10.1016/S0968-0004(03)00166-X. [DOI] [PubMed] [Google Scholar]
- 9.Jackson GS, Murray I, Hosszu LL, Gibbs N, Waltho JP, Clarke AR, et al. Location and properties of metal-binding sites on the human prion protein. Proc Natl Acad Sci U S A. 2001;98:8531–5. doi: 10.1073/pnas.151038498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walter ED, Stevens DJ, Visconte MP, Millhauser GL. The prion protein is a combined zinc and copper binding protein: Zn2+ alters the distribution of Cu2+ coordination modes. J Am Chem Soc. 2007;129:15440–1. doi: 10.1021/ja077146j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garnett AP, Viles JH. Copper binding to the octarepeats of the prion protein. Affinity, specificity, folding, and cooperativity: insights from circular dichroism. J Biol Chem. 2003;278:6795–802. doi: 10.1074/jbc.M209280200. [DOI] [PubMed] [Google Scholar]
- 12.Perera WS, Hooper NM. Ablation of the metal ion-induced endocytosis of the prion protein by disease-associated mutation of the octarepeat region. Curr Biol. 2001;11:519–23. doi: 10.1016/S0960-9822(01)00147-6. [DOI] [PubMed] [Google Scholar]
- 13.Moir RD, Atwood CS, Huang X, Tanzi RE, Bush AI. Mounting evidence for the involvement of zinc and copper in Alzheimer’s disease. Eur J Clin Invest. 1999;29:569–70. doi: 10.1046/j.1365-2362.1999.00472.x. [DOI] [PubMed] [Google Scholar]
- 14.Watt NT, Taylor DR, Kerrigan TL, Griffiths HH, Rushworth JV, Whitehouse IJ, et al. Prion protein facilitates uptake of zinc into neuronal cells. Nat Commun. 2012;3:1134. doi: 10.1038/ncomms2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor DR, Hooper NM. The low-density lipoprotein receptor-related protein 1 (LRP1) mediates the endocytosis of the cellular prion protein. Biochem J. 2007;402:17–23. doi: 10.1042/BJ20061736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taverna FA, Cameron BR, Hampson DL, Wang LY, MacDonald JF. Sensitivity of AMPA receptors to pentobarbital. Eur J Pharmacol. 1994;267:R3–5. doi: 10.1016/0922-4106(94)90161-9. [DOI] [PubMed] [Google Scholar]
- 17.Wong BS, Brown DR, Pan T, Whiteman M, Liu T, Bu X, et al. Oxidative impairment in scrapie-infected mice is associated with brain metals perturbations and altered antioxidant activities. J Neurochem. 2001;79:689–98. doi: 10.1046/j.1471-4159.2001.00625.x. [DOI] [PubMed] [Google Scholar]
- 18.Wong BS, Chen SG, Colucci M, Xie Z, Pan T, Liu T, et al. Aberrant metal binding by prion protein in human prion disease. J Neurochem. 2001;78:1400–8. doi: 10.1046/j.1471-4159.2001.00522.x. [DOI] [PubMed] [Google Scholar]
- 19.Hetz C, Maundrell K, Soto C. Is loss of function of the prion protein the cause of prion disorders? Trends Mol Med. 2003;9:237–43. doi: 10.1016/S1471-4914(03)00069-8. [DOI] [PubMed] [Google Scholar]
- 20.Linden R, Martins VR, Prado MA, Cammarota M, Izquierdo I, Brentani RR. Physiology of the prion protein. Physiol Rev. 2008;88:673–728. doi: 10.1152/physrev.00007.2007. [DOI] [PubMed] [Google Scholar]
- 21.Spevacek AR, Evans EG, Miller JL, Meyer HC, Pelton JG, Millhauser GL. Zinc Drives a Tertiary Fold in the Prion Protein with Familial Disease Mutation Sites at the Interface. Structure. 2013;21:236–46. doi: 10.1016/j.str.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mead S. Prion disease genetics. Eur J Hum Genet. 2006;14:273–81. doi: 10.1038/sj.ejhg.5201544. [DOI] [PubMed] [Google Scholar]
- 23.Kenward AG, Bartolotti LJ, Burns CS. Copper and zinc promote interactions between membrane-anchored peptides of the metal binding domain of the prion protein. Biochemistry. 2007;46:4261–71. doi: 10.1021/bi602473r. [DOI] [PubMed] [Google Scholar]
- 24.Taylor DR, Watt NT, Perera WS, Hooper NM. Assigning functions to distinct regions of the N-terminus of the prion protein that are involved in its copper-stimulated, clathrin-dependent endocytosis. J Cell Sci. 2005;118:5141–53. doi: 10.1242/jcs.02627. [DOI] [PubMed] [Google Scholar]
- 25.Brown DR, Qin K, Herms JW, Madlung A, Manson J, Strome R, et al. The cellular prion protein binds copper in vivo. Nature. 1997;390:684–7. doi: 10.1038/37733. [DOI] [PubMed] [Google Scholar]
- 26.Waggoner DJ, Drisaldi B, Bartnikas TB, Casareno RL, Prohaska JR, Gitlin JD, et al. Brain copper content and cuproenzyme activity do not vary with prion protein expression level. J Biol Chem. 2000;275:7455–8. doi: 10.1074/jbc.275.11.7455. [DOI] [PubMed] [Google Scholar]
- 27.Pushie MJ, Pickering IJ, Martin GR, Tsutsui S, Jirik FR, George GN. Prion protein expression level alters regional copper, iron and zinc content in the mouse brain. Metallomics. 2011;3:206–14. doi: 10.1039/c0mt00037j. [DOI] [PubMed] [Google Scholar]
- 28.Wilson M, Hogstrand C, Maret W. Picomolar concentrations of free zinc(II) ions regulate receptor protein-tyrosine phosphatase β activity. J Biol Chem. 2012;287:9322–6. doi: 10.1074/jbc.C111.320796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamasaki S, Sakata-Sogawa K, Hasegawa A, Suzuki T, Kabu K, Sato E, et al. Zinc is a novel intracellular second messenger. J Cell Biol. 2007;177:637–45. doi: 10.1083/jcb.200702081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fuentes F, Zimmer D, Atienza M, Schottenfeld J, Penkala I, Bale T, et al. Protein tyrosine phosphatase PTP1B is involved in hippocampal synapse formation and learning. PLoS One. 2012;7:e41536. doi: 10.1371/journal.pone.0041536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmitt-Ulms G, Ehsani S, Watts JC, Westaway D, Wille H. Evolutionary descent of prion genes from the ZIP family of metal ion transporters. PLoS One. 2009;4:e7208. doi: 10.1371/journal.pone.0007208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naghibalhossaini F, Stanners CP. Minimal mutations are required to effect a radical change in function in CEA family members of the Ig superfamily. J Cell Sci. 2004;117:761–9. doi: 10.1242/jcs.00903. [DOI] [PubMed] [Google Scholar]
- 33.Ehsani S, Huo H, Salehzadeh A, Pocanschi CL, Watts JC, Wille H, et al. Family reunion--the ZIP/prion gene family. Prog Neurobiol. 2011;93:405–20. doi: 10.1016/j.pneurobio.2010.12.001. [DOI] [PubMed] [Google Scholar]