Abstract
Loss of plasma membrane integrity (LPMI) is a hallmark of necrotic cell death. The involvement of complement and ROS in the development of LPMI during the early stages of murine myocardial ischemia-reperfusion injury was investigated. LPMI developed within 1 hour of reperfusion to a level that was sustained through 24 hours. C3 deposition became significant at 3 hours’ reperfusion and thus contributed little to LPMI prior to this time. SOD1 transgenic mice had significantly less LPMI compared with WT mice at 1 hour of reperfusion but not at later time points. Catalase transgenic mice were not protected from LPMI at 1 hour’s reperfusion compared with WT mice, but had 69% less LPMI at 3 hours’ reperfusion. This protection was transient. At 24 hours’ reperfusion the LPMI of catalase transgenic mice was identical to that of WT mice. The delayed benefits of over-expressed catalase compared with SOD1 are consistent with its antioxidant action downstream of SOD1. The onset of LPMI occurs within 1 hour of reperfusion and is maintained through 24 hours. ROS contribute significantly to LPMI during the first 3 hours of reperfusion, while complement deposition, which becomes significant after 3 hours’ reperfusion, may contribute thereafter.
Keywords: myocardial ischemia, reperfusion injury, necrosis, loss of plasma membrane integrity (LPMI), inflammation, complement, reactive oxygen species (ROS), SOD1, catalase
Introduction
Loss of plasma membrane integrity (LPMI), a hallmark of necrotic cell death, has been studied extensively in cell culture. However, in vivo studies are relatively few due to the lack of effective methods for LPMI identification in vivo. Necrosis of cardiomyocytes occurs in myocardial ischemia, and typically, myocardial necrosis (infarction) is determined histologically using 2,3,5-triphenyltetrazolium chloride (TTC). TTC enters all cells and is reduced to red-fluorescing 1,3,5-triphenylformazan by endogenous dehydrogenases/cofactors when cells are intact. In necrotic cells, when reperfusion following ischemia is extensive, the enzymes/cofactors have been washed out and TTC remains colorless, defining infarction. Since inadequate reperfusion underestimates the extent of infarction (Birnbaum et al., 1997; Ito et al., 1997), effective TTC staining requires at least 3 hours’ reperfusion in the coronary artery occlusion model (Birnbaum et al., 1997). The need for adequate reperfusion limits the use of TTC when focusing on events early in reperfusion. In addition, TTC-stained tissue is not suitable for detection of other markers of injury, obviating a valuable experimental option and requiring study of additional animals.
Propidium iodide (PI), described in three studies using in vivo rodent myocardial ischemia/reperfusion (I/R) models (Ito et al., 1997; Weinbrenner et al., 2004; Wolff et al., 2000), provides an alternative approach for identifying LPMI. PI enters necrotic, but not intact, cells after compromise of plasma membrane integrity, intercalating with DNA to produce red fluorescence. PI can be introduced in vivo and does not require extensive reperfusion. In addition, we recognized that PI-stained tissues can be analyzed for concurrent pathological events.
In the present study, we extend previous PI-based assessments of LPMI occurring during I/R injury (Ito et al., 1997; Weinbrenner et al., 2004; Wolff et al., 2000) by (i), evaluating the early temporal development of LPMI in a murine myocardial I/R model, (ii), working with PI-stained tissue, documenting the innate inflammatory response as denoted by deposition of complement C3, the central molecule in complement pathways associated with myocardial infarction in clinical and animal studies (Weisman et al., 1990), and (iii), using superoxide dismutase 1 (SOD1) and catalase transgenic mice, evaluating the time-dependent contribution of reactive oxygen species (ROS) to necrosis following myocardial I/R.
The ability of PI to assess LPMI early in reperfusion provided an opportunity to re-examine conflicting results using TCC to study the effects of over-expressing or administering SOD1 on reperfusion injury. While several animal studies found that SOD1 could reduce infarction in both regional (Gross et al., 1986; Hangaishi et al., 2001; Kanamasa et al., 2001) and global heart ischemia (Ambrosio and Flaherty, 1992; Nishikawa et al., 1991; Otani et al., 1986; Wang et al., 1998), others found that it failed to protect against infarction in various ischemia models (Gallagher et al., 1986; Jones et al., 2003; Klein et al., 1988; Matsuda et al., 1991; Nejima et al., 1989; Ooiwa et al., 1991; Patel et al., 1990; Przyklenk and Kloner, 1989; Richard et al., 1988; Uraizee et al., 1987; Watanabe et al., 1993). Downey et al. suggested that SOD1 might interfere with the ability of TTC to differentiate between living and dead cells (Downey et al., 1991), with the result that TTC produced an artifactually reduced infarct size in SOD1-treated animals (Shirato et al., 1989).
Methods
Mouse model of myocardial I/R injury
The WT (C57BL/6) mouse strain was obtained from the Jackson Laboratory (Bar Harbor, ME). Transgenic mice over-expressing human SOD1 or catalase and their WT littermates were generously provided by Dr. Arlan Richardson’s group (Chen et al., 2003). All mice were maintained at the Division of Laboratory Animal Resources of SUNY Downstate Medical Center. Genotyping was provided by GeneTyper (New York, NY) using established PCR protocols (Chen et al., 2003; Wessels et al., 1995). All mice were used in compliance with the requirements of the NIH and the SUNY Downstate Institutional Animal Care and Use Committee.
A murine myocardial I/R injury model was used (Zhang et al., 2006). Briefly, adult male mice (10–12 weeks old, average body weight 25–30 grams) were anesthetized with pentobarbital (60mg/kg), intubated and ventilated. Heart rate, core body temperature and ECG were monitored. The left anterior descending artery (LAD) was ligated and occlusion was confirmed by the lack of color of the anterior wall of the left ventricle and the ST elevation on ECG. Following 1 hour of LAD occlusion, the ligature was removed and reperfusion confirmed by return of color to the left ventricle anterior wall and the appropriate ECG changes. After the surgery, analgesia (buprenorphine) was given; the mouse was weaned off the ventilator and allowed to recover under a heat lamp with oxygen via a mask.
To evaluate the early temporal development of LPMI and the concomitant deposition of complement C3, seven groups of WT mice were used. The controls, group (i), were sham-operated, with hearts being harvested 1 hour after sternotomy. Six groups (ii – vii) were subjected to 1 hour LAD occlusion followed by the following reperfusion times: 0, 1, 2, 3, 6 and 24 hours. N=5 mice/group/time point, except for the 24 hrs' group where n=10.
To investigate the contribution of ROS to LPMI during the early stages of reperfusion injury, SOD1 tg mice (n=4 /group/time point) and WT littermates (n=3 /group/time point) were subjected to 1 hour LAD occlusion followed by the following reperfusion times: 1, 3 and 24 hours. Catalase tg mice (n=5 /group/time point) and WT littermates (n=5 /group/time point, except for the 24 hrs’ group where n=3) were subjected to 1 hour LAD occlusion followed by the following reperfusion times: 1, 3 and 24 hours.
Ninety percent of all experimental animals survived the heart surgery (non-survivors were excluded from the final data analyses), the most common cause of surgery-related death being accidental bleeding during the sternotomy and LAD occlusion.
Evaluation of tissue with LPMI and of tissue at risk for LPMI using two fluorescent probes delivered in vivo
LPMI was evaluated using modification of a previously described PI-fluorescent method (Ito et al., 1997; Weinbrenner et al., 2004; Wolff et al., 2000). Fifteen minutes before harvesting, mice were re-anesthetized, intubated and injected with 60 µl PI (5mg/ml) through the retro-orbital sinus to stain cells with LPMI. The chest was re-opened and the LAD re-occluded 1 minute before harvesting. Blue fluorescent microspheres (BFMs, 2 µm, ThermoFisher, PA), 100 µl of a 1% solution (in deionized water with trace amount of surfactant supplied by the vendor), were injected through the aortic arch to delineate non-ischemic tissue. Their absence located ischemic tissue. The heart was harvested, held at −20 °C for 15 minutes to facilitate sectioning, then cut horizontally into four slices, the first at the site of LAD occlusion, the remaining three through the ventricles. Each slice was weighed and imaged on each side under a fluorescent microscope (2x objective lens) using the red fluorescent channel for the PI fluorescent complex, the blue for BFMs.
Weight of tissue with LPMI = (A1 × Wt1) + (A2 × Wt2) + (A3 × Wt3) + (A4 × Wt4),
where A is the percent of the area of a slice exhibiting red fluorescence (average of both sides of a slice) and Wt is the weight of that slice.
Weight of tissue lacking blood flow which is at risk for LPMI (weight of at-risk tissue) = (R1 × Wt1) + (R2 × Wt2) + (R3 × Wt3) + (R4 × Wt4)
where R is percent of the area of a slice lacking blue fluorescence (average of both sides).
The percentage of tissue which is at risk for LPMI and which stains for LPMI (% of at-risk tissue with LPMI) = (weight of tissue with LPMI / weight of at risk tissue) × 100.
Immunohistochemical analysis
Frozen sections were cut from the slices described above, fixed in acetone and stained with an FITC-labeled anti-C3 antibody (Dako, CA). Each section was imaged (2x objective lens) using channel 4 (for all fluorescence) to give total area. The C3 positive area was imaged (10x objective lens) and quantified by Image J software. The percentage of the total area that was C3 positive was determined.
Statistical analysis
All data were expressed as mean ± standard error of the mean (SEM). Levene’s test was used to determine the homogeneity of variances. Statistical significance was assessed by a one-way ANOVA test followed by a post hoc Bonferroni's test (when the equality of variances assumption held) or Dunnett T3 test (when the equality of variances was not met). P<0.05 was considered significant.
Results
LPMI occurred early in myocardial reperfusion
LPMI was studied in 7 groups of WT (C57BL/6) mice (Fig. 1a). Group (i) was sham-operated; groups (ii) - (vii) were subjected to 1 hour of LAD occlusion followed by 0, 1, 2, 3, 6 or 24 hours of reperfusion, respectively (n=5 mice/group/time point, except for the 24 hrs’ group where n=10).
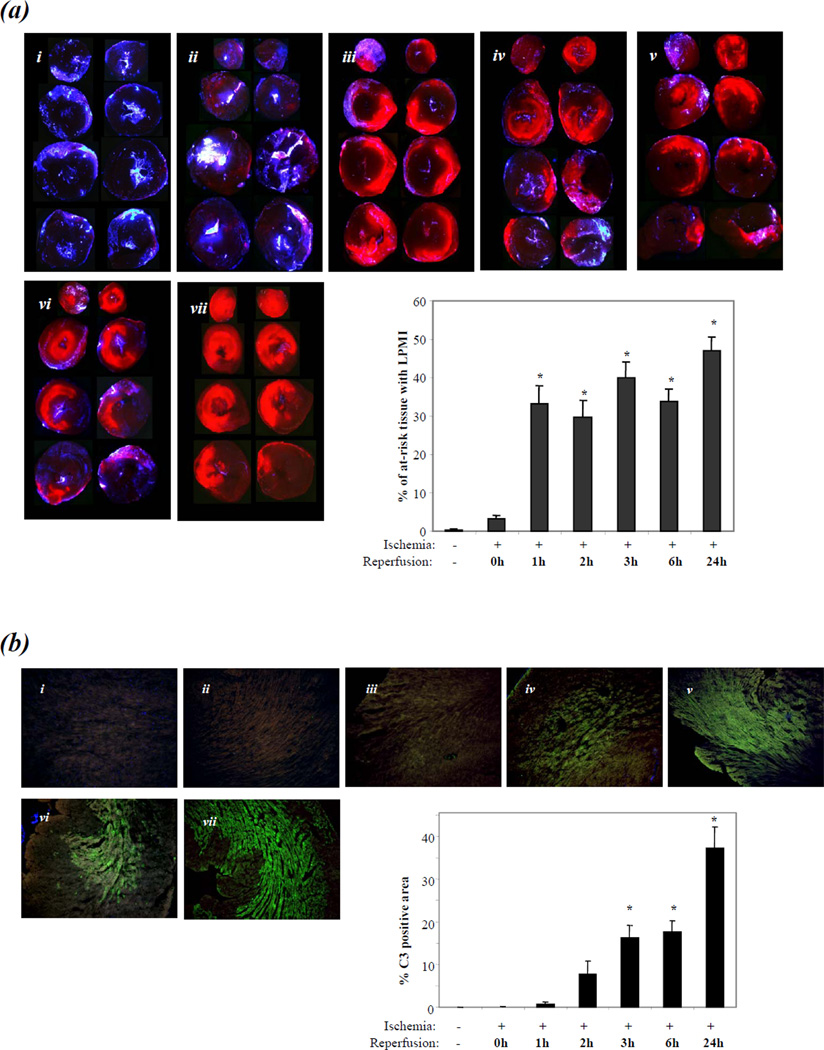
Figure 1. Detection of % of at-risk tissue with LPMI and complement C3 deposition in early myocardial I/R injury.
(a). Panel (i): sham-operated mice. Panels (ii) - (vii): LAD occlusion for 1 hour was followed by reperfusion of 0, 1, 2, 3, 6 and 24 hrs respectively. N=5 mice/group/time point, except for 24 hrs where n=10. Area with LPMI (red fluorescence) was imaged in slices obtained by dividing the ventricles horizontally into four (the top and bottom of a slice are adjacent). Ischemic tissue (tissue at risk for LPMI) was defined by the lack of fluorescence of BFMs. Bar graph: bars indicate % of at-risk tissue with LPMI. Bars represent Means ± SEM (* indicates P<0.05 compared with sham control or 0 hour of reperfusion groups).
(b). Panels (i) - (vii): Cryosections prepared from the heart slices imaged for LPMI in Figure 1(a) were stained with a FITC-tagged anti-C3 antibody. Bar graph: bars indicate the percentage of total area that is C3 positive.
Since the one-way ANOVA test for % of at-risk tissue with LPMI of all groups showed statistical significance (P<0.01), Levene’s test of the homogeneity of variances was applied and indicated P <0.01. Therefore, the Dunnett T3 test was used for post hoc analyses. A small but non-significant % of at-risk tissue with LPMI was detected after 1 hour of ischemia/0 hour of reperfusion compared with the sham-operated group (3±1% versus 0±0%). This increased to a statistically significant level of 33 ± 5% at 1 hour of reperfusion (P<0.05 compared with either the sham-operated or 0 hour of reperfusion groups) and remained elevated during subsequent reperfusion times of 2, 3, 6 and 24 hours (30±4%, 40±4%, 34±3% and 47±4%, respectively; P<0.05 for each result compared with either the sham-operated or 0 hour of reperfusion groups; P>0.05 for comparison of the results obtained at 1, 2, 3, 6 and 24 hours).
Complement C3 deposition lagged the appearance of LPMI in at-risk tissue
To evaluate whether complement-mediated inflammation might be involved in LPMI development, C3 deposition was analyzed in cryosections prepared from the WT heart slices analyzed above (Fig.1b). Since a one-way ANOVA test of C3 deposition with all groups showed statistical significance (P<0.01) and Levene’s test of the homogeneity of variances indicated that P<0.05, the Dunnett T3 test was used for post hoc analyses. C3 deposition became significant at 3 hours of reperfusion (C3 positive area =16±3% of total section area (TSA)) compared with that of sham-operated, or LAD-occluded followed by 0 or 1 hour of reperfusion (0±0 %, 0±0 %, or 1±0 % TSA, respectively, P<0.05). C3 deposition continued to be elevated through 6 and 24 hours (18±3% and 37±5% TSA, respectively, P<0.05 compared with the levels in groups sham-operated, or LAD occluded followed by 0 or 1 hour of reperfusion). While the level at 24 hours is not statistically significant different compared with that at 6 hours, the increase is of interest. Further work is needed to establish whether there is increased inflammation at this time. The C3-significant deposition at 3 hours thus occurs 2 hours after the development of LPMI.
Transgenic mice over-expressing SOD1 or catalase were protected from LPMI at early but not late periods of reperfusion
To investigate the contribution of ROS to LPMI during the early stages of reperfusion injury, we studied transgenic mice over-expressing human SOD1 or catalase in this I/R injury model. SOD1 converts the superoxide anion •O2 −, which is generated excessively during early reperfusion, to H2O2 which is then removed by glutathione peroxidase and catalase (Mates, 2000).
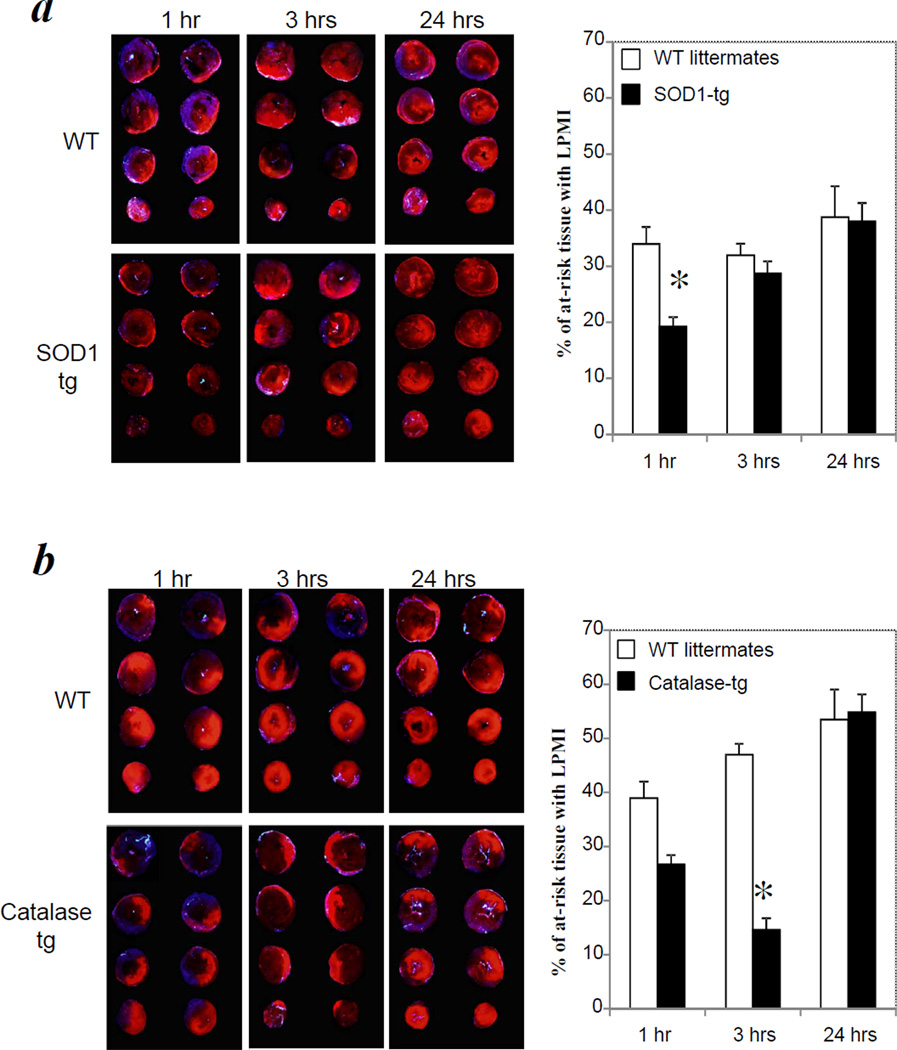
When SOD1 tg mice were subjected to 1 hour of ischemia/1 hour of reperfusion, tissue with LPMI was significantly reduced compared with similarly treated WT littermates (19 ± 2% versus 34 ± 3% of at-risk tissue, P<0.05) (Fig. 2a). In contrast, when SOD1 tg mice were subjected to 1 hour ischemia followed by 3 and 24 hours of reperfusion, the extent of LPMI was not significantly different from that of WT mice.
Figure 2. Transgenic mice over-expressing SOD1 or catalase were protected from LPMI at early but not late periods of reperfusion.
(a). SOD1 tg mice (n = 4/group/time point) and WT littermates (n = 3/group/time point) were subjected to 1 hour of ischemia followed by reperfusion times of 1, 3 or 24 hours. (b). catalase tg mice (n = 5/group/time point) and WT littermates (n=5 /group/time point, except for 24 hrs where n=3) were subjected to 1 hour of ischemia followed by reperfusion times of 1, 3 or 24 hours. LPMI measurement and AAR were determined as described in Figure 1. * indicates P<0.05 compared with WT control at same time point.
When catalase tg mice were subjected to 1 hour of ischemia/1 hour of reperfusion, the LPMI was not significantly reduced compared with WT littermates (27 ± 6% versus 40 ± 2% at-risk tissue, P>0.05) (Fig. 2b). However, at 3 hours of reperfusion, the LPMI was reduced significantly compared with WT controls (15 ± 3% versus 39 ± 2% at-risk tissue, P<0.01). After 24 hours of reperfusion, the LPMI was identical to that of the WT controls. The delayed benefits of over-expressed catalase compared with SOD1 are consistent with its antioxidant action downstream of SOD1.
Discussion
In a murine cardiac I/R model, using in vivo injection of PI to detect LPMI and BFMs to detect ischemic tissue (at risk for necrosis), a significant percent of at-risk tissue with LPMI (33 ± 5%) was detected at the earliest time investigated, 1 hour of reperfusion (Fig.1a). This level of LPMI was maintained through 24 hours’ reperfusion, indicating that the histological boundary of tissue with LPMI was essentially established by the first hour of reperfusion. This is a significant new result, not obtainable using the traditional histochemical approach for identifying necrotic tissue, staining with TTC, which requires at least 3 hours of reperfusion (Birnbaum et al., 1997).
Fluorescent tracking of LPMI with PI permitted examination of the early local inflammatory response in the heart sections stained for LPMI (Fig. 1b). This is the first report in the mouse myocardial I/R injury model demonstrating that C3 deposition becomes significant at 3 hours of reperfusion. A similar result was found for the rat model (Vakeva et al., 1994). The level of C3 deposition was maintained through 6 hours of reperfusion. LPMI was present 2 hours before significant deposition of C3, indicating that mechanisms other than C3 activation lead to the initial development of LPMI.
Transgenic mice over-expressing SOD1 experienced 43% less LPMI than WT littermates at 1 hour of reperfusion but not at later time points (Fig. 2a). Interestingly, catalase tg mice were not protected from LPMI compared with WT littermates at 1 hour of reperfusion (Fig. 2b). However, these mice had significantly less LPMI (69%) than WT littermates at 3 hours of reperfusion. The delayed benefits of over-expressed catalase compared with SOD1 are consistent with its antioxidant action downstream of SOD1. The effect at 3 hours reperfusion was not sustained. At 24 hours of reperfusion, the LPMI of catalase tg mice was identical to that of the WT controls.
The results obtained for tg mice indicate that in WT mice, ROS-initiated mechanisms contribute significantly to LPMI during the first three hours of reperfusion. Specifically, our data suggest that the superoxide anion contributes to the development of LPMI during the first hour of reperfusion, while hydrogen peroxide is important between one and three hours of reperfusion. Since SOD1 and catalase tg mice provided only transient protection against LPMI during early reperfusion, other mechanisms, e.g. complement-mediated inflammation, may contribute to the LPMI after 3 hours of reperfusion.
While many pre-clinical studies have reported a protective role for anti-oxidant enzymes such as SOD1 and catalase in both regional and global heart ischemia, others found that neither enzyme protected against infarction (Supplement Table 1). Careful scrutiny of these studies, however, revealed that the majority with positive results used reperfusion times less than 4 hours, while the majority with negative results used reperfusion times greater than 24 hours. The positive and negative results respectively would be expected from the results of the current study, showing that antioxidant enzymes are effective only in the early stages of reperfusion. Subsequent clinical trials failed to show that infusion of SOD1 improved the recovery of ventricular function in patients with acute myocardial infarction (Flaherty et al., 1994; Murohara et al., 1991). The results of the present study indicate that successful treatment of reperfusion injury needs to target the origins not only of the early but also the late stages of reperfusion injury.
In summary, during development of I/R injury in the murine myocardial model, LPMI is present early, following 1 hour of reperfusion, in approximately 30–45% of the tissue that lacked circulation during ischemia. The extent of at risk tissue with LPMI does not change significantly as reperfusion continues to 24 hours, indicating that the boundary of tissue with LPMI is established at 1 hour of reperfusion. ROS contribute significantly to LPMI during the first 3 hours of reperfusion while complement deposition, significant at 3 hours of reperfusion, may lead to inflammatory reactions that sustain LPMI subsequently.
Supplementary Material
Highlights.
LPMI occurred at 1 hour of myocardial reperfusion and sustained through 24 hours.
Reactive oxygen species contributed to LPMI during the first 3 hours of reperfusion.
Complement deposition was not significant until 3 hours of reperfusion.
Acknowledgements
The authors thank Dr. James Cottrell for continued support and Javi Balroop, Oghomwen Shaka-Idusuyi, EunHee Ko and Danielle Green for technical assistance. We also thank Drs. Arlan Richardson and Holly Van Remmen for providing SOD1 and catalase tg mice. The research was funded in part by NIH grant 1R21HL088527(MZ) and a SUNY-Downstate Dean’s Award (MZ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ambrosio G, Flaherty JT. Effects of the superoxide radical scavenger superoxide dismutase, and of the hydroxyl radical scavenger mannitol, on reperfusion injury in isolated rabbit hearts. Cardiovascular drugs and therapy / sponsored by the International Society of Cardiovascular Pharmacotherapy. 1992;6:623–632. doi: 10.1007/BF00052564. [DOI] [PubMed] [Google Scholar]
- Birnbaum Y, Hale SL, Kloner RA. Differences in reperfusion length following 30 minutes of ischemia in the rabbit influence infarct size, as measured by triphenyltetrazolium chloride staining. Journal of molecular and cellular cardiology. 1997;29:657–666. doi: 10.1006/jmcc.1996.0308. [DOI] [PubMed] [Google Scholar]
- Chen X, Mele J, Giese H, Van Remmen H, Dolle ME, Steinhelper M, Richardson A, Vijg J. A strategy for the ubiquitous overexpression of human catalase and CuZn superoxide dismutase genes in transgenic mice. Mech Ageing Dev. 2003;124:219–227. doi: 10.1016/s0047-6374(02)00161-6. [DOI] [PubMed] [Google Scholar]
- Downey JM, Omar B, Ooiwa H, McCord J. Superoxide dismutase therapy for myocardial ischemia. Free radical research communications. 1991;12–13(Pt 2):703–720. doi: 10.3109/10715769109145850. [DOI] [PubMed] [Google Scholar]
- Flaherty JT, Pitt B, Gruber JW, Heuser RR, Rothbaum DA, Burwell LR, George BS, Kereiakes DJ, Deitchman D, Gustafson N, et al. Recombinant human superoxide dismutase (h-SOD) fails to improve recovery of ventricular function in patients undergoing coronary angioplasty for acute myocardial infarction. Circulation. 1994;89:1982–1991. doi: 10.1161/01.cir.89.5.1982. [DOI] [PubMed] [Google Scholar]
- Gallagher KP, Buda AJ, Pace D, Gerren RA, Shlafer M. Failure of superoxide dismutase and catalase to alter size of infarction in conscious dogs after 3 hours of occlusion followed by reperfusion. Circulation. 1986;73:1065–1076. doi: 10.1161/01.cir.73.5.1065. [DOI] [PubMed] [Google Scholar]
- Gross GJ, Farber NE, Hardman HF, Warltier DC. Beneficial actions of superoxide dismutase and catalase in stunned myocardium of dogs. The American journal of physiology. 1986;250:H372–H377. doi: 10.1152/ajpheart.1986.250.3.H372. [DOI] [PubMed] [Google Scholar]
- Hangaishi M, Nakajima H, Taguchi J, Igarashi R, Hoshino J, Kurokawa K, Kimura S, Nagai R, Ohno M. Lecithinized Cu, Zn-superoxide dismutase limits the infarct size following ischemia-reperfusion injury in rat hearts in vivo. Biochemical and biophysical research communications. 2001;285:1220–1225. doi: 10.1006/bbrc.2001.5319. [DOI] [PubMed] [Google Scholar]
- Ito WD, Schaarschmidt S, Klask R, Hansen S, Schafer HJ, Mathey D, Bhakdi S. Infarct size measurement by triphenyltetrazolium chloride staining versus in vivo injection of propidium iodide. Journal of molecular and cellular cardiology. 1997;29:2169–2175. doi: 10.1006/jmcc.1997.0456. [DOI] [PubMed] [Google Scholar]
- Jones SP, Hoffmeyer MR, Sharp BR, Ho YS, Lefer DJ. Role of intracellular antioxidant enzymes after in vivo myocardial ischemia and reperfusion. American journal of physiology. Heart and circulatory physiology. 2003;284:H277–H282. doi: 10.1152/ajpheart.00236.2002. [DOI] [PubMed] [Google Scholar]
- Kanamasa K, Ishida N, Ishikawa K. Protective effect of PEG-SOD against early coronary reperfusion injury assessed in reperfused and non-reperfused ischaemic areas of the same heart. Acta Cardiol. 2001;56:181–186. doi: 10.2143/AC.56.3.2005638. [DOI] [PubMed] [Google Scholar]
- Klein HH, Pich S, Lindert S, Buchwald A, Nebendahl K, Kreuzer H. Intracoronary superoxide dismutase for the treatment of"reperfusion injury", A blind randomized placebo-controlled trial in ischemic, reperfused porcine hearts. Basic Res Cardiol. 1988;83:141–148. doi: 10.1007/BF01907268. [DOI] [PubMed] [Google Scholar]
- Mates JM. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology. 2000;153:83–104. doi: 10.1016/s0300-483x(00)00306-1. [DOI] [PubMed] [Google Scholar]
- Matsuda M, Fujiwara H, Kawamura A, Ishida M, Takemura G, Kida M, Uegaito T, Fujiwara Y, Fujiwara T, Kawai C. Failure to reduce infarct size by intracoronary infusion of recombinant human superoxide dismutase at reperfusion in the porcine heart: immunohistochemical and histological analysis. Journal of molecular and cellular cardiology. 1991;23:1287–1296. doi: 10.1016/0022-2828(91)90085-z. [DOI] [PubMed] [Google Scholar]
- Murohara Y, Yui Y, Hattori R, Kawai C. Effects of superoxide dismutase on reperfusion arrhythmias and left ventricular function in patients undergoing thrombolysis for anterior wall acute myocardial infarction. Am J Cardiol. 1991;67:765–767. doi: 10.1016/0002-9149(91)90538-v. [DOI] [PubMed] [Google Scholar]
- Nejima J, Knight DR, Fallon JT, Uemura N, Manders WT, Canfield DR, Cohen MV, Vatner SF. Superoxide dismutase reduces reperfusion arrhythmias but fails to salvage regional function or myocardium at risk in conscious dogs. Circulation. 1989;79:143–153. doi: 10.1161/01.cir.79.1.143. [DOI] [PubMed] [Google Scholar]
- Nishikawa Y, Yamamoto S, Ataka K, Nakamura K. The effect of superoxide dismutase and catalase on myocardial reperfusion injury in the isolated rat heart. The Japanese journal of surgery. 1991;21:423–432. doi: 10.1007/BF02470970. [DOI] [PubMed] [Google Scholar]
- Ooiwa H, Stanley A, Felaneous-Bylund AC, Wilborn W, Downey JM. Superoxide dismutase conjugated to polyethylene glycol fails to limit myocardial infarct size after 30 min ischemia followed by 72 h of reperfusion in the rabbit. Journal of molecular and cellular cardiology. 1991;23:119–125. doi: 10.1016/0022-2828(91)90099-8. [DOI] [PubMed] [Google Scholar]
- Otani H, Umemoto M, Kagawa K, Nakamura Y, Omoto K, Tanaka K, Sato T, Nonoyama A, Kagawa T. Protection against oxygen-induced reperfusion injury of the isolated canine heart by superoxide dismutase and catalase. The Journal of surgical research. 1986;41:126–133. doi: 10.1016/0022-4804(86)90017-x. [DOI] [PubMed] [Google Scholar]
- Patel BS, Jeroudi MO, O'Neill PG, Roberts R, Bolli R. Effect of human recombinant superoxide dismutase on canine myocardial infarction. The American journal of physiology. 1990;258:H369–H380. doi: 10.1152/ajpheart.1990.258.2.H369. [DOI] [PubMed] [Google Scholar]
- Przyklenk K, Kloner RA. "Reperfusion injury" by oxygen-derived free radicals? Effect of superoxide dismutase plus catalase, given at the time of reperfusion, on myocardial infarct size, contractile function, coronary microvasculature, and regional myocardial blood flow. Circulation research. 1989;64:86–96. doi: 10.1161/01.res.64.1.86. [DOI] [PubMed] [Google Scholar]
- Richard VJ, Murry CE, Jennings RB, Reimer KA. Therapy to reduce free radicals during early reperfusion does not limit the size of myocardial infarcts caused by 90 minutes of ischemia in dogs. Circulation. 1988;78:473–480. doi: 10.1161/01.cir.78.2.473. [DOI] [PubMed] [Google Scholar]
- Shirato C, Miura T, Ooiwa H, Toyofuku T, Wilborn WH, Downey JM. Tetrazolium artifactually indicates superoxide dismutase-induced salvage in reperfused rabbit heart. Journal of molecular and cellular cardiology. 1989;21:1187–1193. doi: 10.1016/0022-2828(89)90695-0. [DOI] [PubMed] [Google Scholar]
- Uraizee A, Reimer KA, Murry CE, Jennings RB. Failure of superoxide dismutase to limit size of myocardial infarction after 40 minutes of ischemia and 4 days of reperfusion in dogs. Circulation. 1987;75:1237–1248. doi: 10.1161/01.cir.75.6.1237. [DOI] [PubMed] [Google Scholar]
- Vakeva A, Morgan BP, Tikkanen I, Helin K, Laurila P, Meri S. Time course of complement activation and inhibitor expression after ischemic injury of rat myocardium. The American journal of pathology. 1994;144:1357–1368. [PMC free article] [PubMed] [Google Scholar]
- Wang P, Chen H, Qin H, Sankarapandi S, Becher MW, Wong PC, Zweier JL. Overexpression of human copper, zinc-superoxide dismutase (SOD1) prevents postischemic injury. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:4556–4560. doi: 10.1073/pnas.95.8.4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe BI, Premaratne S, Limm W, Mugiishi MM, McNamara JJ. High- and low-dose superoxide dismutase plus catalase does not reduce myocardial infarct size in a subhuman primate model. Am Heart J. 1993;126:840–846. doi: 10.1016/0002-8703(93)90697-8. [DOI] [PubMed] [Google Scholar]
- Weinbrenner C, Schulze F, Sarvary L, Strasser RH. Remote preconditioning by infrarenal aortic occlusion is operative via delta1-opioid receptors and free radicals in vivo in the rat heart. Cardiovascular research. 2004;61:591–599. doi: 10.1016/j.cardiores.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Weisman HF, Bartow T, Leppo MK, Marsh HC, Jr, Carson GR, Concino MF, Boyle MP, Roux KH, Weisfeldt ML, Fearon DT. Soluble human complement receptor type 1: in vivo inhibitor of complement suppressing post-ischemic myocardial inflammation and necrosis. Science. 1990;249:146–151. doi: 10.1126/science.2371562. [DOI] [PubMed] [Google Scholar]
- Wessels MR, Butko P, Ma M, Warren HB, Lage AL, Carroll MC. Studies of group B streptococcal infection in mice deficient in complement component C3 or C4 demonstrate an essential role for complement in both innate and acquired immunity. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:11490–11494. doi: 10.1073/pnas.92.25.11490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff RA, Chien GL, van Winkle DM. Propidium iodide compares favorably with histology and triphenyl tetrazolium chloride in the assessment of experimentally-induced infarct size. Journal of molecular and cellular cardiology. 2000;32:225–232. doi: 10.1006/jmcc.1999.1074. [DOI] [PubMed] [Google Scholar]
- Zhang M, Michael LH, Grosjean SA, Kelly RA, Carroll MC, Entman ML. The role of natural IgM in myocardial ischemia-reperfusion injury. Journal of molecular and cellular cardiology. 2006;41:62–67. doi: 10.1016/j.yjmcc.2006.02.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.