Abstract
Background
Chronic cerebrospinal venous insufficiency (CCSVI) was implicated in the pathophysiology of MS.
Objective
We evaluated neurosonography (NS), magnetic resonance venography (MRV) and transluminal venography (TLV) in subsets of MS patients drawn from a single center, prospective case-control study of 206 MS and 70 non-MS volunteers.
Methods
As previously reported, findings on high resolution B-mode NS imaging with color and spectral Doppler of the extracranial and intracranial venous drainage consistent with CCSVI were similar among MS and non-MS volunteers (3.88% vrs. 7.14%; p=0.266). Ninety-nine MS participants consented to intravascular contrast enhanced 3D MRV to assess their major systemic and intracranial venous circulation, and 40 advanced to TLV that included pressure measurements of the superior vena cava, internal jugular, brachiocephalic, and azygous veins.
Results
NS findings and MRV patterns were discrepant for 26/98 evaluable subjects, including four with abnormal findings on NS that had normal venous anatomy by MRV. In no instance were TLV pressure gradients indicative of clinically significant functional stenosis encountered. The three imaging approaches provided generally consistent data with discrepancies referable to inherent technique properties.
Conclusions
Our findings lend no support for altered venous outflow dynamics as common among MS patients, or likely contribute to the disease process.
Keywords: multiple sclerosis, neurosonography, magnetic resonance venography, venography, cerebral venous outflow, chronic cerebrospinal venous insufficiency
Introduction
MS is generally accepted as an immune-mediated disease triggered by one or more environmental factors, but its precise cause and pathogenesis remain elusive. Chronic cerebrospinal venous insufficiency (CCSVI) was postulated as causally related to MS and disproportionately distributed among clinical MS disease phenotypes.1 Purportedly established by the presence of two or more disordered venous outflow parameters as measured by intra- and extracranial duplex ultrasound,2 CCSVI was originally reported as exclusively associated with the diagnosis of MS and not found in other diseases or normal controls.2, 3 A meta-analysis of subsequent early studies supported the predominance of these findings among subjects with MS compared to those without the disease.4
The frequency of finding evidence of CCSVI by neurosonography (NS) has varied greatly across centers.5 A large single center study found that 56% of patients with MS met ultrasound criteria for CCSVI as did 23% of healthy controls.6 Another noted that while more MS subjects meet criteria for CCSVI than their control subjects, the differences did not reach significance and those MS subjects with and without CCSVI did not clinically differ.7 Other investigators have not found CCSVI,8–10 nor established a cause-effect relationship between CCSVI and clinically isolated syndrome (CIS),11, 12 pediatric onset MS,13 or progressive MS.14 Others who observed CCSVI suggested that it was an age-related secondary phenomena.15 Using magnetic resonance venography (MRV) some have found low rates of obstruction,16 comparable rates in other neurological diseases (OND) or normals,17–20 or were unable to specifically relate their findings to MS.21
In a prospectively acquired series of MS, other neurological disease, and normal volunteers whose cerebral venous drainage systems were studied in blinded fashion with NS using high resolution B-mode imaging with color and spectral Doppler, we found that CCSVI as originally defined was present in only 7.14% of non-MS and 3.88% of MS patients without differences between MS and non-MS subjects for extracranial or intracranial venous flow rates.22 Neither apnea nor Valsalva-induced reflux was detected in the deep intracerebral veins in any subject. Here we compare NS imaging with two other approaches to image the venous drainage system of the brain relevant to the concept of CCSVI, dynamic contrast enhanced magnetic resonance venography (CE-MRV) and transluminal venography (TLV) in a subset of those MS subjects. These operator masked studies were designed to learn to what extent the findings on NS are supported by the other imaging modalities, and to learn the relative merits of CE-MRV of the head, neck, chest, abdomen and pelvis and TLV in the evaluation of the structure and function of the venous anatomy relevant to the CCSVI hypothesis.
Subjects and methods
The design of this study, including recruitment goals, NS methodology and assuring blinding has been detailed.22 In brief, this was a single center, prospective, case-control study that enrolled MS and non-MS volunteers at The University of Texas Health Science Center at Houston. Separate forms were designed to allow subjects to consent first to the concept of the study and the NS evaluation, and then for each subject to reconsent to participate in a subsequent testing stage if invited. Invitations were based on each volunteer’s NS results, the evolving results in the assembled cohort of subjects, and the need to have examples of subjects with and without demonstrated abnormalities at each subsequent level of investigation; only one of the authors (JSW) had access to this information. The original plan was to invite both MS and non-MS subjects to move forward to CE-MRV; only MS subjects would be considered for TLV due to the procedure’s increased inconvenience and possible risk. Given the evolving distribution of findings on NS, selection of subjects for CE-MRV focused to MS volunteers. The selection process was not discussed with any other team members, but was presented to and agreed on by the local executive committee and those at the National Multiple Sclerosis Society overseeing the project. The study was approved by our institution’s Committee for the Protection of Human Subjects.
Imaging: NS
NS was performed and interpreted as previously detailed without knowledge of the subject’s diagnosis.22
Imaging: CE-MRV
While a number of non-contrast MRV techniques and CE-MRV with extracellular gadolinium contrast materials approaches are available,23 but extensive mapping of the venous system of the relevant intracranial and extracranial venous drainage system of the brain and spinal cord remains challenging. Therefore, we adapted the intravascular agent gadofosveset trisodium for dynamic CE-MRV as a potentially better imaging solution in the venous system. Gadofosveset trisodium (Ablavar®, Lantheus Medical Imaging, N. Billerica, MA) has the advantage of a prolonged intravascular half-life due to serum albumin binding. The current approved use of this contrast agent is for peripheral arterial runoff examinations.
Subjects were evaluated supine in a 3T Philips Intera scanner capable of producing maximum gradient amplitude of 80 mT/m. Images were acquired using various combinations of the neurovascular 16 channel and spine 15 channel SENSE coils. Three specific sequences were acquired; a non-contrast 2D time-of-flight (TOF) fast field echo (FFE) acquisition covering the head and neck, a dynamic contrast 3D T1 acquisition covering the skull base to the upper chest, and delayed contrast 3D T1 FFE acquisition covering the chest, abdomen, pelvis and upper neck. The contrast enhanced acquisitions used a single intravenous injection of 0.03 mmol/kg gadofosveset trisodium. A set of precontrast images were obtained to allow background subtraction to be performed on neck, chest, abdomen and pelvis at the operators console to improve recognition and interpretation of the venous structures in these anatomical regions. MRV was performed by an experienced MR technologist unaware of the subject’s diagnosis. The images were transferred without subject identifiers and stored on a password secured server. The images were subsequently reviewed by one of the authors (LAK) unaware of the subject’s diagnosis, demographics or the interpretation of other investigations. Data entry screens constructed before the project’s initiation expected entries as to whether the CE-MRI was consistent with a normal venogram, or fit into one of the four patterns of venous stenosis detailed by Zamboni and colleagues (see supplemental Figure 1 for an example).3 The presence or absence of valves in the internal jugular system was sought, and a comment field was available to describe any critical or unusual findings.
Imaging: TLV
The Seldinger technique was used to access the left or right common femoral vein using a 0.035” guide wire to place a 6 Fr sheath. Catheters of appropriate shape were used to select the iliac veins, superior vena cava, left and right internal jugular veins (IJV) and azygous vein. Isosmolar iodixanol (Visipaque™, GE Healthcare, Inc., Princeton, NJ) was injected through the catheters and digital subtraction angiography (DSA) runs obtained; two views were routinely acquired in different projections for the IJ and azygous veins. Pressure measurements were made through the same catheter; a 5 Fr catheter was used for the azygous. Subjects were evaluated supine. All TLV was performed by one of the authors (AMC) with no access to patient identifiers or the interpretation of other imaging modalities. Fluoroscopic images were transferred in DICOM format without subject identifiers and stored on a password secured server. The images were subsequently re-reviewed by AMC for systematic evaluation and recording of visual impressions and all pressure measurements made during the session using a previously constructed formal data entry screen (see supplemental Figure 2 for an example).
Only after the interpretation of all NS, CE-MRV and TLV were completed, all queries of the data made, and the database locked, were any discussions of the results allowed among the experts at the level of individual subjects. This was done to preserve the blinded and independent evaluation of each test.
Statistical approaches
For comparing demographic and clinical characteristics between the study groups, t tests, Fisher exact tests, and χ2 tests were used. Contingency tests were performed for intra-observer reliability and agreement assessments within and across testing modalities. Statistical analyses were performed using JMP® software (version 10.0.2, SAS Institute Inc., Cary, NC).
Results
The study recruited 276 adult volunteers (206 with MS, and 70 non-MS). The NS results on the entire study population were detailed previously.22 Of the 206 MS subjects that completed NS, all but one was invited to proceed to CE-MRV; 73 refused, 28 were invited but failed to respond, five agreed but could not be scheduled prior to the end of the study, and 99 consented to the procedure. Contrast extravasated during injection in one subject resulting in 98 subjects with CE-MRV sessions valid for interpretation. Of the 99 subjects that attempted CE-MRV, seven were not invited to undergo TLV (not living in reasonable proximity to Houston, excessively immobile, poor venous access, known allergy to iodine based contrast agents), 48 refused, two were invited but failed to respond, one agreed but could not be scheduled prior to the end of the study, and 41 consented. A vasovagal response with bradyarrythmia precluded completion of TLV in one subject. The characteristics of the MS subjects that successfully completed each of the imaging modalities are provided in Table 1.
Table 1.
Demographic data of the participant subgroups at the time of ultrasound.
| NS | NS and MRV | NS and MRV and TLV |
|
|---|---|---|---|
| All MS Subjects | 206 | 98 | 40 |
| Age in years1 | 48.2 ± 9.9 | 47.6 ± 9.9 | 44.5 ± 11.2 |
| % female | 71.4 | 69.4 | 67.5 |
| Duration of Symptoms1 | 13.7 ± 10.0 | 14.2 ± 10.3 | 10.8 ± 10.1 |
| Duration of MS Diagnosis2 |
9.9 ± 7.8 | 9.9 ± 8.2 | 7.6 ± 8.1 |
| % on DMT | 79.7 | 80.6 | 82.5 |
| EDSS at NS3 | 2.6 ± 2.0 2 (0 – 7) |
2.3 ± 1.8 2 (0 – 6.5) |
2.1 ± 1.5 2 (0 – 6.5) |
| CIS | 12 | 6 | 3 |
| RRMS | 128 | 62 | 29 |
| SPMS | 48 | 23 | 8 |
| PRMS | 3 | 2 | 0 |
| PPMS | 15 | 5 | 0 |
| Interval NS to MRV4 | - | 219 ± 143 | - |
| Interval MRV to TLV5 | - | - | 136 ± 109 |
Abbreviations used in order of appearance in Table: NS = neurosonography, MRV = magnetic resonance venography, TLV = transluminal venography, DMT = disease modifying treatment, EDSS = expanded disability status scale, CIS = clinically isolated syndrome, RR = relapsing remitting, SP = secondary progressive, PR = progressive relapsing, PP = primary progressive
in years ± standard deviation at time of consent for NS
in years ± standard deviation at time of consent for NS; excludes CIS group
mean ± standard deviation; median (range)
in days ± standard deviation from performance of NS to completion of MRV
in days ± standard deviation from performance of MRV to completion of TLV
The protocol that we adapted for visualization of the major venous drainage of the brain and spinal cord by CE-MRV provided high quality images for interpretation (Figure 1). Of the 98 MS volunteers with evaluable CE-MRV, the venous draining patterns of the internal jugular veins (IJVs), brachiocephalic veins, azygous vein and superior and inferior vena cava were normal in 75, but ‘atypical’ in 23. The type A pattern of venous stenosis (significant stenosis or obstruction of the proximal azygous and a closed stenosis of one IJV, but a contralateral IJV with ample cross-sectional area) was seen in two (Figure 2), and the type C pattern (bilateral stenosis of both IJVs with a normal azygous system) was found in 21 MS subjects (Figure 3). In the majority of cases with 50% or greater regional stenosis of the IJVs this was located quite high, within 2 – 3 cm of the base of the skull and above the angle of the jaw. This proximal, usually bilateral narrowing of the IJV likely reflects compression of the vein between the posterior belly of the digastric muscle and digastric tubercle of the transverse process of the first cervical vertebral body. In our experience similarpatterns of high IJV stenosis are seen in ~15% of a small series of non-MS subjects including many of the healthy controls recruited for this study (data not shown), and also is considered by others to be a common finding.24
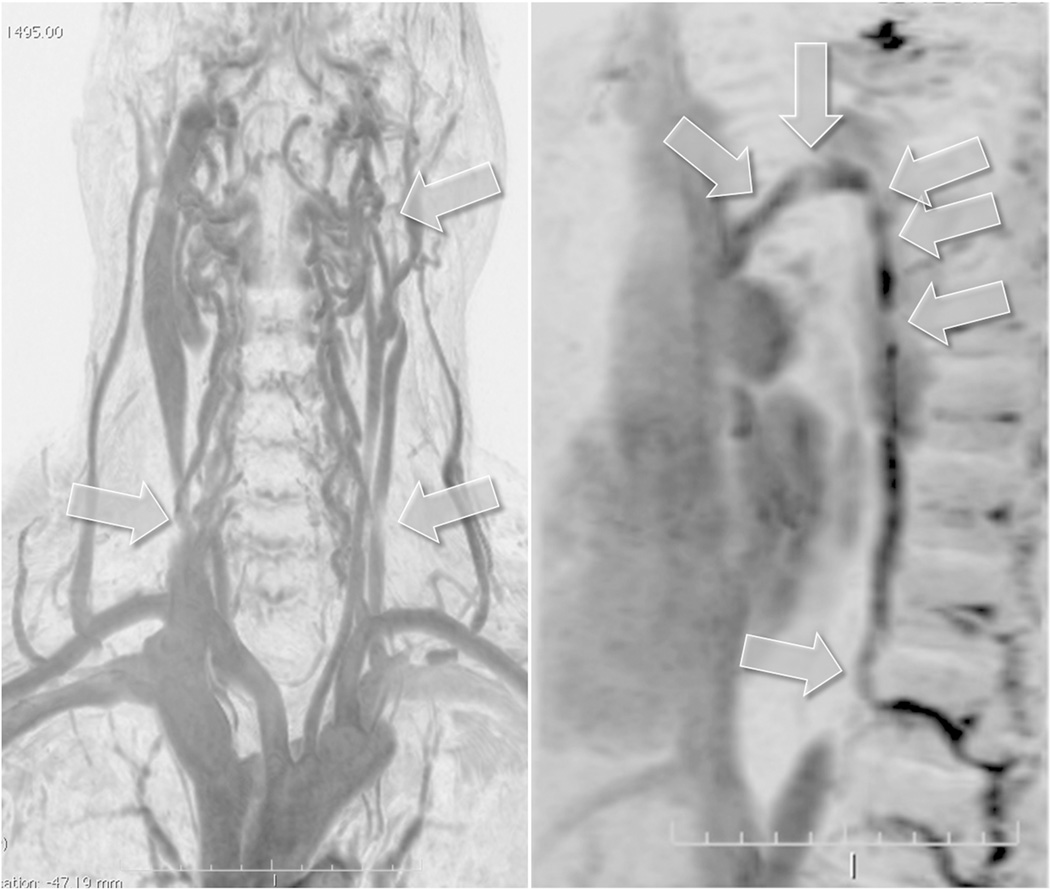
Figure 1.
Montage of representative contrast enhanced magnetic resonance venography (MRV) images obtained by 3D fast field echo sequence and displayed as maximum intensity projection inverted images. The upper left hand panel is a coronal plane through the neck and upper thorax. The lower left hand panel is a sagittal plane through the upper thorax. The right hand spliced panel shows images that span the thorax, abdominal and upper thigh regions. Labeled arrows point to the azygous vein, external and internal jugular veins (EJV, IJV), brachiocephalic vein (BCV), superior and inferior vena cava (SVC, IVC) among other vascular structures readily appreciated in these images.
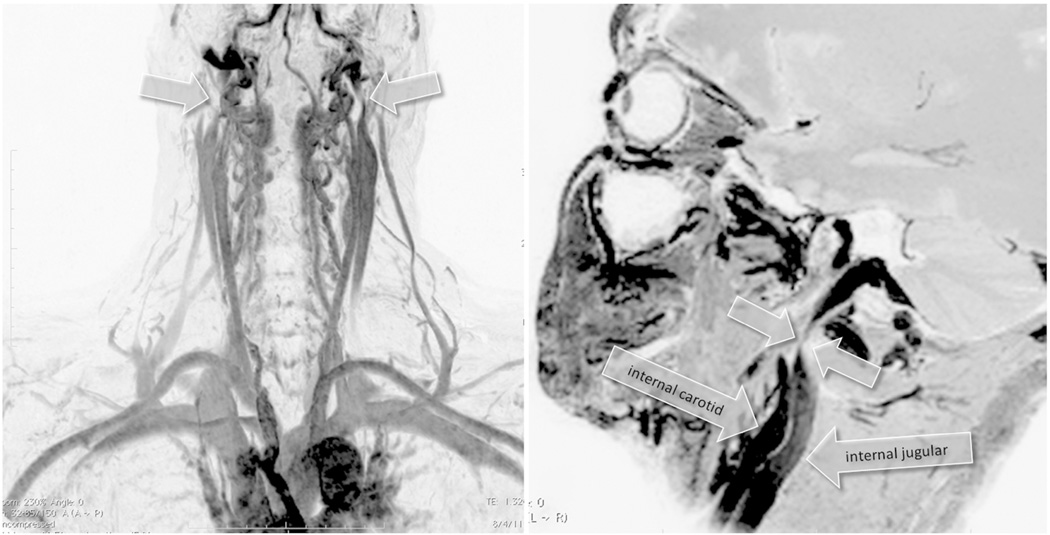
Figure 2.
Example of a Type A pattern as characterized by a stenosis or obstruction of the proximal azygous vein associated with a closed stenosis of one of the two internal jugular veins (IJVs), but with a compensatory contralateral IJV with apparent ample cross-sectional area. Both panels show subtraction masked, maximum intensity projection and inverted images from a 56 year old man with primary progressive multiple sclerosis that were obtained with dynamic contrast enhanced 3D fast field echo sequences. Arrows on the left hand panel point to a proximal left IJV stenosis and distal left and right IJV compression by sternocleidomastoid muscle that are not considered as hemodynamically significant. The right IJV is the dominant vessel with ample cross-sectional area. Arrows on the right hand panel point to multiple regions of narrowing of the azygous vein.
Figure 3.
Example of a Type C pattern characterized by stenosis of both internal jugular veins (IJVs) with a normal azygous system (the latter not shown). The two panels show subtraction masked, maximum intensity projection and inverted images obtained with dynamic contrast enhanced 3D fast field echo sequences. Arrows on the left hand panel point to bilateral proximal IJV stenosis. Also note concordant bilateral distal IJV compression by the sternocleidomastoid muscles. The proximal stenosis is detailed in the sagittal image (upper arrows) on the right hand panel. This pattern of very high IJV stenosis was the most frequent reason for discrepant NS and MRV findings as the stenosis could not be insonated. This finding was present at similar frequency in normal volunteers. Proximal narrowing of the IJV at this site is likely related to IJV compression between the posterior belly of the digastric muscle and digastric tubercle of the transverse process of the C1 vertebra.
We first correlated the findings on NS categorized by the scoring system as originally described by Zamboni and colleagues,2 with the patterns encountered on these same subjects by CE-MRV (Table 2). A score on NS of 2 or more is deemed consistent with CCSVI; none of our subjects had a score above 2. Nor were any subjects encountered with Type B or D patterns by CE-MRV. Neither NS nor CE-MRV was indicative of CCSVI for 71of the 98 subjects. NS findings and MRV patterns were discrepant for 26 MS subjects including those with abnormal findings on NS that were considered to have normal venous anatomy by CE-MRV (see Table 2). The overall agreement between the two tests superficially appeared poor (degree of agreement Kappa coefficient (κ) = −0.014, confidence interval (CI) −0.15 to 0.12; McNemar’s symmetry of disagreement χ2 = 12.5, p = 0.0004). This lead to group discussions following the database lock of the details of the imaging results for the 26 subjects to understand better the reasons, if any, for the discordant findings.
Table II.
Distribution of MS subjects based on neurosonography (NS) score and by pattern of stenosis on dynamic contrast magnetic resonance venography (MRV)
| Pattern on dynamic contrast enhanced MRV |
Zamboni Score by NS | |||
| 0 | 1 | 2 | ||
| Normal | 50 | 21 | 4 | |
| Type A | 2 | 0 | 0 | |
| Type C | 10 | 10 | 1 | |
The major source of disagreement between NS and CE-MRV was in large part related to fundamental differences in the characteristics of the two imaging approaches. The field of view for NS is more restricted for evaluation of the venous system than is CE-MRV. Neither the azygous vein nor the high proximal portion of the IJV above the angle of the jaw and at the base of the skull can be insonated by the approach used. This accounted for the two subjects with a Type A pattern and all ten subjects with the Type C pattern by CE-MRV, but normal findings by NS (Table 2). Of the ten subjects with the Type C pattern on CE-MRV and a score of 1 by NS, four subjects had stenosis in the high proximal portion of the IJV and another four had stenosis in its distal intrathoracic portion which is also not insonated well; the NS score of 1 was the result of a region of stenosis in the IJV noted to be stenotic on both NS and CE-MRV. In two instances, the basis for disagreement between techniques was not readily evident. The four subjects with a score of 2 by NS but normal patterns by CE-MRV related to limitations of MRV to evaluate venous reflux utilized in the criteria for CCSVI.
Conventional TLV was performed on the 40 MS subject subcohort; all had prior evaluations by both NS and MRV. The azygous was accessible in 39 subjects, with minimal narrowing noted in only one subject; there was no measurable pressure gradient across the region of narrowing. Valves in the azygous were encountered in 64% of the subjects; a 1 mm Hg pressure gradient was measured across the valve in five subjects, otherwise no pressure gradients were detected. No webs were visualized in the azygous of 39 subjects. One of the two subjects classified as Type A pattern was also studied by TLV and was among the subjects with azygous vein valves. Of 40 pairs of brachiocephalic veins visualized by TLV, 40% stenosis was encountered in a single right brachiocephalic vein associated with a 2 mm Hg pressure gradient recorded across the region of narrowing; no valves or webs were encountered in these veins. Just over half (55%) of the IJVs studied showed some stenosis that varied from <10% to 90%. Twenty two of the 44 IJVs with any stenosis seen on TLV showed no measurable gradient across the region of stenosis; a single IJV showed a 2 mm Hg gradient. Valves were encountered in nearly all IJVs (74 of 80), but only three were associated with a pressure gradient at 2 mm Hg. A single right IJV had a region of about 80% stenosis above a valve that could be called a web. Thus, despite rating stenosis as present to levels below 10% narrowing, very few of the regions of stenosis, valves or possible webs were associated with a regional pressure gradient as high as 2 mm Hg, and even this magnitude of pressure gradient is not considered indicative of functional stenosis of clinical significance.25
Ratings schema used for the presence or absence of stenosis differed during the independent and masked evaluations of the CE-MRV and TLV, and the venous anatomy visualized by CE-MRV was more extensive than that interrogated by TLV. To harmonize the assessments for comparative purposes, CE-MRV from the 40 subjects who also had TLV were masked and separately reanalyzed by two experienced readers using an ordinal grading system with grade 0 = <50% stenosis, grade 1 = 50% to <75% stenosis, grade 2 = 75% to 99% stenosis, and grade 3 = 100% stenosis; classifications were restricted to levels of the IJV typically studied by TLV and the extent of stenosis reclassified accordingly. Inter-rater correlations for the 195 evaluable veins visualized by CE-MRV were excellent (κ = 0.859, CI 0.731 – 0.986). Inter-test correlations for the 195 evaluable veins visualized by both CE-MRV and TLV were fair (κ = 0.255, CI 0.039 – 0.471). Differences arose exclusively in the IJVs where estimates of stenosis were substantially higher using TLV.
Discussion
Different approaches have been taken by several groups of investigators to explore the postulate that impaired venous outflow from the central nervous system might be causally or secondarily related to the pathogenesis of MS.26 These have included the use of several intrinsically different techniques that variably emphasize functional and structural imaging of the major intracranial and distal venous drainage system; each with its own strengths and limitations. NS has the advantage of non-invasive measurements with the subject in different postures and under different physiologic stresses,27 but has limited access to select proximal and intrathoracic components of the venous drainage system. MRV with or without conventional contrast enhancement of the vasculature provides excellent access to the intracranial and proximal venous drainage system of the brain,28 but due to the irregular architecture of the venous system is not ideal for time of flight visualization below the neck. TLV, while considered a ‘gold standard’, is invasive, and flow artifacts can be induced related to catheter placement and vascular spasm. In this study, we compared all three approaches in a subcohort of MS subjects that participated in a larger study designed to determine the frequency of venous outflow abnormalities seen by NS that are considered to be required for a diagnosis of putative CCSVI. Our approach to MRV was altered to reduce some of possible limitations of the technique through the use of a long lived intravascular contrast agent, 3D image acquisition and post-acquisition image processing and assessment approaches.
As previously detailed, the prevalence of findings on NS among our overall study cohort provided no evidence to suggest that venous outflow patterns from the brain differ between the MS and non-MS subjects, nor were those alterations found to be common. In our hands, both CE-MRV and TLV had advantages for accessing certain regions of the venous system that are poorly or not insonated by ultrasound such as poor temporal bone windows and the azygous system. Both CE-MRV and NS provided inherently different and complementary types of information, with generally congruent results. Dynamic CE-MRV may be preferable to TLV in screening for venous abnormalities because it evaluates the most proximal extent of the IJVs and is less invasive. Most important, our findings on NS, MRV and TLV provide little support for CCSVI as a tenable concept to explain the pathogenesis of MS. Moreover, based on our experience, the use of neurosonology as a screening tool for selecting CCSVI candidates for experimental interventional studies will yield few candidates with patterns consistent with postulated impairment of venous outflow.
Supplementary Material
Acknowledgements
We are indebted to Drs. Susan D. John and James C. Grotta for their service on the project’s local executive committee.
Funding
The study was supported by the National Multiple Sclerosis Society (RC 1019-A-5), with limited personnel support provided through our local Center for Clinical and Translational Sciences funded by the National Center for Research Resources (UL1 RR024148). The content of the paper is solely the responsibility of the authors and does not necessarily represent the official views of the NMSS, NCRR or the NIH. The study sponsors had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding and contributing authors had full access to all the data in the study following database lock and responsibility for the decision to submit for publication.
Footnotes
Authors’ contributions
Dr. Wolinsky served as the overall study principal investigator, was responsible for the study design, securing peer-reviewed support of the investigation, provided semi-annual updates of progress to the local scientific review board, institutional review board and funding agency. He managed the centralized, restricted access subject database and had sole access to the evolving results and unmasked subject demographics. Dr. Brod served as the main study coinvestigator, was responsible for overall recruitment of volunteer healthy controls, subjects with other neurological diseases and the multiple sclerosis volunteers. Dr. Bui performed all of the ultrasound studies without knowledge of the subjects medical condition and provided her images and measurements to the study coordinator for deposit in coded fashion on a password protected, limited access server for storage and subsequent evaluation. Ultrasound data and images were interpreted by Dr. Barreto without any contact with the subject or knowledge of the subject's demographics. Magnetic resonance venography (MRV) was performed in our research imaging center under the direction of Dr. Narayana, and the de-identified images transferred under code to the protected server. The MRV were subsequently reviewed by Dr. Kramer for a structured interpretation performed without access to subject identifiers and then entered into a protected database. Transluminal venography and pressure measurements were performed by Dr. Cohen without access to subject identifiers, and the de-identified images transferred under code to the protected server, and structured interpretation and measurements entered into a protected database. Only after all interpretations of all procedures on all volunteers been completed and the database finalized and locked, were Drs. Barreto, Kramer and Cohen allowed to discuss their results during sessions with the entire team. Mr. Jemelka served as the study coordinator to schedule visits and interact with the subjects. Ms. Ton was the programmer that created the password protected data entry system, monitored the database and ensured that the data on the protected server was only read accessible by those interpreting their specific procedures. All authors had input into the development and various revisions of the manuscript based on their relative expertise and all reviewed the final document.
Conflicts of interest
None of the investigators have any financial interests in the outcome of the study. During the course of the study the investigators received support for other, unrelated activities. ADB: Research support from the National Institutes of Health, and National MS Society. SAB: Consulting agreements or speaker for Acorda, Avanir, Bayer HealthCare, EMD Serono, Genzyme, Pfizer, Questcor, Teva Neurosciences and research or contractual support from the Clayton Foundation for Research, EMD Serono, Pfizer and Questcor. TB & KT: Nothing to declare. JJ: Research support from the National MS Society. LAK: Research or contractual support from the National Institute of Drug Abuse and National MS Society. AMC: Consulting agreement for Medcomp, co-medical director of I-Tomography. JWL: Consulting agreements or speaking for Biogen, EMD Serono, Pfizer, Teva, and research or contractual support from the Clayton Foundation for Research, Teva, National MS Society, and National Institutes of Health. FN: Consulting or speaker for Bayer HealthCare, Biogen Idec, EMD Serono, National MS Society, MS Association of America, Novartis, Sanofi, Teva Neurosciences, and the University of Massachusetts Medical School and research or contractual support from the National Institutes of Health, Novartis and Sanofi-Aventis. PAN: Consulting agreement with Acorda and research or contractual support from the Department of Defense, National Institutes of Health, and Sanofi-Aventis. JSW: Consulting agreements or speaking for Astellas, Bayer HealthCare, Celgene, Consortium of MS Clinics, Eli Lilly, Hoffman LaRoche, Medscape CME, Novartis, sanofi-aventis, Serono Symposia International Foundation, Texas Neurological Society, Teva and Teva Neurosciences, royalties from Millipore [Chemicon International] Corporation, and research or contractual support from the Clayton Foundation for Research, National Institutes of Health, National MS Society and Sanofi.
References
- 1.Zamboni P, Menegatti E, Bartolomei I, et al. Intracranial venous haemodynamics in multiple sclerosis. Curr Neurovasc Res. 2007;4:252–258. doi: 10.2174/156720207782446298. [DOI] [PubMed] [Google Scholar]
- 2.Zamboni P, Menegatti E, Galeotti R, et al. The value of cerebral Doppler venous haemodynamics in the assessment of multiple sclerosis. J Neurol Sci. 2009;282:21–27. doi: 10.1016/j.jns.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 3.Zamboni P, Galeotti R, Menegatti E, et al. Chronic cerebrospinal venous insufficiency in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry. 2009;80:392–399. doi: 10.1136/jnnp.2008.157164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laupacis A, Lillie E, Dueck A, et al. Association between chronic cerebrospinal venous insufficiency and multiple sclerosis: a meta-analysis. CMAJ. 2011;183:E1203–E1212. doi: 10.1503/cmaj.111074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bastianello S, Romani A, Viselner G, et al. Chronic cerebrospinal venous insufficiency in multiple sclerosis: clinical correlates from a multicentre study. BMC Neurol. 2011;11:132. doi: 10.1186/1471-2377-11-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zivadinov R, Marr K, Cutter G, et al. Prevalence, sensitivity, and specificity of chronic cerebrospinal venous insufficiency in MS. Neurology. 2011;77:138–144. doi: 10.1212/WNL.0b013e318212a901. [DOI] [PubMed] [Google Scholar]
- 7.Centonze D, Floris R, Stefanini M, et al. Proposed chronic cerebrospinal venous insufficiency criteria do not predict multiple sclerosis risk or severity. Ann Neurol. 2011;70:52–59. doi: 10.1002/ana.22436. [DOI] [PubMed] [Google Scholar]
- 8.Doepp F, Paul F, Valdueza JM, Schmierer K, Schreiber SJ. No cerebrocervical venous congestion in patients with multiple sclerosis. Ann Neurol. 2010;68:173–183. doi: 10.1002/ana.22085. [DOI] [PubMed] [Google Scholar]
- 9.Tsivgoulis G, Mantatzis M, Bogiatzi C, et al. Extracranial venous hemodynamics in multiple sclerosis. Neurology. 2011;77:1241–1245. doi: 10.1212/WNL.0b013e318230a149. [DOI] [PubMed] [Google Scholar]
- 10.Auriel E, Karni A, Bornstein NM, Nissel T, Gadoth A, Hallevi H. Extra-cranial venous flow in patients with multiple sclerosis. J Neurol Sci. 2011;309:102–104. doi: 10.1016/j.jns.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Baracchini C, Perini P, Calabrese M, Causin F, Rinaldi F, Gallo P. No evidence of chronic cerebrospinal venous insufficiency at multiple sclerosis onset. Ann Neurol. 2011;69:90–99. doi: 10.1002/ana.22228. [DOI] [PubMed] [Google Scholar]
- 12.Chambers B, Chambers J, Cameron H, Macdonell R. Chronic cerebrospinal venous insufficiency is not more prevalent in patients with mild multiple sclerosis: a sonographer-blinded, case-control ultrasound study. Multiple Sclerosis Journal. 2012 doi: 10.1177/1352458512459986. in press. [DOI] [PubMed] [Google Scholar]
- 13.Amato MP, Saia V, Hakiki B, et al. No association between chronic cerebrospinal venous insufficiency and pediatric-onset multiple sclerosis. Mult Scler. 2012;18:1791–1796. doi: 10.1177/1352458512445943. [DOI] [PubMed] [Google Scholar]
- 14.Baracchini C, Perini P, Causin F, Calabrese M, Rinaldi F, Gallo P. Progressive multiple sclerosis is not associated with chronic cerebrospinal venous insufficiency. Neurology. 2011;77:844–850. doi: 10.1212/WNL.0b013e31822c6208. [DOI] [PubMed] [Google Scholar]
- 15.Van den Berg PJ, Van den Berg GB, Westerhuis LW, Visser LH. Occurrence of CCSVI in patients with MS and its relationship with iron metabolism and varicose veins. Eur J Neurol. 2013;20:649–660. doi: 10.1111/ene.12010. [DOI] [PubMed] [Google Scholar]
- 16.Doepp F, Würfel JT, Pfueller CF, et al. Venous drainage in multiple sclerosis: A combined MRI and ultrasound study. Neurology. 2011;77:1745–1751. doi: 10.1212/WNL.0b013e318236f0ea. [DOI] [PubMed] [Google Scholar]
- 17.Ertl-Wagner B, Koerte I, Kümpfel T, et al. Non-specific alterations of craniocervical venous drainage in multiple sclerosis revealed by cardiac-gated phase-contrast MRI. Multiple Sclerosis Journal. 2012;18:1000–1007. doi: 10.1177/1352458511432742. [DOI] [PubMed] [Google Scholar]
- 18.Sundstrom P, Wahlin A, Ambarki K, Birgander R, Eklund A, Malm J. Venous and cerebrospinal fluid flow in multiple sclerosis: A case-control study. Ann Neurol. 2010;68:255–259. doi: 10.1002/ana.22132. [DOI] [PubMed] [Google Scholar]
- 19.Wattjes MP, van Oosten BW, de Graaf WL, et al. No association of abnormal cranial venous drainage with multiple sclerosis: a magnetic resonance venography and flow-quantification study. J Neurol Neurosurg Psychiatry. 2011;82:429–435. doi: 10.1136/jnnp.2010.223479. [DOI] [PubMed] [Google Scholar]
- 20.Blinkenberg M, Åkeson P, Sillesen H, et al. Chronic cerebrospinal venous insufficiency and venous stenoses in multiple sclerosis. Acta Neurol Scand. 2012;126:421–427. doi: 10.1111/j.1600-0404.2012.01671.x. [DOI] [PubMed] [Google Scholar]
- 21.Garaci FG, Marziali S, Meschini A, et al. Brain hemodynamic changes associated with chronic cerebrospinal venous insufficiency are not specific to multiple sclerosis and do not increase its severity. Radiology. 2012;265:233–239. doi: 10.1148/radiol.12112245. [DOI] [PubMed] [Google Scholar]
- 22.Barreto AD, Brod SA, Bui T-T, et al. Chronic cerebrospinal venous insufficiency: case-control neurosonography results. Ann Neurol. 2013 doi: 10.1002/ana.23839. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirchhof K, Welzel T, Jansen O, Sartor K. More reliable noninvasive visualization of the cerebral veins and dural sinuses: comparison of three MR angiographic techniques. Radiology. 2002;224:804–810. doi: 10.1148/radiol.2243011019. [DOI] [PubMed] [Google Scholar]
- 24.Zivadinov R, Galeotti R, Hojnacki D, et al. Value of MR venography for detection of internal jugular vein anomalies in multiple sclerosis: a pilot longitudinal study. AJNR Am J Neuroradiol. 2011;32:938–946. doi: 10.3174/ajnr.A2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Labropoulos N, Borge M, Pierce K, Pappas PJ. Criteria for defining significant central vein stenosis with duplex ultrasound. J Vasc Surg. 2007;46:101–107. doi: 10.1016/j.jvs.2007.02.062. [DOI] [PubMed] [Google Scholar]
- 26.Mancini M, Morra VB, Di Donato O, et al. Multiple sclerosis: cerebral circulation time. Radiology. 2012;262:947–955. doi: 10.1148/radiol.11111239. [DOI] [PubMed] [Google Scholar]
- 27.McDonald S, Iceton JB. The use of Doppler ultrasound in the diagnosis of chronic cerebrospinal venous insufficiency. Tech Vasc Interv Radiol. 2012;15:113–120. doi: 10.1053/j.tvir.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Utriainen D, Feng W, Elias S, Latif Z, Hubbard D, Haacke EM. Using magnetic resonance imaging as a means to study chronic cerebral spinal venous insufficiency in multiple sclerosis patients. Tech Vasc Interv Radiol. 2012;15:101–112. doi: 10.1053/j.tvir.2012.02.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.