Abstract
Longitudinal analyses allow us to understand how genetic risk unfolds across development, in a way that is not possible with cross-sectional analyses of individuals at different ages. This has received little attention in genetic association analyses. In this study, we test for genetic effects of GABRA2, a gene previously associated with alcohol dependence, on trajectories of drunkenness from age 14 to 25. We use data from 1070 individuals who participated in the prospective sample of the Collaborative Study on the Genetics of Alcoholism (COGA), in order to better understand the unfolding of genetic risk across development. Piecewise linear growth models were fit to model the influence of genotype on rate of increase in drunkenness from early adolescence to young adulthood (14–18 years), the change in drunkenness during the transition to adulthood (18–19 years), and the rate of change in drunkenness across young adulthood (≥ 19 years). Variation in GABRA2 was associated with an increase in drunkenness that occurred at the transition between adolescence and adulthood. The genotypic effect was more pronounced in females. These analyses illustrate the importance of longitudinal data to characterize how genetic effects unfold across development. The findings suggest that transitions across important developmental periods may alter the relative importance of genetic effects on patterns of alcohol use. The findings also suggest the importance of considering gender when evaluating genetic effects on drinking patterns in males and females.
Keywords: alcohol, COGA, GABRA2, genetic association, longitudinal, trajectories
Introduction
Most large-scale genetic association studies have tested for genetic effects on lifetime clinical diagnoses of substance use and psychiatric disorders. For example, there are large-scale efforts underway to identify genes involved in alcohol dependence (Bierut, Agrawal et al. 2009; Edenberg, Koller et al. 2010), schizophrenia (Ripke, Sanders et al. 2011; Lee, DeCandia et al. 2012), major depression (Consortium 2012; Wray, Pergadia et al. 2012), bipolar disorder (Chen, Jiang et al. 2011), autism (Chahrour, Yu et al. 2012; Sanders, Murtha et al. 2012), and attention-deficit hyperactivity disorder (Elia, Glessner et al. 2012; Stergiakouli, Hamshere et al. 2012; Williams, Franke et al. 2012). There has also been interest in expanding gene identification efforts to include phenotypes additional to clinical diagnoses. This has involved studying: behavioral components related to psychiatric and substance use disorders (e.g., drinking patterns) (Dick, Plunkett et al. 2006); subtypes that may have more homogeneous etiologies (e.g., early onset alcohol dependence) (Edenberg, Xuei et al. 2007); and broader phenotypes that may jointly reflect a shared etiology (e.g., by studying general externalizing or internalizing disorders) (Hettema, An et al. 2006; Dick, Aliev et al. 2008). The study of endophenotypes, or intermediary phenotypes thought to be closer to the underlying genetic architecture than are clinical diagnoses, has also received great attention (Almasy and Blangero 2001; Gottesman and Gould 2003; Cannon and Keller 2006; Dick, Jones et al. 2006). However, one area that has remained relatively neglected is the evaluation of longitudinal phenotypes, that is, patterns of change in behavior and physiology over specified time periods. Here we report analyses that use longitudinal measures of alcohol-related outcomes to characterize how genetic risk unfolds across developmental stages.
We focus on GABRA2, because polymorphisms in GABRA2 have been associated with adult alcohol dependence across multiple independent studies (Covault, Gelernter et al. 2004; Edenberg, Dick et al. 2004; Fehr, Sander et al. 2006; Enoch 2008; Soyka, Preuss et al. 2008). However, the two reports that have tested for association with alcohol dependence in younger samples have not found evidence for this association (Dick, Bierut et al. 2006; Sakai, Stallings et al. 2010). These findings are consistent with twin data in which there is significant heritability for alcohol dependence in adulthood (in the range of 50–60%) (Dick Prescott et al. 2009); but little evidence of genetic influence on alcohol dependence symptoms in early adolescence (< age 15) (Rose, Dick et al. 2004; Knopik, Heath et al. 2009). A major limitation of the literature on GABRA2 is that no studies of which we are aware have used longitudinal data to explicitly test the unfolding of risk associated with GABRA2 across development. In the current analyses, we used longitudinal data from individuals with up to three assessments between the ages of 14 and 25 in order to examine the effects of GABRA2 on trajectories of drunkenness from adolescence to young adulthood. We used drunkenness as an index of risky drinking behavior, as we hypothesized that genetic effects may be evident for drinking patterns/subclinical indices of drinking problems earlier in the developmental history than for diagnostic level alcohol dependence problems. Accordingly, understanding how genetic influences impact changes in drunkenness across adolescence and into young adulthood may help us understand the unfolding of risk across this critical transitional period.
Methods
Sample
The data presented here are from the Phase IV Prospective Study of the subjects in the Collaborative Study of the Genetics of Alcoholism (COGA) sample. As approved by IRBs at each participating center, COGA is a large family study with the goal of identifying genes affecting alcohol dependence and related phenotypes; the Prospective Study is focused on understanding how genetic risk unfolds across adolescence into young adulthood. COGA families were identified through probands in inpatient or outpatient alcohol treatment programs at six sites across the United States. Additional “community comparison” families were obtained through a variety of sources such as driver’s license registries and dental clinics; alcohol dependence and other psychiatric disorders were not exclusionary criteria for the comparison families. More details about the basic COGA study have been published previously (Begleiter, Reich et al. 1995; Foroud, Edenberg et al. 2000).
Recruitment for the Prospective Study began in December 2004 among adolescents (age between 12 and 17 years) and young adults (age between 18 and 21 years) who were part of a current COGA family. Recruited participants had at least one parent who was interviewed in one of the previous phases of COGA. Individuals were interviewed using the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA-IV), for adults 18 years or older, and using its adolescent version (C-SSAGA-IV) for subjects under 18 years (Reich, Herjanic et al. 1982). Here we focus on responses to the item “How often did you get drunk during the last 12 months?”. Responses are grouped into 13 categories, from “NEVER” to “EVERY DAY”, and the responses were converted into numeric values by taking the midpoint of each response category. For example, the category “EVERYDAY” corresponds to 365/year, and the category “2 DAYS PER WEEK (100 – 149 DAYS)” corresponds to 124.5/year in the converted variable.
After the initial assessment, the participants are followed up at approximately biennial intervals. To avoid potential effects associated with population stratification, we limited our analyses here to the European American subset of the sample. Individuals who answered “No” to the screening question about lifetime drinking at all assessments were excluded from the analysis, as were individuals who had missing information on drunkenness at all assessments. We used data from ages 14 to 25 due to sparseness of data at earlier/later timepoints. This yielded a final N of 1070 individuals (49% male). Because data collection remains on-going, 545 individuals had 3 assessments (51%) at the time analyses were conducted, 378 had two assessments (35%), and 147 had one assessment. Individuals contribute to the mean and trajectory parameters that encompass the age period for which their data is available; this allows all individuals to contribute to the analyses.
Genotyping
We tested 6 SNPs in GABRA2 (major and minor alleles, respectively, shown in parentheses, along with minor allele frequencies): rs497068 (T-C; 0.42), rs279871 (A-G; 0.43), rs279867 (T-G; 0.43), rs279858 (A-G; 0.43), rs279845 (T-A; 0.45), and rs279836 (T-A; 0.43). These SNPs were selected for being among the most significantly associated with alcohol dependence in previous COGA analyses (Edenberg, Dick et al. 2004). The SNPs are highly correlated with each other, with r2 values ranging from 0.82 to 0.99, mean = 0.89; accordingly we expect consistency of results across SNPs. Genotyping was performed at the Genome Technology Access Center at Washington University School of Medicine in St. Louis (http://gtac.wustl.edu/) using an Illumina GoldenGate custom array as part of a larger set of 384 SNPs. All 6 SNPs had a genotyping rate greater than 98% and were in Hardy-Weinberg equilibrium. Because of the relatively few number of SNPs genotyped, we used both PLINK and PREST to examine IBD relationship.
Statistical model
We fit a piecewise linear growth model to evaluate different growth trajectories for the adolescent (≤ 18 years) and adult (≥ 19 years) periods by estimating different intercepts and slopes for each period. The transition from adolescence to adulthood is a period of considerable theoretical interest with important developmental changes associated with the attainment of adult status. Therefore, we modeled the transition from adolescence to adulthood as the discrepancy between the expected values at 19 based on the growth trajectories of adolescence (data from 14 to 18 years) and young adulthood (data from 19 to 25 years). As seen in Figure 1, the mean trajectory of drunkenness shows a steadily increasing trend up to age 18, a substantial increase in the average response between 18 and 19 (referred to here as the “jump”), and a leveling off in drunkenness that occurs after that period. Differences in each of these parameters (slope of drunkenness across adolescence, transition point (“jump”), and slope of drunkenness across young adulthood) were tested by genotype. The model was fit in the framework of hierarchical linear modeling (Byrk and Raudenbush 1992) given varying numbers and times of assessments across individuals. Unlike conventional growth curve models using a structural equation modeling approach, hierarchical linear modeling does not have equality assumptions on the numbers and times of follow-up assessments of individuals (Mehta and West 2000). Model fitting and parameter estimation were conducted using the Mixed procedure, Version 9.3 of SAS.
Figure 1.
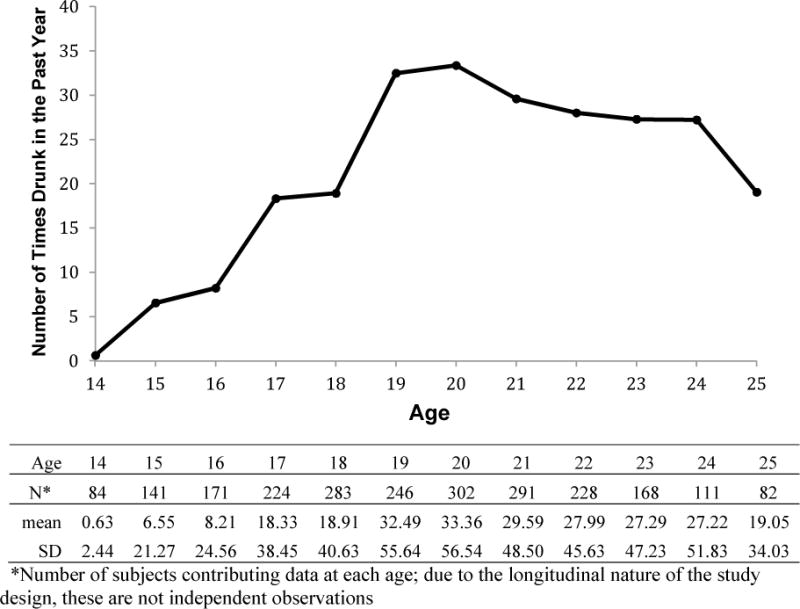
Means and standard deviations of drunkenness (days per year)
Genotype was coded as a categorical covariate with minor allele count 1 as the reference category. This is a model-free approach that does not assume a linear, recessive, or dominant effect of genotype, and was chosen because different genetic models have been indicated across different samples (Dick, Bierut et al. 2006).
A p-value of 0.025 was used to indicate study-wide significance. This is based on a multiple testing correction performed using the web-based software SNPSpD (Nyholt 2004), which takes into account the number of SNPs genotyped and linkage disequilibrium (LD) structure between them. Based on this test, the effective number of independent marker loci for our analyses was 2.0, resulting in the adjusted significance level of 0.05/2.0 = 0.025.
Results
Table 1 shows the p-values for each of the six SNPs for the different growth trajectories. After correcting for multiple testing, the only consistently significant difference across the six SNPs is the difference in the jump between minor allele count 0 and 1, with all SNPs yielding p < 0.005. None of the differences between minor allele count 1 and 2 were significant.
Table 1.
p-values of the tests of genotype effects for GABRA2 SNPs
| Parameters | Comparisons | rs497068 | rs279871 | rs279867 | rs279858 | rs279845 | rs279836 |
|---|---|---|---|---|---|---|---|
| Slope before 19 | Minor allele count 0 vs. 1 |
0.42 | 0.33 | 0.33 | 0.32 | 0.19 | 0.22 |
| Minor allele count 2 vs. 1 |
0.33 | 0.30 | 0.30 | 0.30 | 0.70 | 0.33 | |
| Jump | Minor allele count 0 vs. 1 |
0.0032* | 0.0017* | 0.0017* | 0.0017* | 0.0012* | 0.0002* |
| Minor allele count 2 vs. 1 |
0.75 | 0.36 | 0.36 | 0.36 | 0.65 | 0.23 | |
| Slope after 19 | Minor allele count 0 vs. 1 |
0.13 | 0.11 | 0.12 | 0.14 | 0.28 | 0.07 |
| Minor allele count 2 vs. 1 |
0.78 | 0.79 | 0.80 | 0.83 | 0.95 | 0.74 |
p-values significant after correction for multiple testing (Nyholt, 2004).
In order to test whether the jump in drunkenness was specific to the transition between ages 18 and 19, we also tested whether the genotypic effect was significant if the transition point was moved forward or backward one year in time. This allowed us to define the window across which the observed genotypic effect was evident. The effect was found to be specific to age 18–19; the genotypic effect associated with the homozygous major allele was not significant at age 17–18 (p = 0.14) or age 19–20 (p = 0.61).
The pattern of results was similar across all SNPs. We present in Figure 2 results for rs279858 since it has been one of the most widely studied and replicated SNPs in GABRA2 (Covault, Gelernter et al. 2004; Edenberg, Dick et al. 2004; Lappalainen, Krupitsky et al. 2005; Lind, Macgregor et al. 2008; Bierut, Agrawal et al. 2010). Figure 2 shows the estimated growth trajectories for each of the different genotypes. As illustrated in the figure, the trajectory of the A-A genotype (the major allele homozygote) is characterized by a significantly greater jump in drunkenness between 18 to 19 years of age. None of the slope differences for rs279858 were significant after correction for multiple testing.
Figure 2.
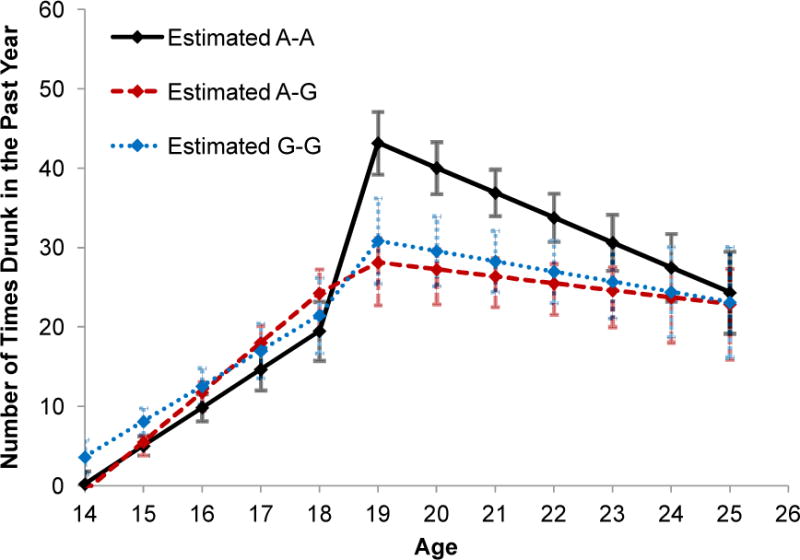
Observed mean trajectories of drunkenness by genotype (rs279858) for European Americans
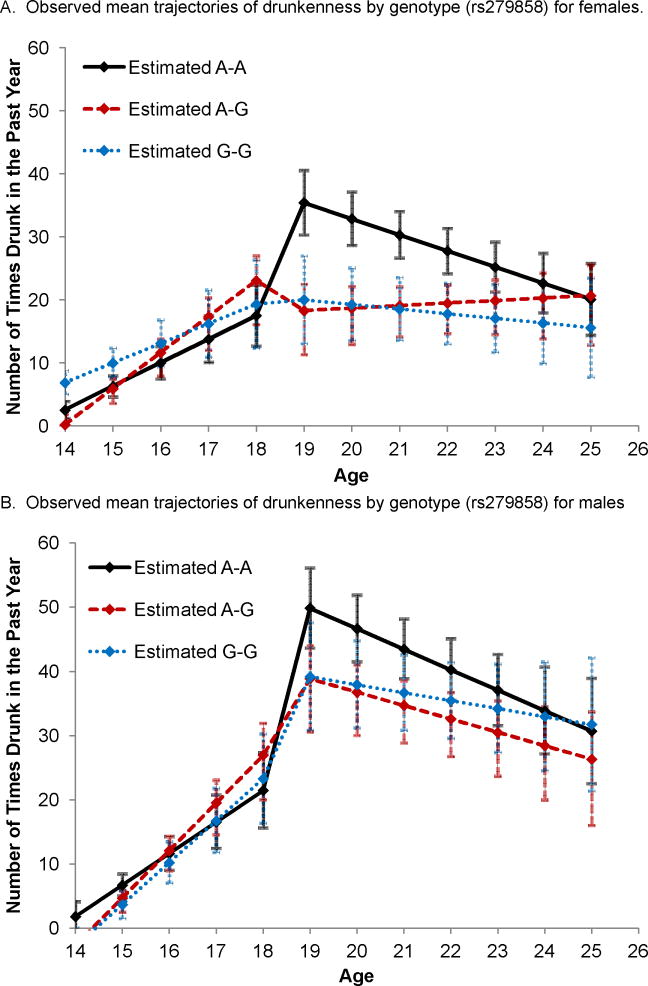
Posthoc analyses were run to test for potential sex effects by fitting the models to the data for females and males separately (Tables 2 and 3). Although similar trends are observed for both females and males, the effect of genotype on the jump in drunkenness observed between adolescence and adulthood was significant only in females. Only those females who were homozygous for the major allele showed a large jump in drunkenness from 18 to 19 years (Figure 3A). Males who were homozygous for the major allele also showed the highest levels of drunkenness (Table 3; Figure 3B), but it was only significant with one SNP. Males overall showed higher levels of drunkenness, contributing to the lack of significant differences by genotype.
Table 2.
p-values of the tests of genotype effects for GABRA2 SNPs in females
| Parameters | Comparisons | rs497068 | rs279871 | rs279867 | rs279858 | rs279845 | rs279836 |
|---|---|---|---|---|---|---|---|
| Slope before 19 | Minor allele count 0 vs. 1 |
0.34 | 0.25 | 0.25 | 0.24 | 0.44 | 0.35 |
| Minor allele count 2 vs. 1 |
0.13 | 0.22 | 0.22 | 0.22 | 0.91 | 0.35 | |
| Jump | Minor allele count 0 vs. 1 |
0.0044* | 0.0028* | 0.0026* | 0.0027* | 0.0040* | 0.0022* |
| Minor allele count 2 vs. 1 |
0.49 | 0.43 | 0.42 | 0.42 | 0.81 | 0.32 | |
| Slope after 19 | Minor allele count 0 vs. 1 |
0.13 | 0.10 | 0.10 | 0.10 | 0.12 | 0.10 |
| Minor allele count 2 vs. 1 |
0.95 | 0.61 | 0.62 | 0.61 | 0.48 | 0.51 |
p-values significant after correction for multiple testing (Nyholt, 2004).
Table 3.
p-values of the tests of genotype effects for GABRA2 SNPs in males
| Parameters | Comparisons | rs497068 | rs279871 | rs279867 | rs279858 | rs279845 | rs279836 |
|---|---|---|---|---|---|---|---|
| Slope before 19 | Minor allele count 0 vs. 1 |
0.31 | 0.27 | 0.27 | 0.27 | 0.06 | 0.12 |
| Minor allele count 2 vs. 1 |
0.93 | 0.73 | 0.71 | 0.73 | 0.45 | 0.62 | |
| Jump | Minor allele count 0 vs. 1 |
0.16 | 0.06 | 0.06 | 0.07 | 0.04 | 0.0124* |
| Minor allele count 2 vs. 1 |
0.66 | 0.66 | 0.64 | 0.68 | 0.78 | 0.53 | |
| Slope after 19 | Minor allele count 0 vs. 1 |
0.93 | 0.54 | 0.51 | 0.65 | 1.00 | 0.36 |
| Minor allele count 2 vs. 1 |
0.57 | 0.85 | 0.97 | 0.77 | 0.47 | 0.85 |
p-values significant after correction for multiple testing (Nyholt, 2004).
Figure 3.
Discussion
This study assesses the longitudinal, developmental trajectory of risk associated with a specific genotype in a large prospective cohort of adolescents with drinking patterns characterized from adolescence to young adulthood. Understanding risk across this period is a particularly critical area of study in the alcohol field, as there has been a discrepancy between genetic findings for alcohol related outcomes in adolescent and adult samples (Dick, Bierut et al. 2006; Sakai, Stallings et al. 2010). With longitudinal data we demonstrate that genotype effects are associated with the transition to adulthood: genetic differences emerge as a jump in drunkenness between age 18 and 19. This is obviously a rich developmental phase that is associated with a number of milestones, such as leaving home, entering college, and building new social networks (White, Xie et al. 2001; Borsari, Murphy et al. 2007). These milestones reflect enhanced independence associated with the attainment of adult status. Twin data have indicated that environments that exert less social control and/or provide greater opportunity to engage in alcohol use allow for greater expression of genetic predispositions (Dick and Kendler 2012). This has been shown, for example, with respect to low parental monitoring (Dick, Purcell et al. 2006), higher peer deviance (Dick, Pagan et al. 2007; Harden, Hill et al. 2008; Button, Stallings et al. 2009), and communities with higher alcohol sales and less stability (Dick, Rose et al. 2001), all of which are associated with greater genetic variance. Here, we demonstrate that the effect of a specific gene on high risk alcohol use becomes evident during the transition to adulthood. Although we did not directly measure environmental characteristics in this study, this transition is generally associated with the attainment of greater autonomy and fits in the broader theoretical mechanism suggested by the gene environment interaction literature (Shanahan and Hofer 2005; Dick and Kendler 2012).
Secondary analyses reveal that the findings are more significant in females. All males, irrespective of genotype, showed a greater increase in drunkenness associated with the transition to adulthood. A number of previous studies have documented higher rates of risky drinking behaviors in males as compared to females at this age (White, Kraus et al. 2006; White and Swartzwelder 2009), and our data support this. It is possible that this leads to a heightened culture of accepted drunken behavior in males, and that under these environmental pressures, genotypic effects are attenuated. None of the twin studies, reviewed above, showing that genetic effects are more evident in environments with less social control, have tested for sex differences. Our study illustrates the complexity of understanding how genetic predispositions interact with contextual influences and suggests that examining environmental circumstances and pressures that may differ for males and females at the transition to adulthood is an important area to pursue.
Our findings also complement other studies that have tested for genetic effects across adolescents/young adults of different ages. In at least two independent studies examining other genes thought to be involved in alcohol related outcomes, genetic effects that were present at later ages were not evident earlier in adolescence. Using data from the Add Health study on individuals ages 13–26, genetic effects associated with five monoamine genes and patterns of alcohol consumption were only evident among individuals age 19 or older, not among younger individuals (Guo, Wilhelmsen et al. 2007). Similarly, associations between ALDH2 and alcohol consumption among Asian-Americans also showed a parallel pattern with genetic effects becoming evident late in adolescence/young adulthood (Irons, Iacono et al. 2012). These studies together point toward a more global picture of genetic effects on alcohol related outcomes emerging in young adulthood. However we note that the increase in drunkenness observed at the transition to adulthood in our sample is not sustained. There are no significant differences in rate of change in drunkenness after age 19, and mean levels of drunkenness do not differ significantly by genotype by the mid-20s, as illustrated by the overlapping error bars in the figures. Other analyses exploring the association between GABRA2 and a variety of impulsivity phenotypes in this sample show that this gene is associated with subclinical levels of externalizing behavior, as measured by the Achenbach Externalizing Scale, but not with clinical-level DSM symptom counts of alcohol dependence, other drug dependence, or antisocial behavior, as in the older adult COGA samples (Dick Aliev et al. 2013). These findings illustrate that genes can impact different outcomes across different developmental stages and underscore the importance of understanding the longitudinal pathways of risk associated with particular genes.
There has been inconsistency in the genetic model associated with GABRA2 in different studies and in different age groups (Dick, Bierut et al. 2006); accordingly, we coded the genotypes so as to not assume a specific genetic model. In these analyses, we found a greater jump in drunkenness during the transition from adolescence to adulthood among individuals carrying 0 copies of the minor allele, with no difference observed among individuals heterozygous or homozygous for the minor allele. This is the same genetic model (risk associated with carrying 0 copies of the minor allele, alternately referred to as two copies of the major allele) that was associated with elevated alcohol problems in our previous cross-sectional studies of adults. In other words, the genotype originally associated with adult alcohol dependence in the parental generation of COGA is the same genotype associated with the sharper increase in drunkenness from 18 to 19 found among these prospectively followed children of COGA families.
Although we have focused these analyses on self-reports of drunkenness, as an index of high risk drinking behavior, we note that this measure correlated highly with other indices of drinking that were assessed, including frequency of drinking any alcoholic beverages (r = 0.69) and frequency of drinking 5 or more drinks in a 24 hour period (r = 0.83). The trajectory analyses of GABRA2 yielded parallel results with these other indices of drinking, providing further support for the robustness of the effect, and alleviating concern that may exist about the subjective nature of what constitutes “drunkenness”.
Our study should be interpreted in the context of several limitations. Although the age range covered encompassed 14 to 25 years, participants did not have data across this entire range. Since data collection was not age-standardized, each participant contributes to the portion of the overall trajectory for which they have data, but different time points have somewhat different individuals contributing. The number of individuals contributing to each age point is included in Figure 1. In addition, although we assume that the jump in drunkenness found between ages 18 and 19 is attributable to changing environmental circumstances associated with the transition to adulthood, we have not explicitly included any measured aspects of the environment in the model. Finally, the analyses reported here are limited to the European American subset of a study that selected families rich in alcohol dependent subjects, and it is not clear if similar results would be seen in other subgroups. There are known allele frequency differences between populations and we did not want this to lead to spurious findings, however, we note that the overall pattern of results was similar when the entire sample (of which European Americans were 62%) was analyzed jointly.
In summary, our analyses illustrate the importance of longitudinal data to characterize how genetic effects unfold across development. This allows us to go beyond simple studies of means and characterize genetic effects on patterns of use across time. By modeling patterns of high risk drinking behavior across time, our analyses suggest that the effect of this gene becomes evident during the transition to young adulthood, as evidenced by a jump in drunkenness evident between age 18 and 19 that is associated with GABRA2 genotype. These findings fit within the broader literature suggesting that environments that exert less social control and/or allow greater opportunity to engage in alcohol use also allow for increased expression of genetic effects (Shanahan and Hofer 2005). Interestingly, our findings are more significant in females. Males overall showed larger increases in drunkenness from 18 to 19 years; accordingly, the effect of genotype was attenuated. This underscores the potential importance of studying how etiological factors may differentially impact alcohol use in males and females at this important developmental juncture. Understanding how genetic risk unfolds across development has important implications for prevention and intervention efforts.
Acknowledgments
The Collaborative Study on the Genetics of Alcoholism (COGA), Principal Investigators B. Porjesz, V. Hesselbrock, H. Edenberg, L. Bierut, includes ten different centers: University of Connecticut (V. Hesselbrock); Indiana University (H.J. Edenberg, J. Nurnberger Jr., T. Foroud); University of Iowa (S. Kuperman, J. Kramer); SUNY Downstate (B. Porjesz); Washington University in St. Louis (L. Bierut, A. Goate, J. Rice, K. Bucholz); University of California at San Diego (M. Schuckit); Rutgers University (J. Tischfield); Southwest Foundation (L. Almasy), Howard University (R. Taylor) and Virginia Commonwealth University (D. Dick). Other COGA collaborators include: L. Bauer (University of Connecticut); D. Koller, S. O’Connor, L. Wetherill, X. Xuei (Indiana University); Grace Chan (University of Iowa); N. Manz, M. Rangaswamy (SUNY Downstate); A. Hinrichs, J. Rohrbaugh, J-C Wang (Washington University in St. Louis); A. Brooks (Rutgers University); and F. Aliev (Virginia Commonwealth University). A. Parsian and M. Reilly are the NIAAA Staff Collaborators. We continue to be inspired by our memories of Henri Begleiter and Theodore Reich, founding PI and Co-PI of COGA, and also owe a debt of gratitude to other past organizers of COGA, including Ting-Kai Li, currently a consultant with COGA, P. Michael Conneally, Raymond Crowe, and Wendy Reich, for their critical contributions. This national collaborative study is supported by NIH Grant U10AA008401 from the National Institute on Alcohol Abuse and Alcoholism (NIAAA) and the National Institute on Drug Abuse (NIDA). This work is also supported by K02AA018755 (DMD). Drs. LJ Bierut, AM Goate, AJ Hinrichs, J Rice and JC Wang are listed as inventors on the patent “Markers for Addiction” (US 20070258898) covering the use of certain SNPs in determining the diagnosis, prognosis, and treatment of addiction. No other authors have any conflicts of interest to declare.
Footnotes
To be submitted to Addiction Biology
Authors Contribution
DMD and SBC were responsible for the study concept and design. SBC, SJL, and FA performed the statistical analyses. DMD and SBC drafted the manuscript. All co-authors aided with interpretation of the results and provided critical revision of the manuscript for important intellectual content. All authors critically reviewed content and approved final version for publication.
References
- Almasy L, Blangero J. Endophenotypes as quantitative risk factors for psychiatric disease: rationale and study design. American Journal of Medical Genetics. 2001;105:42–44. [PubMed] [Google Scholar]
- Begleiter H, Reich T, et al. The Collaborative Study on the Genetics of Alcoholism. Alcohol Health & Research World. 1995;19:228–236. [PMC free article] [PubMed] [Google Scholar]
- Bierut L, Agrawal A, et al. A genome-wide association study of alcohol dependence. under review 2009 [Google Scholar]
- Bierut LJ, Agrawal A, et al. A genome-wide association study of alcohol dependence. Proc Natl Acad Sci U S A. 2010;107(11):5082–5087. doi: 10.1073/pnas.0911109107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsari B, Murphy JG, et al. Predictors of alcohol use during the first year of college: Implications for prevention. Addictive Behaviors. 2007;32(10):2062–2086. doi: 10.1016/j.addbeh.2007.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button TM, Stallings MC, et al. Perceived peer delinquency and the genetic predisposition for substance dependence vulnerability. Drug & Alcohol Dependence. 2009;100:1–8. doi: 10.1016/j.drugalcdep.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrk A, Raudenbush SW. Hierarchical linear models for social and behavioral research: applications and data analysis methods. Newbury Park: Sage Publications; 1992. [Google Scholar]
- Cannon TD, Keller MC. Endophenotypes in the genetic analyses of mental disorders. Annual Review of Clinical Psychology. 2006;2:267–290. doi: 10.1146/annurev.clinpsy.2.022305.095232. [DOI] [PubMed] [Google Scholar]
- Chahrour MH, Yu TW, et al. Whole-exome sequencing and homozygosity analysis implicate depolarization-regulated neuronal genes in autism. PLoS Genet. 2012;8(4):e1002635. doi: 10.1371/journal.pgen.1002635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DT, Jiang X, et al. Genome-wide association study meta-analysis of European and Asian-ancestry samples identifies three novel loci associated with bipolar disorder. Mol Psychiatry. 2011 doi: 10.1038/mp.2011.157. [DOI] [PubMed] [Google Scholar]
- Consortium, M. D. D. W. G. o. t. P. G. A mega-analysis of genome-wide association studies for major depressive disorder. Molecular Psychatry. 2012 doi: 10.1038/mp.2012.21. Epub ahead of print (Apr 3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covault J, Gelernter J, et al. Allelic and haplotypic association of GABRA2 with alcohol dependence. American Journal of Medical Genetics (Neuropsychiatric Genetics) 2004;129B:104–109. doi: 10.1002/ajmg.b.30091. [DOI] [PubMed] [Google Scholar]
- Dick DM, Aliev F, et al. How phenotype and developmental stage affect the genes we find: GABRA2 and impulsivity. Twin Research and Human Genetics. 2013 doi: 10.1017/thg.2013.20. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Aliev F, et al. Using Dimensional Models of Externalizing Psychopathology to Aid in Gene Identification. Archives of General Psychiatry. 2008;65(3):310–318. doi: 10.1001/archpsyc.65.3.310. [DOI] [PubMed] [Google Scholar]
- Dick DM, Bierut L, et al. The role of GABRA2 in risk for conduct disorder and alcohol and drug dependence across developmental stages. Behav Genet. 2006;36(4):577–590. doi: 10.1007/s10519-005-9041-8. [DOI] [PubMed] [Google Scholar]
- Dick DM, Bierut L, et al. The role of GABRA2 in risk for conduct disorder and alcohol and drug dependence across developmental stages. Behavior Genetics. 2006;36:577–590. doi: 10.1007/s10519-005-9041-8. [DOI] [PubMed] [Google Scholar]
- Dick DM, Jones K, et al. Endophenotypes Successfully Lead to Gene Identification: Results from the Collaborative Study on the Genetics of Alcoholism. Behavior Genetics. 2006;36:77–86. doi: 10.1007/s10519-005-9001-3. [DOI] [PubMed] [Google Scholar]
- Dick DM, Kendler KS. The impact of gene environment interaction on alcohol use disorders. Alcohol Research & Health. 2012 in press. [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Pagan JL, et al. Changing Environmental Influences on Substance Use Across Development. 2007;10:315–326. doi: 10.1375/twin.10.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Plunkett J, et al. Association between GABRA1 and Drinking Behaviors in the Collaborative Study on the Genetics of Alcoholism Sample. Alcoholism: Clinical and Experimental Research. 2006;30:1101–1110. doi: 10.1111/j.1530-0277.2006.00136.x. [DOI] [PubMed] [Google Scholar]
- Dick DM, Prescott C, et al. The Genetics of Substance Use and Substance Use Disorders. Y. K. Kim, Springer; 2009. [Google Scholar]
- Dick DM, Purcell S, et al. Parental Monitoring Moderates the Importance of Genetic and Environmental Influences on Adolescent Smoking. Journal of Abnormal Psychology. 2006 doi: 10.1037/0021-843X.116.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Rose RJ, et al. Exploring gene-environment interactions: Socioregional moderation of alcohol use. Journal of Abnormal Psychology. 2001;110:625–632. doi: 10.1037//0021-843x.110.4.625. [DOI] [PubMed] [Google Scholar]
- Edenberg H, Koller DL, et al. Genome-wide association study of alcohol dependence implicates a region on chromosome 11. Alcoholism: Clinical and Experimental Research. 2010 doi: 10.1111/j.1530-0277.2010.01156.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg HJ, Dick DM, et al. Variations in GABRA2, encoding the ‡2 subunit of the GABA-A receptor are associated with alcohol dependence and with brain oscillations. American Journal of Human Genetics. 2004;74:705–714. doi: 10.1086/383283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg HJ, Dick DM, et al. Variations in GABRA2, encoding the alpha 2 subunit of the GABA(A) receptor, are associated with alcohol dependence and with brain oscillations. Am J Hum Genet. 2004;74(4):705–714. doi: 10.1086/383283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg HJ, Xuei X, et al. Association of NFKB1, which encodes a subunit of the transcription factor NF-{kappa}B, with Alcohol Dependence. Human Molecular Genetics. 2007;ddm368 doi: 10.1093/hmg/ddm368. [DOI] [PubMed] [Google Scholar]
- Elia J, Glessner JT, et al. Genome-wide copy number variation study associates metabotropic glutamate receptor gene networks with attention deficit hyperactivity disorder. Nat Genet. 2012;44(1):78–84. doi: 10.1038/ng.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA. The role of GABA(A) receptors in the development of alcoholism. Pharmacol Biochem Behav. 2008;90(1):95–104. doi: 10.1016/j.pbb.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr C, Sander T, et al. Confirmation of association of the GABRA2 gene with alcohol dependence by subtype-specific analysis. Psychiatric Genetics. 2006;16:9–17. doi: 10.1097/01.ypg.0000185027.89816.d9. [DOI] [PubMed] [Google Scholar]
- Foroud T, Edenberg HJ, et al. Alcoholism susceptibility loci: Confirmation studies in a replicate sample and further mapping. Alcoholism: Clinical and Experimental Research. 2000;24:933–945. [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. American Journal of Psychiatry. 2003;160:1–10. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Guo G, Wilhelmsen K, et al. Gene-lifecourse interaction for alcohol consumption in adolescence and young adulthood: five monoamine genes. Am J Med Genet B Neuropsychiatr Genet. 2007;144B(4):417–423. doi: 10.1002/ajmg.b.30340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden KP, Hill JE, et al. Gene-environment correlation and interaction in peer effects on adolescent alcohol and tobacco use. Behavior Genetics. 2008;38:339–347. doi: 10.1007/s10519-008-9202-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettema JM, An SS, et al. Association between glutamic acid decarboxylase genes and anxiety disorders, major depression, and neuroticism. Molecular Psychiatry. 2006;11:752–762. doi: 10.1038/sj.mp.4001845. [DOI] [PubMed] [Google Scholar]
- Irons D, Iacono WG, et al. Developmental trajectory and environmental moderation of the effect of ALDH2 polymorphism on alcohol use. Alcoholism: Clinical and Experiemental Research. 2012;36:1882–1891. doi: 10.1111/j.1530-0277.2012.01809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopik V, Heath A, et al. Genetic and environmental influences on externalizing behavior and alcohol problems in adolescence: A female twin study. Pharmacology, Biochemistry, and Behavior. 2009;93:313–321. doi: 10.1016/j.pbb.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappalainen J, Krupitsky E, et al. Association between alcoholism and Gamma-Amino Butyric Acid alpha2 receptor subtype in a Russian population. Alcoholism: Clinical and Experimental Research. 2005;29:493–498. doi: 10.1097/01.alc.0000158938.97464.90. [DOI] [PubMed] [Google Scholar]
- Lee SH, DeCandia TR, et al. Estimating the proportion of variation in susceptibility to schizophrenia captured by common SNPs. Nat Genet. 2012;44(3):247–250. doi: 10.1038/ng.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind PA, Macgregor S, et al. The role of GABRA2 in alcohol dependence, smoking, and illicit drug use in an Australian population sample. Alcohol Clin Exp Res. 2008;32(10):1721–1731. doi: 10.1111/j.1530-0277.2008.00768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta PD, West SG. Putting the individual back into individual growth curves. Psychological Methods. 2000;5:23–45. doi: 10.1037/1082-989x.5.1.23. [DOI] [PubMed] [Google Scholar]
- Nyholt DR. A simple correction for multiple testing for single-nucleotide polymorphisms in linkage disequilibrium with each other. American Journal of Human Genetics. 2004;74:765–769. doi: 10.1086/383251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich W, Herjanic B, et al. Development of a structured psychiatric interview for children: Aggreement on diagnosis comparing child and parent interviews. Journal of Abnormal Child Psychology. 1982;10:325–326. doi: 10.1007/BF00912325. [DOI] [PubMed] [Google Scholar]
- Ripke S, Sanders AR, et al. Genome-wide association study identifies five new schizophrenia loci. Nat Genet. 2011;43(10):969–976. doi: 10.1038/ng.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose RJ, Dick DM, et al. Genetic and environmental effects on conduct disorder, alcohol dependence symptoms, and their covariation at age 14. Alcoholism: Clinical and Experimental Research. 2004;28:1541–1548. doi: 10.1097/01.alc.0000141822.36776.55. [DOI] [PubMed] [Google Scholar]
- Sakai JT, Stallings MC, et al. Test of association between GABRA2 (SNP rs279871) and adolescent conduct/alcohol use disorders utilizing a sample of clinic referred youth with serious substance and conduct problems, controls and available first degree relatives. Drug Alcohol Depend. 2010;106(2–3):199–203. doi: 10.1016/j.drugalcdep.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders SJ, Murtha MT, et al. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature. 2012 doi: 10.1038/nature10945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanahan MJ, Hofer SM. Social context in gene-environment interactions: retrospect and prospect. J Gerontol B Psychol Sci Soc Sci. 2005;60 Spec No 1:65–76. doi: 10.1093/geronb/60.special_issue_1.65. [DOI] [PubMed] [Google Scholar]
- Soyka M, Preuss UW, et al. GABA-A2 receptor subunit gene (GABRA2) polymorphisms and risk for alcohol dependence. Journal of Psychiatric Research. 2008;42(3):184–191. doi: 10.1016/j.jpsychires.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Stergiakouli E, Hamshere M, et al. Investigating the contribution of common genetic variants to the risk and pathogenesis of ADHD. Am J Psychiatry. 2012;169(2):186–194. doi: 10.1176/appi.ajp.2011.11040551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AM, Kraus CL, et al. Many college freshmen drink at levels far beyond the binge threshold. Alcoholism-Clinical and Experimental Research. 2006;30(6):1006–1010. doi: 10.1111/j.1530-0277.2006.00122.x. [DOI] [PubMed] [Google Scholar]
- White AM, Swartzwelder HS. Inbound college students drink heavily during the summer before their freshman year: implications for education and prevention efforts. American Journal of Health Education. 2009;40(2):7. [Google Scholar]
- White HR, Xie M, et al. Psychopathology as a predictor of adolescent drug use trajectories. Psychology of Addictive Behaviors. 2001;15(3):210–218. [PubMed] [Google Scholar]
- Williams NM, Franke B, et al. Genome-wide analysis of copy number variants in attention deficit hyperactivity disorder: the role of rare variants and duplications at 15q13.3. Am J Psychiatry. 2012;169(2):195–204. doi: 10.1176/appi.ajp.2011.11060822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray NR, Pergadia ML, et al. Genome-wide association study of major depressive disorder: new results, meta-analysis, and lessons learned. Mol Psychiatry. 2012;17(1):36–48. doi: 10.1038/mp.2010.109. [DOI] [PMC free article] [PubMed] [Google Scholar]