Abstract
The human protein siderocalin (Scn) inhibits bacterial iron acquisition by binding catechol siderophores. Several pathogenic bacteria respond by making stealth siderophores that are not recognized by Scn. Fluvibactin and vibriobactin, respectively of Vibrio fluvialis and Vibrio cholerae, include an oxazoline adjacent to a catechol. This chelating unit binds iron either in a catecholate or a phenolate oxazoline coordination mode. The latter has been suggested to make vibriobactin a stealth siderophore without directly identifying the coordination mode in relation to Scn binding. We use Scn binding assays with the two siderophores and two oxazoline substituted analogs and the crystal structure of Fe fluvibactin:Scn to show that the oxazoline does not prevent Scn binding; hence, vibriobactin is not a stealth siderophore. We show that the phenolate oxazoline coordination mode is present at physiological pH and is not bound by Scn. However, Scn binding shifts the coordination to the catecholate mode and thereby inactivates this siderophore.
A bacterial pathogen and its host engage in a battle for iron. The host restricts iron levels to inhibit infection, and the pathogen steals iron from the host to support growth.(1) Bacteria use several strategies to acquire iron, especially the secretion of small molecule ferric chelators called siderophores. The human immune system defends against siderophore mediated iron acquisition by expressing the protein siderocalin (Scn).(2) Scn is a member of the lipocalin family of proteins that displays an evolutionarily conserved fold consisting of an eight stranded, anti parallel β barrel which forms a broad, positively charged binding pocket. It predominately recognizes catechol siderophores including enterobactin (Fig. S1) which it binds with a subnanomolar dissociation constant (Kd).(3, 4) Such high affinity is achieved through cation-π and Coulombic interactions.(5) Bacterial pathogens have responded to the Scn defense by using stealth siderophores that are not bound by Scn.(6) Three stealth strategies have been observed. The first is to use siderophores with no aromatic groups such as aerobactin of pathogenic strains of Escherichia coli.(4) The second is to add sugars or lipids to the siderophore for increased bulk as observed with the salmochelins of Salmonella enterica(7) and the carboxymycobactins of Mycobacterium tuberculosis.(8) The third is for metal coordination to change the shape of the siderophore as observed with petrobactin of Bacillus anthracis.(6)
The last two stealth strategies allow a pathogen to use catechol siderophores and avoid Scn recognition. Masking catechol siderophores is advantageous because they have the highest affinity for iron and fast kinetics of iron removal from transferrin.(9, 10) This prompted us to look further into how other catechol siderophores avoid Scn recognition.
Vibrio fluvialis and Vibrio cholerae each synthesize and use triscatechol siderophores that have at least one catechol attached to a five membered heterocycle oxazoline. V. fluvialis makes fluvibactin (10) with one catechol oxazoline unit.(11) V. cholerae makes vibriobactin (17) with two oxazoline catechol units (Scheme 1).(12) The catechol oxazoline units are capable of coordinating iron either in a catecholate mode or a phenolate oxazoline mode, and the two modes resemble the catecholate and salicylate modes of catechol units (Fig. 1).(13) Siderophores in a salicylate mode are not recognized by Scn,(14) and, by analogy, the Vibrio siderophores may avoid Scn recognition by coordinating iron in a phenolate oxazoline mode.
Scheme 1.
Convergent synthesis of the four siderophores fluvibactin, fluvibactin A, vibriobactin, and vibriobactin A.
Figure 1.
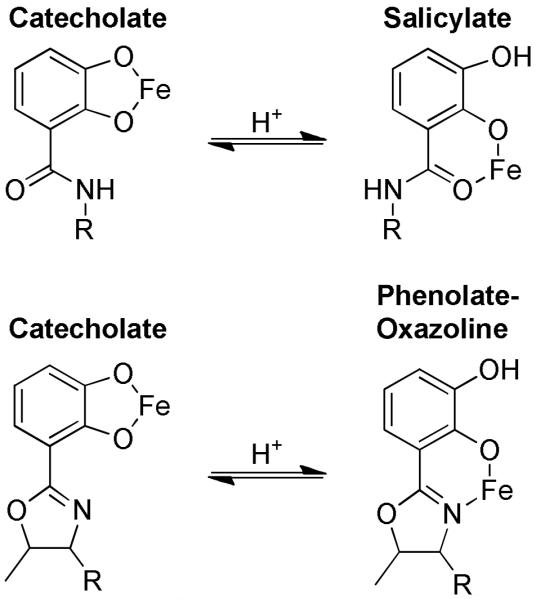
Ferric coordination modes of the catechol and catechol oxazoline units of siderophores. Catechol units may coordinate iron in either a catecholate mode or a salicylate mode. Catechol oxazoline units may coordinate iron in either a catecholate mode or a phenolate oxazoline mode. Both transitions are proton dependent.
Recently, Li et al. reported that Fe vibriobactin is a stealth siderophore, and they used indirect evidence from a crystal structure of a periplasmic protein bound to Fe vibriobactin to suggest that the phenolate oxazoline coordination mode provides the stealth character.(15) However, the coordination mode was not directly identified in relation to Scn binding. In this report, we characterize the effect of the oxazolines of fluvibactin and vibriobactin on Scn recognition with particular focus on the phenolate oxazoline iron coordination mode, and we have found that the phenolate oxazoline unit does not give fluvibactin or vibriobactin stealth character.
The oxazolines in fluvibactin and vibriobactin are cyclized threonine residues.(16) Substituting linear threonine residues for each oxazoline gives fluvibactin A (13) or vibriobactin A (19), named by following the convention previoulsy used to distinguish the oxazoline siderophore agrobactin from the ring opened agrobactin A (Fig. S1).(17) The complete series of natural siderophores and oxazoline substituted siderophores, including fluvibactin, fluvibactin A, vibriobactin, and vibriobactin A were synthesized and used to identify the effects of the oxazoline on Scn recognition (Scheme 1).
The siderophore series has only three unique catechol arms defined by the linker that attaches it to the backbone including a catechol amide, a catechol oxazoline, and a catechol threonine. A synthetic building block for each of the arms was prepared and incorporated in the synthesis of the siderophores (Scheme 1).
The syntheses of all three catechol building blocks begins with the starting material 2,3 dihydroxybenzoic acid (1), which was converted into an acid chloride (4). Combining 4 with 2 mercaptothiazoline made the first building block (5) which was used to install the catechol amide units in all four siderophores. The 1,3 thiazilodine 2 thione functionality selectively couples to the primary amines. It was stirred with symmetric norspermidine to give symmetric diamide 6 without using amine protection strategies. Benzyloxycarbonyl (Cbz) threonine was then coupled to the free secondary amine to give 7. The secondary amine of 6 is particularly unreactive,(18) but the coupling agent 2 (1H 7 azabenzotriazol 1 yl) 1,1,3,3 tetramethyl uronium hexafluorophosphate (HATU) allowed the reaction to proceed. The HATU coupling was later used to form the tertiary amide in each of the siderophores. All of the protecting groups present in 7 are easily removed by hydrogenolysis to yield 8. Finally, the catechol oxazoline building block (9) was condensed with 8 as reported by Bergeron et al. to produce fluvibactin (10).(19)
Secondary amine 6 was condensed with the catechol threonine synthetic building block (11) using HATU to give 12, which was then deprotected to give fluvibactin A (13).
For the vibriobactin series, desymmetrizing norspermidine was performed with mono Boc protection (20) and incorporation of the catechol amide to give 14 (Scheme S1). Monoamide 14 was combined with two equivalents of Cbz threonine using HATU (15), deprotected (16), and condensed with 9 to yield vibriobactin (17).(21) Lastly, two equivalents of 11 were combined with 14 to give 18 which was deprotected to give vibriobactin A (19). This modular and convergent synthetic design produced four different siderophores while using the same building blocks, reagents, and minimally modified reactions.
The synthesized siderophores were used as metal free ligands (apo) or ferric complexes in fluorescence quenching (FQ) titrations with Scn at physiological pH (7.4) to quantify the affinity of the protein for the siderophores. Fitting a one to one binding model (Fig. S2) gave the Kds listed in Table 1.(22) All of the apo and Fe siderophores have submicromolar Kds with Scn, and the high affinity of Fe fluvibactin and Fe vibriobactin shows that incorporation of catechol oxazoline units in a siderophore structure is not a stealth strategy. The three catechol units of fluvibactin and vibriobactin provide the key interactions for binding with Scn. The design of the Scn binding pocket to recognize the catechol units of a siderophore despite large variations in the backbone allows it to bind many siderophores with catechol as the only common feature including fluviabactin and vibriobactin.(4)
Table 1.
Dissociation constants of Scn and siderophores at pH 7.4
| Siderophore | Apo- Kd (nM) |
Fe- Kd (nM) |
|---|---|---|
| Fluvibactin | 34(4) | 4.4(8) |
| Fluvibactin A | 12(2) | 1.0(3) |
| Vibriobactin | 56(6) | 18(3) |
| Vibriobactin A | 18(2) | 8(1) |
Although each of the four siderophores is bound by Scn, the affinity varies. Three major trends reveal the effect of catechol oxazoline on Scn recognition. The first trend is that Scn has higher affinity for Fe siderophores than apo siderophores. The Fe siderophores are more negatively charged and thus have greater Coulombic interactions with the positive binding pocket. This trend has been observed in all siderophores bound by Scn, (5, 14) and the shape constraints of the catechol oxazoline units do not reverse it.
The second trend is that Scn has lower affinity for the vibriobactin series than for the fluvibactin series. Two linkers between the backbone and catechol, whether oxazoline or threonine, fit less well in the protein binding pocket, and the effect is more pronounced for the Fe siderophores than for the apo siderophores.
The third trend is that Scn has higher affinity for the catechol threonine siderophores than for the catechol oxazoline siderophores in both apo and Fe siderophores. For apo and Fe siderophores the trend arises because the catechol threonine may rotate and fold to fit in the binding pocket, but the catechol oxazoline has relatively limited flexibility because of the rigidity of the heterocycle. Notably, the Kd for Fe vibriobactin is significantly larger than the Kd for the other Fe siderophores and infers the contribution of another factor beyond the sterics of the oxazoline linker.
We suggest that the additional factor affecting Scn affinity is the way in which the coordination mode influences the shape of the Fe siderophores. The catechol threonine siderophores fluvibactin A and vibriobactin A only coordinate iron in the catecholate mode because the salicylate mode only occurs below pH 5.(9) As expected, the Kd for each is close to the Kd for triscatecholate Fe enterobactin. The identification of the coordination mode of Fe fluvibactin and Fe vibriobactin is not possible from FQ titrations, and the change from catecholate to phenolate oxazoline modes had not been previously characterized. Therefore, other methods were used to identify the coordination mode at physiological conditions and when bound by Scn.
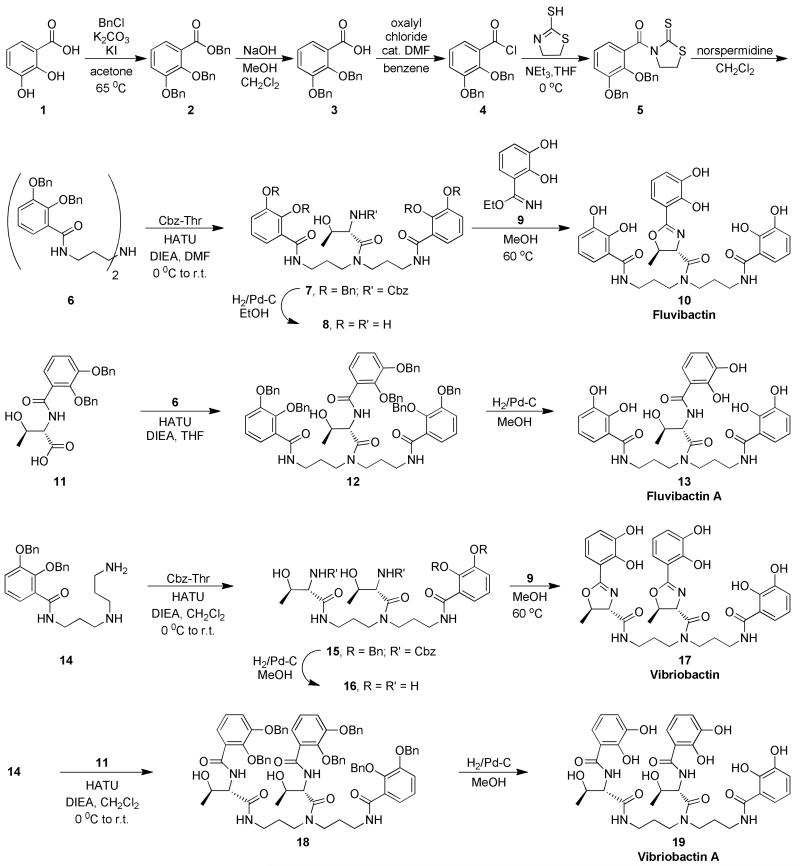
Structural characterization of Scn with Fe siderophores has been used extensively to elucidate protein ligand interactions. The crystal structure of Scn:Fe fluvibactin shows that the protein interacts with the siderophore similar to other catecholate Fe siderophores such as enterobactin (Fig. 2a).(3) The three catechols fit into three subpockets formed by the positive residues (R81, K125, and K134) of the binding pocket. Although Fe fluvibactin was only modeled in a single conformation, it is clear from the electron density that the catechol oxazoline unit is never seen in pocket 1, consistent with it being the key pocket for recognition. The catechol oxazoline unit of Fe fluvibactin is in a catecholate coordination mode (Fig. 2b). The catechol oxygen atoms average about 2 Å from the iron center, which is an expected Fe O bond distance.(23) The oxazoline nitrogen is over 4 Å from the metal which is much larger than a Fe N bonding interaction. This coordination mode is observed inside Scn even when the crystals were grown at pH 4.5, and at such low pH it is more likely that protonation of Fe fluvibactin would increase the stability of the phenolate oxazoline mode. If Fe fluvibactin were to adopt the phenolate oxazoline mode, then it appears that one of the catechols would clash with the protein wall and prevent binding as was observed while docking salicylate models of enterobactin into the Scn binding pocket.(14) The lowered affinity for Fe fluvibactin relative to Fe fluvibactin A observed in the fluorescence quenching titrations is due to the steric constraints of the oxazoline but not due to a phenolate oxazoline iron coordination.
Figure 2.
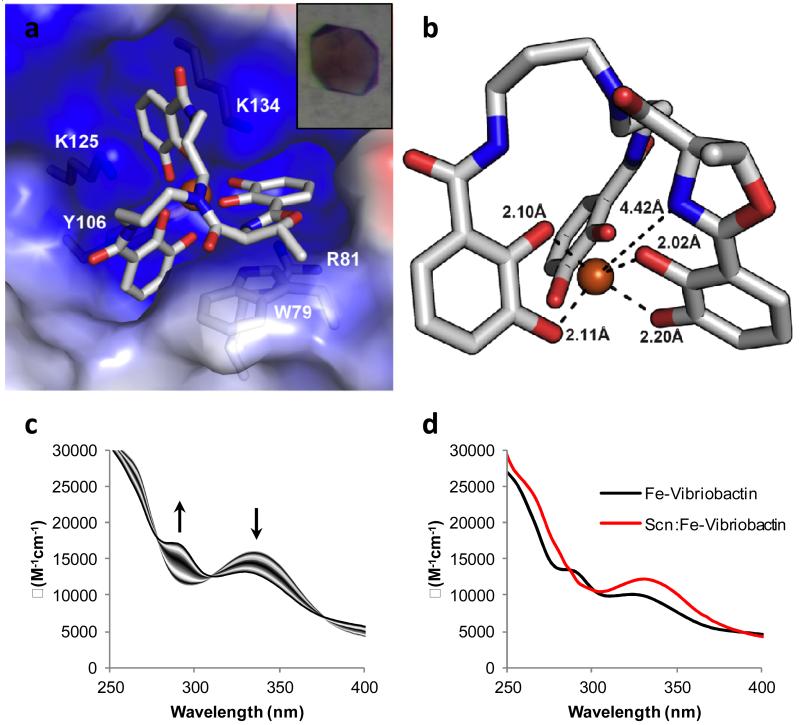
The coordination mode of Fe fluvibactin and Fe vibriobactin when bound by Scn. a) Crystal structure of Fe fluvibactin in the binding pocket of Scn. Insert shows complex crystals of Scn:Fe fluvibactin. b) Another view of Fe fluvibactin from the Scn binding pocket. The atom atom distances show that three catechols are coordinated to iron with Fe O distances near 2.0 Å (only two shown for clarity). The oxazoline has a N Fe distance greater than 4.4 Å, and it is not coordinated to the metal. c) Spectrophotometric titration of Fe vibriobactin showing the transition from catecholate mode at pH 10 to phenolate oxazoline mode at pH 7. The arrows indicate the change in the spectra as the pH of the solution was lowered. d) Absorbance spectra of Fe vibriobactin alone and with Scn. The presence of Scn induces a change in the spectrum at pH 7 that matches the spectrum of Fe vibriobactin at high pH. Scn binding causes a coordination change from phenolate oxazoline to catecholate mode.
We were unable to obtain diffraction quality crystals of Fe vibriobactin with Scn, so spectroscopic methods were used to identify the coordination mode of the siderophore during binding. The change in coordination mode from catecholate to phenolate oxazoline is proton dependent. Addition of a proton to a Fe catecholate complex blocks metal coordination through the meta oxygen, the most basic site while the ortho oxygen remains coordinated to the metal, and the oxazoline nitrogen becomes the new coordination site (Fig. 1). Spectrophotometric pH titrations of Fe vibriobactin were performed to measure the protonation constant. The electronic spectra of the complex were recorded as the solution was changed from pH 10 to pH 7 (Fig. 2c). At high pH a major absorbance is observed at 336 nm. It decreases in intensity while moving to 330 nm as the pH was lowered, and a new peak grows in at 284 nm. The changes in the spectra were fit to give a protonation constant (logK) of 8.21. The first protonation of Fe vibriobactin occurs well above physiological pH, and a speciation calculation shows that 87% of the Fe vibriobactin is protonated at the pH of the Scn binding assay (Fig. S3). Thus, one of the catechol oxazoline units of Fe vibriobactin is predominately in a phenolate oxazoline coordination mode. The spectral change from 336 to 330 nm is similar to that observed for the salicylate shift in enterobactin.(9, 14) The first protonation of Fe fluvibactin occurs at a much lower pH than Fe vibriobactin meaning that the phenolate oxazoline coordination mode of this siderophore does not affect Scn binding at physiological pH (Fig S4).
In addition to measuring the protonation constant of Fe vibriobactin, the spectrophotometric titrations gave the absorbance spectrum of the catecholate and phenolate oxazoline modes of Fe vibriobactin. This made it possible to use UV vis spectroscopy to identify the coordination mode of Fe vibriobactin when bound by Scn. The absorbance spectrum of Fe vibriobactin with Scn was measured at pH 7 and compared to the spectrum of Fe vibriobactin at the same pH without Scn (Fig. 2d). Without Scn, the phenolate oxazoline mode is observed. The spectrum with Scn resembles the triscatecholate Fe vibriobactin that is observed only at high pH without Scn. Therefore, all three catechol units of Fe vibriobactin are in the catecholate mode when bound by Scn, and Scn binding provides the energy for a coordination shift by stabilizing the catecholate mode. Similar stabilization had also been observed with Fe enterobactin where the salicylate shift was not observed when bound by Scn even at pH 3.(14)
The spectrophotometric titrations and absorbance spectra show that Fe vibriobactin is in two coordination modes at pH 7.4, and that Scn binds the less abundant triscatecholate mode. The Scn binding assay measures the apparent affinity of Scn for a mixture of Fe vibriobactin species but not the affinity for triscatecholate Fe vibriobactin. The binding model used to quantify the FQ titrations was modified to include the protonation equilibrium of Fe vibriobactin with the assumption that Scn only binds triscatecholate Fe vibriobactin to give a Kd of 2.5 nM. The affinity of Scn for triscatecholate Fe vibriobactin is similar to the affinity of Scn for other triscatecholate siderophores. Thus, the Fe triscatecholate structure is recognized with high affinity by Scn which outwits the coordination chemistry of V. cholerae.
Contrary to this, Li et al. reported that Fe vibriobactin is a stealth siderophore, and that it is not bound because one catechol unit is in a phenolate oxazoline coordination mode.(15) The evidence presented for the coordination mode was from a crystal structure of the periplasmic binding protein ViuP bound to Fe vibriobactin. For several reasons this does not characterize Scn binding to vibriobactin. The ViuP:vibriobactin crystals were formed at pH 4, well below the logK of Fe vibriobactin protonation. Then the structure of protonated Fe vibriobactin was excised from ViuP and docked into the binding pocket of Scn. Unsurprisingly, phenolate oxazoline coordination makes the siderophore clash with the Scn binding pocket walls. ViuP may bind Fe vibriobactin in a phenolate oxazoline mode which is predominant at physiological pH. Transposing the Fe vibriobactin structure from ViuP to Scn is not proof of the coordination mode or explanation for the low affinity observed in the FQ titrations with Scn that Li et al. performed. However, Scn is optimized to bind triscatecholate complexes with high affinity, and the protein binding provides the energy needed to shift the coordination. The FQ results reported here, which directly contradict Li et al., are supported by the FQ results of analogs. As additional evidence that Scn binds Fe vibriobactin, addition of the protein to the siderophore solution significantly changes the absorbance spectrum. The other data support the result of the FQ titration that Fe vibriobactin is not a stealth siderophore.
Since vibriobactin and fluvibactin are bound by Scn, V. fluvialis and V. cholerae would likely have trouble infecting the host in locations where Scn is expressed if they exclusively depend on these siderophores. V. cholerae infects the small intestine and Scn is expression is upregulated by V. cholerae infection,(24) and V. fluvialis also most frequently infects the gastrointestinal tract.(25) Vibriobactin has been associated with increased virulence in the small intestine of a murine model, especially with small inoculum sizes, but additional iron transport systems, including systems for transporting heme or the siderophores of other bacteria, can provide the iron for growth even if vibriobactin is not available.(26) V. fluvialis also has heme and xenosiderophore uptake systems.(27, 28) Although the catechol siderophores are not critical to virulence in the small intestine, they may be more important during extra gastrointestinal infections. The virulence of another Vibrio pathogen, Vibrio vulnificus, depends on the catechol siderophore vulnibactin to acquire iron from transferrin.(29) Vulnibactin is identical to vibriobactin except that the catechol oxazolines are exchanged for phenol oxazolines which solely bind iron in a phenolate oxazoline mode (Fig. S1).(30) As we now understand the phenolate oxazoline mode in Scn recognition, we expect that vulnibactin is a stealth siderophore.
METHODS
The methods are reported in the supporting information.
Supplementary Material
ACKNOWLEDGEMENTS
We thank M. Sturzbecher Hoehne and T. Pham for help with the spectrophotometric titrations and A. Sia for reviewing the manuscript. This work was funded by National Institutes of Health Grants R01AI11744 and R01DK73462.
Footnotes
Matthew C. Clifton current address: Emerald Bio, 3 Preston Court, Bedford, MA 01730.
The supporting information contains supplemental figures and methods. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Ratledge C. Iron metabolism and infection. Food Nutr. Bull. 2007;28:S515–523. doi: 10.1177/15648265070284S405. [DOI] [PubMed] [Google Scholar]
- 2.Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, Akira S, Aderem A. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432:917–921. doi: 10.1038/nature03104. [DOI] [PubMed] [Google Scholar]
- 3.Goetz DH, Holmes MA, Borregaard N, Bluhm ME, Raymond KN, Strong RK. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore mediated iron acquisition. Mol. Cell. 2002;10:1033–1043. doi: 10.1016/s1097-2765(02)00708-6. [DOI] [PubMed] [Google Scholar]
- 4.Holmes MA, Paulsene W, Jide X, Ratledge C, Strong RK. Siderocalin (Lcn 2) Also Binds Carboxymycobactins, Potentially Defending against Mycobacterial Infections through Iron Sequestration. Structure. 2005;13:29–41. doi: 10.1016/j.str.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 5.Hoette TM, Abergel RJ, Xu J, Strong RK, Raymond KN. The role of electrostatics in siderophore recognition by the immunoprotein siderocalin. J. Am. Chem. Soc. 2008;130:17584–17592. doi: 10.1021/ja8074665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abergel RJ, Wilson MK, Arceneaux JEL, Hoette TM, Strong RK, Byers BR, Raymond KN. Anthrax pathogen evades the mammalian immune system through stealth siderophore production. Proc. Natl. Acad. Sci. U.S.A. 2006;103:18499–18503. doi: 10.1073/pnas.0607055103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischbach MA, Lin H, Zhou L, Yu Y, Abergel RJ, Liu DR, Raymond KN, Wanner BL, Strong RK, Walsh CT, Aderem A, Smith KD. The pathogen associated iroA gene cluster mediates bacterial evasion of lipocalin 2. Proc. Natl. Acad. Sci. U.S.A. 2006;103:16502–16507. doi: 10.1073/pnas.0604636103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoette TM, Clifton MC, Zawadzka AM, Holmes MA, Strong RK, Raymond KN. Immune interference in Mycobacterium tuberculosis intracellular iron acquisition through siderocalin recognition of carboxymycobactins. ACS Chem. Biol. 2011;6:1327–1331. doi: 10.1021/cb200331g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loomis LD, Raymond KN. Solution equilibria of enterobactin and metal enterobactin complexes. Inorg. Chem. 1991;30:906–911. [Google Scholar]
- 10.Rodgers SJ, Raymond KN. Ferric ion sequestering agents. 11 Synthesis and kinetics of iron removal from transferrin of catechoyl derivatives of desferrioxamine B. J. Med. Chem. 1983;26:439–442. doi: 10.1021/jm00357a022. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto S, Okujo N, Fujita Y, Saito M, Yoshida T, Shinoda S. Structures of two polyamine containing catecholate siderophores from Vibrio fluvialis. J. Biochem. 1993;113:538–544. doi: 10.1093/oxfordjournals.jbchem.a124079. [DOI] [PubMed] [Google Scholar]
- 12.Griffiths GL, Sigel SP, Payne SM, Neilands JB. Vibriobactin, a siderophore from Vibrio cholerae. J. Biol. Chem. 1984;259:383–385. [PubMed] [Google Scholar]
- 13.Cass ME, Garrett TM, Raymond KN. The salicylate mode of bonding in protonated ferric enterobactin analogs. J. Am. Chem. Soc. 1989;111:1677–1682. [Google Scholar]
- 14.Abergel RJ, Clifton MC, Pizarro JC, Warner JA, Shuh DK, Strong RK, Raymond KN. The siderocalin/enterobactin interaction: a link between mammalian immunity and bacterial iron transport. J. Am. Chem. Soc. 2008;130:11524–11534. doi: 10.1021/ja803524w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li N, Zhang C, Li B, Liu X, Huang Y, Xu S, Gu L. Unique iron coordination in iron chelating molecule vibriobactin helps Vibrio cholerae evade mammalian siderocalin mediated immune response. J. Biol. Chem. 2012;287:8912–8919. doi: 10.1074/jbc.M111.316034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keating TA, Marshall CG, Walsh CT. Reconstitution and characterization of the Vibrio cholerae vibriobactin synthetase from VibB, VibE, VibF, and VibH. Biochemistry. 2000;39:15522–15530. doi: 10.1021/bi0016523. [DOI] [PubMed] [Google Scholar]
- 17.Ong SA, Peterson T, Neilands JB. Agrobactin, a siderophore from Agrobacterium tumefaciens. J. Biol. Chem. 1979;254:1860–1865. [PubMed] [Google Scholar]
- 18.Sakakura A, Umemura S, Ishihara K. Convergent total syntheses of fluvibactin and vibriobactin using molybdenum(VI) oxide catalyzed dehydrative cyclization as a key step. Chem. Commun. 2008:3561–3563. doi: 10.1039/b805880f. [DOI] [PubMed] [Google Scholar]
- 19.Bergeron RJ, McManis JS, Dionis J, Garlich JR. An efficient total synthesis of agrobactin and its gallium(III) chelate. J. Org. Chem. 1985;50:2780–2782. [Google Scholar]
- 20.Krapcho AP, Kuell CS. Mono protected diamines. N tert butoxycarbonyl α,ω alkanediamines from α,ω alkanediamines. Synth. Commun. 1990;20:2559. [Google Scholar]
- 21.Bergeron RJ, Garlich JR, McManis JS. Total synthesis of vibriobactin. Tetrahedron. 1985;41:507–510. [Google Scholar]
- 22.Kuzmic P. Program DYNAFIT for the analysis of enzyme kinetic data: application to HIV proteinase. Anal. Biochem. 1996;237:260–273. doi: 10.1006/abio.1996.0238. [DOI] [PubMed] [Google Scholar]
- 23.Raymond KN, Isied SS, Brown LD, Fronczek FR, Nibert JH. Coordination isomers of biological iron transport compounds. VI. Models of the enterobactin coordination site. A crystal field effect in the structure of potassium tris(catecholato)chromate(III) and ferrate(III) sesquihydrates, K3[M(O2C6H4)3]*1.5H2O, M = Cr, Fe. J. Am. Chem. Soc. 1976;98:1767–1774. doi: 10.1021/ja00423a022. [DOI] [PubMed] [Google Scholar]
- 24.Flach CF, Qadri F, Bhuiyan TR, Alam NH, Jennische E, Lönnroth I, Holmgren J. Broad up regulation of innate defense factors during acute cholera. Infect. Immun. 2007;75:2343–2350. doi: 10.1128/IAI.01900-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Igbinosa EO, Okoh AI. Vibrio fluvialis: an unusual enteric pathogen of increasing public health concern. Int. J. Environ. Res. Public Health. 2010;7:3628–3643. doi: 10.3390/ijerph7103628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henderson DP, Payne SM. Vibrio cholerae iron transport systems: roles of heme and siderophore iron transport in virulence and identification of a gene associated with multiple iron transport systems. Infect. Immun. 1994;62:5120–5125. doi: 10.1128/iai.62.11.5120-5125.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahn SH, Han JH, Lee JH, Park KJ, Kong IS. Identification of an iron regulated hemin binding outer membrane protein, HupO, in Vibrio fluvialis: effects on hemolytic activity and the oxidative stress response. Infect. Immun. 2005;73:722–729. doi: 10.1128/IAI.73.2.722-729.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldberg MB, Boyko SA, Butterton JR, Stoebner JA, Payne SM, Calderwood SB. Characterization of a Vibrio cholerae virulence factor homologous to the family of TonB dependent proteins. Mol. Microbiol. 1992;6:2407–2418. doi: 10.1111/j.1365-2958.1992.tb01415.x. [DOI] [PubMed] [Google Scholar]
- 29.Kim CM, Park RY, Park JH, Sun HY, Bai YH, Ryu PY, Kim SY, Rhee JH, Shin SH. Vibrio vulnificus vulnibactin, but not metalloprotease VvpE, is essentially required for iron uptake from human holotransferrin. Biol. Pharm. Bull. 2006;29:911–918. doi: 10.1248/bpb.29.911. [DOI] [PubMed] [Google Scholar]
- 30.Okujo N, Saito M, Yamamoto S, Yoshida T, Miyoshi S, Shinoda S. Structure of vulnibactin, a new polyamine containing siderophore from Vibrio vulnificus. Biometals. 1994;7:109–116. doi: 10.1007/BF00140480. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.