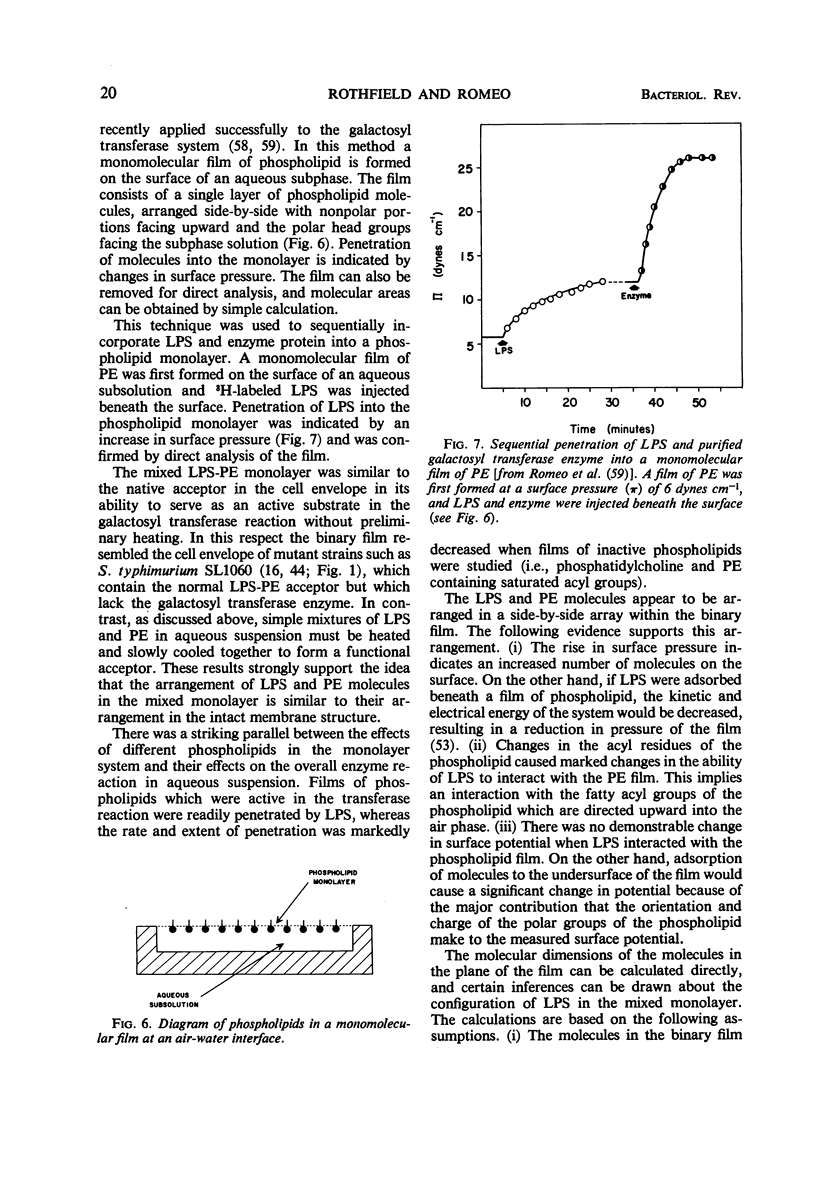
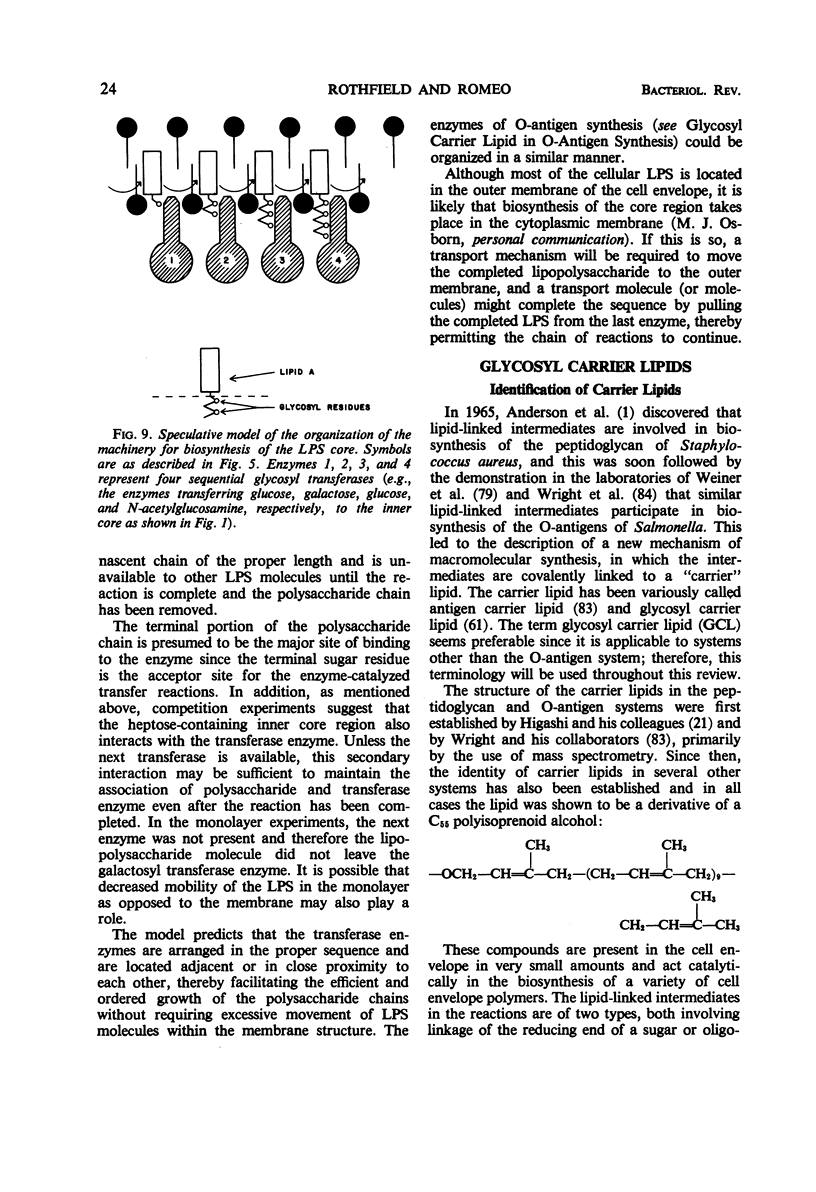
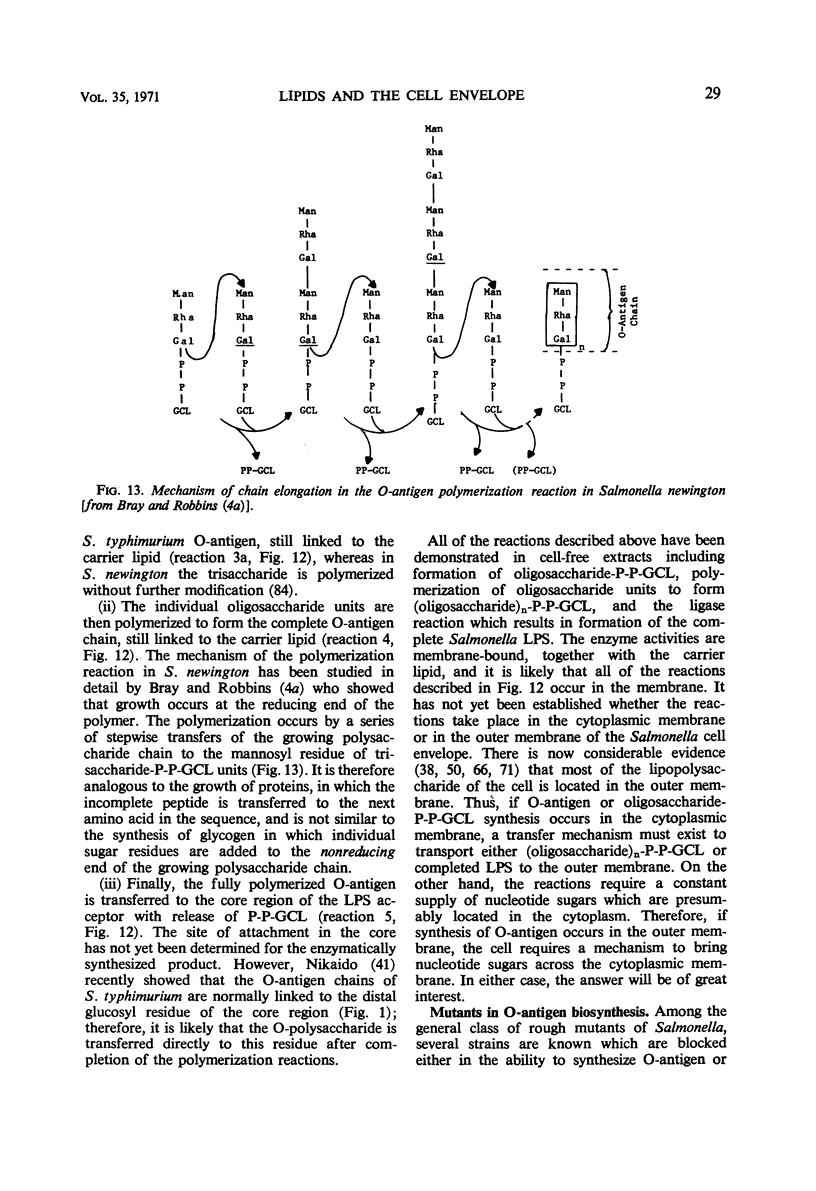
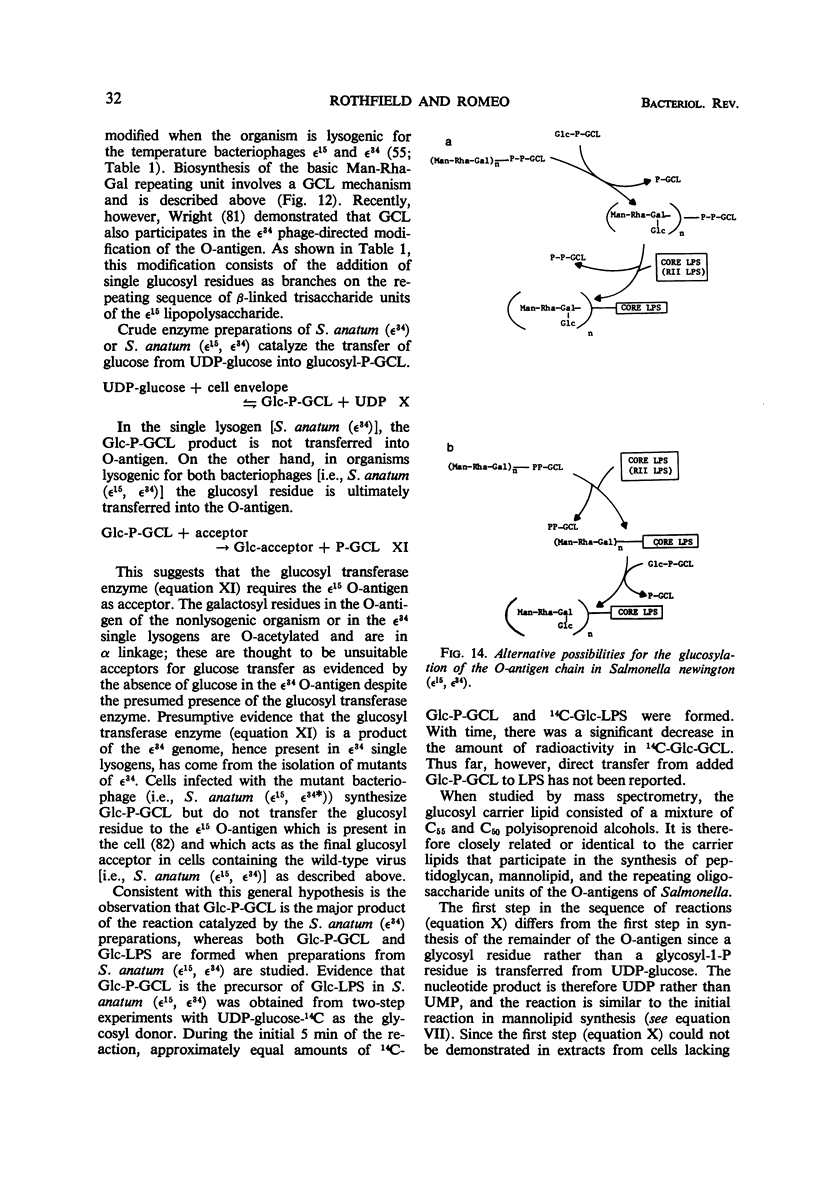
Full text
PDF




















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON J. S., MATSUHASHI M., HASKIN M. A., STROMINGER J. L. LIPID-PHOSPHOACETYLMURAMYL-PENTAPEPTIDE AND LIPID-PHOSPHODISACCHARIDE-PENTAPEPTIDE: PRESUMED MEMBRANE TRANSPORT INTERMEDIATES IN CELL WALL SYNTHESIS. Proc Natl Acad Sci U S A. 1965 Apr;53:881–889. doi: 10.1073/pnas.53.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BECKMANN I., SUBBAIAH T. V., STOCKER B. A. ROUGH MUTANTS OF SALMONELLA TYPHIMURIUM. II. SEROLOGICAL AND CHEMICAL INVESTIGATIONS. Nature. 1964 Mar 28;201:1299–1301. doi: 10.1038/2011299a0. [DOI] [PubMed] [Google Scholar]
- BURGER M. M., GLASER L., BURTON R. M. THE ENZYMATIC SYNTHESIS OF A RHAMNOSE-CONTAINING GLYCOLIPID BY EXTRACTS OF PSEUDOMONAS AERUGINOSA. J Biol Chem. 1963 Aug;238:2595–2602. [PubMed] [Google Scholar]
- BURGER M. M., GLASER L. THE SYNTHESIS OF TEICHOIC ACIDS. I. POLYGLYCEROPHOSPHATE. J Biol Chem. 1964 Oct;239:3168–3177. [PubMed] [Google Scholar]
- BURGER M., GLASER L., BURTON R. M. The synthesis of a rhamnolipid by enzyme preparations from Pseudomonas aeruginosa. Biochim Biophys Acta. 1962 Jan 1;56:172–174. doi: 10.1016/0006-3002(62)90544-9. [DOI] [PubMed] [Google Scholar]
- Behrens N. H., Leloir L. F. Dolichol monophosphate glucose: an intermediate in glucose transfer in liver. Proc Natl Acad Sci U S A. 1970 May;66(1):153–159. doi: 10.1073/pnas.66.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray D., Robbins P. W. Mechanism of epsilon-15 conversion studies with bacteriphage mutants. J Mol Biol. 1967 Dec 28;30(3):457–475. doi: 10.1016/0022-2836(67)90362-2. [DOI] [PubMed] [Google Scholar]
- Bray D., Robbins P. W. The direction of chain growth in Salmonella anatum O-antigen biosynthesis. Biochem Biophys Res Commun. 1967 Aug 7;28(3):334–339. doi: 10.1016/0006-291x(67)90314-2. [DOI] [PubMed] [Google Scholar]
- Brooks D., Baddiley J. A lipid intermediate in the synthesis of a poly-(N-acetylglucosamine 1-phosphate) from the wall of Staphylococcus lactis N.C.T.C. 2102. Biochem J. 1969 Nov;115(2):307–314. doi: 10.1042/bj1150307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burge R. E., Draper J. C. The structure of the cell wall of the Gram-negative bacterium Proteus vulgaris. 3. A lipopolysaccharide "unit membrane". J Mol Biol. 1967 Sep 14;28(2):205–210. doi: 10.1016/s0022-2836(67)80003-2. [DOI] [PubMed] [Google Scholar]
- CARDINI C. E., LELOIR L. F., CHIRIBOGA J. The biosynthesis of sucrose. J Biol Chem. 1955 May;214(1):149–155. [PubMed] [Google Scholar]
- CHATTERJEE A. N., PARK J. T. BIOSYNTHESIS OF CELL WALL MUCOPEPTIDE BY A PARTICULATE FRACTION FROM STAPHYLOCOCCUS AUREUS. Proc Natl Acad Sci U S A. 1964 Jan;51:9–16. doi: 10.1073/pnas.51.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccam J. F., Jackson J. J., Eylar E. H. The biosynthesis of mannose-containing glycoproteins: a possible lipid intermediate. Biochem Biophys Res Commun. 1969 May 22;35(4):505–511. doi: 10.1016/0006-291x(69)90375-1. [DOI] [PubMed] [Google Scholar]
- Christenson J. G., Gross S. K., Robbins P. W. Enzymatic synthesis of the antigen carrier lipid. J Biol Chem. 1969 Oct 25;244(20):5436–5439. [PubMed] [Google Scholar]
- Douglas L. J., Baddiley J. A lipid intermediate in the biosynthesis of a teichoic acid. FEBS Lett. 1968 Aug;1(2):114–116. doi: 10.1016/0014-5793(68)80034-1. [DOI] [PubMed] [Google Scholar]
- Edstrom R. D., Heath E. C. Sugar nucleotide transferases in Escherichia coli lipopolysaccharide biosynthesis. Biochem Biophys Res Commun. 1964 Aug 11;16(6):576–581. doi: 10.1016/0006-291x(64)90195-0. [DOI] [PubMed] [Google Scholar]
- Endo A., Rothfield L. Studies of a phospholipid-requiring bacterial enzyme. I. Purification and properties of uridine diphosphate galactose: lipopolysaccharide alpha-3-galactosyl transferase. Biochemistry. 1969 Sep;8(9):3500–3507. doi: 10.1021/bi00837a003. [DOI] [PubMed] [Google Scholar]
- Endo A., Rothfield L. Studies of a phospholipid-requiring bacterial enzyme. II. The role of phospholipid in the uridine diphosphate galactose: lipopolysaccharide alpha-3-galactosyl transferase reaction. Biochemistry. 1969 Sep;8(9):3508–3515. doi: 10.1021/bi00837a004. [DOI] [PubMed] [Google Scholar]
- FRAENKEL D., OSBORN M. J., HORECKER B. L., SMITH S. M. Metabolism and cell wall structure of a mutant of Salmonella typhimurium deficient in phosphoglucose isomerase. Biochem Biophys Res Commun. 1963 Jun 20;11:423–428. doi: 10.1016/0006-291x(63)90086-x. [DOI] [PubMed] [Google Scholar]
- FUKASAWA T., JOKURA K., KURAHASHI K. A new enzymic defect of galactose metabolism in Escherichia coli K-12 mutants. Biochem Biophys Res Commun. 1962 Apr 3;7:121–125. doi: 10.1016/0006-291x(62)90158-4. [DOI] [PubMed] [Google Scholar]
- FUKASAWA T., NIKAIDO H. Galactose-sensitive mutants of Salmonella. II. Bacteriolysis induced by galactose. Biochim Biophys Acta. 1961 Apr 15;48:470–483. doi: 10.1016/0006-3002(61)90045-2. [DOI] [PubMed] [Google Scholar]
- Gemski P., Jr, Stocker B. A. Transduction by bacteriophage P22 in nonsmooth mutants of Salmonella typhimurium. J Bacteriol. 1967 May;93(5):1588–1597. doi: 10.1128/jb.93.5.1588-1597.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi Y., Strominger J. L., Sweeley C. C. Structure of a lipid intermediate in cell wall peptidoglycan synthesis: a derivative of a C55 isoprenoid alcohol. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1878–1884. doi: 10.1073/pnas.57.6.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaki K., Matsuhashi M., Strominger J. L. Glycopeptide transpeptidase and D-alanine carboxypeptidase: penicillin-sensitive enzymatic reactions. Proc Natl Acad Sci U S A. 1966 Mar;55(3):656–663. doi: 10.1073/pnas.55.3.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUFMAN B., KUNDIG D., DISTLER J., ROSEMAN S. ENZYMATIC SYNTHESIS AND STRUCTURE OF TWO GLYCOLIPIDS FROM TYPE XIV PNEUMOCOCCUS. Biochem Biophys Res Commun. 1965 Feb 3;18:312–318. doi: 10.1016/0006-291x(65)90705-9. [DOI] [PubMed] [Google Scholar]
- Katz W., Matsuhashi M., Dietrich C. P., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. IV. Incorporation of glycine in Micrococcus lysodeikticus. J Biol Chem. 1967 Jul 10;242(13):3207–3217. [PubMed] [Google Scholar]
- Kauss H. A plant mannosyl-lipid acting in reversible transfer of mannose. FEBS Lett. 1969 Sep;5(1):81–84. doi: 10.1016/0014-5793(69)80298-x. [DOI] [PubMed] [Google Scholar]
- Kent J. L., Osborn M. J. Properties of the O-specific hapten formed in vivo by mutant strains of Salmonella typhimurium. Biochemistry. 1968 Dec;7(12):4396–4408. doi: 10.1021/bi00852a036. [DOI] [PubMed] [Google Scholar]
- LELOIR L. F., CARDINI C. E. The biosynthesis of sucrose phosphate. J Biol Chem. 1955 May;214(1):157–165. [PubMed] [Google Scholar]
- Lahav M., Chiu T. H., Lennarz W. J. Studies on the biosynthesis of mannan in Micrococcus lysodeikticus. II. The enzymatic synthesis of mannosyl-l-phosphoryl-undecaprenol. J Biol Chem. 1969 Nov 10;244(21):5890–5898. [PubMed] [Google Scholar]
- Lopes J., Inniss W. E. Electron microscopic study of lipopolysaccharide from an avian strain of Escherichia coli O18. J Bacteriol. 1970 Jul;103(1):238–243. doi: 10.1128/jb.103.1.238-243.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losick R., Robbins P. W. Mechanism of epsilon-15 conversion studies with a bacterial mutant. J Mol Biol. 1967 Dec 28;30(3):445–455. doi: 10.1016/0022-2836(67)90361-0. [DOI] [PubMed] [Google Scholar]
- Lüderitz O., Staub A. M., Westphal O. Immunochemistry of O and R antigens of Salmonella and related Enterobacteriaceae. Bacteriol Rev. 1966 Mar;30(1):192–255. doi: 10.1128/br.30.1.192-255.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malchow D., Lüderitz O., Kickhöfen B., Westphal O., Gerisch G. Polysaccharides in vegetative and aggregation-competent amoebae of Dictyostelium discoideum. 2. Purification and characterization of amoeba-degraded bacterial polysaccharides. Eur J Biochem. 1969 Jan;7(2):239–246. doi: 10.1111/j.1432-1033.1969.tb19598.x. [DOI] [PubMed] [Google Scholar]
- Matsuhashi M., Dietrich C. P., Strominger J. L. Incorporation of glycine into the cell wall glycopeptide in Staphylococcus aureus: role of sRNA and lipid intermediates. Proc Natl Acad Sci U S A. 1965 Aug;54(2):587–594. doi: 10.1073/pnas.54.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadow P. M., Anderson J. S., Strominger J. L. Enzymatic polymerization of UDP-acetylmuramyl.L-ala.D-glu.L-lys.D-ala.D-ala and UDP-acetylglucosamine by a particulate enzyme from Staphylococcus aureus and its inhibition by antibiotics. Biochem Biophys Res Commun. 1964;14:382–387. doi: 10.1016/s0006-291x(64)80014-0. [DOI] [PubMed] [Google Scholar]
- Mergenhagen S. E., Bladen H. A., Hsu K. C. Electron microscopic localization of endotoxic lipopolysaccharide in gram-negative organisms. Ann N Y Acad Sci. 1966 Jun 30;133(2):279–291. doi: 10.1111/j.1749-6632.1966.tb52371.x. [DOI] [PubMed] [Google Scholar]
- NAIDE Y., NIKAIDO H., MAEKELAE P. H., WILKINSON R. G., STOCKER B. A. SEMIROUGH STRAINS OF SALMONELLA. Proc Natl Acad Sci U S A. 1965 Jan;53:147–153. doi: 10.1073/pnas.53.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIKAIDO H. Galactose-sensitive mutants of Salmonella. I. Metabolism of galactose. Biochim Biophys Acta. 1961 Apr 15;48:460–469. doi: 10.1016/0006-3002(61)90044-0. [DOI] [PubMed] [Google Scholar]
- NIKAIDO H. Studies on the biosynthesis of cell-wall polysaccharide in mutant strains of Salmonella. I. Proc Natl Acad Sci U S A. 1962 Aug;48:1337–1341. doi: 10.1073/pnas.48.8.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Nikaido K., Mäkelä P. H. Genetic determination of enzymes synthesizing O-specific sugars of Salmonella lipopolysaccharides. J Bacteriol. 1966 Mar;91(3):1126–1135. doi: 10.1128/jb.91.3.1126-1135.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H. Structure of cell wall lipopolysaccharide from Salmonella typhimurium. I. Linkage between o side chains and R core. J Biol Chem. 1969 Jun 10;244(11):2835–2845. [PubMed] [Google Scholar]
- OSBORN M. J., ROSEN S. M., ROTHFIELD L., HORECKER B. L. Biosynthesis of bacterial lipopolysaccharide. I. Enzymatic incorporation of galactose in a mutant strain of Salmonella. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1831–1838. doi: 10.1073/pnas.48.10.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J. Biochemical characterization of mutants of Salmonella typhimurium lacking glucosyl or galactosyl lipopolysaccharide transferases. Nature. 1968 Mar 9;217(5132):957–960. doi: 10.1038/217957a0. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., D'Ari L. Enzymatic incorporation of N-acetylglucosamine into cell wall lipopolysaccharide in a mutant strain of Salmonella typhimurium. Biochem Biophys Res Commun. 1964 Aug 11;16(6):568–575. doi: 10.1016/0006-291x(64)90194-9. [DOI] [PubMed] [Google Scholar]
- Osborn M. J. Structure and biosynthesis of the bacterial cell wall. Annu Rev Biochem. 1969;38:501–538. doi: 10.1146/annurev.bi.38.070169.002441. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Tze-Yuen R. Y. Biosynthesis of bacterial lipopolysaccharide. VII. Enzymatic formation of the first intermediate in biosynthesis of the O-antigen of Salmonella typhimurium. J Biol Chem. 1968 Oct 10;243(19):5145–5152. [PubMed] [Google Scholar]
- Osborn M. J., Weiner I. M. Biosynthesis of a bacterial lipopolysaccharide. VI. Mechanism of incorporation of abequose into the O-antigen of Salmonella typhimurium. J Biol Chem. 1968 May 25;243(10):2631–2639. [PubMed] [Google Scholar]
- Petit J. F., Strominger J. L., Söll D. Biosynthesis of the peptidoglycan of bacterial cell walls. VII. Incorporation of serine and glycine into interpeptide bridges in Staphylococcus epidermidis. J Biol Chem. 1968 Feb 25;243(4):757–767. [PubMed] [Google Scholar]
- ROBBINS P. W., UCHIDA T. Studies on the chemical basis of the phage conversion of O-antigens in the E-group Salmonellae. Biochemistry. 1962 Mar;1:323–335. doi: 10.1021/bi00908a020. [DOI] [PubMed] [Google Scholar]
- ROTHFIELD L., HORECKER B. L. THE ROLE OF CELL-WALL LIPID IN THE BIOSYNTHESIS OF BACTERIAL LIPOPOLYSACCHARIDE. Proc Natl Acad Sci U S A. 1964 Oct;52:939–946. doi: 10.1073/pnas.52.4.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTHFIELD L., OSBORN M. J., HORECKER B. L. BIOSYNTHESIS OF BACTERIAL LIPOPOLYSACCHARIDE. II. INCORPORATION OF GLUCOSE AND GALACTOSE CATALYZED BY PARTICULATE AND SOLUBLE ENZYMES IN SALMONELLA. J Biol Chem. 1964 Sep;239:2788–2795. [PubMed] [Google Scholar]
- Roberts W. S., Petit J. F., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. 8. Specificity in the utilization of L-alanyl transfer ribonucleic acid for interpeptide bridge synthesis in Arthrobacter crystallopoietes. J Biol Chem. 1968 Feb 25;243(4):768–772. [PubMed] [Google Scholar]
- Roberts W. S., Strominger J. L., Söll D. Biosynthesis of the peptidoglycan of bacterial cell walls. VI. Incorporation of L-threonine into interpeptide bridges in Micrococcus roseus. J Biol Chem. 1968 Feb 25;243(4):749–756. [PubMed] [Google Scholar]
- Romeo D., Girard A., Rothfield L. Reconstitution of a functional membrane enzyme system in a monomolecular film. I. Formation of a mixed monolayer of lipopolysaccharide and phospholipid. J Mol Biol. 1970 Nov 14;53(3):475–490. doi: 10.1016/0022-2836(70)90078-1. [DOI] [PubMed] [Google Scholar]
- Romeo D., Hinckley A., Rothfield L. Reconstitution of a functional membrane enzyme system in a monomolecular film. II. Formation of a functional ternary film of lipopolysaccharide, phospholipid and transferase enzyme. J Mol Biol. 1970 Nov 14;53(3):491–501. doi: 10.1016/0022-2836(70)90079-3. [DOI] [PubMed] [Google Scholar]
- Rosen S. M., Zeleznick L. D., Fraenkel D., Wiener I. M., Osborn M. J., Horecker B. L. Characterization of the cell wall lipopolysaccharide of a mutant of Salmonella typhimurium lacking phosphomannose isomerase. Biochem Z. 1965 Aug 19;342(4):375–386. [PubMed] [Google Scholar]
- Rothfield L., Finkelstein A. Membrane biochemistry. Annu Rev Biochem. 1968;37:463–496. doi: 10.1146/annurev.bi.37.070168.002335. [DOI] [PubMed] [Google Scholar]
- Rothfield L., Horne R. W. Reassociation of purified lipopolysaccharide and phospholipid of the bacterial cell envelope: electron microscopic and monolayer studies. J Bacteriol. 1967 May;93(5):1705–1721. doi: 10.1128/jb.93.5.1705-1721.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothfield L., Pearlman-Kothencz M. Synthesis and assembly of bacterial membrane components. A lipopolysaccharide-phospholipid-protein complex excreted by living bacteria. J Mol Biol. 1969 Sep 28;44(3):477–492. doi: 10.1016/0022-2836(69)90374-x. [DOI] [PubMed] [Google Scholar]
- Rothfield L., Pearlman M. The role of cell envelope phospholipid in the enzymatic synthesis of bacterial lipopolysaccharide. Structural requirements of the phospholipid molecule. J Biol Chem. 1966 Mar 25;241(6):1386–1392. [PubMed] [Google Scholar]
- Rothfield L., Takeshita M., Pearlman M., Horne R. W. Role of phospholipids in the enzymatic synthesis of the bacterial cell envelope. Fed Proc. 1966 Sep-Oct;25(5):1495–1502. [PubMed] [Google Scholar]
- STRUVE W. G., NEUHAUS F. C. EVIDENCE FOR AN INITIAL ACCEPTOR OF UDP-NAC-MURAMYL-PENTAPEPTIDE IN THE SYNTHESIS OF BACTERIAL MUCOPEPTIDE. Biochem Biophys Res Commun. 1965 Jan 4;18:6–12. doi: 10.1016/0006-291x(65)90873-9. [DOI] [PubMed] [Google Scholar]
- SUNDARARAJAN T. A., RAPIN A. M., KALCKAR H. M. Biochemical observations on E. coli mutants defective in uridine diphosphoglucose. Proc Natl Acad Sci U S A. 1962 Dec 15;48:2187–2193. doi: 10.1073/pnas.48.12.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher M., Lennarz W. J. Studies on the biosynthesis of mannan in Micrococcus lysodeikticus. I. Characterization of mannan-14C formed enzymatically from mannosyl-1-phosphoryl-undecaprenol. J Biol Chem. 1969 May 25;244(10):2777–2789. [PubMed] [Google Scholar]
- Scher M., Lennarz W. J., Sweeley C. C. The biosynthesis of mannosyl-1-phosphoryl-polyisoprenol in Micrococcus lysodeikticus and its role in mannan synthesis. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1313–1320. doi: 10.1073/pnas.59.4.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shands J. W. Localization of somatic antigen on gram-negative bacteria using ferritin antibody conjugates. Ann N Y Acad Sci. 1966 Jun 30;133(2):292–298. doi: 10.1111/j.1749-6632.1966.tb52372.x. [DOI] [PubMed] [Google Scholar]
- Siewert G., Strominger J. L. Bacitracin: an inhibitor of the dephosphorylation of lipid pyrophosphate, an intermediate in the biosynthesis of the peptidoglycan of bacterial cell walls. Proc Natl Acad Sci U S A. 1967 Mar;57(3):767–773. doi: 10.1073/pnas.57.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siewert G., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. XI. Formation of the isoglutamine amide group in the cell walls of Staphylococcus aureus. J Biol Chem. 1968 Feb 25;243(4):783–790. [PubMed] [Google Scholar]
- Tanner W. A lipid intermediate in mannan biosynthesis in yeast. Biochem Biophys Res Commun. 1969 Apr 10;35(1):144–150. doi: 10.1016/0006-291x(69)90496-3. [DOI] [PubMed] [Google Scholar]
- Troy F. A., Frerman F. E., Heath E. C. The biosynthesis of capsular polysaccharide in Aerobacter aerogenes. J Biol Chem. 1971 Jan 10;246(1):118–133. [PubMed] [Google Scholar]
- Weiner I. M., Higuchi T., Rothfield L., Saltmarsh-Andrew M., Osborn M. J., Horecker B. L. Biosynthesis of bacterial lipopolysaccharide. V. Lipid-linked intermediates in the biosynthesis of the O-antigen groups of Salmonella typhimurium. Proc Natl Acad Sci U S A. 1965 Jul;54(1):228–235. doi: 10.1073/pnas.54.1.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser M. M., Rothfield L. The reassociation of lipopolysaccharide, phospholipid, and transferase enzymes of the bacterial cell envelope. Isolation of binary and ternary complexes. J Biol Chem. 1968 Mar 25;243(6):1320–1328. [PubMed] [Google Scholar]
- Wright A., Barzilai N. Isolation and haracterization nonconverting mutants of bacteriophage epsilon 34. J Bacteriol. 1971 Mar;105(3):937–939. doi: 10.1128/jb.105.3.937-939.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright A., Dankert M., Fennessey P., Robbins P. W. Characterization of a polyisoprenoid compound functional in O-antigen biosynthesis. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1798–1803. doi: 10.1073/pnas.57.6.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright A., Dankert M., Robbins P. W. Evidence for an intermediate stage in the biosynthesis of the Salmonella O-antigen. Proc Natl Acad Sci U S A. 1965 Jul;54(1):235–241. doi: 10.1073/pnas.54.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright A. Mechanism of conversion of the salmonella O antigen by bacteriophageepsilon 34. J Bacteriol. 1971 Mar;105(3):927–936. doi: 10.1128/jb.105.3.927-936.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]



