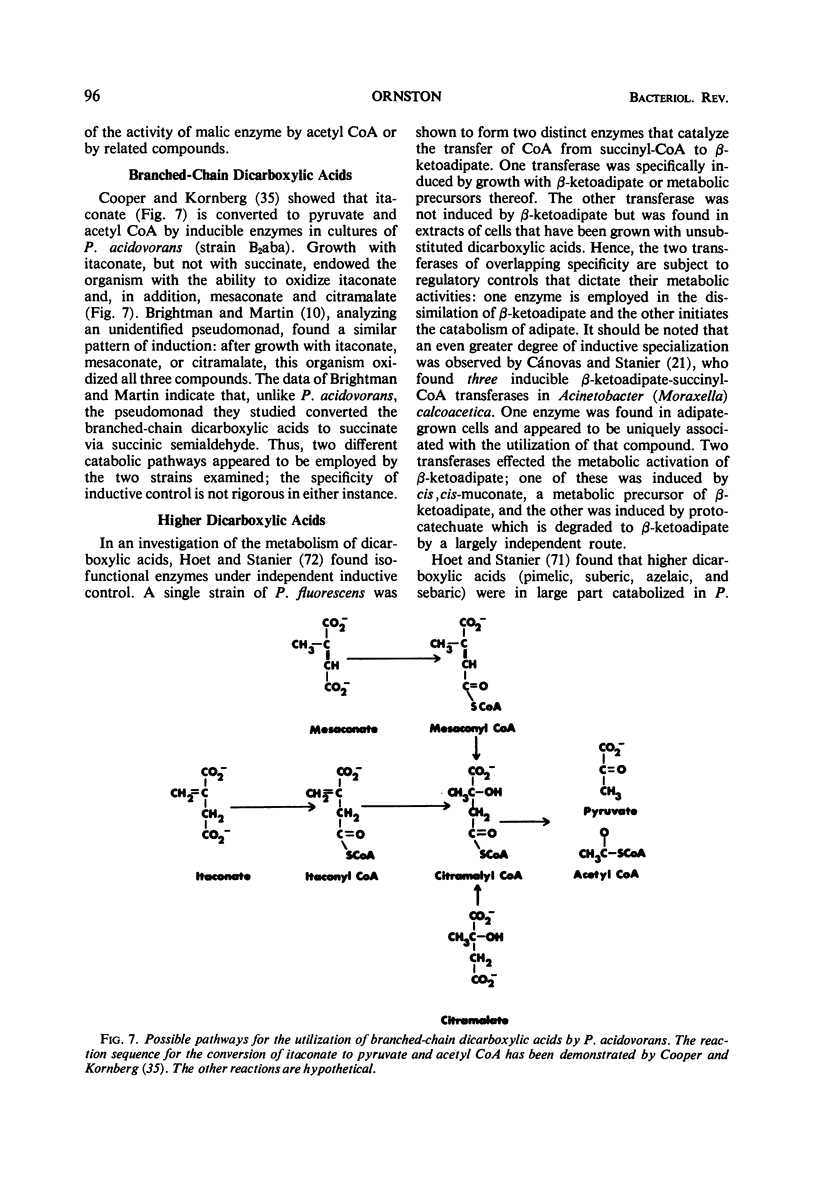
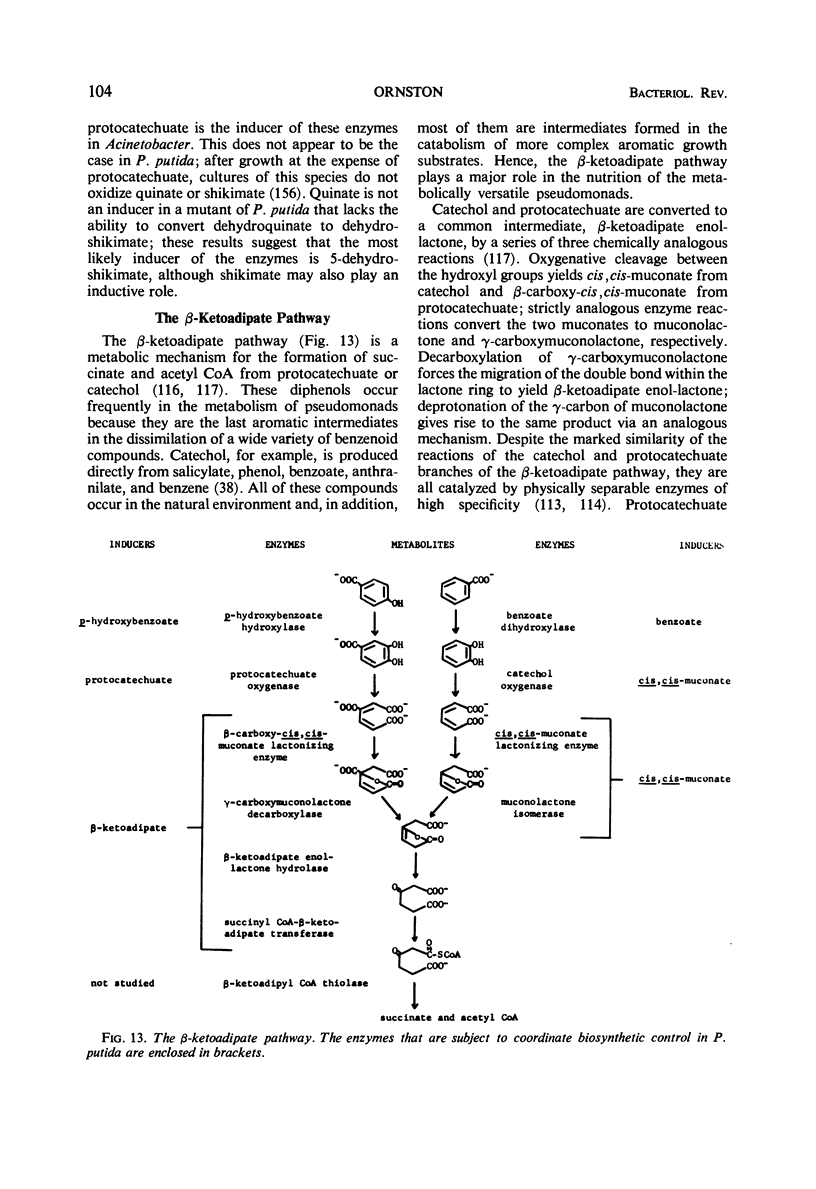
Full text
PDF



























Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AZOULAY E., SENEZ J. C. [Bacterial degradation of paraffin hydrocarbons. II. Determination of intermediary products by the simultaneous adaptation method]. Ann Inst Pasteur (Paris) 1960 Jun;98:868–879. [PubMed] [Google Scholar]
- Adams E., Rosso G. Alpha-ketoglutaric semialdehyde dehydrogenase of Pseudomonas. Properties of the purified enzyme induced by hydroxyproline and of the glucarate-induced and constitutive enzymes. J Biol Chem. 1967 Apr 25;242(8):1802–1814. [PubMed] [Google Scholar]
- BEHRMAN E. J. Tryptophan metabolism in Pseudomonas. Nature. 1962 Oct 13;196:150–152. doi: 10.1038/196150a0. [DOI] [PubMed] [Google Scholar]
- BURSTEIN C., COHN M., KEPES A., MONOD J. R OLE DU LACTOSE ET DE SES PRODUITS M'ETABOLIQUES DANS L'INDUCTION DE L'OP'ERON LACTOSE CHEZ ESCHERICHIA COLI. Biochim Biophys Acta. 1965 Apr 19;95:634–639. [PubMed] [Google Scholar]
- BUTTIN G. M'ECANISMES R'EGULATEURS DANS LA BIOSYNTH'ESE DES ENZYMES DU M'ETABOLISME DU GALACTOSE CHEZ ESCHERICHIA COLI K12. I. LA BIOSYNTH'ESE INDUITE DE LA GALACTOKINASE ET L'INDUCTION SIMULTAN'EE DE LA S'EQUENCE ENZYMATIQUE. J Mol Biol. 1963 Aug;7:164–182. doi: 10.1016/s0022-2836(63)80044-3. [DOI] [PubMed] [Google Scholar]
- Bannerjee D., Sanders L. E., Sokatch J. R. Properties of purified methylmalonate semialdehyde dehydrogenase of Pseudomonas aeruginosa. J Biol Chem. 1970 Apr 10;245(7):1828–1835. [PubMed] [Google Scholar]
- Bayly R. C., Dagley S., Gibson D. T. The metabolism of cresols by species of Pseudomonas. Biochem J. 1966 Nov;101(2):293–301. doi: 10.1042/bj1010293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird J. A., Cain R. B. cis-cis-Muconate, the product inducer of catechol 1,2-oxygenase in Pseudomonas aeruginosa. Biochem J. 1968 Sep;109(3):479–481. doi: 10.1042/bj1090479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakley E. R. The metabolism of aromatic compounds with different side chains by a pseudomonas. Can J Microbiol. 1967 Jul;13(7):761–769. doi: 10.1139/m67-101. [DOI] [PubMed] [Google Scholar]
- Brammar W. J., Clarke P. H., Skinner A. J. Biochemical and genetic studies with regulator mutants of the Pseudomonas aeruginosa 8602 amidase system. J Gen Microbiol. 1967 Apr;47(1):87–102. doi: 10.1099/00221287-47-1-87. [DOI] [PubMed] [Google Scholar]
- Brightman V., Martin W. R. PATHWAY FOR THE DISSIMILATION OF ITACONIC AND MESACONIC ACIDS. J Bacteriol. 1961 Sep;82(3):376–382. doi: 10.1128/jb.82.3.376-382.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill W. J., Magasanik B. Genetic and metabolic control of histidase and urocanase in Salmonella typhimurium, strain 15-59. J Biol Chem. 1969 Oct 10;244(19):5392–5402. [PubMed] [Google Scholar]
- Brown J. E., Brown P. R., Clarke P. H. Butyramide-utilizing mutants of Pseudomonas aeruginosa 8602 which produce an amidase with altered substrate specificity. J Gen Microbiol. 1969 Aug;57(2):273–285. doi: 10.1099/00221287-57-2-273. [DOI] [PubMed] [Google Scholar]
- CANOVAS J. L., KORNBERG H. L. FINE CONTROL OF PHOSPHOPYRUVATE CARBOXYLASE ACTIVITY IN ESCHERICHIA COLI. Biochim Biophys Acta. 1965 Jan;96:169–172. doi: 10.1016/0005-2787(65)90624-6. [DOI] [PubMed] [Google Scholar]
- COHN M. Contributions of studies on the beta-galactosidase of Escherichia coli to our understanding of enzyme synthesis. Bacteriol Rev. 1957 Sep;21(3):140–168. doi: 10.1128/br.21.3.140-168.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN M., MONOD J., POLLOCK M. R., SPIEGELMAN S., STANIER R. Y. Terminology of enzyme formation. Nature. 1953 Dec 12;172(4389):1096–1096. doi: 10.1038/1721096a0. [DOI] [PubMed] [Google Scholar]
- Cain R. B., Farr D. R. Metabolism of arylsulphonates by micro-organisms. Biochem J. 1968 Feb;106(4):859–877. doi: 10.1042/bj1060859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazzulo J. J., Sundaram T. K., Kornberg H. L. Regulation of pyruvate carboxylase formation from the apo-enzyme and biotin in a thermophilic bacillus. Nature. 1969 Sep 13;223(5211):1137–1138. doi: 10.1038/2231137a0. [DOI] [PubMed] [Google Scholar]
- Chakrabarty A. M., Gunsalus C. F., Gunsalus I. C. Transduction and the clustering of genes in fluorescent Pseudomonads. Proc Natl Acad Sci U S A. 1968 May;60(1):168–175. doi: 10.1073/pnas.60.1.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty A. M., Gunsalus I. C. Autonomous replication of a defective transducing phage in Pseudomonas putida. Virology. 1969 May;38(1):92–104. doi: 10.1016/0042-6822(69)90131-7. [DOI] [PubMed] [Google Scholar]
- Chakrabarty A. M., Gunsalus I. C. Defective phage and chromosome mobilization in Pseudomonas putida. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1217–1223. doi: 10.1073/pnas.64.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty A. M., Gunsalus I. C. Transduction and genetic homology between Pseudomonas species putida and aeruginosa. J Bacteriol. 1970 Sep;103(3):830–832. doi: 10.1128/jb.103.3.830-832.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty A. M., Niblack J. F., Gunsalus I. C. A phage-initiated polysaccharide depolymerase in Pseudomonas putida. Virology. 1967 Jul;32(3):532–534. doi: 10.1016/0042-6822(67)90305-4. [DOI] [PubMed] [Google Scholar]
- Chasin L. A., Magasanik B. Induction and repression of the histidine-degrading enzymes of Bacillus subtilis. J Biol Chem. 1968 Oct 10;243(19):5165–5178. [PubMed] [Google Scholar]
- Clarke P. H., Houldsworth M. A., Lilly M. D. Catabolite repression and the induction of amidase synthesis by Pseudomonas aeruginosa 8602 in continuous culture. J Gen Microbiol. 1968 Apr;51(2):225–234. doi: 10.1099/00221287-51-2-225. [DOI] [PubMed] [Google Scholar]
- Cooper R. A., Kornberg H. L. The utilization of itaconate by Pseudomonas sp. Biochem J. 1964 Apr;91(1):82–91. doi: 10.1042/bj0910082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cánovas J. L., Johnson B. F. Regulation of the enzymes of the beta-ketoadipate pathway in Moraxella calcoacetica. 4. Constitutive synthesis of beta-ketoadipate succinyl-CoA transferases II and 3. Eur J Biochem. 1968 Jan;3(3):312–317. doi: 10.1111/j.1432-1033.1968.tb19531.x. [DOI] [PubMed] [Google Scholar]
- Cánovas J. L., Johnson B. F., Wheelis M. L. Regulation of the enzymes of the beta-ketoadipate pathway in Moraxella calcoacetica. 3. Effects of 3-hydroxy-4-methylbenzoate on the synthesis of enzymes of the protocatechuate branch. Eur J Biochem. 1968 Jan;3(3):305–311. doi: 10.1111/j.1432-1033.1968.tb19530.x. [DOI] [PubMed] [Google Scholar]
- Cánovas J. L., Kornberg H. L. Properties and regulation of phosphopyruvate carboxylase activity in Escherichia coli. Proc R Soc Lond B Biol Sci. 1966 Aug 16;165(999):189–205. doi: 10.1098/rspb.1966.0064. [DOI] [PubMed] [Google Scholar]
- Cánovas J. L., Ornston L. N., Stanier R. Y. Evolutionary significance of metabolic control systems. The beta-ketoadipate pathway provides a case history in bacteria. Science. 1967 Jun 30;156(3783):1695–1699. doi: 10.1126/science.156.3783.1695. [DOI] [PubMed] [Google Scholar]
- Cánovas J. L., Stanier R. Y. Regulation of the enzymes of the beta-ketoadipate pathway in Moraxella calcoacetica. 1. General aspects. Eur J Biochem. 1967 May;1(3):289–300. doi: 10.1007/978-3-662-25813-2_40. [DOI] [PubMed] [Google Scholar]
- Cánovas J. L., Wheelis M. L., Stanier R. Y. Regulation of the enzymes of the beta-ketoadipate pathway in Moraxella calcoacetica. 2. The role of protocatechuate as inducer. Eur J Biochem. 1968 Jan;3(3):293–304. doi: 10.1111/j.1432-1033.1968.tb19529.x. [DOI] [PubMed] [Google Scholar]
- DAGLEY S., CHAPMAN P. J., GIBSON D. T., WOOD J. M. DEGRADATION OF THE BENZENE NUCLEUS BY BACTERIA. Nature. 1964 May 23;202:775–778. doi: 10.1038/202775a0. [DOI] [PubMed] [Google Scholar]
- DAGLEY S., EVANS W. C., RIBBONS D. W. New pathways in the oxidative metabolism of aromatic compounds by microorganisms. Nature. 1960 Nov 12;188:560–566. doi: 10.1038/188560a0. [DOI] [PubMed] [Google Scholar]
- DAGLEY S., GIBSON D. T. THE BACTERIAL DEGRADATION OF CATECHOL. Biochem J. 1965 May;95:466–474. doi: 10.1042/bj0950466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAGLEY S., TRUDGILL P. W. THE METABOLISM OF GALACTARATE, D-GLUCARATE AND VARIOUS PENTOSES BY SPECIES OF PSEUDOMONAS. Biochem J. 1965 Apr;95:48–58. doi: 10.1042/bj0950048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAGLEY S., TRUDGILL P. W. THE METABOLISM OF TARTARIC ACID BY A PSEUDOMONAS. A NEW PATHWAY. Biochem J. 1963 Oct;89:22–31. doi: 10.1042/bj0890022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. I., Evans W. C. Oxidative metabolism of naphthalene by soil pseudomonads. The ring-fission mechanism. Biochem J. 1964 May;91(2):251–261. doi: 10.1042/bj0910251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENGLESBERG E. Enzymatic characterization of 17 L-arabinose negative mutants of Escherichia coli. J Bacteriol. 1961 Jun;81:996–1006. doi: 10.1128/jb.81.6.996-1006.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENTNER N., DOUDOROFF M. Glucose and gluconic acid oxidation of Pseudomonas saccharophila. J Biol Chem. 1952 May;196(2):853–862. [PubMed] [Google Scholar]
- ENTNER N., STANIER R. Y. Studies on the oxidation of glucose by Pseudomonas fluorescens. J Bacteriol. 1951 Aug;62(2):181–186. doi: 10.1128/jb.62.2.181-186.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVANS W. C. THE MICROBIOLOGICAL DEGRADATION OF AROMATIC COMPOUNDS. J Gen Microbiol. 1963 Aug;32:177–184. doi: 10.1099/00221287-32-2-177. [DOI] [PubMed] [Google Scholar]
- Englesberg E., Irr J., Power J., Lee N. Positive control of enzyme synthesis by gene C in the L-arabinose system. J Bacteriol. 1965 Oct;90(4):946–957. doi: 10.1128/jb.90.4.946-957.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARGIE B., HOLLOWAY B. W. ABSENCE OF CLUSTERING OF FUNCTIONALLY RELATED GENES IN PSEUDOMONAS AERUGINOSA. Genet Res. 1965 Jul;6:284–299. doi: 10.1017/s0016672300004158. [DOI] [PubMed] [Google Scholar]
- Farr D. R., Cain R. B. Catechol oxygenase induction in Pseudomonas aeruginosa. Biochem J. 1968 Feb;106(4):879–885. doi: 10.1042/bj1060879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feist C. F., Hegeman G. D. Phenol and benzoate metabolism by Pseudomonas putida: regulation of tangential pathways. J Bacteriol. 1969 Nov;100(2):869–877. doi: 10.1128/jb.100.2.869-877.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feist C. F., Hegeman G. D. Regulation of the meta cleavage pathway for benzoate oxidation by Pseudomonas putida. J Bacteriol. 1969 Nov;100(2):1121–1123. doi: 10.1128/jb.100.2.1121-1123.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUNSALUS C. F., STANIER R. Y., GUNSALUS I. C. The enzymatic conversion of mandelic acid to benzoic acid. III. Fractionation and properties of the soluble enzymes. J Bacteriol. 1953 Nov;66(5):548–553. doi: 10.1128/jb.66.5.548-553.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUNTER S. E. The enzymatic oxidation of p-hydroxymandelic acid to p-hydroxybenzoic acid. J Bacteriol. 1953 Sep;66(3):341–346. doi: 10.1128/jb.66.3.341-346.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson D. T. Microbial degradation of aromatic compounds. Science. 1967 Sep 13;161(3846):1093–1097. [PubMed] [Google Scholar]
- Gilbert W., Müller-Hill B. Isolation of the lac repressor. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1891–1898. doi: 10.1073/pnas.56.6.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryder R. M., Adams E. Inducible degradation of hydroxyproline in Pseudomonas putida: pathway regulation and hydroxyproline uptake. J Bacteriol. 1969 Jan;97(1):292–306. doi: 10.1128/jb.97.1.292-306.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunsalus I. C., Bertland A. U., 2nd, Jacobson L. A. Enzyme induction and repression in anabolic and catabolic pathways. Arch Mikrobiol. 1967;59(1):113–122. doi: 10.1007/BF00406322. [DOI] [PubMed] [Google Scholar]
- HAYAISHI O., STANIER R. Y. The bacterial oxidation of tryptophan. III. Enzymatic activities of cell-free extracts from bacteria employing the aromatic pathway. J Bacteriol. 1951 Dec;62(6):691–709. doi: 10.1128/jb.62.6.691-709.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLOWAY B. W. Genetic recombination in Pseudomonas aeruginosa. J Gen Microbiol. 1955 Dec;13(3):572–581. doi: 10.1099/00221287-13-3-572. [DOI] [PubMed] [Google Scholar]
- HOLLOWAY B. W., HODGINS L., FARGIE B. UNLINKED LOCI AFFECTING RELATED BIOSYNTHETIC STEPS IN PSEUDOMONAS AERUGINOSA. Nature. 1963 Aug 31;199:926–927. doi: 10.1038/199926a0. [DOI] [PubMed] [Google Scholar]
- HOLLOWAY B. W., MONK M. Transduction in Pseudomonas aeruginosa. Nature. 1959 Oct 31;184(Suppl 18):1426–1427. doi: 10.1038/1841426b0. [DOI] [PubMed] [Google Scholar]
- HURLBERT R. E., JAKOBY W. B. TARTARIC ACID METABOLISM. I. SUBUNITS OF L(+)-TARTARIC ACID DEHYDRASE. J Biol Chem. 1965 Jul;240:2772–2777. [PubMed] [Google Scholar]
- Hayaishi O. Crystalline oxygenases of pseudomonads. Bacteriol Rev. 1966 Dec;30(4):720–731. doi: 10.1128/br.30.4.720-731.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegeman G. D., Rosenberg E. Y., Kenyon G. L. Mandelic acid racemase from Pseudomonas putida. Purification and properties of the enzyme. Biochemistry. 1970 Oct 13;9(21):4029–4036. doi: 10.1021/bi00823a001. [DOI] [PubMed] [Google Scholar]
- Hegeman G. D., Rosenberg S. L. The evolution of bacterial enzyme systems. Annu Rev Microbiol. 1970;24:429–462. doi: 10.1146/annurev.mi.24.100170.002241. [DOI] [PubMed] [Google Scholar]
- Hegeman G. D. Synthesis of the enzymes of the mandelate pathway by Pseudomonas putida. 3. Isolation and properties of constitutive mutants. J Bacteriol. 1966 Mar;91(3):1161–1167. doi: 10.1128/jb.91.3.1161-1167.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegeman G. D. Synthesis of the enzymes of the mandelate pathway by Pseudomonas putida. I. Synthesis of enzymes by the wild type. J Bacteriol. 1966 Mar;91(3):1140–1154. doi: 10.1128/jb.91.3.1140-1154.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegeman G. D. Synthesis of the enzymes of the mandelate pathway by Pseudomonas putida. II. Isolation and properties of blocked mutants. J Bacteriol. 1966 Mar;91(3):1155–1160. doi: 10.1128/jb.91.3.1155-1160.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoet P. P., Stanier R. Y. Existence and functions of two enzymes with beta-ketoadipate: succinyl-CoA transferase activity in Pseudomonas florescens. Eur J Biochem. 1970 Mar 1;13(1):71–76. doi: 10.1111/j.1432-1033.1970.tb00900.x. [DOI] [PubMed] [Google Scholar]
- Hoet P. P., Stanier R. Y. The dissimilation of higher dicarboxylic acids by Pseudomonas fluorscens. Eur J Biochem. 1970 Mar 1;13(1):65–70. doi: 10.1111/j.1432-1033.1970.tb00899.x. [DOI] [PubMed] [Google Scholar]
- Holloway B. W. Genetics of Pseudomonas. Bacteriol Rev. 1969 Sep;33(3):419–443. doi: 10.1128/br.33.3.419-443.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz N. H. On the Evolution of Biochemical Syntheses. Proc Natl Acad Sci U S A. 1945 Jun;31(6):153–157. doi: 10.1073/pnas.31.6.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa K. Regulation of synthesis of early enzymes of p-hydroxybenzoate pathway in Pseudomonas putida. J Biol Chem. 1970 Oct 25;245(20):5304–5308. [PubMed] [Google Scholar]
- Hug D. H., Roth D., Hunter J. Regulation of histidine catabolism by succinate in Pseudomonas putida. J Bacteriol. 1968 Aug;96(2):396–402. doi: 10.1128/jb.96.2.396-402.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ICHIHARA A., ICHIHARA E. A. Metabolism of L-lysine by bacterial enzymes. V. Glutaric semialdehyde dehydrogenase. J Biochem. 1961 Feb;49:154–157. doi: 10.1093/oxfordjournals.jbchem.a127272. [DOI] [PubMed] [Google Scholar]
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- Jacobson L. A., Bartholomaus R. C., Gunsalus I. C. Repression of malic enzyme by acetate in Pseudomonas. Biochem Biophys Res Commun. 1966 Sep 22;24(6):955–960. doi: 10.1016/0006-291x(66)90343-3. [DOI] [PubMed] [Google Scholar]
- Jeffcoat R., Hassall H., Dagley S. The metabolism of D-glucarate by Pseudomonas acidovorans. Biochem J. 1969 Dec;115(5):969–976. doi: 10.1042/bj1150969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLY M., CLARKE P. H. An inducible amidase produced by a strain of Pseudomonas aeruginosa. J Gen Microbiol. 1962 Feb;27:305–316. doi: 10.1099/00221287-27-2-305. [DOI] [PubMed] [Google Scholar]
- Kemp M. B., Hegeman G. D. Genetic control of the beta-ketoadipate pathway in Pseudomonas aeruginosa. J Bacteriol. 1968 Nov;96(5):1488–1499. doi: 10.1128/jb.96.5.1488-1499.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn L. D., Jakoby W. B. Tartaric acid metabolism. 3. The formation of glyceric acid. J Biol Chem. 1968 May 25;243(10):2465–2471. [PubMed] [Google Scholar]
- Kohn L. D., Jakoby W. B. Tartaric acid metabolism. IV. Crystalline L-malic dehydrogenase from Pseudomonas acidovorans. J Biol Chem. 1968 May 25;243(10):2472–2478. [PubMed] [Google Scholar]
- Kohn L. D., Jakoby W. B. Tartaric acid metabolism. VI. Crystalline oxaloglycolate reductive decarboxylase. J Biol Chem. 1968 May 25;243(10):2486–2493. [PubMed] [Google Scholar]
- Kohn L. D., Jakoby W. B. Tartaric acid metabolism. VII. Crystalline hydroxypyruvate reductase (D-glycerate dehydrogenase). J Biol Chem. 1968 May 25;243(10):2494–2499. [PubMed] [Google Scholar]
- Kohn L. D., Packman P. M., Allen R. H., Jakoby W. B. Tartaric acid metabolism. V. Crystalline tartrate dehydrogenase. J Biol Chem. 1968 May 25;243(10):2479–2485. [PubMed] [Google Scholar]
- LERNER S. A., WU T. T., LIN E. C. EVOLUTION OF A CATABOLIC PATHWAY IN BACTERIA. Science. 1964 Dec 4;146(3649):1313–1315. doi: 10.1126/science.146.3649.1313. [DOI] [PubMed] [Google Scholar]
- LEWIS E. B. Pseudoallelism and gene evolution. Cold Spring Harb Symp Quant Biol. 1951;16:159–174. doi: 10.1101/sqb.1951.016.01.014. [DOI] [PubMed] [Google Scholar]
- LOUTIT J. S. A transduction-like process within a single strain of Pseudomonas aeruginosa. J Gen Microbiol. 1958 Apr;18(2):315–319. doi: 10.1099/00221287-18-2-315. [DOI] [PubMed] [Google Scholar]
- Laishley E. J., Bernlohr R. W. Regulation of arginine and proline catabolism in Bacillus licheniformis. J Bacteriol. 1968 Aug;96(2):322–329. doi: 10.1128/jb.96.2.322-329.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessie T. G., Neidhardt F. C. Formation and operation of the histidine-degrading pathway in Pseudomonas aeruginosa. J Bacteriol. 1967 Jun;93(6):1800–1810. doi: 10.1128/jb.93.6.1800-1810.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessie T., Neidhardt F. C. Adenosine triphosphate-linked control of Pseudomonas aeruginosa glucose-6-phosphate dehydrogenase. J Bacteriol. 1967 Apr;93(4):1337–1345. doi: 10.1128/jb.93.4.1337-1345.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loutit J. S., Pearce L. E., Marinus M. G. Investigation of the mating system of Pseudomonas aeruginosa strain 1. I. Kinetic studies. Genet Res. 1968 Aug;12(1):29–36. doi: 10.1017/s0016672300011587. [DOI] [PubMed] [Google Scholar]
- MAAS W. K., MCFALL E. GENETIC ASPECTS OF METABOLIC CONTROL. Annu Rev Microbiol. 1964;18:95–110. doi: 10.1146/annurev.mi.18.100164.000523. [DOI] [PubMed] [Google Scholar]
- MANDELSTAM J., JACOBY G. A. INDUCTION AND MULTI-SENSITIVE END-PRODUCT REPRESSION IN THE ENZYMIC PATHWAY DEGRADING MANDELATE IN PSEUDOMONAS FLUORESCENS. Biochem J. 1965 Mar;94:569–577. doi: 10.1042/bj0940569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN W. R., FOSTER J. W. Adaptation patterns in the utilization of the stereo-isomers of tartaric acid by a pseudomonad. J Bacteriol. 1957 May;73(5):683–684. doi: 10.1128/jb.73.5.683-684.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCFALL E., MANDELSTAM J. SPECIFIC METABOLIC REPRESSION OF THREE INDUCED ENZYMES IN ESCHERICHIA COLI. Biochem J. 1963 Nov;89:391–398. doi: 10.1042/bj0890391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan M. J., Kornberg H. L. Regulation of sugar accumulation by Escherichia coli. FEBS Lett. 1969 Apr;3(1):53–56. doi: 10.1016/0014-5793(69)80095-5. [DOI] [PubMed] [Google Scholar]
- Newell C. P., Lessie T. G. Induction of histidine-degrading enzymes in Pseudomonas aeruginosa. J Bacteriol. 1970 Oct;104(1):596–598. doi: 10.1128/jb.104.1.596-598.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORNSTON L. N., STANIER R. Y. MECHANISM OF BETA-KETOADIPATE FORMATION BY BACTERIA. Nature. 1964 Dec 26;204:1279–1283. doi: 10.1038/2041279a0. [DOI] [PubMed] [Google Scholar]
- Ornston L. N., Stanier R. Y. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. J Biol Chem. 1966 Aug 25;241(16):3776–3786. [PubMed] [Google Scholar]
- Ornston L. N. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. 3. Enzymes of the catechol pathway. J Biol Chem. 1966 Aug 25;241(16):3795–3799. [PubMed] [Google Scholar]
- Ornston L. N. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. II. Enzymes of the protocatechuate pathway. J Biol Chem. 1966 Aug 25;241(16):3787–3794. [PubMed] [Google Scholar]
- Ornston L. N. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. IV. Regulation. J Biol Chem. 1966 Aug 25;241(16):3800–3810. [PubMed] [Google Scholar]
- PALLERONI N. J., STANIER R. Y. REGULATORY MECHANISMS GOVERNING SYNTHESIS OF THE ENZYMES FOR TRYPTOPHAN OXIDATION BY PSEUDOMONAS FLUORESCENS. J Gen Microbiol. 1964 May;35:319–334. doi: 10.1099/00221287-35-2-319. [DOI] [PubMed] [Google Scholar]
- Ptashne M. ISOLATION OF THE lambda PHAGE REPRESSOR. Proc Natl Acad Sci U S A. 1967 Feb;57(2):306–313. doi: 10.1073/pnas.57.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIBBONS D. W., EVANS W. C. Oxidative metabolism of protocatechuic acid by certain soil pseudomonads: a new ring-fission mechanism. Biochem J. 1962 Jun;83:482–492. doi: 10.1042/bj0830482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitz M. S., Rodwell V. W. Alpha-hydroxyglutarate oxidoreductase of Pseudomonas putida. J Bacteriol. 1969 Nov;100(2):708–714. doi: 10.1128/jb.100.2.708-714.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribbons D. W. Metabolism of omicron-cresol by Pseudomonas aeruginosa strain T1. J Gen Microbiol. 1966 Aug;44(2):221–231. doi: 10.1099/00221287-44-2-221. [DOI] [PubMed] [Google Scholar]
- Riggs A. D., Bourgeois S. On the assay, isolation and characterization of the lac repressor. J Mol Biol. 1968 Jul 14;34(2):361–364. doi: 10.1016/0022-2836(68)90260-x. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. L., Hegeman G. D. Clustering of functionally related genes in Pseudomonas aeruginosa. J Bacteriol. 1969 Jul;99(1):353–355. doi: 10.1128/jb.99.1.353-355.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld H., Feigelson P. Product induction in Pseudomonas acidovorans of a permease system which transports L-tryptophan. J Bacteriol. 1969 Feb;97(2):705–714. doi: 10.1128/jb.97.2.705-714.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld H., Feigelson P. Synergistic and product induction of the enzymes of tryptophan metabolism in Pseudomonas acidovorans. J Bacteriol. 1969 Feb;97(2):697–704. doi: 10.1128/jb.97.2.697-704.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Amil M., Aparicio M. L., Canovas J. L. Regulation of the synthesis of glyceraldehyde-3-phosphate dehydrogenase in Pseudomonas putida. FEBS Lett. 1969 Apr;3(1):65–67. doi: 10.1016/0014-5793(69)80098-0. [DOI] [PubMed] [Google Scholar]
- SHILO M. The enzymic conversion of the tartaric acids to oxaloacetic acid. J Gen Microbiol. 1957 Apr;16(2):472–481. doi: 10.1099/00221287-16-2-472. [DOI] [PubMed] [Google Scholar]
- STANIER R. Y. Enzymatic adaptation in bacteria. Annu Rev Microbiol. 1951;5:35–56. doi: 10.1146/annurev.mi.05.100151.000343. [DOI] [PubMed] [Google Scholar]
- STANIER R. Y., GUNSALUS I. C., GUNSALUS C. F. The enzymatic conversion of mandelic acid to benzoic acid. II. Properties of the particulate fractions. J Bacteriol. 1953 Nov;66(5):543–547. doi: 10.1128/jb.66.5.543-547.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STANIER R. Y., HAYAISHI O., TSUCHIDA M. The bacterial oxidation of tryptophan. I. A general survey of the pathways. J Bacteriol. 1951 Oct;62(4):355–366. doi: 10.1128/jb.62.4.355-366.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STANIER R. Y., HAYAISHI O. The bacterial oxidation of tryptophan; a study in comparative biochemistry. Science. 1951 Sep 28;114(2961):326–330. doi: 10.1126/science.114.2961.326. [DOI] [PubMed] [Google Scholar]
- Sakaki Y., Kageyama M., Egami F. Effects of mitomycin C and other antibiotics on the inducible synthesis of protocatechuate 3,4-oxygenase in Pseudomonas aeruginosa. Z Allg Mikrobiol. 1969;9(2):143–152. doi: 10.1002/jobm.3630090207. [DOI] [PubMed] [Google Scholar]
- Sanwal B. D. Allosteric controls of amphilbolic pathways in bacteria. Bacteriol Rev. 1970 Mar;34(1):20–39. doi: 10.1128/br.34.1.20-39.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger S., Magasanik B. Imidazolepropionate, a nonmetabolizable inducer for the histidine-degrading enzymes in Aerobacter aerogenes. J Biol Chem. 1965 Nov;240(11):4325–4330. [PubMed] [Google Scholar]
- Schlesinger S., Scotto P., Magasanik B. Exogenous and endogenous induction of the histidine-degrading enzymes in Aerobacter aerogenes. J Biol Chem. 1965 Nov;240(11):4331–4337. [PubMed] [Google Scholar]
- Seidman M. M., Toms A., Wood J. M. Influence of side-chain substituents on the position of cleavage of the benzene ring by Pseudomonas fluorescens. J Bacteriol. 1969 Mar;97(3):1192–1197. doi: 10.1128/jb.97.3.1192-1197.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro J., Machattie L., Eron L., Ihler G., Ippen K., Beckwith J. Isolation of pure lac operon DNA. Nature. 1969 Nov 22;224(5221):768–774. doi: 10.1038/224768a0. [DOI] [PubMed] [Google Scholar]
- Shuster C. W., Doudoroff M. Purification of 2-keto-3-deoxy-6-phosphohexonate aldolases of Pseudomonas saccharophila. Arch Mikrobiol. 1967;59(1):279–286. doi: 10.1007/BF00406341. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y. Simultaneous Adaptation: A New Technique for the Study of Metabolic Pathways. J Bacteriol. 1947 Sep;54(3):339–348. doi: 10.1128/jb.54.3.339-348.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y. The Oxidation of Aromatic Compounds by Fluorescent Pseudomonads. J Bacteriol. 1948 Apr;55(4):477–494. doi: 10.1128/jb.55.4.477-494.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Wachter D., Gasser C., Wilson A. C. Comparative immunological studies of two Pseudomonas enzymes. J Bacteriol. 1970 May;102(2):351–362. doi: 10.1128/jb.102.2.351-362.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanisich V., Holloway B. W. Conjugation in Pseudomonas aeruginosa. Genetics. 1969 Feb;61(2):327–339. doi: 10.1093/genetics/61.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson I. L., Mandelstam J. Induction and multi-sensitive end-product repression in two converging pathways degrading aromatic substances in Pseudomonas fluorescens. Biochem J. 1965 Aug;96(2):354–362. doi: 10.1042/bj0960354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari N. P., Campbell J. J. Enzymatic control of the metabolic activity of Pseudomonas aeruginosa grown in glucose or succinate media. Biochim Biophys Acta. 1969 Dec 30;192(3):395–401. doi: 10.1016/0304-4165(69)90388-2. [DOI] [PubMed] [Google Scholar]
- Tresguerres M. E., de Torrontegui G., Ingledew W. M., Cánovas J. L. Regulation of the enzymes of the beta-ketoadipate pathway in Moraxella. Control of quinate oxidation by protocatechuate. Eur J Biochem. 1970 Jul;14(3):445–450. doi: 10.1111/j.1432-1033.1970.tb00309.x. [DOI] [PubMed] [Google Scholar]
- Wheelis M. L., Stanier R. Y. The genetic control of dissimilatory pathways in Pseudomonas putida. Genetics. 1970 Oct;66(2):245–266. doi: 10.1093/genetics/66.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C. A., Gunsalus I. C. Monoxygenases. VII. Camphor ketolactonase I and the role of three protein components. J Biol Chem. 1969 Nov 25;244(22):6149–6152. [PubMed] [Google Scholar]


