Abstract
Th9 cells are a new subset of helper T cells, and the signature cytokine for Th9 cells is IL-9. Both Th9 cells and Th9 products are implicated in multiple disease settings. Thus, a clear understanding of how Th9 cells are induced and controlled is an important and clinically relevant issue. There are different molecular pathways identified thus far in the induction of Th9 cells, and activation of such diverse pathways requires integration of signals from TGF-β and IL-4 cytokine receptors as well as costimulatory molecules. These signals converge on the induction of multiple transcription factors that collectively drive the development of Th9 cells.
Keywords: allergy, IL-9, inflammation, Th9
Introduction
IL-9 was originally cloned in 1989 from murine helper T-cell clones (1), so it is by no means a newly discovered cytokine. IL-9 per se has been extensively studied; IL-9 belongs to a family of cytokines that use the common IL-2Rγc for signal transduction, and similar to other family members (i.e. IL-2, IL-4, IL-7, IL-15 and IL-21), IL-9 was believed to be a T-cell growth factor and its chief function was to drive T-cell proliferation. But later studies showed that IL-9 has a weak effect in proliferation of primary T cells (2), despite the fact that proliferation of certain T-cell clones can be strongly stimulated by IL-9. Instead, IL-9 exhibits other functions, most noticeably in proliferation of mast cells, goblet cells and airway mucin-producing cells. Thus, in many ways, IL-9 is different from other γc cytokines as a T-cell growth factor. IL-9 signals through the JAK/STAT system. Specifically, upon binding to its cell surface receptor, which consists of a private IL-9Rα chain and the common γc, IL-9 induces recruitment of JAK1 and JAK3 to the IL-9Rα chain and the common γc, respectively, followed by cross-phosphorylation and activation of JAK1 and JAK3. This leads to the activation of STAT1, STAT3 and STAT5. Consequently, STAT1 and STAT5 form homodimers, while STAT1 and STAT3 form heterodimers, and such dimeric complexes translocate to the nucleus to drive transcription of IL-9-inducible genes (3). These gene products are involved in cell survival, proliferation and secretion of inflammatory mediators.
IL-9 is often seen in the context of Th2 cells in vitro or Th2-associated inflammatory conditions in vivo, especially in allergic inflammation (4, 5). Thus, for a long time, IL-9 was considered just another Th2 cytokine and thought to be redundant among other Th2 cytokines (i.e. IL-4, IL-5 and IL-13) (6, 7). Furthermore, IL-9 is not confined to Th2 cells, and other cell types including mast cells, NKT cells, Th17 cells or even Treg cells can become IL-9 producers (1, 8–15). Moreover, a recent study demonstrated by using IL-9-Cre reporter mice that even innate lymphoid cells are significant producers of IL-9 (16). So, IL-9 seems to be one cytokine of many sources, and therefore, interest in IL-9 biology and in its significance is diluted, and study of IL-9 has lagged behind that of others. The recent discovery that IL-9-producing cells are in fact a unique subset of CD4+ helper T cells that is different from other subsets, with distinct features and transcriptional controls, generates renewed interest in the field.
In this review, we summarize the latest advances in the study of Th9 cells, discuss the evolving conditions that promote their differentiation as well as the in vivo relevance of Th9 cells and finally we highlight some outstanding issues that remain to be resolved.
Defining Th9 cells
Naive CD4+ T cells can be further specialized into functionally different subsets upon activation (e.g. Th1, Th2, Th17, Th22 and Treg cells), which are often measured by the distinct cytokine profiles they express (17–20). Subset specialization is driven primarily by the texture of cytokines in the local environment where naive the T cells are activated, with the induction of lineage-specific transcription factors as a critical event in further development of specific subsets (21). Th9 cells are a recently described new helper T-cell subset; the signature cytokine for Th9 cells is IL-9 (without IL-4). Th9 cells, together with other helper T-cell subsets, form a complex array of effector mechanisms in the immune system.
In many aspects, Th9 cells are a unique helper T-cell subset. For example, in most studies, the frequency of Th9 cells is very low (~5%), even under optimal polarizing conditions in vitro (22). This often casts considerable concerns over whether Th9 cells are truly a distinct helper T-cell subset. Also, Th9 cells are closely associated with Th2 cells, as Th2 cells co-express both IL-4 and IL-9 in the early phase of Th2 differentiation, and the Th2 cytokine IL-4 provides one of the key signals for Th9 induction (23).
Furthermore, some of the transcription factors in Th2 development are also involved in Th9 induction. A clear example is that STAT6 knockout CD4+ T cells fail to develop to Th2 cells; they also fail to become Th9 cells (24). However, Th9 cells are not Th2 cells. As discussed below, the culture conditions and cytokine milieu that lead to Th2 and Th9 cells are very different. In some Th2 cultures, CD4+ T cells that express IL-4 (Th2 cells) and IL-9 (Th9 cells) are completely segregated in that only those that lose the ability to express IL-4 will become IL-9 producers (23). Interestingly, only a small fraction of Th2 cells acquire the ability to continually express IL-9. Importantly, the transcriptional regulation mechanisms of Th9 and Th2 cells are strikingly different from each other, thus clearly setting Th9 and Th2 cells apart (23).
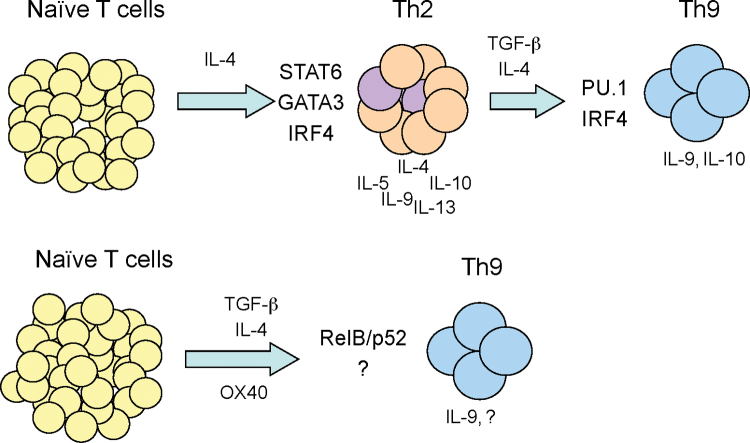
Are Th9 cells progeny of Th2 cells? In most reports showing low levels of Th9 cells under TGF-β and IL-4 culture conditions, Th9 cells often co-express IL-10, which is another Th2 cytokine (25). It is likely that such Th9 cells are derivatives of Th2 cells as a consequence of induction of additional transcription factors such as PU.1 (purine-rich box 1) and IRF4 (interferon regulatory factor 4) (26–29), which shut off IL-4 and turn on IL-9 (see below). In this setting, Th2 cells are likely intermediaries that may further differentiate to Th9 cells. However, our own studies suggest another pathway of Th9 induction in which naive CD4+ T cells can be directly converted to Th9 cells at high levels (up to 80% of the CD4+ T cells) by TGF-β and IL-4 when OX40 costimulation is engaged (30). Furthermore, we demonstrated that the non-canonical NF-κB (RelB–p52) pathway (the canonical pathway involves RelA–p50) rather than PU.1 and IRF4 is essential to Th9 induction (Fig. 1).
Fig. 1.
Pathways of Th9 induction. Naive CD4+ T cells can be converted to IL-9-producing Th9 cells via different molecular pathways. Depending on the presence or absence of OX40 costimulation, Th9 cells can develop from a subset of Th2 cells (shown in purple) or directly from CD4+ T-cell precursors under TGF-β/IL-4-polarizing conditions.
Th9 induction
Cytokines
A complex cytokine milieu is required for Th9 induction, and integration of multiple cytokine signals is critical to optimal Th9 development. The best cytokine mixture for Th9 induction is a combination of TGF-β and IL-4, which contrasts sharply to the role of individual cytokines. TGF-β alone without IL-4 promotes Treg cells by inducing Foxp3, whereas IL-4 alone without TGF-β supports Th2 induction. This highlights the complexity of Th9 induction and also places Th9 cells as a unique subset that is different from Th2 and Treg cells. IL-4 activates STAT6 and IRF4, whereas TGF-β activates PU.1 and represses T-bet (T-box expressed in T cells) and GATA3 (GATA-binding protein 3), and the integration of those events eventually drives IL-9 expression (18, 31–35).
In some models, IL-1 favors induction of Th9 cells; so does IL-25 or IL-33 (16, 36–38), although the exact mechanism remains to be defined. On the other hand, IFN-γ and IL-23 are potent inhibitors of Th9 induction. Also, cytokines that stimulate IFN-γ production such as IL-12 and IL-18 also inhibit the induction of Th9 cells. These cytokines most likely act through the induction of T-bet, which promotes Th1 cells and opposes other helper T-cell lineages including Th9 cells. Thus, the texture of cytokines fine-tunes the production of different helper T-cell subsets.
Costimulatory signals
T-cell costimulatory signals control not only the status of T-cell activation but also the character of the T-cell response. We recently showed that OX40, a costimulatory molecule in the TNFR superfamily (TNFRSF), is surprisingly potent in promoting Th9 cells (30), thus emphasizing the importance of costimulation, in addition to cytokines, in Th9 induction. OX40 is expressed by activated, but not resting, T cells, especially activated CD4+ T cells, and plays an important role in cell survival and proliferation (39). Specifically, we found that under Th9-polarizing conditions, OX40 ligation on naive CD4+ T cells resulted in a remarkable increase in Th9 induction. Such Th9 cells did not express detectable levels of IL-4 or IL-10 (30). Furthermore, OX40 ligation under Treg- and Th17-polarizing conditions potently inhibited the induction of Foxp3+ cells and IL-17-producing cells (30). Thus, the effect of OX40 on Th9 induction seems to be specific.
The role of OX40 in the induction of Th9 cells was also observed in vivo where OX40L transgenic mice or injection of agonist anti-OX40 antibody induced allergic airway inflammation, as demonstrated by goblet-cell metaplasia and eosinophil infiltration (30). Mechanistically, OX40 signaling activates and sustains induction of the non-canonical NF-κB pathway (RelB–p52), which is critical to IL-9 transcription. This is further confirmed using both gain-of-function assays and loss-of-function assays. Under Th9-polarizing conditions, overexpression of RelB–p52 in CD4+ T cells led to much greater Th9 induction by TGF-β and IL-4, and knockout of p52 drastically reduced Th9 induction (30), which places RelB–p52 as a center piece in the induction of Th9 cells. While interesting, these data also raise several questions on the role of other TNFRSF members in the induction of Th9 cells, especially those that activate the non-canonical NF-κB pathway. Studies in this area deserve more attention.
Other costimulatory molecules that are known to affect Th9 induction include the CD28 and Notch pathways. It has been shown that conditional deletion of Notch1 and Notch2 markedly decreased IL-9 production in Th9 cultures (40). There are multiple ligands for the Notch receptors, and Jagged2 but not Delta-like 1 was shown to induce IL-9 production under TGF-β-based polarizing conditions. In an experimental auto-immune encephalitis (EAE) model, Jagged2-mediated IL-9 production was involved the EAE pathology, and conditional deletion of Notch1 and Notch2 in T cells attenuated the disease (40).
The exact mechanism of how Notch promotes the generation of Th9 cells remains unclear. Notch signaling is known to favor Th2 cells (41, 42), which may indirectly promote Th9 cells. This notion is supported by the finding that exogenous IL-4 could overcome the effects of Notch1 and Notch 2 deficiency. Other studies indicate that Notch may modulate TGF-β signaling by acting on Smad (small/mothers against decapentaplegic) proteins. For example, in humans, Smad3 binds Notch 1, whereas Jagged2 and Delta-like 1 bind Notch 2 (43). It should be noted that Th9 cells induced upon Notch stimulation are also at low levels, but PU.1 and IRF4 appear not critical to Notch-mediated induction of Th9 cells.
Transcription machinery
Cytokine signals and costimulatory signals converge to activate the transcriptional apparatus that eventually drives the differentiation of Th9 cells. Unlike other helper T-cell subsets, there is a hierarchy of transcription factors involved in both induction and differentiation of Th9 cells. But a single ‘master’ transcription factor, as shown in other subsets, has not been identified thus far for Th9 cells.
The IL-9 promoter region plus two additional regions of conserved non-coding sequences upstream of the promoter region form the cis- and trans-regulatory elements collectively regulating IL-9 gene expression (26, 35). Sequence analysis identified binding sites for a plethora of transcription factors, which include PU.1, IRF4, STATs, NFAT (nuclear factor of activated T cells), GATA1, GATA3, Smads and Notch as well as NF-κB and AP-1 (activator protein 1) (35), highlighting the complexity of IL-9 gene regulation. In a broad sense, the transcriptional control of Th9 cells induced by polarizing cytokines (TGF-β and IL-4) and the polarizing cytokines plus costimulatory signals appears to be very different.
Under TGF-β- and IL-4-polarizing conditions, PU.1 and IRF4 have been identified as key transcription factors in the induction of Th9 cells (26, 27). Overexpression of PU.1 in CD4+ T cells facilitates Th9 induction by TGF-β and IL-4, and deficiency of PU.1 inhibits the induction of Th9 cells. Furthermore, PU.1 knockout mice exhibit reduced allergic lung inflammation in which Th9 cells are known to be involved (26). Using a similar experimental strategy, IRF4 was shown to display the same effect on Th9 development as PU.1 (27). Mechanistically, both transcription factors have been shown to bind to the promoter region of IL-9 and are capable of promoting IL-9 gene expression.
It should be noted that Th9 development requires signals from both TGF-β and IL-4 cytokine receptors. In the absence of IL-4, TGF-β promotes Treg cells, and in absence of TGF-β, IL-4 leads to the development of Th2 cells. As Th9 cells are closely related to Th2 cells, IL-4-mediated induction of STAT6 and the STAT6 target gene GATA3 are both required for Th9 development (24, 25). However, it is not clear how STAT6 and GATA3 function to promote Th9 cells at the cost of Th2 cells, nor it is clear how STAT6 and GATA3 collaborate with PU.1 and/or IRF4 in Th9 induction. In addition, the target molecules downstream of TGF-β signaling pathways that are critical to Th9 development are incompletely defined. An intriguing point is that TGF-β and IL-4 only convert a small fraction of Th2 cells to Th9 cells (26); what renders some Th2 cells responsive to the switch while other cells are resistant under the same Th9-polarizing conditions warrants further clarification. Interestingly, a recent study from Chen Dong’s group uncovered the importance of cytokine-induced SH2 protein (CIS) in the control of Th2 and Th9 differentiation (44). CIS is induced by IL-4 and suppresses the activation of STAT3, STAT5 and STAT6 in T cells. They found that STAT5 and STAT6 promote IL-9 expression by directly binding to the IL-9 promoter, and therefore, CIS-deficient T cells exhibit enhanced differentiation into Th9 cells. Consequently, CIS-deficient mice spontaneously develop airway inflammation in which Th9 cells are required (44).
In our own studies, we identified a new molecular pathway by which Th9 cells develop, and this pathway is triggered by OX40-mediated costimulation (30). One striking feature is that when OX40 costimulation is delivered to CD4+ T cells, up to 80% of the CD4+ T cells can be converted to IL-9-producing Th9 cells by TGF-β and IL-4. Unlike previously reported Th9 cells, such Th9 cells have no detectable levels of IL-10 and are highly pathogenic in a mouse model of allergic lung inflammation (30). Thus, we believe that Th9 cells developed under OX40 costimulation are bona fide Th9 cells. However, OX40 must act in concert with polarizing cytokines in Th9 development. We showed that OX40 signaling blocks the induction of inducible Treg cells and Th17 cells and selectively diverts the cells to a Th9 phenotype. However, without the polarizing cytokines, OX40 signaling instead supports Th1 and Th2 development (30).
Mechanistically, OX40 ligation activates, and most strikingly sustains, activities of the non-canonical NF-κB pathway (RelB–p52), which potently mediates Th9 induction by TGF-β and IL-4. In fact, the promoter region of IL-9 has multiple NF-κB binding sites and RelB–p52 is directly involved in IL-9 transcription (30). These studies uncover additional complexities in IL-9 transcription and further suggest that, for Th9 cells, additional signals besides those downstream of cytokine receptors are critically important. Our studies also raise other questions. For example, are other costimulatory molecules that also activate the non-canonical NF-κB pathway involved in Th9 development? RelB–p52 is not acting alone, and PU.1 and IRF4 are not involved in OX40-mediated induction of Th9 cells (30). Thus, what are the molecular partners downstream of TGF-β and IL-4 receptors that conspire with RelB–p52 in driving development of Th9 cells? Are there any roles for the classical Th2 transcription factors STAT6 and GATA3 in this process? Clearly, more studies are required to further clarify these questions.
Clinical relevance
What are Th9 cells made for? There are several clinical settings in which Th9 cells are implicated in the disease process. This suggests that intervention of Th9 development may be therapeutically important. Studies from many laboratories including our own highlight the importance of Th9 cells in allergic lung inflammation (30, 45, 46). However, the inflammatory response in the lung also involves other cell types besides Th9 cells, and most prominently Th2 cells. The interactions among different cell types in development and progression of the disease remain unclear, but Th9 cells appear to play a particularly important role in airway epithelial alterations, which include goblet-cell hyperplasia, mucus production and infiltration of the airspace by mast cells and eosinophils. Indeed, blocking Th9 cells markedly reduced the airway pathology while that in the lung parenchyma was not significantly affected (30), suggesting that Th9 cells are a part, but may not the entirety, of allergic lung inflammation.
The role of Th9 cells in other inflammatory conditions, especially chronic inflammation, remains to be determined. There are reports supporting a role for Th9 cells in certain autoimmune diseases including EAE (40), suggesting that targeting Th9 cells may provide an additional approach in treatment of such autoimmune conditions. There are other conditions where promotion of Th9 cells might be therapeutically beneficial. It has been shown that IL-9 from mast cells promotes transplant tolerance to skin allografts by recruiting Foxp3+ Treg cells to the grafts (15). Thus, neutralizing IL-9 resulted in failure of tolerance induction (15). By the same token, Th9 cells might also be tolerogenic in transplant settings. However, considering the inflammatory nature of mast cells, Th9 cells and cells recruited by IL-9, the exact role of Th9 cells and Th9 cell products in immunity and immune tolerance deserves careful clarification.
Another area that Th9 cells recently attracted considerable attention is cancer therapy. Two laboratories independently reported that Th9 cells exhibit remarkable therapeutic efficacy in cancer models (47, 48). In a highly aggressive B16 melanoma model, it has been shown that induction of Th9 cells is associated with potent anti-cancer effects and favorable outcomes of cancer-bearing mice (47, 48). This is a significant area, considering the growing incidence of cancers and the limited choices in treatment of cancer patients.
Conclusions
Th9 cells are a new and evolving subset of helper T cells. There are different molecular pathways identified thus far supporting the development of Th9 cells, and integration of multiple signaling pathways downstream of cytokine receptors and costimulatory molecules is essential for specification and induction of Th9 cells. The greatest effect on Th9 induction is achieved by engaging OX40 under TGF-β- and IL-4-polarizing conditions. Th9 cells and Th9 products are highly pathogenic in allergic lung inflammation as well as in some autoimmune conditions, but they may be therapeutically desirable in other conditions such as cancer therapies. However, compared with other T helper subsets, Th9 cells are less well studied. Many questions regarding Th9 induction, the molecular machinery involved, their relationships with other helper T-cell subsets, especially Th2 cells, and the exact role of Th9 cells in immunity and immune pathology deserve further attention in future studies.
Funding
National Institutes of Health (R01 AI057409, R01 AI070315).
References
- 1. Van Snick J., Goethals A., Renauld J. C., et al. 1989. Cloning and characterization of a cDNA for a new mouse T cell growth factor (P40). J. Exp. Med. 169:363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li X. C., Schachter A. D., Zand M. S., et al. 1998. Differential expression of T-cell growth factors in rejecting murine islet and human renal allografts: conspicuous absence of interleukin (IL)-9 despite expression of IL-2, IL-4, IL-7, and IL-15. Transplantation 66:265. [DOI] [PubMed] [Google Scholar]
- 3. Knoops L., Renauld J. C. 2004. IL-9 and its receptor: from signal transduction to tumorigenesis. Growth Factors 22:207. [DOI] [PubMed] [Google Scholar]
- 4. Soroosh P., Doherty T. A. 2009. Th9 and allergic disease. Immunology 127:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kaplan M. H. 2013. Th9 cells: differentiation and disease. Immunol. Rev. 252:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dugas B., Renauld J. C., Pène J., et al. 1993. Interleukin-9 potentiates the interleukin-4-induced immunoglobulin (IgG, IgM and IgE) production by normal human B lymphocytes. Eur. J. Immunol. 23:1687. [DOI] [PubMed] [Google Scholar]
- 7. Petit-Frere C., Dugas B., Braquet P., Mencia-Huerta J. M. 1993. Interleukin-9 potentiates the interleukin-4-induced IgE and IgG1 release from murine B lymphocytes. Immunology 79:146. [PMC free article] [PubMed] [Google Scholar]
- 8. Hültner L., Druez C., Moeller J., et al. 1990. Mast cell growth-enhancing activity (MEA) is structurally related and functionally identical to the novel mouse T cell growth factor P40/TCGFIII (interleukin 9). Eur. J. Immunol. 20:1413. [DOI] [PubMed] [Google Scholar]
- 9. Renauld J. C., Vink A., Louahed J., Van Snick J. 1995. Interleukin-9 is a major anti-apoptotic factor for thymic lymphomas. Blood 85:1300. [PubMed] [Google Scholar]
- 10. Uyttenhove C., Simpson R. J., Van Snick J. 1988. Functional and structural characterization of P40, a mouse glycoprotein with T-cell growth factor activity. Proc. Natl Acad. Sci. USA 85:6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stephens G. L., Swerdlow B., Benjamin E., et al. 2011. IL-9 is a Th17-derived cytokine that limits pathogenic activity in organ-specific autoimmune disease. Eur. J. Immunol. 41:952. [DOI] [PubMed] [Google Scholar]
- 12. Eller K., Wolf D., Huber J. M., et al. 2011. IL-9 production by regulatory T cells recruits mast cells that are essential for regulatory T cell-induced immune suppression. J. Immunol. 186:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Noelle R. J., Nowak E. C. 2010. Cellular sources and immune functions of interleukin-9. Nat. Rev. Immunol. 10:683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nowak E. C., Weaver C. T., Turner H., et al. 2009. IL-9 as a mediator of Th17-driven inflammatory disease. J. Exp. Med. 206:1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lu L. F., Lind E. F., Gondek D. C., et al. 2006. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature 442:997. [DOI] [PubMed] [Google Scholar]
- 16. Wilhelm C., Hirota K., Stieglitz B., et al. 2011. An IL-9 fate reporter demonstrates the induction of an innate IL-9 response in lung inflammation. Nat. Immunol. 12:1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harrington L. E., Hatton R. D., Mangan P. R., et al. 2005. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 6:1123. [DOI] [PubMed] [Google Scholar]
- 18. Murphy K. M., Reiner S. L. 2002. The lineage decisions of helper T cells. Nat. Rev. Immunol. 2:933. [DOI] [PubMed] [Google Scholar]
- 19. Duhen T., Geiger R., Jarrossay D., Lanzavecchia A., Sallusto F. 2009. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat. Immunol. 10:857. [DOI] [PubMed] [Google Scholar]
- 20. Hori S., Nomura T., Sakaguchi S. 2003. Control of regulatory T cell development by the transcription factor Foxp3. Science 299:1057. [DOI] [PubMed] [Google Scholar]
- 21. O’Shea J. J., Paul W. E. 2010. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science 327:1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tan C., Gery I. 2012. The unique features of Th9 cells and their products. Crit. Rev. Immunol. 32:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Veldhoen M., Uyttenhove C., van Snick J., et al. 2008. Transforming growth factor-beta ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat. Immunol. 9:1341. [DOI] [PubMed] [Google Scholar]
- 24. Goswami R., Jabeen R., Yagi R., et al. 2012. STAT6-dependent regulation of Th9 development. J. Immunol. 188:968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dardalhon V., Awasthi A., Kwon H., et al. 2008. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(−) effector T cells. Nat. Immunol. 9:1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chang H. C., Sehra S., Goswami R., et al. 2010. The transcription factor PU.1 is required for the development of IL-9-producing T cells and allergic inflammation. Nat. Immunol. 11:527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Staudt V., Bothur E., Klein M., et al. 2010. Interferon-regulatory factor 4 is essential for the developmental program of T helper 9 cells. Immunity 33:192. [DOI] [PubMed] [Google Scholar]
- 28. Chang H. C., Han L., Jabeen R., Carotta S., Nutt S. L., Kaplan M. H. 2009. PU.1 regulates TCR expression by modulating GATA-3 activity. J. Immunol. 183:4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chang H. C., Zhang S., Thieu V. T., et al. 2005. PU.1 expression delineates heterogeneity in primary Th2 cells. Immunity 22:693. [DOI] [PubMed] [Google Scholar]
- 30. Xiao X., Balasubramanian S., Liu W., et al. 2012. OX40 signaling favors the induction of T(H)9 cells and airway inflammation. Nat. Immunol. 13:981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ansel K. M., Djuretic I., Tanasa B., Rao A. 2006. Regulation of Th2 differentiation and Il4 locus accessibility. Annu. Rev. Immunol. 24:607. [DOI] [PubMed] [Google Scholar]
- 32. Szabo S. J., Kim S. T., Costa G. L., Zhang X., Fathman C. G., Glimcher L. H. 2000. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 100:655. [DOI] [PubMed] [Google Scholar]
- 33. Weaver C. T., Hatton R. D., Mangan P. R., Harrington L. E. 2007. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu. Rev. Immunol. 25:821. [DOI] [PubMed] [Google Scholar]
- 34. Sakaguchi S., Yamaguchi T., Nomura T., Ono M. 2008. Regulatory T cells and immune tolerance. Cell 133:775. [DOI] [PubMed] [Google Scholar]
- 35. Perumal N. B., Kaplan M. H. 2011. Regulating Il9 transcription in T helper cells. Trends Immunol. 32:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schmitt E., Beuscher H. U., Huels C., et al. 1991. IL-1 serves as a secondary signal for IL-9 expression. J. Immunol. 147:3848. [PubMed] [Google Scholar]
- 37. Angkasekwinai P., Chang S. H., Thapa M., Watarai H., Dong C. 2010. Regulation of IL-9 expression by IL-25 signaling. Nat. Immunol. 11:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Blom L., Poulsen B. C., Jensen B. M., Hansen A., Poulsen L. K. 2011. IL-33 induces IL-9 production in human CD4+ T cells and basophils. PLoS One 6:e21695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li X. C., Rothstein D. M., Sayegh M. H. 2009. Costimulatory pathways in transplantation: challenges and new developments. Immunol. Rev. 229:271. [DOI] [PubMed] [Google Scholar]
- 40. Elyaman W., Bassil R., Bradshaw E. M., et al. 2012. Notch receptors and Smad3 signaling cooperate in the induction of interleukin-9-producing T cells. Immunity 36:623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Amsen D., Antov A., Jankovic D., et al. 2007. Direct regulation of Gata3 expression determines the T helper differentiation potential of Notch. Immunity 27:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fang T. C., Yashiro-Ohtani Y., Del Bianco C., Knoblock D. M., Blacklow S. C., Pear W. S. 2007. Notch directly regulates Gata3 expression during T helper 2 cell differentiation. Immunity 27:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fu Y., Chang A., Chang L., et al. 2009. Differential regulation of transforming growth factor beta signaling pathways by Notch in human endothelial cells. J. Biol. Chem. 284:19452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yang X. O., Zhang H., Kim B. S., et al. 2013. The signaling suppressor CIS controls proallergic T cell development and allergic airway inflammation. Nat. Immunol. 14:732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nicolaides N. C., Holroyd K. J., Ewart S. L., et al. 1997. Interleukin 9: a candidate gene for asthma. Proc. Natl Acad. Sci. USA 94:13175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jones C. P., Gregory L. G., Causton B., Campbell G. A., Lloyd C. M. 2012. Activin A and TGF-β promote T(H)9 cell-mediated pulmonary allergic pathology. J. Allergy Clin. Immunol. 129:1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lu Y., Hong S., Li H., et al. 2012. Th9 cells promote antitumor immune responses in vivo . J. Clin. Invest. 122:4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Purwar R., Schlapbach C., Xiao S., et al. 2012. Robust tumor immunity to melanoma mediated by interleukin-9-producing T cells. Nat. Med. 18:1248. [DOI] [PMC free article] [PubMed] [Google Scholar]