Abstract
Neonates show an impaired anti-microbial host defence, but the underlying immune mechanisms are not understood fully. Myeloid-derived suppressor cells (MDSCs) represent an innate immune cell subset characterized by their capacity to suppress T cell immunity. In this study we demonstrate that a distinct MDSC subset with a neutrophilic/granulocytic phenotype (Gr-MDSCs) is highly increased in cord blood compared to peripheral blood of children and adults. Functionally, cord blood isolated Gr-MDSCs suppressed T cell proliferation efficiently as well as T helper type 1 (Th1), Th2 and Th17 cytokine secretion. Beyond T cells, cord blood Gr-MDSCs controlled natural killer (NK) cell cytotoxicity in a cell contact-dependent manner. These studies establish neutrophilic Gr-MDSCs as a novel immunosuppressive cell subset that controls innate (NK) and adaptive (T cell) immune responses in neonates. Increased MDSC activity in cord blood might serve as key fetomaternal immunosuppressive mechanism impairing neonatal host defence. Gr-MDSCs in cord blood might therefore represent a therapeutic target in neonatal infections.
Keywords: cord blood, Gr-MDSC, MDSC, myeloid-derived suppressor cells, neonatal, neutrophilic, NK cells, Th17
Introduction
In the neonatal period, the human immune system adapts from the fetal to the postnatal life. The immune system has to transform from an immunosuppressive state in pregnancy to a neonatal immune response that protects the infant from various bacterial, viral and fungal pathogens [1]. This transition is critical, as almost 40% of overall childhood mortality occurs in this time-period. Previous studies investigating neonatal immune responses provided evidence that both innate and adaptive immune systems are impaired during the postnatal period, rendering neonates prone to invasive infections [1–4]. Despite several mechanisms proposed to play a role in neonatal immunity, such as a T helper type 2 (Th2) cell-biased cytokine profile [5,6], reduced CD8+ T cell expansion and interferon (IFN)-gamma response [7], differences in regulatory T cells [8] and immaturity of dendritic cells [9], the underlying mechanisms mediating both innate and adaptive immune cell suppression in neonates are still not defined fully.
Myeloid-derived suppressor cells (MDSCs) represent a heterogeneous innate immune cell subset generated in tumour, infective and proinflammatory environments [10–16]. In mice, as well as in humans, MDSCs can be dichotomized into neutrophilic/granulocytic (Gr-MDSC) and monocytic MDSC (Mo-MDSC) subsets [10,11]. MDSCs are characterized by their capacity to potently suppress T cell and natural killer (NK) cell responses and thereby induce an immunocompromised state, which favours infection susceptibility [10,17–19]. We hypothesized that neonates show increased MDSCs as underlying factor of their impaired innate (NK cells) and adaptive (T cell) immune response. Our studies demonstrate for the first time that neutrophilic Gr-MDSCs are increased strikingly in neonatal cord blood and decrease to adult values early in infancy. Functionally, cord blood Gr-MDSCs efficiently suppress T cell proliferation, Th1, Th2 and Th17 responses as well as NK cell cytotoxicity. These studies establish Gr-MDSCs as a novel immunosuppressive cell subset that controls innate and adaptive immunity in the neonatal period.
Methods
Study subjects
The study was conducted at the University Children's Hospital Tübingen (Germany). MDSCs were analysed in cord blood from healthy term neonatal donors (n = 58) and in peripheral blood from healthy individuals (n = 33). Informed written consent was obtained from all subjects included in the study or their legal representatives, and all study methods were approved by the local ethics committee. The peripheral blood samples from the children were taken through a routinely inserted intravenous line before undergoing elective surgery for multiple diagnoses. At the time of blood sampling, all subjects were without signs of infection, inflammation or neoplasia.
Cell isolation and flow cytometry
Gr-MDSCs were characterized and isolated as described previously [16]. Fresh peripheral blood mononuclear cells (PBMC) were prepared from heparinized blood samples by Ficoll density gradient sedimentation (lymphocyte separation medium; Biochrom, Cambridge, UK) and washed twice in RPMI-1640 medium. Trypan blue staining solution at 0·5% differentiated between viable and nonviable cells and showed viability > 90% in all samples. After Ficoll density gradient sedimentation, Gr-MDSCs were characterized as CD66bhighCD33highinterleukin (IL)-4Rαinterhuman leucoyte antigen D-related (HLA-DR)neg low-density neutrophilic cells in the PBMC fraction [12,14,16] (Fig. 1a,b) with the typical morphological characteristics of neutrophilic granulocytes (see Supporting information, Fig. S1). For Gr-MDSC isolation, cells were obtained from the PBMC fraction and labelled with anti-CD66b-fluorescein isothiocyanate (FITC) followed by two sequential anti-FITC magnetic bead separation steps (Miltenyi Biotech, Bergisch Gladbach, Germany), according to the manufacturer's protocol. The purity of the CD66b+ cells after separation was >95% as tested by flow cytometry. A complete characterization of the CD66b-positive cells isolated by magnetic bead separation is shown in the Supporting information, Fig. S3. Antibodies against CD3, CD4, CD8, CD14, CD16, CD66b, HLA-DR and CD124 (IL-4Rα) were purchased from BD Pharmingen (Heidelberg, Germany). Antibodies against CD11b, CD33 were purchased from Miltenyi Biotec. Mouse immunoglobulin (Ig)G1-FITC, mouse IgM-FITC, mouse IgG1-phycoerythrin (PE) and mouse IgG1-allophycocyanin (APC) (BD Pharmingen) were used as isotype controls. Results were expressed as percentage of positive cells and mean fluorescence intensity (MFI). Calculations were performed with BD CellQuestPro analysis software.
Fig. 1.
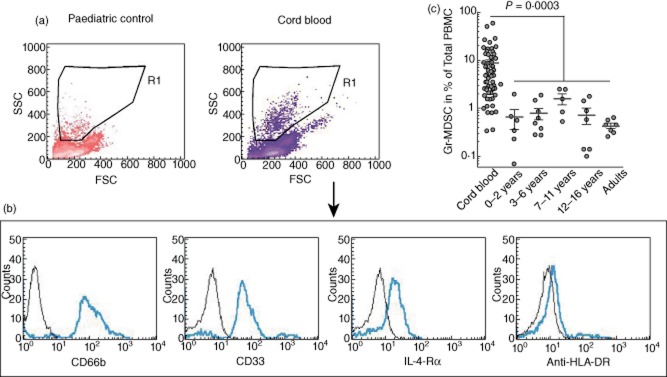
Characterization and quantification of human granulocytic/neutrophilic myeloid-derived suppressor cells (Gr-MDSCs) in cord blood and different age groups. (a) Gr-MDSCs are characterized in the forward-/side-scatter (FSC/SCC) area as a granulocytic cell population in the peripheral blood mononuclear cells (PBMC) fraction (low-density neutrophils) (R1), as published previously [12,14,42]. This population was distinct from lymphocytes, monocytes, erythrocytes or debris. Representative scatter-plots are shown. (b) Within R1, Gr-MDSCs were identified as CD66bhighCD33highinterleukin IL-4Rαinterhuman leucocyte antigen D-related (HLA-DR)neg expressing neutrophilic MDSC population. Representative histograms are shown. (c) Log scale illustration of human Gr-MDSC numbers in cord blood and different age groups of healthy children and adults.
T cell suppression assay
Target PBMCs were obtained from cord blood and adult healthy volunteers and stained with carboxyfluoresceinsuccinimidyl ester (CFSE), according to the manufacturer's protocol (Invitrogen, Carlsbad, CA, USA). PBMCs were stimulated with 100 U/ml IL-2 (R&D Systems, Abingdon, UK) and 1 μg/ml muromonab-CD3 (OKT3) (Janssen Cilag, High Wycombe, UK). In a standardized manner, 60 000 PBMCs per well in RPMI-1640 (Biochrom) were seeded in a 96-well microtitre plate and 10 000 to 30 000 Gr-MDSC in RPMI-1640 or as control magnetic affinity cell sorter (MACS)-isolated conventional high-density non-MDSC neutrophils in RPMI-1640 or RPMI-1640 only was added. The cell culture was supplemented with 10% heat-inactivated human serum, 2 mM glutamine, 100 IU/ml penicillin and 100 mg/ml streptomycin. After 96 h of incubation in a humidified atmosphere at 37°C and 5% CO2, cells were harvested and supernatants were frozen at −20°C. CFSE fluorescence intensity was analysed by flow cytometry to determine polycloncal T cell proliferation. We used the ratio of T cell proliferation with the addition of Gr-MDSCs/T cell proliferation without Gr-MDSCs for statistical analysis. T cell proliferation without Gr-MDSC was set to a fixed value of 1.
Cytokine analysis
Cytokine analysis in supernatants was performed using the Bioplex system (Bio-Rad, Munich, Germany) for IFN-γ, IL-5 and IL-17 and ELISA kits from R&D Systems for IL-4 and IL-13, according to the manufacturer's recommendations.
NK cell cytotoxicity assay
NK cells were separated with anti-CD56 beads (Miltenyi Biotech) by MACS and co-incubated with Gr-MDSCs overnight in a 1:1 ratio. Afterwards cytolytic activity of NK cells against K562 tumour cell line was tested in a bis(acetoxymethyl)2,2:6,2-terpyridine-6,6-dicarboxylate (BATDA) europium release assay, as published earlier [20]. The effector : target (E : T) ratio was 2·5:1. We used the ratios of NK cell cytotoxicity with the addition of Gr-MDSCs/NK cell cytotoxicity without Gr-MDSCs for statistical analysis. NK cell cytotoxicity without Gr-MDSCs was set to a fixed value of 1.
Quantitiative reverse transcription–polymerase chain reaction (qRT–PCR)
qRT–PCR was performed in MACS-isolated Gr-MDSCs. mRNA was isolated with the RNeasy Mini/Micro Kit (Qiagen, Hilden, Germany), cDNA was synthesized with the iScript Advanced Synthesis kit (Bio-Rad) and real-time RT–PCR was performed by using the Power SYBR Green Master Mix (Applied Biosystems, Darmstadt, Germany) and a ViiA7 Real-time PCR cycler (Applied Biosystems), according to the manufacturers' protocols. Primer sequences are given in the Supporting information, Table S1.
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 5.0. Differences between groups were determined by Student's t-test. A P value of <0·05 was considered to be significant.
Results
Neutrophilic Gr-MDSCs accumulate in cord blood
We applied previously published criteria [12–14,16] to characterize MDSCs in neonates, infants and adults. Accordingly, MDSCs were identified based on surface marker expression profiles as well as on their capacity to suppress T cell proliferation. Given these criteria, neutrophilic (CD66b+CD33b+HLA-DRlow) Gr-MDSCs were quantitatively predominant over monocytic (CD14+CD33b+HLA-DRlow) Mo-MDSC subsets, providing the rationale to focus upon Gr-MDSCs in our studies. Studying a large representative cohort of neonates (n = 58), we found that percentages of Gr-MDSCs were increased highly in cord blood (Gr-MDSC percentages: mean 8·66%; median 4·77%) compared to percentages of Gr-MDSCs found in peripheral blood from healthy children of all age groups and to peripheral blood from adults (n = 33; mean 0·79%; median 0·57%). Beyond the neonatal period, percentages of Gr-MDSCs over the different paediatric age groups did not differ significantly and were similar to numbers observed in healthy adults (Fig. 1c). Scatter-plot analyses further revealed a wide range of Gr-MDSC percentages among the neonates analysed (Fig. 1c and Supporting information, Fig. S2). Using qRT–PCR technology we analysed several factors discussed previously as being involved in MDSC induction and/or functionality [9]. These studies revealed that several of these candidate genes were expressed considerably in cord blood Gr-MDSCs [in descending order of expression levels: S100A9, NOX2, arginase-1, transforming growth factor (TGF)-β, signal transducer and activator of transcription 3 (STAT)-3, CCAAT/enhancer-binding protein beta (C/EBP-β), cyclo-oxygenase (COX)2; see Supporting information, Table S2] and could be involved in generation of Gr-MDSCs in cord blood. When viewed in combination, Gr-MDSCs accumulate selectively in cord blood and decrease rapidly after the neonatal period down to adult values, suggesting a key role for this innate immune cell subset within this critical time-frame.
Cord blood Gr-MDSCs potently suppress T cell proliferation and cytokine production
To assess the functional capacities of cord blood-derived Gr-MDSCs, we isolated these cells using density gradient centrifugation and sequential CD66b magnetic bead isolation and studied Gr-MDSC–T cell interactions ex vivo. These functional studies, using T cell CFSE labelling, demonstrated that cord blood Gr-MDSCs potently suppressed polyclonal T cell proliferation of both CD4+ and CD8+ T cell subsets in a dose-dependent manner (Fig. 2a,b). On a cell-to-cell basis, there were no significant differences in the suppressive capacity between cord blood Gr-MDSCs and Gr-MDSCs isolated from healthy adults (adult Gr-MDSCs) mediated on cord blood target PBMCs. However, when using PBMCs from healthy adults as the target cell population, adult Gr-MDSCs showed a significantly more potent suppression than did cord blood Gr-MDSCs. These suppressive effects were partly cell contact-dependent, as shown by Transwell assays (Fig. 2c). Importantly, as control, neither cord blood nor adult blood-derived CD66b-positive magnetic bead isolated cells from the Ficoll high-density neutrophil fraction (conventional cord blood or peripheral blood adult neutrophils) showed any suppressive activity on T cells (Fig. 2d), indicating that neutrophilic Gr-MDSCs represent a population functionally distinct from conventional neonatal or adult neutrophils.
Fig. 2.
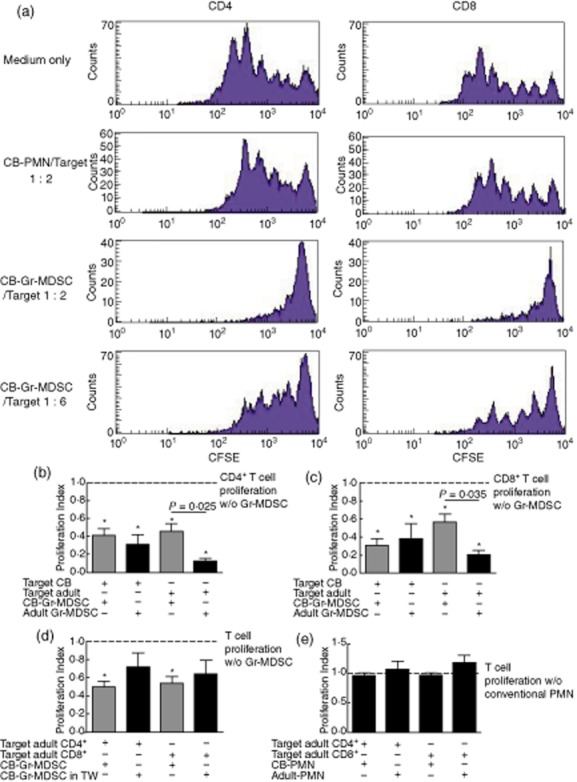
Cord blood granulocytic/neutrophilic myeloid-derived suppressor cells (CB-Gr-MDSCs) suppress T cell proliferation. The T cell suppressive capacities of cord blood Gr-MDSCs are shown. For proliferation assays, peripheral blood mononuclear cells (PBMC) were stimulated with interleukin (IL)-2 (100 U/ml) and muromonab-CD3 (OKT3) (1 μg/ml). The suppressive effects of CD66b+-magnetic-activated cell sorting (MACS)-isolated Gr-MDSCs or as a control CD66b+-MACS-isolated conventional high-density polymorphonuclear leucocytes (PMNs) were analysed on CD4+ and CD8+ T cell subsets using the carboxyfluorescein succinimidyl ester (CFSE) polyclonal proliferation assay. A minimum of three independent CFSE assays was conducted. Asterisks indicate significant differences in proliferation indices under addition of Gr-MDSCs compared to target cells only (a–e). (a) Representative CFSE histograms. (b) Effect of cord blood and adult Gr-MDSCs on CD4+ T cell proliferation. (c) Effect of cord blood and adult Gr-MDSCs on CD8+ T cell proliferation. (d) Transwell assays indicating cell contact-dependent effects of Gr-MDSCs. (e) No effect of conventional cord blood or adult PMNs on lymphocyte proliferation. CB: cord blood; TW: Transwell.
Aside from polyclonal T cell proliferation, we investigated the impact of neonatal Gr-MDSCs on IL-2 and muromonab-CD3 (OKT3)-induced T cell cytokine production. These studies demonstrated that neutrophilic cord blood Gr-MDSCs suppressed IFN-γ, IL-5 and IL-17 cytokine protein production efficiently (Fig. 3), pointing to a broad suppressive effect of cord blood Gr-MDSCs on Th1, Th2 and Th17 lymphocytes, in line with previous studies on adult MDSCs [21,22]. When viewed in combination, these studies demonstrate that neutrophilic Gr-MDSCs enriched in cord blood, in contrast to conventional neutrophils, potently suppress the proliferation and cytokine production by CD4+ and CD8+ T cell subsets.
Fig. 3.
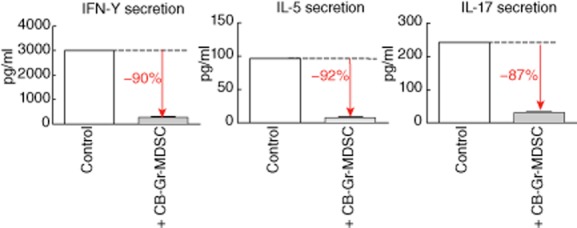
Cord blood granulocytic/neutrophilic myeloid-derived suppressor cells (Gr-MDSCs) inhibit T helper cell cytokine secretion. Cord blood Gr-MDSCs were added to interleukin (IL)-2 (100 U/ml) and muromonab-CD3 (OKT3) (1 μg/ml)-stimulated peripheral blood mononuclear cells (PBMC) in a 1 : 2 ratio and the effect on interferon (IFN)-γ, IL-5 and IL-17 concentrations in supernatants was measured using a Bioplex system.
Cord blood Gr-MDSCs suppress NK cell function
Because NK cells have been recognized as key anti-infectious effector cells of the innate immune system and previous publications have shown that this cell type has a particular relevance in neonates [23] where NK cells are increased [24–27], and recent publications on MDSC also showed suppressive effects on these innate immune cells [19,28], we investigated whether isolated adult and cord blood Gr-MDSCs are capable of functionally modulating NK cell responses. These studies demonstrated a significant reduction of NK cell cytotoxicity against the K562 target tumour cell line by both adult and cord blood Gr-MDSCs (Fig. 4). Transwell assays again showed that this effect was partially contact-dependent, as also observed for the effect of neonatal Gr-MDSCs on T cells. In summary, these experiments provide evidence that Gr-MDSCs in cord blood modulate both adaptive (T cell) and innate (NK) cell responses and indicate that this mechanism is, at least partially, cell contact-dependent.
Fig. 4.
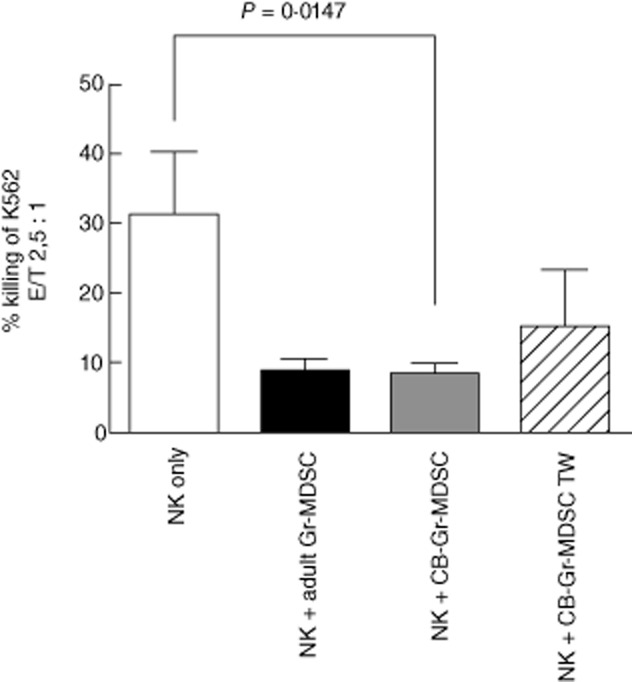
Cord blood granulocytic/neutrophilic myeloid-derived suppressor cells (Gr-MDSCs) suppress natural killer (NK cell cytotoxicity in a cell contact-dependent manner. NK cell cytotoxicity against K562 tumour cell line with or without addition of cord-blood Gr-MDSCs in a 1:1 ratio was measured in a europium release assay. Effector : target (E : T) ratio was 2·5:1 (NK cells : K562 cells). CB: cord blood; TW: Transwell.
Discussion
We show here for the first time, to our knowledge, that neutrophilic Gr-MDSCs are enriched abundantly in cord blood compared to paediatric and adult control cohorts. This finding has functional relevance, as cord blood Gr-MDSCs potently suppressed Th1, Th2 and Th17 responses and modulated NK cell cytotoxicity in a partially cell contact-dependent manner. These functional capacities were unique to neutrophilic Gr-MDSCs, as conventional neutrophils had no effect on T or NK cell suppression. Mechanistically, these findings suggest that neonatal Gr-MDSCs impair both adaptive (T cellular) and innate (NK cell) immune responses and thereby contribute to an immunocompromised state of the neonate.
To sustain pregnancy, T helper cell effector reponses need to be controlled and dampened to avoid a harmful cytotoxic allograft reaction against the fetus. This immunosuppressive micromilieu has been attributed previously to be generated and/or maintained by an interplay of a Th2 shift [5], dysbalanced cytokine responses [26], naive regulatory T cells [8], reduced NK T cells, soluble factors produced by the placenta and others [1], with differences between term and preterm infants [29] and an association with the infection status [30]. In particular, both T cell and NK cell activation were found to be impaired in neonates [31,32]. This study extends this concept by demonstrating that neutrophilic Gr-MDSCs accumulate in cord blood and efficiently dampen both T cell and NK cell responses. Given previous reports on the effect of MDSCs on regulatory T cells in humans in vitro [22] and mice in vivo [33], we speculate that, in line with these findings, neutrophilic Gr-MDSC represent an early innate immune cell population that orchestrates the establishment of an immunosuppressive state in the peri- and postnatal periods. Immunologically, MDSCs probably precede the occurrence of regulatory T cells, as they have been found to recruit regulatory T cells through the chemokine receptor CCR5 [33]. Beyond this, human MDSCs were found to drive the differentiation of naive CD4+ T cells into regulatory T cells [22,34], further supporting this immunosuppressive alliance.
Physiologically, cord blood Gr-MDSCs could be critical for maintaining intrauterine feto–maternal tolerance by controlling/suppressing T cell responses [1,5]. Cord blood is used increasingly as a stem cell source for haematopoietic stem cell transplantation [34]. As MDSCs have been shown to reduce the risk for developing graft-versus-host disease (GVHD) in vivo [35], neutrophilic cord blood Gr-MDSCs might also be involved in the reduced GVHD risk, as observed in cord blood transplantations compared to bone marrow transplantations [34,36], an issue requiring further investigation. In our study, analyses of cord blood MDSCs in individual newborns yielded a wide range of Gr-MDSCs, suggesting underlying immunological and/or microenvironmental factors regulating intrauterine Gr-MDSC generation. By analysing a study subcohort, we found that Gr-MDSC percentages from vaginally delivered neonates tended to be higher compared to neonates delivered by caesarean section (data not shown). We speculate that stress during spontaneous labour and/or interaction of the fetus with the maternal microbial microenvironment could play a role – an issue worth future investigation. We are currently performing a longitudinal study on cord blood Gr-MDSC with a special focus on the course of pregnancy and the early postnatal period. Candidate molecules already described previously as being involved in the induction and/or functionality of MDSCs include growth factors and haematological differentiation factors, such as vascular endothelial growth factor (VEGF), granulocyte–macrophage colony-stimulating factor (GM-CSF) and granulocyte colony-stimulating factor (G-CSF) [37,38], proinflammatory mediators such as IL-6, IL-1β or S100A9 and other enzymes and transcription factors, particularly arginase-1, indoleamine 2,3-dioxygenase (IDO) and STAT-3 [13,37,39]. We performed qPCR studies to analyse the mRNA expression profile of MDSC candidate genes and found that neonatal Gr-MDSCs displayed a distinct gene expression signature characteristic for MDSCs, with the highest relative gene expression found for S100A9. The functional contribution of this and other pathways to the generation and inhibitory capacity of neonatal Gr-MDSCs awaits further investigation.
Functionally, Gr-MDSCs were equally potent in suppressing cord blood T cells, irrespective of whether or not the Gr-MDSCs were isolated from cord or adult blood. Intriguingly, however, adult Gr-MDSCs showed a significantly more potent suppressive capacity than cord blood Gr-MDSCs when using adult T cells as the target cell population. Currently, we have no explanation for this phenomenon, but speculate that the interaction between Gr-MDSCs and their target T cell population is affected by cell maturity or by as yet unidentified paracrine factors released differentially by cord blood versus adult responder T cells. In the search for candidate cytokines mediating immune modulation by cord blood Gr-MDSC we focused our analyses on the Th2 cytokines IL-4 and IL-13, as the neonatal T cell immune system is Th2-biased [5,6] and MDSCs are supposed to favour Th2 over Th1 answers [40]. However, we did not find significant differences in Th2 cytokine release between cord blood Gr-MDSCs and conventional cord blood PMNs (Supporting information, Fig. S4), pointing to other molecules responsible for the immune modulation.
Although percentages of NK cells were found to be increased in cord blood versus adult blood [24–27], cord blood NK cells appear to be distinct in their phenotypical as well as functional behaviour [25,41]. These studies demonstrated that cord blood NK cells, while equipped with the lytic molecules perforin and granzyme B, were impaired in their lytic activity against K562 cells compared to NK cells derived from adult peripheral blood [41]. Further studies also showed that neonatal NK cells were inefficient in killing target cells and were found with higher percentages of immature and CD56bright NK cell subsets in cord blood [25]. Based on our findings, we conclude that Gr-MDSCs might represent an underlying factor, abundantly present in cord blood, that impairs NK cell functionality through a cell contact-dependent mechanism.
When viewed in combination, these studies demonstrate that neutrophilic myeloid-derived suppressor cells accumulate in cord blood and modulate innate and adaptive immune responses efficiently. Physiologically, neonatal Gr-MDSCs may have a dual role: on one hand, they dampen potentially harmful Th1 responses and thereby support feto–maternal tolerance. On the other hand, they weaken cellular anti-microbial host defence responses and could contribute to the increased infection susceptibility in neonates. Based on these studies, we propose neutrophilic Gr-MDSC as a novel immunosuppressive mechanism in neonates and potential therapeutic target in neonatal infections. As arginase and nitric oxide (NO) have been implicated in the functionality of MDSCs [10,13,14,37], interfering with these molecules might represent a future therapeutic strategy in neonatal infections.
Acknowledgments
Supported by the German Research Foundation (DFG, Emmy Noether Programme HA 5274/3-1 to D.H.), the German Society of Pediatric Pneumology (D.H.), the Novartis Foundation (D.H.) and the Ernest-Solvay-Foundation (D.H.).
Disclosure
The authors declare no conflicts of interest.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Fig. S1. Cytospins of granulocytic/neutrophilic myeloid-derived suppressor cells (Gr-MDSCs). Isolated neutrophils or Gr-MDSCs were fixed with cytospin centrifugation and stained with May–Gruenwald–Giemsa. Images were acquired on a Carl Zeiss Fotomicroscope (×40 Planapo oil objective, Carl Zeiss) using a Canon EOS 500 camera (Canon) and Adobe Photoshop CS3 software (Adobe).
Fig. S2. Granulocytic/neutrophilic myeloid-derived suppressor cells (Gr-MDSCs) in cord blood and different age groups. Linear scale illustration of human Gr-MDSC numbers in cord blood and different age groups of healthy children and adults.
Fig. S3. Immunophenotyping of magnetic-activated cell sorting (MACS)-isolated human cord blood granulocytic/neutrophilic myeloid-derived suppressor cells (Gr-MDSCs).
The immunophenotype of MACS-isolated cord blood Gr-MDSCs is CD66bhighCD33high CD11bhighIL-4RαinterHLA-DRnegCD14neg. Representative histograms are shown.
Fig. S4. Interleukin (IL)-13 secretion of magnetic-activated cell sorting (MACS)-isolated human cord blood polymorphonuclear leucocytes (PMN) and granulocytic/neutrophilic myeloid-derived suppressor cells (Gr-MDSCs). IL-13 quantification was performed in culture supernatants of MACS-isolated human cord blood PMN and Gr-MDSCs cultured over 4 days in complete medium using standard enzyme-linked immunosorbent assay (ELISA) technique.
Table S1. Primer sequences for quantitative reverse transcription–polymerase chain reaction (qRT–PCR).
Table S2. RNA expression of (MDSC)-related genes. Quantitative reverse transcription–polymerase chain reaction (qRT–PCR) was performed in magnetic-activated cell sorting (MACS)-isolated granulocytic/neutrophilic myeloid-derived suppressor cells (Gr-MDSCs). Ct values of the housekeeping gene β-actin and 10 established MDSC-related genes are shown (higher Ct value implies lower gene expression).
References
- 1.Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. 2007;7:379–390. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- 2.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4:553–564. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- 3.Gille C, Spring B, Tewes LJ, et al. Diminished response to interleukin-10 and reduced antibody-dependent cellular cytotoxicity of cord blood monocyte-derived macrophages. Pediatr Res. 2006;60:152–157. doi: 10.1203/01.pdr.0000228345.58509.7b. [DOI] [PubMed] [Google Scholar]
- 4.Marchant A, Goldman M. T cell-mediated immune responses in human newborns: ready to learn? Clin Exp Immunol. 2005;141:10–18. doi: 10.1111/j.1365-2249.2005.02799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marodi L. Innate cellular immune responses in newborns. Clin Immunol. 2006;118:137–144. doi: 10.1016/j.clim.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Zaghouani H, Hoeman CM, Adkins B. Neonatal immunity: faulty T-helpers and the shortcomings of dendritic cells. Trends Immunol. 2009;30:585–591. doi: 10.1016/j.it.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandez MA, Evans IA, Hassan EH, Carbone FR, Jones CA. Neonatal CD8+ T cells are slow to develop into lytic effectors after HSV infection in vivo. Eur J Immunol. 2008;38:102–113. doi: 10.1002/eji.200636945. [DOI] [PubMed] [Google Scholar]
- 8.Fritzsching B, Oberle N, Pauly E, et al. Naive regulatory T cells: a novel subpopulation defined by resistance toward CD95L-mediated cell death. Blood. 2006;108:3371–3378. doi: 10.1182/blood-2006-02-005660. [DOI] [PubMed] [Google Scholar]
- 9.Hunt DW, Huppertz HI, Jiang HJ, Petty RE. Studies of human cord blood dendritic cells: evidence for functional immaturity. Blood. 1994;84:4333–4343. [PubMed] [Google Scholar]
- 10.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peranzoni E, Zilio S, Marigo I, et al. Myeloid-derived suppressor cell heterogeneity and subset definition. Curr Opin Immunol. 2010;22:238–244. doi: 10.1016/j.coi.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 12.Brandau S, Trellakis S, Bruderek K, et al. Myeloid-derived suppressor cells in the peripheral blood of cancer patients contain a subset of immature neutrophils with impaired migratory properties. J Leukoc Biol. 2011;89:311–317. doi: 10.1189/jlb.0310162. [DOI] [PubMed] [Google Scholar]
- 13.Lechner MG, Liebertz DJ, Epstein AL. Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells. J Immunol. 2010;185:2273–2284. doi: 10.4049/jimmunol.1000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodriguez PC, Ernstoff MS, Hernandez C, et al. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 2009;69:1553–1560. doi: 10.1158/0008-5472.CAN-08-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen S, Akbar SM, Abe M, Hiasa Y, Onji M. Immunosuppressive functions of hepatic myeloid-derived suppressor cells of normal mice and in a murine model of chronic hepatitis B virus. Clin Exp Immunol. 2011;166:134–142. doi: 10.1111/j.1365-2249.2011.04445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rieber N, Brand A, Hector A, et al. Flagellin induces myeloid-derived suppressor cells: implications for Pseudomonas aeruginosa infection in cystic fibrosis lung disease. J Immunol. 2013;190:1276–1284. doi: 10.4049/jimmunol.1202144. [DOI] [PubMed] [Google Scholar]
- 17.Elkabets M, Ribeiro VS, Dinarello CA, et al. IL-1beta regulates a novel myeloid-derived suppressor cell subset that impairs NK cell development and function. Eur J Immunol. 2010;40:3347–3357. doi: 10.1002/eji.201041037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poschke I, Mougiakakos D, Hansson J, Masucci GV, Kiessling R. Immature immunosuppressive CD14+HLA-DR–/low cells in melanoma patients are Stat3hi and overexpress CD80, CD83, and DC-sign. Cancer Res. 2010;70:4335–4345. doi: 10.1158/0008-5472.CAN-09-3767. [DOI] [PubMed] [Google Scholar]
- 19.Li H, Han Y, Guo Q, Zhang M, Cao X. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-beta 1. J Immunol. 2009;182:240–249. doi: 10.4049/jimmunol.182.1.240. [DOI] [PubMed] [Google Scholar]
- 20.Lang P, Barbin K, Feuchtinger T, et al. Chimeric CD19 antibody mediates cytotoxic activity against leukemic blasts with effector cells from pediatric patients who received T-cell-depleted allografts. Blood. 2004;103:3982–3985. doi: 10.1182/blood-2003-05-1735. [DOI] [PubMed] [Google Scholar]
- 21.Ioannou M, Alissafi T, Lazaridis I, et al. Crucial role of granulocytic myeloid-derived suppressor cells in the regulation of central nervous system autoimmune disease. J Immunol. 2012;188:1136–1146. doi: 10.4049/jimmunol.1101816. [DOI] [PubMed] [Google Scholar]
- 22.Hoechst B, Gamrekelashvili J, Manns MP, Greten TF, Korangy F. Plasticity of human Th17 cells and iTregs is orchestrated by different subsets of myeloid cells. Blood. 2011;117:6532–6541. doi: 10.1182/blood-2010-11-317321. [DOI] [PubMed] [Google Scholar]
- 23.Sundstrom Y, Nilsson C, Lilja G, Karre K, Troye-Blomberg M, Berg L. The expression of human natural killer cell receptors in early life. Scand J Immunol. 2007;66:335–344. doi: 10.1111/j.1365-3083.2007.01980.x. [DOI] [PubMed] [Google Scholar]
- 24.Dalle JH, Menezes J, Wagner E, et al. Characterization of cord blood natural killer cells: implications for transplantation and neonatal infections. Pediatr Res. 2005;57:649–655. doi: 10.1203/01.PDR.0000156501.55431.20. [DOI] [PubMed] [Google Scholar]
- 25.Luevano M, Daryouzeh M, Alnabhan R, et al. The unique profile of cord blood natural killer cells balances incomplete maturation and effective killing function upon activation. Hum Immunol. 2012;73:248–257. doi: 10.1016/j.humimm.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 26.Peoples JD, Cheung S, Nesin M, et al. Neonatal cord blood subsets and cytokine response to bacterial antigens. Am J Perinatol. 2009;26:647–657. doi: 10.1055/s-0029-1220788. [DOI] [PubMed] [Google Scholar]
- 27.Perez A, Gurbindo MD, Resino S, Aguaron A, Munoz-Fernandez MA. NK cell increase in neonates from the preterm to the full-term period of gestation. Neonatology. 2007;92:158–163. doi: 10.1159/000101567. [DOI] [PubMed] [Google Scholar]
- 28.Fortin C, Huang X, Yang Y. NK cell response to vaccinia virus is regulated by myeloid-derived suppressor cells. J Immunol. 2012;189:1843–1849. doi: 10.4049/jimmunol.1200584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berrington JE, Barge D, Fenton AC, Cant AJ, Spickett GP. Lymphocyte subsets in term and significantly preterm UK infants in the first year of life analysed by single platform flow cytometry. Clin Exp Immunol. 2005;140:289–292. doi: 10.1111/j.1365-2249.2005.02767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Juretic E, Juretic A, Uzarevic B, Petrovecki M. Alterations in lymphocyte phenotype of infected preterm newborns. Biol Neonate. 2001;80:223–227. doi: 10.1159/000047147. [DOI] [PubMed] [Google Scholar]
- 31.Gasparoni A, Ciardelli L, Avanzini A, et al. Age-related changes in intracellular TH1/TH2 cytokine production, immunoproliferative T lymphocyte response and natural killer cell activity in newborns, children and adults. Biol Neonate. 2003;84:297–303. doi: 10.1159/000073638. [DOI] [PubMed] [Google Scholar]
- 32.Leibson PJ, Hunter-Laszlo M, Douvas GS, Hayward AR. Impaired neonatal natural killer-cell activity to herpes simplex virus: decreased inhibition of viral replication and altered response to lymphokines. J Clin Immunol. 1986;6:216–224. doi: 10.1007/BF00918701. [DOI] [PubMed] [Google Scholar]
- 33.Schlecker E, Stojanovic A, Eisen C, et al. Tumor-infiltrating monocytic myeloid-derived suppressor cells mediate CCR5-dependent recruitment of regulatory T cells favoring tumor growth. J Immunol. 2012;189:5602–5611. doi: 10.4049/jimmunol.1201018. [DOI] [PubMed] [Google Scholar]
- 34.Brown JA, Boussiotis VA. Umbilical cord blood transplantation: basic biology and clinical challenges to immune reconstitution. Clin Immunol. 2008;127:286–297. doi: 10.1016/j.clim.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Highfill SL, Rodriguez PC, Zhou Q, et al. Bone marrow myeloid-derived suppressor cells (MDSCs) inhibit graft-versus-host disease (GVHD) via an arginase-1-dependent mechanism that is up-regulated by interleukin-13. Blood. 2010;116:5738–5747. doi: 10.1182/blood-2010-06-287839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim Y-J, Broxmeyer HE. Immune regulatory cells in umbilical cord blood and their potential roles in transplantation tolerance. Crit Rev Oncol Hematol. 2011;79:112–126. doi: 10.1016/j.critrevonc.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Condamine T, Gabrilovich DI. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol. 2011;32:19–25. doi: 10.1016/j.it.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luyckx A, Schouppe E, Rutgeerts O, et al. G-CSF stem cell mobilization in human donors induces polymorphonuclear and mononuclear myeloid-derived suppressor cells. Clin Immunol. 2012;143:83–87. doi: 10.1016/j.clim.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 39.Cheng P, Corzo CA, Luetteke N, et al. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med. 2008;205:2235–2249. doi: 10.1084/jem.20080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gabitass RF, Annels NE, Stocken DD, Pandha HA, Middleton GW. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother. 2011;60:1419–1430. doi: 10.1007/s00262-011-1028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gaddy J, Broxmeyer HE. Cord blood CD16+56- cells with low lytic activity are possible precursors of mature natural killer cells. Cell Immunol. 1997;180:132–142. doi: 10.1006/cimm.1997.1175. [DOI] [PubMed] [Google Scholar]
- 42.Movahedi K, Guilliams M, Van den Bossche J, et al. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111:4233–4244. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


