Abstract
Surgical treatment of epilepsy is a challenge for patients with non-contributive brain magnetic resonance imaging. However, surgery is feasible if the seizure-onset zone is precisely delineated through intracranial electroencephalography recording. We recently described a method, volumetric imaging of epileptic spikes, to delineate the spiking volume of patients with focal epilepsy using magnetoencephalography. We postulated that the extent of the spiking volume delineated with volumetric imaging of epileptic spikes could predict the localizability of the seizure-onset zone by intracranial electroencephalography investigation and outcome of surgical treatment. Twenty-one patients with non-contributive magnetic resonance imaging findings were included. All patients underwent intracerebral electroencephalography investigation through stereotactically implanted depth electrodes (stereo-electroencephalography) and magnetoencephalography with delineation of the spiking volume using volumetric imaging of epileptic spikes. We evaluated the spatial congruence between the spiking volume determined by magnetoencephalography and the localization of the seizure-onset zone determined by stereo-electroencephalography. We also evaluated the outcome of stereo-electroencephalography and surgical treatment according to the extent of the spiking volume (focal, lateralized but non-focal or non-lateralized). For all patients, we found a spatial overlap between the seizure-onset zone and the spiking volume. For patients with a focal spiking volume, the seizure-onset zone defined by stereo-electroencephalography was clearly localized in all cases and most patients (6/7, 86%) had a good surgical outcome. Conversely, stereo-electroencephalography failed to delineate a seizure-onset zone in 57% of patients with a lateralized spiking volume, and in the two patients with bilateral spiking volume. Four of the 12 patients with non-focal spiking volumes were operated upon, none became seizure-free. As a whole, patients having focal magnetoencephalography results with volumetric imaging of epileptic spikes are good surgical candidates and the implantation strategy should incorporate volumetric imaging of epileptic spikes results. On the contrary, patients with non-focal magnetoencephalography results are less likely to have a localized seizure-onset zone and stereo electroencephalography is not advised unless clear localizing information is provided by other presurgical investigation methods.
Keywords: partial seizures, intracranial EEG, epileptogenic zone, epilepsy surgery, EEG, MEG
Introduction
Up to 30% of patients with focal epilepsy develop persistent seizures refractory to pharmacotherapy (Kwan and Brodie, 2000). In these patients, cortical resection is the most effective treatment to eliminate seizures.
Despite technical improvements in MRI sequences and post-processing, best-practice MRI is unable to reveal a potential epileptogenic lesion in up to 50% of surgical candidates (Berg et al., 2003; McGonigal et al., 2007).
The outcome of epilepsy surgery in patients without a relevant MRI lesion is generally poorer than when a lesion is clearly discernible, with seizure freedom only obtained in 30–60% of cases (Spencer and Huh, 2008; Tellez-Zenteno et al., 2010; Wiebe, 2011). Intracranial EEG recordings are usually mandatory before surgery in this situation. Numerous studies have shown that intracranial EEG can precisely map the seizure-onset zone (McGonigal et al., 2007; Holtkamp et al., 2012). However, intracranial EEG must be guided by non-invasive investigations as brain coverage by intracranial EEG electrodes is limited (Knowlton et al., 2008a; Sutherling et al., 2008). Non-invasive investigations are also expected to provide information regarding the probability of successful seizure-onset zone localization by intracranial EEG recording, thus contributing to the optimal selection of surgical candidates.
In the past few years, recent prospective studies have shown that magnetoencephalography (MEG) can successfully guide depth electrode placements, leading to a correct identification of the epileptogenic zone in 50–80% of cases (Stefan et al., 2003; Knowlton et al., 2006, 2008a). Moreover, the presence of a focal MEG focus increases the probability of successful seizure-onset zone localization through intracranial EEG and thus, of a good surgical outcome (Knowlton et al., 2008b).
However, the studies published so far included both MRI-negative patients and patients with known focal epileptogenic lesions, such as focal cortical dysplasia, which clearly orient the strategy of intracranial EEG exploration when invasive recordings are needed (Stefan et al., 2003; Knowlton et al., 2008a, b; Sutherling et al., 2008). Therefore it remains unknown whether MEG does help to accurately localize the seizure-onset zone and to predict the outcome of intracranial EEG recordings in the specific subgroup of patients with non-contributive MRI.
To address this issue, we performed a retrospective study including 21 patients with normal, subtle or non-focal MRI findings who had been investigated with MEG and intracranial EEG. We modelled the sources of epileptic spikes using a recently described method that allows a delineation of the brain spiking volume, volumetric imaging of epileptic spikes (VIES) (Bouet et al., 2012). We hypothesized that the presence of a focal MEG interictal spiking volume would predict a successful localization of the seizure-onset zone by intracranial EEG investigation and a subsequent favourable surgical outcome.
Materials and methods
Patients
We retrospectively included all patients with focal epilepsy who had undergone presurgical evaluation in the Epilepsy Department of the Neurological Hospital, Lyon, France between 2006 and 2010 and having the following inclusion criteria: (i) intracranial EEG recordings with stereotactically implanted electrodes (stereo-encephalography, SEEG) (Bancaud et al., 1969); (ii) MEG modelling of the sources of epileptic spikes; and (iii) negative findings on brain MRI. Patients were considered MRI-negative if MRI disclosed no abnormalities, or only subtle abnormalities of unclear significance, based on visual inspection by an expert neuroradiologist blind to the clinical history of patients and validated by the epilepsy multidisciplinary group that includes the epileptologists and neurosurgeons of our department. Patients were excluded if MRI identified epileptogenic lesions such as tumours, hippocampal atrophy, vascular malformations or suggested the presence of focal cortical dysplasia through findings such as blurring of the grey–white matter junction. Patients were not excluded if the only findings on visual analysis were mild sulcal variation, diffuse atrophy, or non-specific white matter signal changes. The study was approved by the local ethics committee, and written consent was obtained.
Ten male and 11 female patients with a mean age of 15.6 years (range 4–55 years) were included. After presurgical evaluation, 11 patients underwent surgery with a ‘tailored’ resection of the seizure-onset zone based on SEEG recordings (see Table 1 for the clinical features of the patients). Nine were not offered surgery because the seizure-onset zone was too large or not defined with sufficient precision to delineate the resection, and for one patient surgery was contraindicated because of a functional risk for language. Of the 11 operated patients, six had a favourable outcome following surgery (Engel Class I) (Engel Jr and Rasmussen, 1993) after a median follow-up of 24 months (range 10–39 months), and five had a poor outcome (Engel Class III or IV) after a median follow-up of 20 months (range 5–32 months).
Table 1.
Overview of the clinical features, presurgical investigations and surgical outcome of the 21 MRI-negative patients
| Patient | Age at SEEG, years | Video-EEG | MEG | SEEG anatomy-based seizure-onset zone | Overlap MEG/ SEEG | Surgical procedure | Surgery outcome | Pathology |
|---|---|---|---|---|---|---|---|---|
| 1 | 17 | Left frontal | Focal | Left medial OFC, left lateral OFC lateral, left F3 | Yes | Left lateral OFC, left F3 | Engel I | FCDIIa |
| 2 | 20 | Right frontal | Lateralizing | Right posterior T2/right posterior T1/right superior parietal lobule, right inferior parietal lobule, right central | Yes | NA | NA | NA |
| 3 | 12 | Left front | No spike | Left pre-SMA, left medial F1, left lateral OFC 1 | No spike | NA | NA | NA |
| 4 | 36 | Right front | Focal | Right T2, right T1, right temporal pole/right medial temporal lobe | Yes | Right temporal pole, right amygdala, right hippocampus, right T5 | Engel I | Neuronal loss |
| 5 | 4 | Right front | Non lateralizing | Right lateral F1, right F2, right lateral OFC/right SMA | Yes | Right frontal deconnexion (F1-F2-pole), resection: right F3 resection and right OFC, anterior callosotomy | Engel IV | Mild MCD |
| 6 | 24 | Right frontal | Lateralizing | Right frontal pole, right medial F1 | Yes | Right medial F1, right SMA, right frontal pole | Engel IV | Mild MCD |
| 7 | 15 | Left frontal | Non lateralizing | Left SMA, left pre-SMA, left lateral F2, left lateral F1, left lateral OFC, left posterior cingulate gyrus | Yes | Left frontal pole, left anterior F1, Left SMA, Left anterior cingulate gyrus | Engel III | Normal |
| 8 | 55 | Left frontal | Lateralizing | Left SMA, left pre-SMA/left F1, left F2 | Yes | NA | NA | NA |
| 9 | 19 | Right temporal | Focal | Right lateral OFC | Yes | Right OFC | Engel I | Normal |
| 10 | 36 | Right temporal | Focal | Right OFC, right anterior T1, right anterior T2 | Yes | Right OFC, right temporal pole, right amygdala, right hippocampus, right parahippocampus | Engel I | FCDI |
| 11 | 32 | Left frontal | Focal | Left frontal operculum, left precentral gyrus, left OFC | Yes | NA | NA | NA |
| 12 | 11 | Left frontal | Lateralizing | Left posterior cingulate gyrus, left medial parietal, left lateral parietal | Yes | NA | NA | NA |
| 13 | 18 | Left temporal-occipital | Lateralizing | Left basal temporal, left occipital, left parietal | Yes | NA | NA | NA |
| 14 | 25 | Left temporal | No spike | Left anterior medial temporal, left posterior medial temporal | No spike | NA | NA | NA |
| 15 | 10 | Right parietal/occipital | Lateralizing | Right superior parietal lobule, right inferior parietal lobule, right basal temporal | Yes | Right medial parietal, right posterior cingulate gyrus | Engel III | Normal |
| 16 | 16 | Left posterior perisylvian | Focal | Left posterior T1, left posterior T2, left posterior insula, left temporal pole | Yes | Left posterior T1, left posterior T2 | Engel I | Normal |
| 17 | 17 | Left posterior perisylvian | Focal | Right temporo-parietal junction, right parietal operculum, right posterior insula | Yes | Right parietal operculum, right posterior T1, right posterior insula | Engel III | Normal |
| 18 | 6 | Left posterior perisylvian | Focal | Left frontal operculum, left parietal operculum | Yes | Left frontal operculum, left parietal operculum | Engel I | FCDIIa |
| 19 | 25 | Left frontal | No spike | Left temporal pole, left anterior T1/left anterior T2, left amygdala | No spike | NA | Engel III | Normal |
| 20 | 8 | Right central | No spike | Right medial parietal, right posterior central gyrus, right superior parietal lobule, right central | No spike | NA | NA | No |
| 21 | 11 | Left posterior perisylvian | Lateralizing | Left F1, left F2, frontal operculum, left temporal pole, left anterior insula, left posterior insula | Yes | NA | NA | NA |
Video-EEG: the main lobar hypothesis regarding the location of the seizure-onset zone is provided; MEG: the extent of the MEG spiking volume determined by VIES is summarized in three categories (see ‘Material and methods’ section).
FCD = focal cortical dysplasia; F1 = superior frontal gyrus; F2 = middle frontal gyrus; F3 = inferior frontal gyrus; MCD = malformation of cortical development; NA = not applicable; OFC = orbito-frontal cortex; SMA = supplementary motor area; T1 = superior temporal gyrus; T2 = middle temporal gyrus; T5 = lingual gyrus.
At the time of inclusion, MEG was considered as a complementary investigation under validation in our department so that MEG data had no influence on the decision to proceed to intracranial EEG recordings. Moreover, VIES data were not used for clinical purposes. However, when dipole modelling of epileptic spikes provided focal results, MEG data were sometimes considered in the choice of depth electrode placements for SEEG recordings. As a first step, the implantation scheme was done by authors P.R. or J.I. who were blind to MEG data, according to interictal EEG, video-scalp-EEG ictal data, interictal 18F-fluorodeoxyglucose-PET, interictal and ictal single photon emission tomography; as a second step, MEG data were included in the final decision. The total number of electrodes added to the original scheme when MEG data were included is of one electrode for four patients and null for the remaining patients. Moreover, the whole set of depth electrodes (between 8 and 15 depth electrodes per patient) sampled the neural activity of a brain volume larger than the MEG spiking volume (the number of electrodes sampling the MEG volume divided by the total set of electrodes was between 1 over 12 and 5 over 10 per patient).
Depth stereo–electroencephalography recordings
18F-Fluorodeoxyglucose-PET, ictal and interictal intracranial video-SEEG recordings were obtained in all 21 patients. Intracranial EEG sampling was guided by analysis of seizure symptoms, fluorodeoxyglucose-PET and MEG data.
SEEG explorations were carried out during long-term video-EEG monitoring. Recordings were performed using intracerebral electrodes with multiple contacts (10–15 contacts, length: 2 mm, electrode diameter: 0.8 mm, inter-contact spacing 1.5 mm) placed according to Talairach’s stereotactic method (Engel Jr and Rasmussen, 1993). The number of electrodes implanted per patient varied between eight and 15, with a total number of recording contacts between 102 and 128 per patient. Electrodes were left in place between 1 and 3 weeks.
Regarding the localizability of the seizure-onset zone, we defined two categories based on the results of the SEEG recordings. The seizure-onset zone was considered as localized when few SEEG contacts within a small cortical patch (one or contiguous sublobar regions of the brain) disclosed high frequency activity, while the other surrounding contacts disclosed lower frequency in the 5 s preceding the seizure or in the 5 s after the clinical onset of seizures (Bancaud et al., 1969). When ictal discharges at seizure onset were spatially widespread or multifocal, the seizure-onset zone was considered as non-localized.
Magnetic resonance imaging acquisition
In all patients, brain MRI was performed using a 3 T MR scanner (Philips Achieva, Philips), and included the following sequences in all patients: (i) 3D anatomical T1-weighted covering the whole brain volume with 1 mm3 cubic voxels, in the bihippocampal and coronal planes; (ii) 6-mm thick turbo-spin echo T2-weighted acquired in the bihippocampal plane; (iii) turbo-spin echo T2 perpendicular to the bihippocampal plane; (iv) FLAIR in the same plane as the turbo-spin echo T2 sequence; and (v) inversion-recovery. In all patients included in the present study brain MRI was considered as non-contributive; it was entirely normal in 19 patients, showed subtle abnormalities of unclear significance in one patient (Patient 1; mild sulcal abnormality in the frontal lobe) and diffuse left hemispheric atrophy in another patient (Patient 4).
In the 24 h after electrode implantation, a second MRI 3D T1 sequence was obtained to show the exact location of recording contacts.
Magnetoencephalography acquisition
MEG signals were recorded on a CTF Omega 275 channel whole head system (VSM MedTech Ltd.). For each patient, 45 min of continuous MEG signal were recorded at a sampling frequency of 1200 Hz using a third-order spatial gradient noise. Patients were investigated at rest with their eyes closed.
Three fiducial coils (nasion, left and right pre-auricular points) were placed for each patient to determine the head position within the MEG helmet, and to provide co-registration with the anatomical magnetic resonance images.
Magnetoencephalography modelling of the sources of interictal spikes
MEG data were processed using VIES, a pipeline of analysis previously described and validated. VIES aims at localizing the brain spiking volume, which generates high frequency activities (>20 Hz) associated with interictal spikes [see Bouet et al. (2012) for a full description of the method].
In VIES, spikes are first visually identified on raw MEG signals. Secondly, the brain sources of spike-related high frequency activity (>20 Hz) are located using a beam forming technique (Dynamic Imaging of Coherent Sources; Gross et al., 2001). Lastly, the MEG maps are thresholded using a method described in Bouet et al. (2012), providing a MEG spiking volume map for each patient.
Congruence between magnetoencephalography spiking volume and the seizure-onset zone
For each patient, we determined the seizure onset-zone based on visual analysis of SEEG data. The seizure-onset zone is the brain volume encompassing all bipolar derivations presenting high frequency activity (mostly in the beta or gamma range) at the beginning of seizures. We then plotted the MEG spiking volume onto the patient’s co-registered post-implantation MRI, and determined whether there was a spatial overlap between the seizure-onset zone and MEG spiking volume. The MEG spiking volume was considered congruent to the seizure-onset zone if at least five bipolar derivations included in the seizure-onset zone were located within the MEG spiking volume. In addition, for patients with localized spiking volume on MEG analysis (see below), we determined: (i) the proportion of implanted depth electrodes within the MEG spiking volume that showed at least five bipolar derivations with high frequency activity at seizure onset; and (ii) the proportion of implanted depth electrodes with at least five bipolar derivations presenting high frequency activity at seizure onset that were included in the MEG spiking volume.
Determining the spiking volume with magnetoencephalography
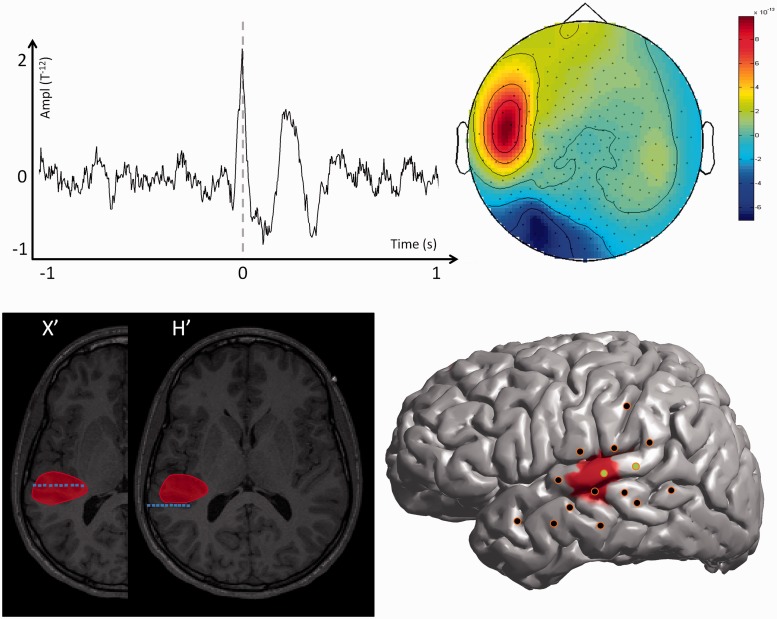
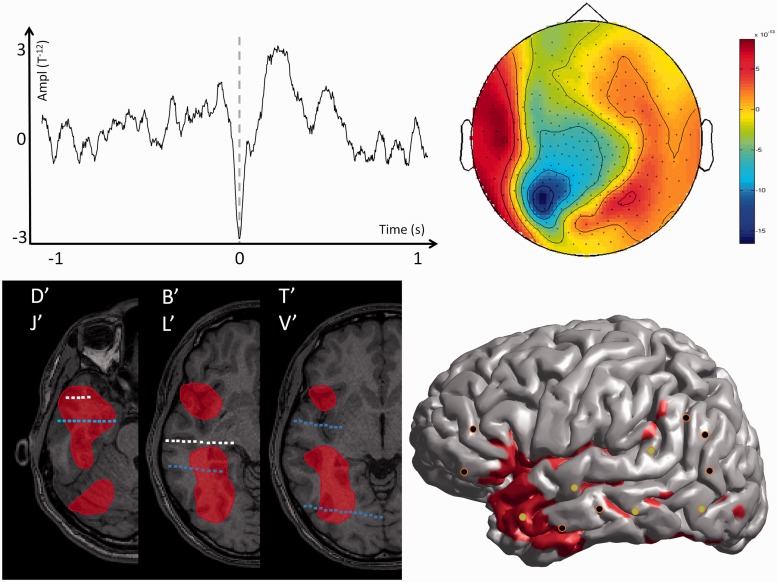
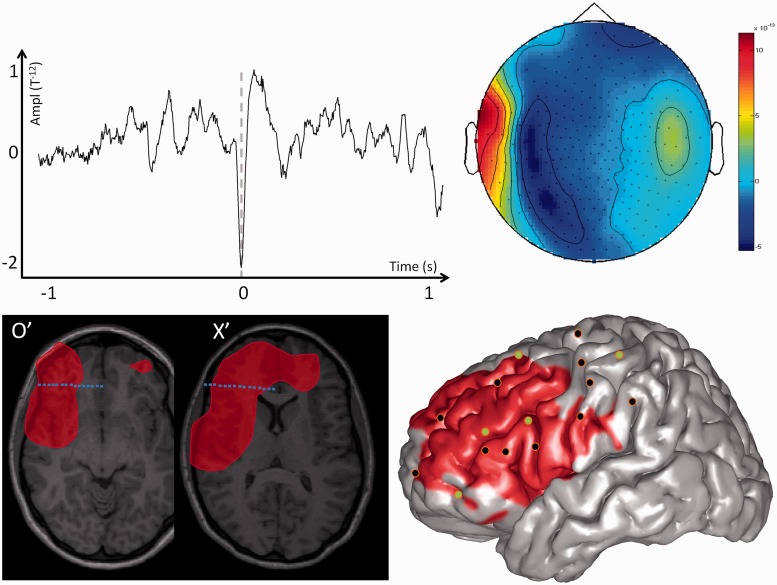
For each patient, the spatial distribution of the MEG spiking volume was categorized in three groups according to its spatial extent. We considered that the MEG spiking volume was localized when it was located within only one brain lobe, lateralized when it extended into several brain lobes of the same hemisphere and non-lateralized when it extended over both hemispheres. Typical examples of patients with localized, lateralized and non-lateralized spiking volumes are shown in Figs 1, 2 and 3. Short clinical reports of those three cases are provided in the Supplementary material.
Figure 1.
MEG modelling of epileptic spikes and SEEG localization of the seizure-onset zone in a patient with a focal spiking volume (Patient 16). Top left: Example of one prototypical spike recorded with MEG for one single channel. Top right: Topographic map of the magnetic field recorded for all MEG channels at the main peak of the prototypical spike. Bottom left: The MEG spiking volume determined with VIES (red volume) is shown on representative MRI slices. On the same slices, the SEEG electrodes showing clear signal changes at seizure-onset are shown in blue. Bottom right: SEEG implantation scheme of Patient 16. The location of the penetrating points of intracranial electrodes is determined by co-registering the patient’s post-implantation MRI onto the cortical mesh extracted from the MRI. The MEG spiking volume projected onto the cortical surface is shown as a red area. SEEG electrodes showing clear signal change at seizure onset are shown in green and electrodes without ictal changes are shown in black. Notice that the spiking volume is spatially restricted and co-extensive with the seizure-onset zone determined with SEEG. The patient underwent surgery with a resection of the posterior part of the left temporal neocortex (superior and middle temporal gyrus) and is seizure free postoperatively after 22 months of follow-up.
Figure 2.
MEG modelling of epileptic spikes and SEEG localization of the seizure-onset zone in a patient with a lateralized spiking volume (Patient 13). Top left: Example of one prototypical spike recorded with MEG for one single channel of Patient 13. Top right: Topographic map of the magnetic field recorded for all MEG channels at the main peak of the prototypical spike. Bottom left: The MEG spiking volume determined with VIES (red volume) is shown on representative MRI slices. On the same slices, the SEEG electrodes showing clear signal changes at seizure-onset are shown in blue, and electrodes without clear ictal activity are in white. Bottom right: SEEG implantation scheme of Patient 13. The location of the penetrating points of intracranial electrodes is determined by co-registering the patient’s post-implantation MRI onto the cortical mesh extracted from the MRI. The MEG spiking volume projected onto the cortical surface is shown as a red area. SEEG electrodes showing clear signal changes at seizure-onset are shown in green and electrodes without ictal changes are shown in black. Notice that the spiking volume is lateralized but not focal and only partly co-extensive with the seizure-onset zone determined with SEEG (several lobes are included in the MEG spiking volume). Surgery was contraindicated for Patient 13 as the seizure-onset zone was considered as non-localized based on SEEG.
Figure 3.
MEG modelling of epileptic spikes and SEEG localization of the seizure-onset zone in a patient with a non-lateralized spiking volume. Top left: Example of one prototypical spike recorded with MEG for one single channel of Patient 7. Top right: Topographic map of the magnetic field recorded for all MEG channels at the main peak of the prototypical spike. Bottom left: The MEG spiking volume determined with VIES (red volume) is shown on representative MRI slices. On the same slices, the SEEG electrodes showing clear signal changes at seizure-onset are shown in blue, and electrodes without clear ictal activity are in white. Bottom right: SEEG implantation scheme of Patient 7. The location of the penetrating points of intracranial electrodes is determined by co-registering the patient’s post-implantation MRI onto the cortical mesh extracted from the MRI. The MEG spiking volume projected onto the cortical surface is shown as a red area. SEEG electrodes showing clear signal changes at seizure-onset are shown in green and electrodes without ictal changes are shown in black. Notice that the spiking volume is spatially very extensive and partly co-extensive with the seizure-onset zone determined with SEEG. The seizure-onset zone was considered as not perfectly delineated. However, because the epilepsy was very severe, the patient underwent surgical resection of left antero-mesial frontal cortex [frontal pole + anterior superior frontal gyrus (F1) + supplementary motor area and anterior cingulate gyrus] with an unsatisfactory surgical outcome (Engel III).
Magnetoencephalography prediction of stereo-electroencephalography outcome
For each MEG group, we determined the proportion of patients with localized seizure-onset zone and non-localized seizure-onset zone. We compared those proportions using Fischer’s exact test, setting the significance threshold to P ≤ 0.05.
Magnetoencephalography prediction of surgical outcome
Lastly, we determined the proportion of patients with good surgical outcome (Engel Class I) and poor surgical outcome (Engel Class III, Engel Class IV) for each MEG group, and those proportions using Fischer’s exact test, setting the significance threshold to P ≤ 0.05.
Results
Magnetoencephalography delineation of the spiking volume
Among the 21 patients, 17 had unambiguous epileptic spikes during MEG recordings, whereas four had no spikes. The mean sensitivity of MEG in the whole group of patients was therefore 81%. For the 17 patients with interictal spikes, VIES showed significant spike-related high frequency activity >20 Hz within a brain volume of variable extent. The mean number of epileptic spikes recorded per patient was 32 ± 20.4. The mean frequency of the spikes themselves was variable across patients (30 ± 11.46 Hz). In 8 of 17 patients (47%), the MEG spiking volume was localized; in 7 of 17 (41%) it was lateralized and in 2 of 17 (12%) non-lateralized. The mean spiking volume for patients with localized spiking volume was 32.8 cm3, 95.2 cm3 for patients with lateralized spiking volume and 165.1 cm3 for patients with non-lateralized spiking volume. In 9 of 17 (53%), the voxel with maximal spike-related activity was located in the frontal lobe, in 6 of 17 (35%) in the temporal lobe, and in 2 of 17 (12%) in the parietal lobe.
Congruence between magnetoencephalography spiking volume and the seizure-onset zone
Across the 17 patients with MEG spikes, visual analysis of seizures recorded with SEEG showed that 11 patients (65%) had a localized seizure-onset zone, whereas six patients (35%) had a non-localized seizure-onset zone. For each of the 17 patients, there was spatial overlap between the seizure-onset zone and the MEG spiking volume. In addition, for the eight patients with localized spiking volume with MEG, 68% of the depth electrodes implanted in the MEG spiking volume showed high frequency activity at seizure onset, and 66% of the depth electrodes with high frequency activity at seizure onset were included in the MEG spiking volume. Figures 1, 2 and 3 show examples of the congruence of SEEG and MEG in three patients with different extents of the spiking volume.
Magnetoencephalography prediction of stereo-electroencephalography outcome
In the localized MEG spiking volume group (n = 8 patients), all eight (100%) had also localized seizure-onset zone. In the lateralized MEG spiking volume group (n = 7 patients), three (43%) had localized seizure-onset zone. In the non-lateralized MEG spiking volume group (n = 2 patients), none (0%) had localized seizure-onset zone (Table 2). Across the 17 patients with MEG spikes, the proportion of patients with localized SEEG differed significantly according to the extent of spiking volume determined by MEG (Fischer’s exact test, P < 0.05).
Table 2.
Outcome of SEEG investigation and surgical outcome of the patients according to MEG spiking volume
| MEG SV | SEEG |
Surgery |
SEEG congruence |
|||
|---|---|---|---|---|---|---|
| Non-localized n (%) | Localized n (%) | Good outcome n (%) | Poor outcome n (%) | Lateralized-epileptogenic zone (%) | Non-lateralized- epileptogenic zone (%) | |
| Focal | 0 (0) | 8 (100) | 6 (86) | 1 (14) | 65 | 35 |
| Lateralized | 4 (57) | 3 (43) | 0 (0) | 2 (100) | ||
| Non-lateralized | 2 (100) | 0 (0) | 0 (0) | 2 (100) | ||
Good outcome = number (percentage) of patients with Engel Score I; poor outcome = number (percentage) of patients with Engel Score other than I.
EZ = epileptogenic zone; SV = spiking volume.
Magnetoencephalography predictions of surgical outcomes
In the group with a localized MEG spiking volume, seven patients were operated upon, of whom six (86%) had a good surgical outcome (Table 2). In the group with a lateralized MEG spiking volume (n = 2 patients operated), as well as in the non-lateralized MEG spiking volume group (n = 2 patients operated), no patient had a good surgical outcome. Across the 11 patients with MEG spikes who were operated upon, the proportion of patients with good surgical outcome differed significantly according to the extent of the spiking volume determined by MEG (Fischer’s exact test, P < 0.05).
Relationships between magnetoencephalography spiking volume, surgical outcome and pathology
Among the patients who were operated with a good surgical outcome (n = 6), five had focal cortical dysplasia and one a normal pathological examination. Conversely, we found that for all patients with a poor surgical outcome (n = 5), the pathological examination was either normal (n = 3) or showed a mild malformation of cortical development (n = 2).
Discussion
Epilepsy surgery in patients with non-contributive magnetic resonance imaging
Several studies have shown that brain MRI is a strong predictor of surgical outcome (Berg et al., 2003; Spencer and Huh, 2008; Tellez-Zenteno et al., 2010; Wiebe, 2011). In our sample of 21 patients, 11 patients have been operated, with six of them (54%) achieving a seizure-free postoperative outcome. Careful presurgical evaluation including MEG modelling of epileptic spikes, fluorodeoxyglucose PET, and invasive intracranial SEEG when needed, provides objective clues to localize the epileptogenic zone, which is a prerequisite for obtaining a high rate of seizure freedom after surgery (Knowlton et al., 2006, 2008b, 2009; McGonigal et al., 2007). It is noticeable that a high proportion of patients were discarded from surgery in our group (12 of 21 patients), despite having undergone invasive SEEG. This suggests that the selection of patients who are candidates for SEEG needs to be optimized by non-invasive explorations able to evaluate the probability of success of SEEG when brain MRI is non-contributive. Our study suggests that MEG modelling of spikes could be useful for this purpose.
Methodological issues
Before addressing the questions of the clinical relevance of MEG for seizure-onset localization in MRI-negative patients, several issues regarding data analysis and interpretation deserve comments.
Stereo-electroencephalography as a gold standard for the seizure-onset zone determination
SEEG enables the recording of neural activity not only in superficial cortical areas but also in deep or sulcal cortices and is usually considered as the gold-standard for seizure-onset zone localization. However, the brain sampling of neural activity provided by SEEG electrodes is necessarily limited, implying that the exact spatial extent of the seizure-onset zone cannot be perfectly determined. Consequently, the quantitative spatial overlap between the MEG spiking volume and the true seizure-onset zone cannot be directly calculated, and is derived from the analysis of neural recordings provided by some depth EEG electrodes optimally sampling the seizure-onset zone and the MEG maps. For patients with focal MEG maps, the degree of overlap between MEG and the seizure-onset zone can be more precisely evaluated as MEG volumes are better sampled by SEEG electrodes. For those patients, the congruence of MEG and seizure-onset zone was represented by two measures: the proportion of implanted depth electrodes within the MEG spiking volume presenting ictal activity and the proportion of depth electrodes presenting ictal activity that were included in the MEG spiking volume. For patients with non-focal MEG maps, several parts of the MEG spiking volume are not explored by SEEG electrodes. In that situation, the only conclusion that can be reached is whether there is or not a spatial overlap (represented here by a limited number of common electrode contacts) between MEG maps and the SEEG seizure-onset zone, whatever its degree.
The place of magnetoencephalography modelling during presurgical workup of patients
At the time of inclusion of the patients, only the modelling of epileptic spikes with equivalent current dipoles was used (and not VIES modelling) for clinical decisions. As reported above, MEG results influenced the strategy of implantation only for patients with focal cluster of spikes, and the specific influence of MEG was limited in those cases. In theory, the results of our MEG modelling method should not influence the strategy of implantation of SEEG electrodes (the gold-standard method in our case) in order to be perfectly evaluable. However, for several reasons discussed below, we argue that in our study, this potential bias does not invalidate the main results.
For patients with focal cluster of spikes, the total number of electrodes specifically guided by MEG data was of one electrode for four patients and nil for the remaining patients. Moreover, the whole set of depth electrodes (between 8 and 15 depth electrodes per patient) sampled the neural activity of a brain volume larger than the MEG spiking volume. In this type of patient, electrodes located remotely from the focal MEG spiking volume are implanted for safety in order to exclude the possibility of a seizure onset zone larger than that predicted. We do not claim that the seizure-onset zones were strictly overlapping the MEG spiking volume maps. It remains that these electrodes could have pointed out non-focal seizure onset zone, but this was never observed. Secondly, most of the patients with focal MEG spiking volume have been surgically treated with good surgical outcome. This point constitutes a second argument in favour of a truly focal seizure-onset zone. Lastly, for ethical reasons, it was impossible to hide MEG results when establishing the implantation strategy of depth electrodes because their clinical relevance have been shown by numerous studies.
For patients with non-focal MEG results, a non-focal MEG result could still hide a focal seizure onset. It is not known whether the extent of the MEG spiking volume is directly related to the true extent of the seizure-onset. However, in simulation studies, it has been shown that the volumetric images produced by beam-formers typically show a peak centred around the electrical source location with a width depending on true source extent and on the signal-to-noise ratio of the data (Barnes and Hillebrand, 2003; Hillebrand and Barnes, 2011). It is thus likely that the extent of MEG spiking volume is at least partially correlated with the true intracranial spiking volume (as shown by our previous study; Bouet et al., 2012) and with the extent of the seizure-onset zone. Secondly, from a clinical point of view, the main message is that the likelihood of finding a focal seizure-onset zone in SEEG using standard presurgical investigation tools (video-EEG, fluorodeoxyglucose-PET) is greatly reduced when the MEG spiking volume is not focal. We believe that the prognostic information provided by MEG is in itself relevant, independently of the exact significance of the MEG findings.
The role of magnetoencephalography for patients with non-contributive magnetic resonance imaging
It is now well established that MEG is a robust method to precisely localize the brain sources of epileptic spike (Fischer et al., 2005; Agirre-Arrizubieta et al., 2009; Mauguière et al., 2009). Because the spiking areas often spatially overlap with the seizure-onset zone, the brain sources of MEG spikes are a good indicator of the seizure-onset zone (Knowlton et al., 2006, 2008a). In our series of patients, we found indeed that the intracranial EEG seizure-onset zone always overlapped with the MEG spiking volume. However, the extent of the spiking volume also showed large variations across patients and we show that the extent of the MEG spiking volume carries meaningful clinical implications regarding the surgical prognosis.
For patients with a focal spiking volume (n = 8), intracranial EEG always showed a localized seizure-onset zone and most of the patients were operated with good surgical outcome (six of seven). In this situation, it is probable that the true seizure-onset zone was spatially restricted and well mapped by intracranial EEG electrodes so that patients were good candidates for surgery. It is usually considered that the main role of MEG during the presurgical evaluation is to guide the placement of depth electrodes (Sutherling et al., 2008; Knowlton et al., 2009; Stefan et al., 2011). Our data confirm that for patients with focal spiking volume, MEG guided implantation of depth electrodes led to successful localization of the seizure-onset zone. Moreover, we found that most of the patients had focal cortical dysplasia on pathological examination, showing that MEG is a useful investigation to detect occult focal cortical dysplasia in patients with unremarkable brain MRI. This finding has been suggested by a previous study (Funke et al., 2011), which did not use SEEG to validate MEG findings. As a whole, our results suggest that a focal MEG spiking volume is predictive of a successful presurgical evaluation and subsequent surgery.
In contrast, when the spiking volume was not focal (n = 9), none of the four operated patients had a seizure-free surgical outcome. Within this group, SEEG did not provide a clear delineation of the seizure-onset zone in most patients, and surgery was discarded in five of nine cases. The failure of SEEG to delineate a focal seizure-onset zone in this context could be due to two causes: (i) there was no clear focal focus generating the seizures which were instead triggered by a widespread epileptogenic network; and/or (ii) the seizure onset zone was not detected by SEEG due to inadequate spatial sampling of the implanted depth electrodes. It is not possible to disentangle those two possibilities in the absence of a gold standard method able to localize the seizure-onset zone with certainty. Whatever the correct explanation, the extent of the spiking volume determined by the VIES method proved to be an important prognostic factor regarding the outcomes of SEEG and surgery. Moreover, we found that all patients who were finally operated upon based on SEEG data (four patients) in spite of a non-focal MEG spiking volume had a poor surgical outcome. Taken together our results suggest that a non-focal spiking volume in patients with non-contributive brain MRI is predictive of an unsuccessful or a potentially misleading SEEG interpretation, and of a poor outcome following surgery.
Lastly, four patients had no spikes recorded during MEG acquisition. None had good surgical outcomes. It is possible that the absence of information provided by MEG, and thus not contributing to the strategy of implantation, might decrease the likelihood of finding a focal seizure-onset zone during SEEG. Large cohort studies comparing the surgical outcome for patients with positive and negative MEG results should clarify directly this point.
Conclusion
Our study shows that spiking volume MEG modelling using VIES is a promising tool to determine the surgical prognosis for MRI-negative patients. Based on the results of that study, which require confirmation on a larger cohort of patients, we consider that the added value of MEG modelling using VIES in MRI-negative patients can be summarized as follows: patients having focal MEG results with VIES are good surgical candidates and the implantation strategy should incorporate VIES results. On the contrary, patients with non-focal MEG results are less likely to have localized seizure-onset zone and SEEG is not advised unless clear localizing information is provided by other presurgical investigation methods (focal interictal hypometabolism using fluorodeoxyglucose-PET or focal ictal hyperperfusion using single PET).
Supplementary Material
Acknowledgements
Dr Jung thanks the NeuroDis foundation for excellent technical assistance. Dr Jung and Dr Bouet have full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Dr Jung, Dr Bouet, Dr Delpuech, Dr Ryvlin, Dr Isnard, Dr Guenot, Dr Bertrand, Dr Hammers, Dr Mauguière report no disclosures.
Glossary
Abbreviations
- MEG
magnetoencephalography
- SEEG
stereo-encephalography
- VIES
volumetric imaging of epileptic spikes
Funding
Support to this study was provided through grants from the PHRC 27-11 (Programme Hospitalier de Recherche Clinique) ‘High Frequency oscillations in Magneto-EncephaloGraphy (MEG): diagnostic usefulness in presurgical assessment of partial epilepsies’, and grants from the FFRE (French Foundation for Research on Epilepsy). This work was also performed within the framework of the LABEX CORTEX (ANR-11-LABX-0042) of Université de Lyon, within the program “Invetsissements d'Avenir” (ANR-11-IDEX-0007) operated by the French National Research Agency (ANR).
Supplementary material
Supplementary material is available at Brain online.
References
- Agirre-Arrizubieta Z, Huiskamp GJ, Ferrier CH, van Huffelen AC, Leijten FS. Interictal magnetoencephalography and the irritative zone in the electrocorticogram. Brain. 2009;132(Pt 11):3060–71. doi: 10.1093/brain/awp137. [DOI] [PubMed] [Google Scholar]
- Bancaud J, Angelergues R, Bernouilli C, Bonis A, Bordas-Ferrer M, Bresson M, et al. [Functional stereotaxic exploration (stereo-electroencephalography) in epilepsies] Rev Neurol (Paris) 1969;120:448. [PubMed] [Google Scholar]
- Barnes GR, Hillebrand A. Statistical flattening of MEG beamformer images. Hum Brain Mapp. 2003;18:1–12. doi: 10.1002/hbm.10072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg AT, Vickrey BG, Langfitt JT, Sperling MR, Walczak TS, Shinnar S, et al. The multicenter study of epilepsy surgery: recruitment and selection for surgery. Epilepsia. 2003;44:1425–33. doi: 10.1046/j.1528-1157.2003.24203.x. [DOI] [PubMed] [Google Scholar]
- Bouet R, Jung J, Delpuech C, Ryvlin P, Isnard J, Guenot M, et al. Towards source volume estimation of interictal spikes in focal epilepsy using magnetoencephalography. Neuroimage. 2012;59:3955–66. doi: 10.1016/j.neuroimage.2011.10.052. [DOI] [PubMed] [Google Scholar]
- Engel J, Jr, Rasmussen TB. Outcome with respect to epileptic seizures. Surgical Treatment of the Epilepsies. New York, NY: Raven Press; 1993. pp. 609–21. [Google Scholar]
- Fischer MJ, Scheler G, Stefan H. Utilization of magnetoencephalography results to obtain favourable outcomes in epilepsy surgery. Brain. 2005;128(Pt 1):153–7. doi: 10.1093/brain/awh333. [DOI] [PubMed] [Google Scholar]
- Funke ME, Moore K, Orrison WW, Jr, Lewine JD. The role of magnetoencephalography in ‘nonlesional’ epilepsy. Epilepsia. 2011;52(Suppl 4):10–14. doi: 10.1111/j.1528-1167.2011.03144.x. [DOI] [PubMed] [Google Scholar]
- Gross J, Kujala J, Hamalainen M, Timmermann L, Schnitzler A, Salmelin R. Dynamic imaging of coherent sources: studying neural interactions in the human brain. Proc Natl Acad Sci USA. 2001;98:694–9. doi: 10.1073/pnas.98.2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillebrand A, Barnes GR. Practical constraints on estimation of source extent with MEG beamformers. Neuroimage. 2011;54:2732–40. doi: 10.1016/j.neuroimage.2010.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtkamp M, Sharan A, Sperling MR. Intracranial EEG in predicting surgical outcome in frontal lobe epilepsy. Epilepsia. 2012;53:1739–45. doi: 10.1111/j.1528-1167.2012.03600.x. [DOI] [PubMed] [Google Scholar]
- Knowlton RC, Elgavish R, Howell J, Blount J, Burneo JG, Faught E, et al. Magnetic source imaging versus intracranial electroencephalogram in epilepsy surgery: a prospective study. Ann Neurol. 2006;59:835–42. doi: 10.1002/ana.20857. [DOI] [PubMed] [Google Scholar]
- Knowlton RC, Elgavish RA, Bartolucci A, Ojha B, Limdi N, Blount J, et al. Functional imaging: II. Prediction of epilepsy surgery outcome. Ann Neurol. 2008a;64:35–41. doi: 10.1002/ana.21419. [DOI] [PubMed] [Google Scholar]
- Knowlton RC, Elgavish RA, Limdi N, Bartolucci A, Ojha B, Blount J, et al. Functional imaging: I. Relative predictive value of intracranial electroencephalography. Ann Neurol. 2008b;64:25–34. doi: 10.1002/ana.21389. [DOI] [PubMed] [Google Scholar]
- Knowlton RC, Razdan SN, Limdi N, Elgavish RA, Killen J, Blount J, et al. Effect of epilepsy magnetic source imaging on intracranial electrode placement. Ann Neurol. 2009;65:716–23. doi: 10.1002/ana.21660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342:314–9. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- Mauguière F, Merlet I, Jung J. Experimental neurophysiological techniques. In: Shorvon SD, Perucca E, Engel J, editors. The treatment of epilepsy. 3rd edn. Oxford, UK: Wiley-Blackwell; 2009. , doi: 10.1002/9781444316667.ch66. [Google Scholar]
- McGonigal A, Bartolomei F, Regis J, Guye M, Gavaret M, Trebuchon-Da Fonseca A, et al. Stereoelectroencephalography in presurgical assessment of MRI-negative epilepsy. Brain. 2007;130(Pt 12):3169–83. doi: 10.1093/brain/awm218. [DOI] [PubMed] [Google Scholar]
- Spencer S, Huh L. Outcomes of epilepsy surgery in adults and children. Lancet Neurol. 2008;7:525–37. doi: 10.1016/S1474-4422(08)70109-1. [DOI] [PubMed] [Google Scholar]
- Stefan H, Hummel C, Scheler G, Genow A, Druschky K, Tilz C, et al. Magnetic brain source imaging of focal epileptic activity: a synopsis of 455 cases. Brain. 2003;126(Pt 11):2396–405. doi: 10.1093/brain/awg239. [DOI] [PubMed] [Google Scholar]
- Stefan H, Rampp S, Knowlton RC. Magnetoencephalography adds to the surgical evaluation process. Epilepsy Behav. 2011;20:172–7. doi: 10.1016/j.yebeh.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Sutherling WW, Mamelak AN, Thyerlei D, Maleeva T, Minazad Y, Philpott L, et al. Influence of magnetic source imaging for planning intracranial EEG in epilepsy. Neurology. 2008;71:990–6. doi: 10.1212/01.wnl.0000326591.29858.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellez-Zenteno JF, Hernandez Ronquillo L, Moien-Afshari F, Wiebe S. Surgical outcomes in lesional and non-lesional epilepsy: a systematic review and meta-analysis. Epilepsy Res. 2010;89:310–8. doi: 10.1016/j.eplepsyres.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Wiebe S. Epilepsy. Outcome patterns in epilepsy surgery—the long-term view. Nat Rev Neurol. 2011;8:123–4. doi: 10.1038/nrneurol.2012.9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.