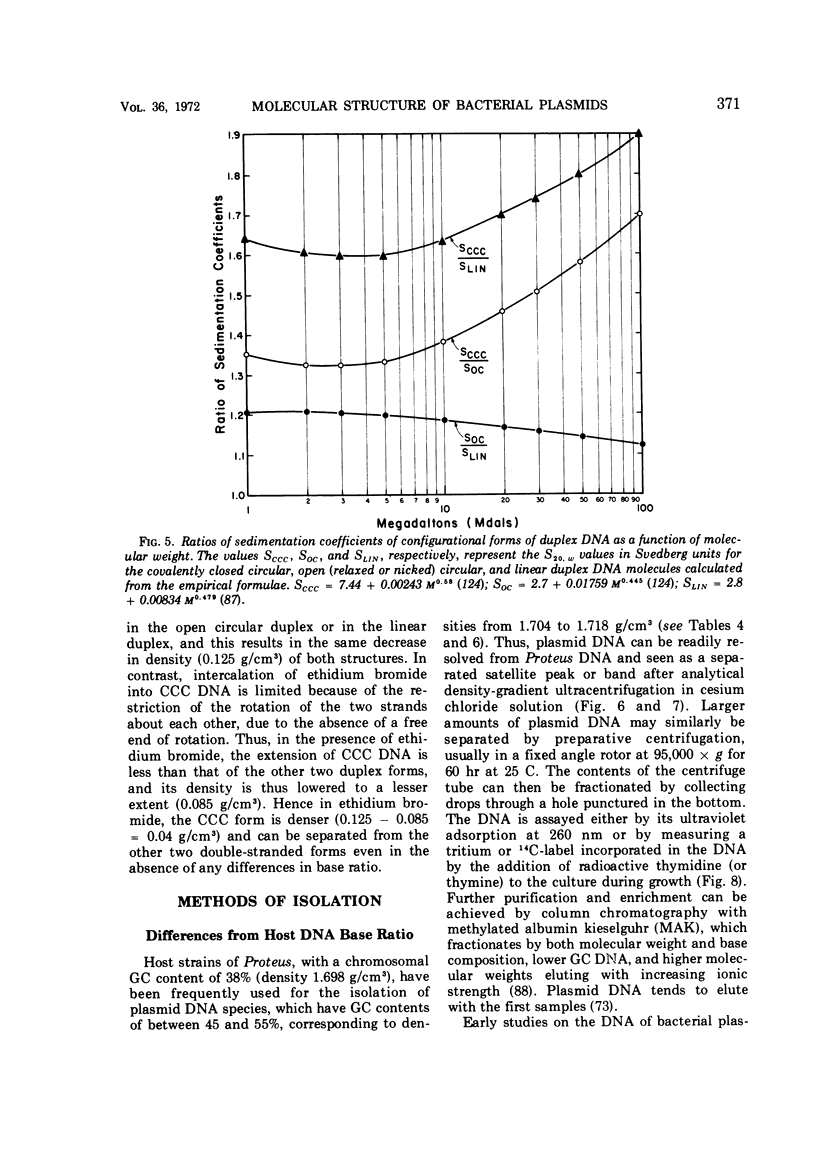
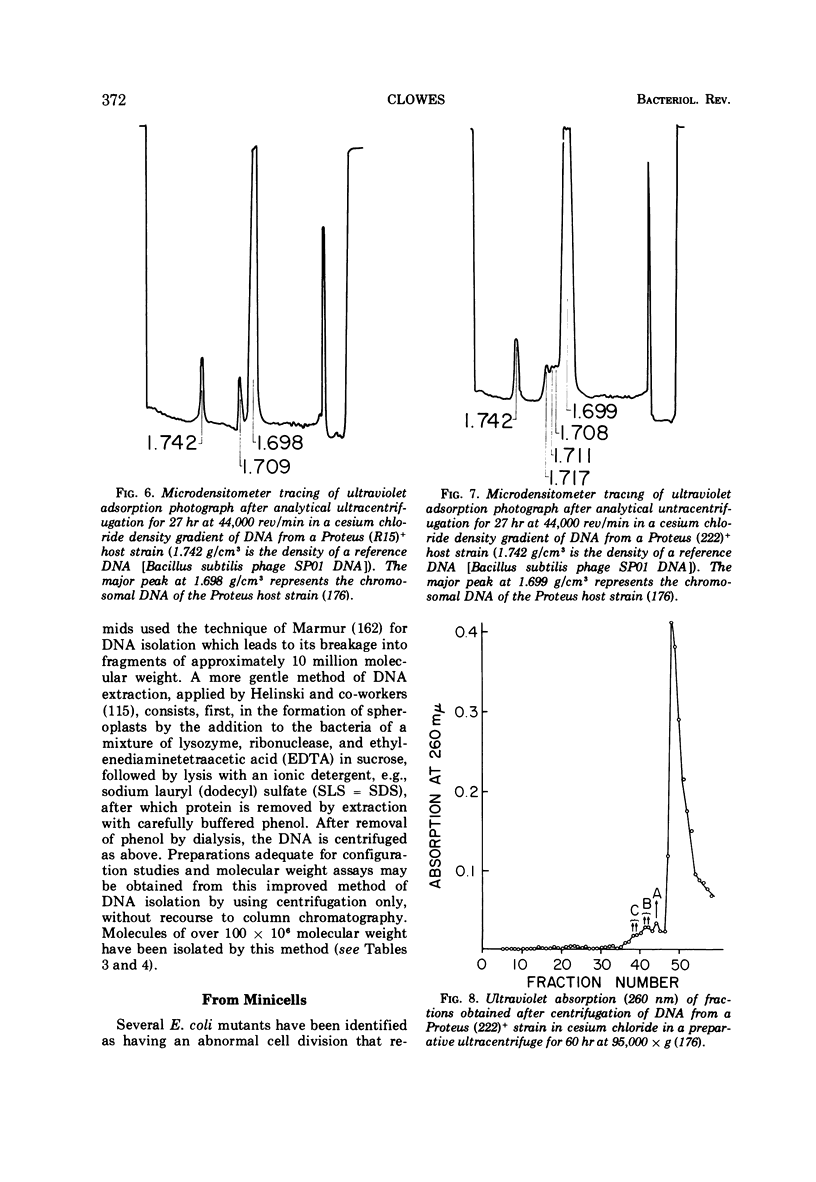
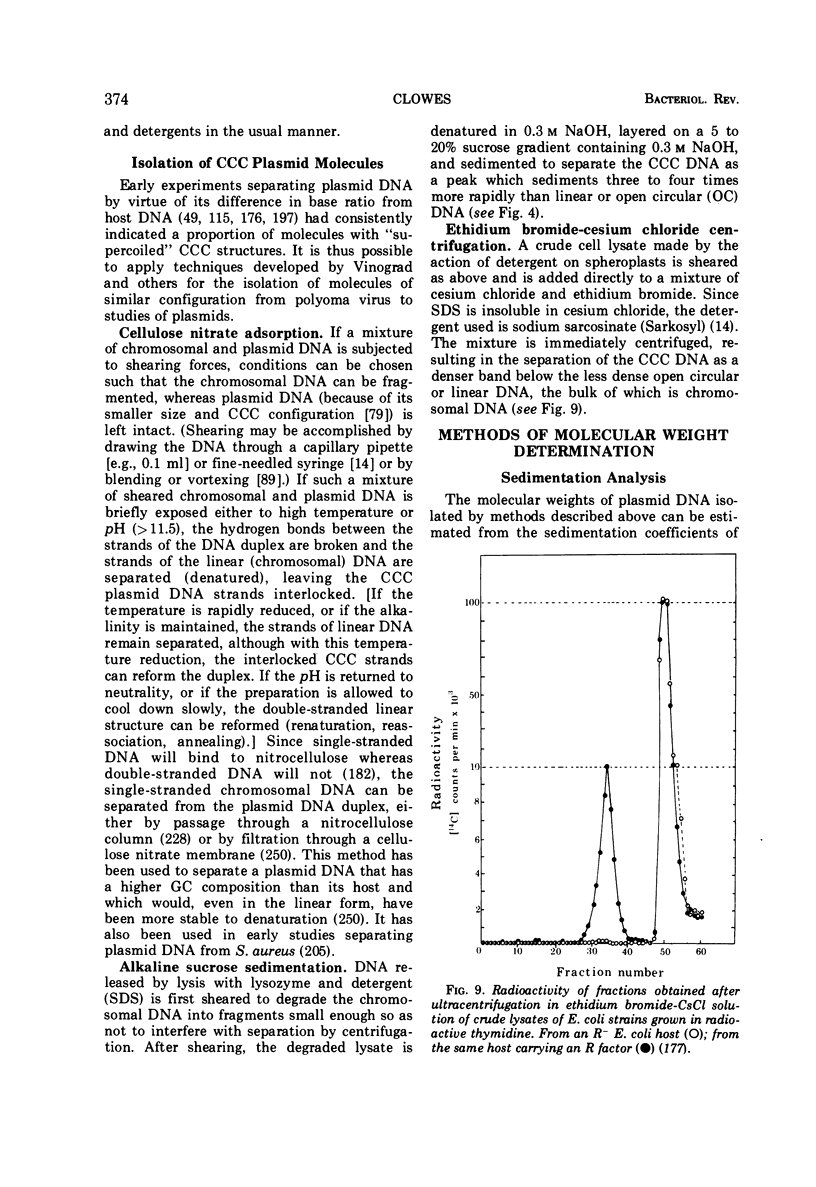
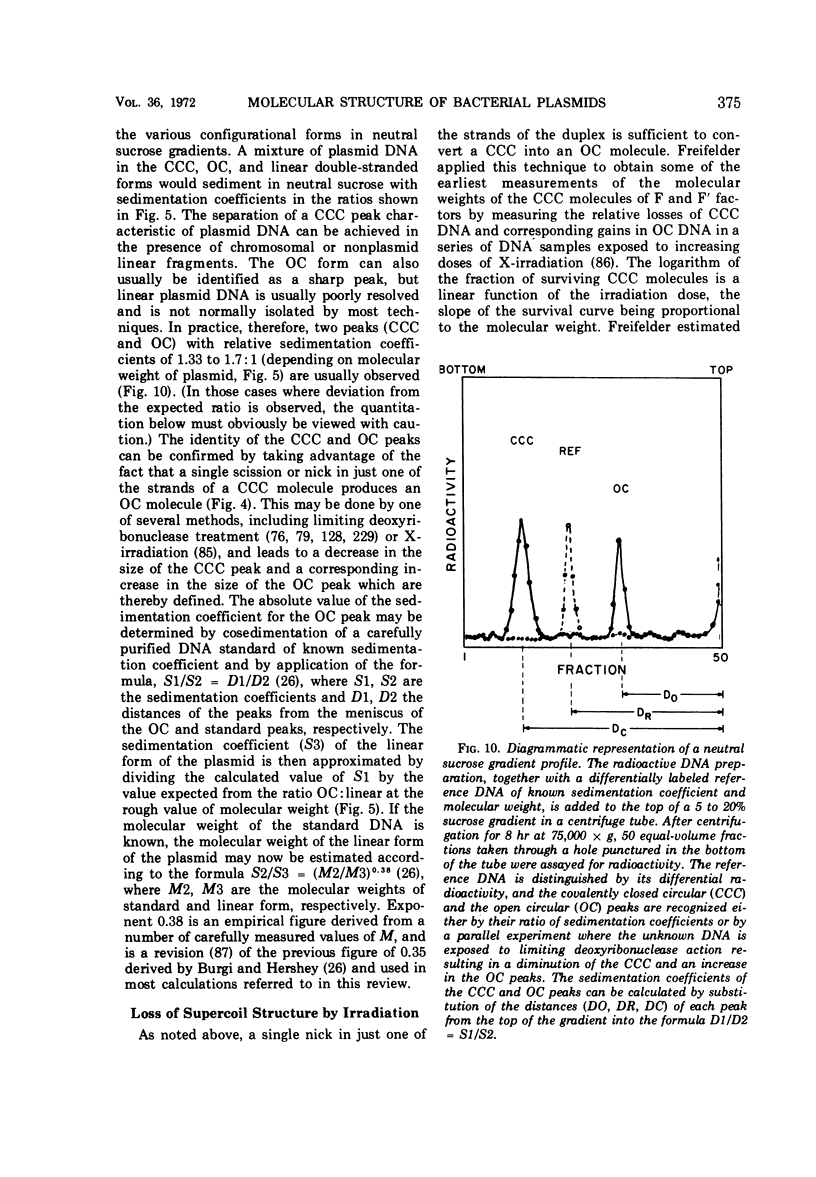
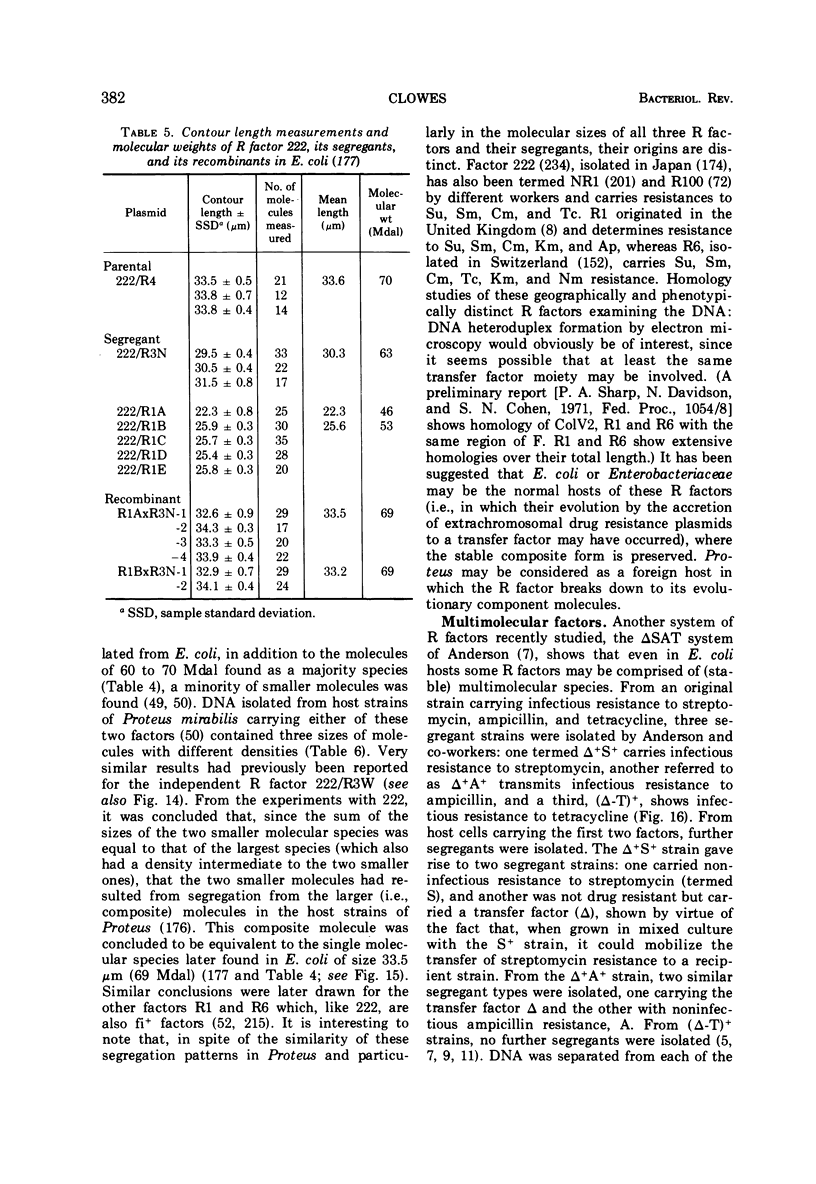
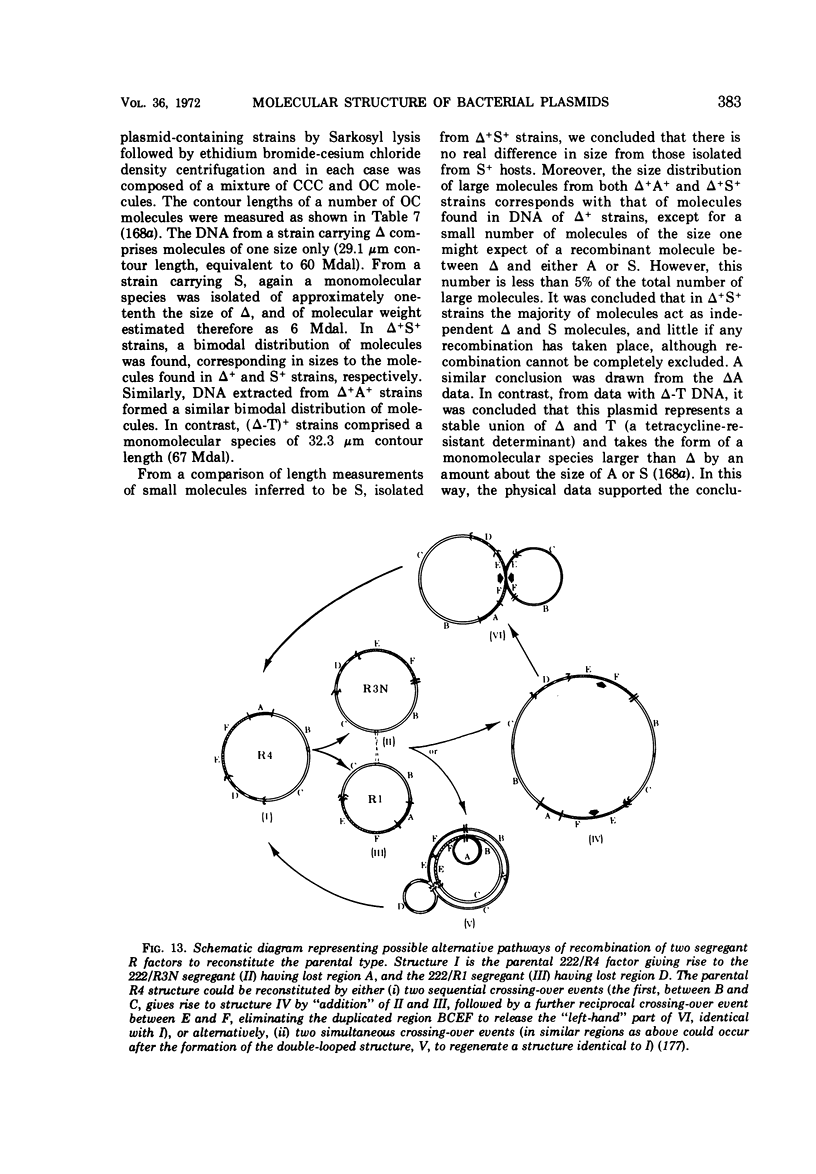
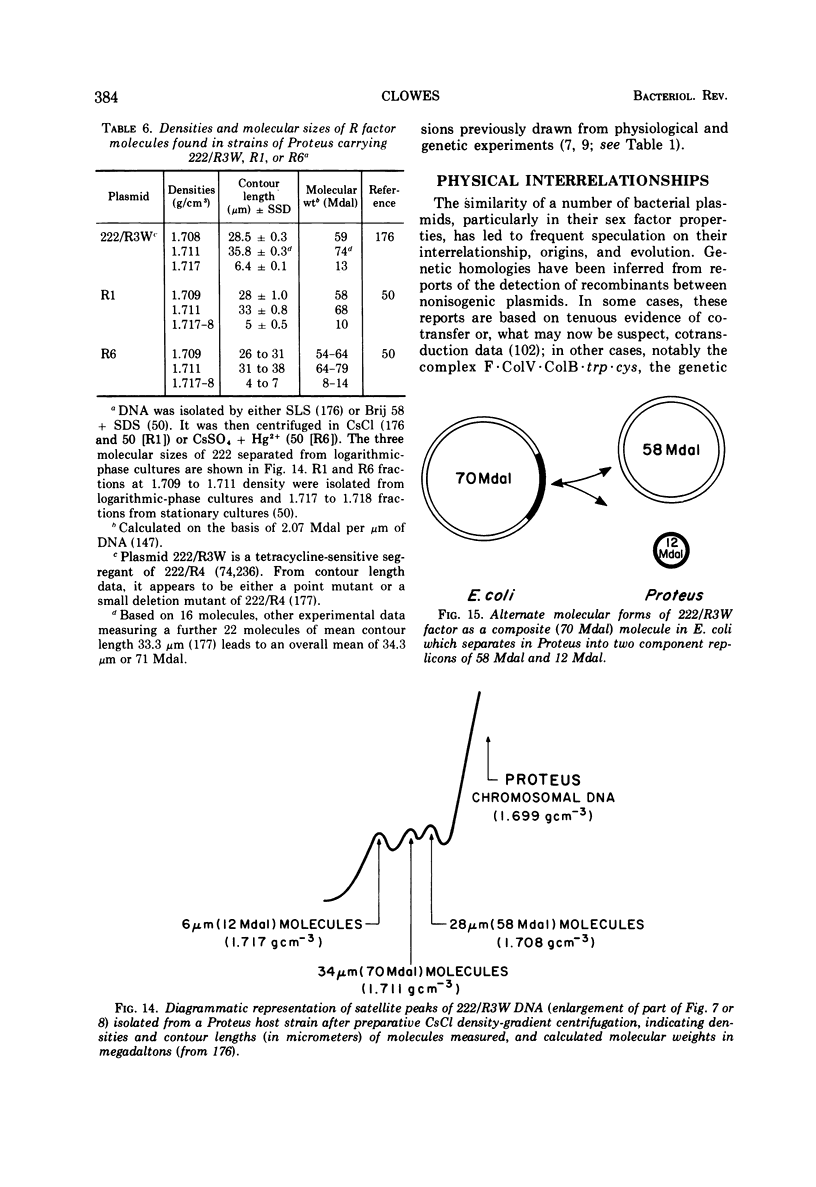
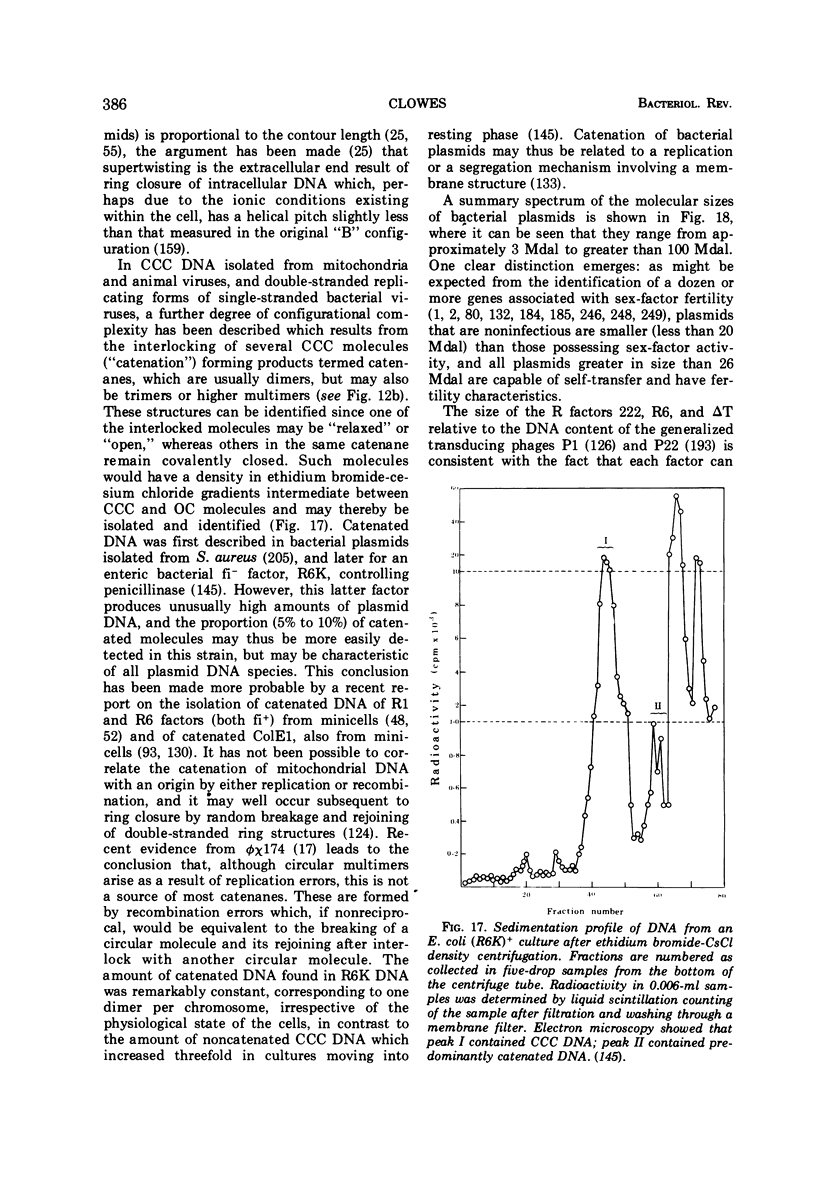
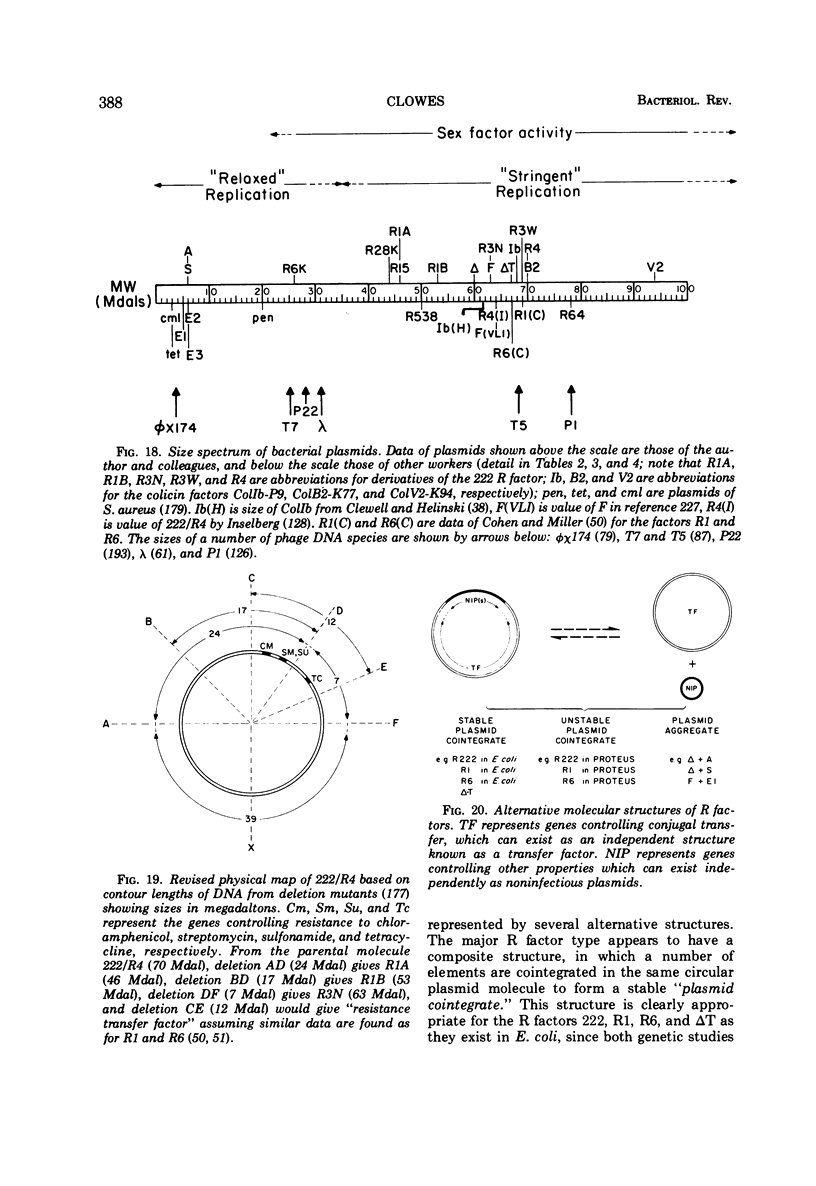
Full text
PDF







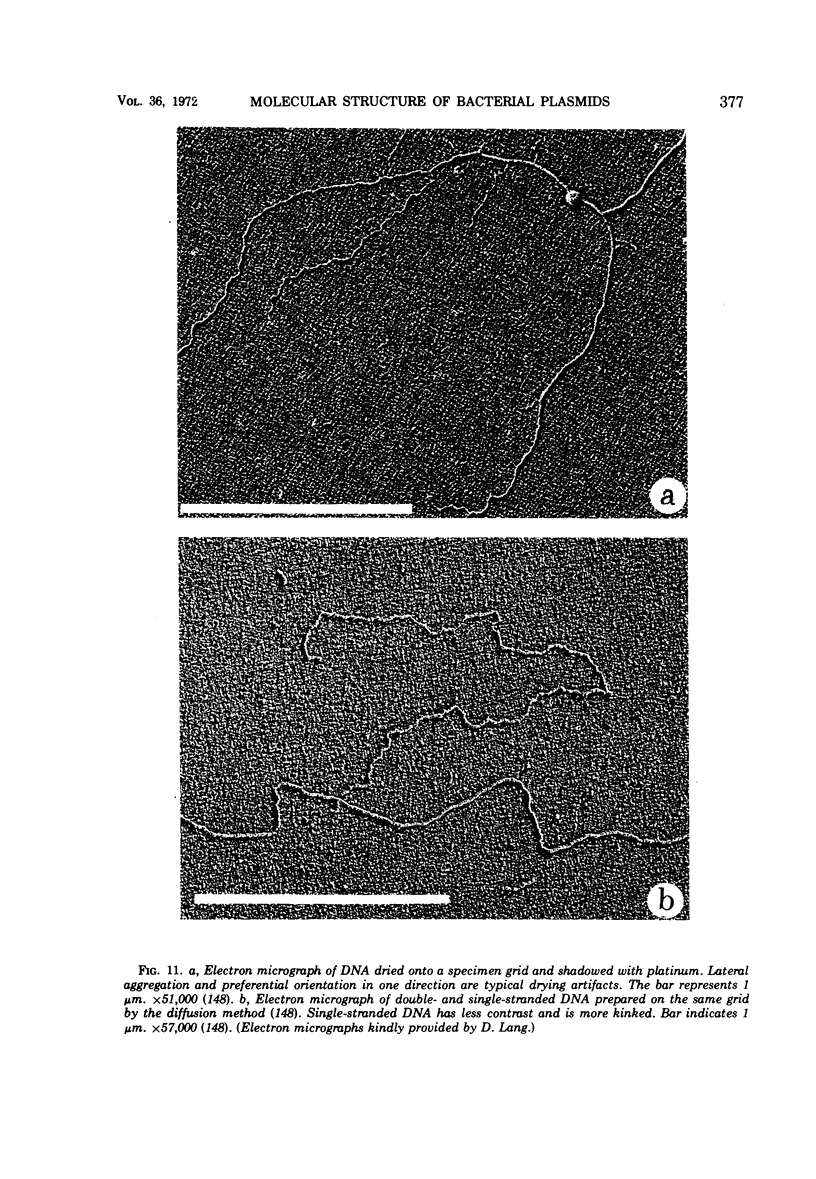
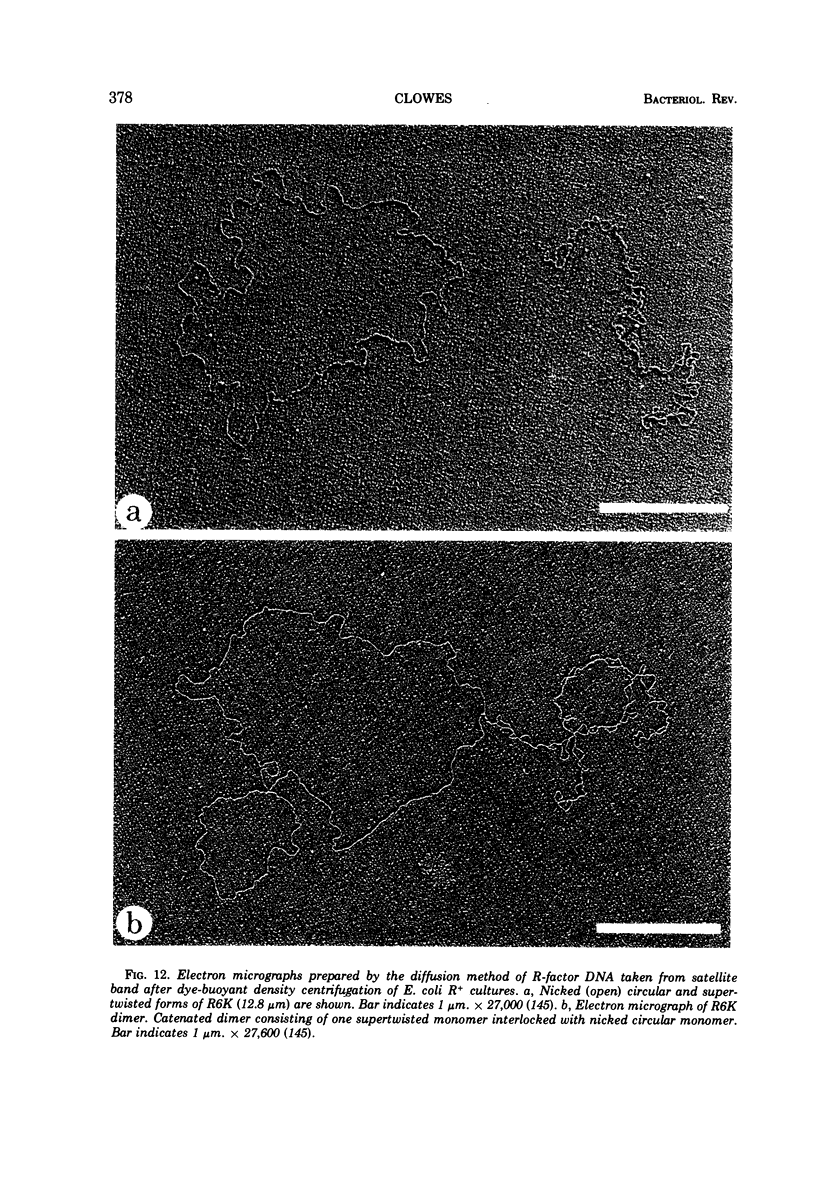





















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALFOLDI L., JACOB F., WOLLMAN E. L., MAZE R. Sur le déterminisme génétique de la colicinogénie. C R Hebd Seances Acad Sci. 1958 Jun 23;246(25):3531–3533. [PubMed] [Google Scholar]
- ANDERSON E. S., DATTA N. RESISTANCE TO PENICILLINS AND ITS TRANSFER IN ENTEROBACTERIACEAE. Lancet. 1965 Feb 20;1(7382):407–409. doi: 10.1016/s0140-6736(65)90004-8. [DOI] [PubMed] [Google Scholar]
- ARBER W. Transduction of chromosomal genes and episomes in Escherichia coli. Virology. 1960 May;11:273–288. doi: 10.1016/0042-6822(60)90066-0. [DOI] [PubMed] [Google Scholar]
- Achtman M., Willetts N., Clark A. J. Beginning a genetic analysis of conjugational transfer determined by the F factor in Escherichia coli by isolation and characterization of transfer-deficient mutants. J Bacteriol. 1971 May;106(2):529–538. doi: 10.1128/jb.106.2.529-538.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtman M., Willetts N., Clark A. J. Conjugational complementation analysis of transfer-deficient mutants of Flac in Escherichia coli. J Bacteriol. 1972 Jun;110(3):831–842. doi: 10.1128/jb.110.3.831-842.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler H. I., Fisher W. D., Cohen A., Hardigree A. A. MINIATURE escherichia coli CELLS DEFICIENT IN DNA. Proc Natl Acad Sci U S A. 1967 Feb;57(2):321–326. doi: 10.1073/pnas.57.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson E. S. Drug resistance in Salmonella typhimurium and its implications. Br Med J. 1968 Aug 10;3(5614):333–339. doi: 10.1136/bmj.3.5614.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson E. S. Influence of the delta transfer factor on the phage sensitivity of salmonellae. Nature. 1966 Nov 19;212(5064):795–799. doi: 10.1038/212795a0. [DOI] [PubMed] [Google Scholar]
- Anderson E. S., Lewis M. J. Characterization of a transfer factor associated with drug resistance in Salmonella typhimurium. Nature. 1965 Nov 27;208(5013):843–849. doi: 10.1038/208843a0. [DOI] [PubMed] [Google Scholar]
- Anderson E. S., Natkin E. Transduction of resistance determinants and R factors of the transfer systems by phage Plkc. Mol Gen Genet. 1972;114(3):261–265. doi: 10.1007/BF01788895. [DOI] [PubMed] [Google Scholar]
- Anderson E. S., Perret D. Mobilization of transduced tetracycline resistance by the delta transfer factor in Salmonella typhimurium and S. typhi. Nature. 1967 May 20;214(5090):810–811. doi: 10.1038/214810b0. [DOI] [PubMed] [Google Scholar]
- BOLTON E. T., McCARTHY B. J. A general method for the isolation of RNA complementary to DNA. Proc Natl Acad Sci U S A. 1962 Aug;48:1390–1397. doi: 10.1073/pnas.48.8.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURGI E., HERSHEY A. D. Sedimentation rate as a measure of molecular weight of DNA. Biophys J. 1963 Jul;3:309–321. doi: 10.1016/s0006-3495(63)86823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer W., Vinograd J. The interaction of closed circular DNA with intercalative dyes. I. The superhelix density of SV40 DNA in the presence and absence of dye. J Mol Biol. 1968 Apr 14;33(1):141–171. doi: 10.1016/0022-2836(68)90286-6. [DOI] [PubMed] [Google Scholar]
- Bazaral M., Helinski D. R. Circular DNA forms of colicinogenic factors E1, E2 and E3 from Escherichia coli. J Mol Biol. 1968 Sep 14;36(2):185–194. doi: 10.1016/0022-2836(68)90374-4. [DOI] [PubMed] [Google Scholar]
- Bazaral M., Helinski D. R. Replication of a bacterial plasmid and an episome in Escherichia coli. Biochemistry. 1970 Jan 20;9(2):399–406. doi: 10.1021/bi00804a029. [DOI] [PubMed] [Google Scholar]
- Bazzicalupo P., Tocchini-Valentini G. P. Curing of an Escherichia coli episome by rifampicin (acridine orange-F + -F - -Hfr-lac). Proc Natl Acad Sci U S A. 1972 Feb;69(2):298–300. doi: 10.1073/pnas.69.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benbow R. M., Eisenberg M., Sinsheimer R. L. Multiple length DNA molecules of bacteriophage phi-X174. Nat New Biol. 1972 May 31;237(74):141–144. doi: 10.1038/newbio237141a0. [DOI] [PubMed] [Google Scholar]
- Blair D. G., Clewell D. B., Sheratt D. J., Helinski D. R. Strand-specific supercoiled DNA-protein relaxation complexes: comparison of the complexes of bacterial plasmids ColE1 and ColE2. Proc Natl Acad Sci U S A. 1971 Jan;68(1):210–214. doi: 10.1073/pnas.68.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouanchaud D. H., Chabbert Y. A. Stable coexistence of three resistance factors (fi-) in Salmonella panama and Escherichia coli K12. J Gen Microbiol. 1969 Sep;58(1):107–113. doi: 10.1099/00221287-58-1-107. [DOI] [PubMed] [Google Scholar]
- Bouanchaud D. H., Scavizzi M. R., Chabbert Y. A. Elimination by ethidium bromide of antibiotic resistance in enterobacteria and staphylococci. J Gen Microbiol. 1968 Dec;54(3):417–425. doi: 10.1099/00221287-54-3-417. [DOI] [PubMed] [Google Scholar]
- Brenner D. J., Falkow S. Genetics of the Enterobacteriaceae. C. Molecular relationships among members of the Enterobacteriaceae. Adv Genet. 1971;16:81–118. doi: 10.1016/s0065-2660(08)60355-7. [DOI] [PubMed] [Google Scholar]
- Brinton C. C., Jr The structure, function, synthesis and genetic control of bacterial pili and a molecular model for DNA and RNA transport in gram negative bacteria. Trans N Y Acad Sci. 1965 Jun;27(8):1003–1054. doi: 10.1111/j.2164-0947.1965.tb02342.x. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Brutlag D., Schekman R., Kornberg A. A possible role for RNA polymerase in the initiation of M13 DNA synthesis. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2826–2829. doi: 10.1073/pnas.68.11.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujard H. Studies on circular DNA. II. Number of tertiary turns in papilloma DNA. J Mol Biol. 1968 Apr 28;33(2):503–505. doi: 10.1016/0022-2836(68)90207-6. [DOI] [PubMed] [Google Scholar]
- Burton A., Sinsheimer R. L. The process of infection with bacteriophage phi-X174 VII. Ultracentrifugal analysis of the replicative form. J Mol Biol. 1965 Dec;14(2):327–347. doi: 10.1016/s0022-2836(65)80185-1. [DOI] [PubMed] [Google Scholar]
- CLARK A. J., MARGULIES A. D. ISOLATION AND CHARACTERIZATION OF RECOMBINATION-DEFICIENT MUTANTS OF ESCHERICHIA COLI K12. Proc Natl Acad Sci U S A. 1965 Feb;53:451–459. doi: 10.1073/pnas.53.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLOWES R. C. Colicine factors as fertility factors in bacteria: Escherichia coli K-12. Nature. 1961 Jun 10;190:988–989. doi: 10.1038/190988a0. [DOI] [PubMed] [Google Scholar]
- CLWES R. C., MOODY E. E., PRITCHARD R. H. THE ELIMINATION OF EXTRACHROMOSOMAL ELEMENTS IN THYMINELESS STRAINS OF ESCHERICHIA COLI K12. Genet Res. 1965 Feb;6:147–152. doi: 10.1017/s0016672300004018. [DOI] [PubMed] [Google Scholar]
- Carlton B. C., Helinski D. R. Heterogeneous circular DNA elements in vegetative cultures of Bacillus megaterium. Proc Natl Acad Sci U S A. 1969 Oct;64(2):592–599. doi: 10.1073/pnas.64.2.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro L. G., Berg C. M. Chromosome replication in some strains of Escherichia coli K12. Cold Spring Harb Symp Quant Biol. 1968;33:559–573. doi: 10.1101/sqb.1968.033.01.063. [DOI] [PubMed] [Google Scholar]
- Chakrabarty A. M., Gunsalus I. C. Defective phage and chromosome mobilization in Pseudomonas putida. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1217–1223. doi: 10.1073/pnas.64.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Evenchik B., Cranston J. W. Direct inhibition of Col E 1 plasmid DNA replication in Escherichia coli by rifampicin. Nat New Biol. 1972 May 3;237(70):29–31. doi: 10.1038/newbio237029a0. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. E. Existence of the colicinogenic factor-sex factor ColI-b-P9 as a supercoiled circular DNA-protein relaxation complex. Biochem Biophys Res Commun. 1970 Oct 9;41(1):150–156. doi: 10.1016/0006-291x(70)90481-x. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Effect of growth conditions on the formation of the relaxation complex of supercoiled ColE1 deoxyribonucleic acid and protein in Escherichia coli. J Bacteriol. 1972 Jun;110(3):1135–1146. doi: 10.1128/jb.110.3.1135-1146.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Evidence for the existence of the colicinogenic factors E2 and E3 as supercoiled circular DNA-protein relaxation complexes. Biochem Biophys Res Commun. 1970 Aug 11;40(3):608–613. doi: 10.1016/0006-291x(70)90947-2. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Properties of a supercoiled deoxyribonucleic acid-protein relaxation complex and strand specificity of the relaxation event. Biochemistry. 1970 Oct 27;9(22):4428–4440. doi: 10.1021/bi00824a026. [DOI] [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowes R. C., Moody E. E. Chromosomal transfer from "recombination-deficient" strains of Escherichia coli K-12. Genetics. 1966 Apr;53(4):717–726. doi: 10.1093/genetics/53.4.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A., Fisher W. D., Curtiss R., 3rd, Adler H. I. The properties of DNA transferred to minicells during conjugation. Cold Spring Harb Symp Quant Biol. 1968;33:635–641. doi: 10.1101/sqb.1968.033.01.071. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Miller C. A. Multiple molecular species of circular R-factor DNA isolated from Escherichia coli. Nature. 1969 Dec 27;224(5226):1273–1277. doi: 10.1038/2241273a0. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Miller C. A. Non-chromosomal antibiotic resistance in bacteria. 3. Isolation of the discrete transfer unit of the R-factor R1. Proc Natl Acad Sci U S A. 1970 Oct;67(2):510–516. doi: 10.1073/pnas.67.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Miller C. A. Non-chromosomal antibiotic resistance in bacteria. II. Molecular nature of R-factors isolated from Proteus mirabilis and Escherichia coli. J Mol Biol. 1970 Jun 28;50(3):671–687. doi: 10.1016/0022-2836(70)90092-6. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Silver R. P., McCoubrey A. E. Isolation of catenated forms of R factor DNA from minicells. Nat New Biol. 1971 Jun 23;231(25):249–252. doi: 10.1038/newbio231249a0. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Silver R. P., Sharp P. A., McCoubrey A. E. The problems of drug-resistant pathogenic bacteria. Studies on the molecular nature of R factors. Ann N Y Acad Sci. 1971 Jun 11;182:172–187. doi: 10.1111/j.1749-6632.1971.tb30655.x. [DOI] [PubMed] [Google Scholar]
- Cooper S., Helmstetter C. E. Chromosome replication and the division cycle of Escherichia coli B/r. J Mol Biol. 1968 Feb 14;31(3):519–540. doi: 10.1016/0022-2836(68)90425-7. [DOI] [PubMed] [Google Scholar]
- Cozzarelli N. R., Kelly R. B., Kornberg A. A minute circular DNA from Escherichia coli 15. Proc Natl Acad Sci U S A. 1968 Jul;60(3):992–999. doi: 10.1073/pnas.60.3.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford L. V., Waring M. J. Supercoiling of polyoma virus DNA measured by its interaction with ethidium bromide. J Mol Biol. 1967 Apr 14;25(1):23–30. doi: 10.1016/0022-2836(67)90276-8. [DOI] [PubMed] [Google Scholar]
- Curtiss R., 3rd Bacterial conjugation. Annu Rev Microbiol. 1969;23:69–136. doi: 10.1146/annurev.mi.23.100169.000441. [DOI] [PubMed] [Google Scholar]
- Cuzin F., Jacob F. Existence chez Escherichia coli K12 d'une unité génétique de transmission formée de différents réplicons. Ann Inst Pasteur (Paris) 1967 May;112(5):529–545. [PubMed] [Google Scholar]
- DUBNAU E., STOCKER B. A. GENETICS OF PLASMIDS IN SALMONELLA TYPHIMURIUM. Nature. 1964 Dec 12;204:1112–1113. doi: 10.1038/2041112a0. [DOI] [PubMed] [Google Scholar]
- Datta N., Hedges R. W. Compatibility groups among fi - R factors. Nature. 1971 Nov 26;234(5326):222–223. doi: 10.1038/234222a0. [DOI] [PubMed] [Google Scholar]
- Datta N., Hedges R. W. Host ranges of R factors. J Gen Microbiol. 1972 May;70(3):453–460. doi: 10.1099/00221287-70-3-453. [DOI] [PubMed] [Google Scholar]
- Datta N., Hedges R. W., Shaw E. J., Sykes R. B., Richmond M. H. Properties of an R factor from Pseudomonas aeruginosa. J Bacteriol. 1971 Dec;108(3):1244–1249. doi: 10.1128/jb.108.3.1244-1249.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. E., Rownd R. Transmissible multiple drug resistance in Enterobacteriaceae. Science. 1972 May 19;176(4036):758–768. doi: 10.1126/science.176.4036.758. [DOI] [PubMed] [Google Scholar]
- Davies J., Brzezinska M., Benveniste R. The problems of drug-resistant pathogenic bacteria. R factors: biochemical mechanisms of resistance to aminoglycoside antibiotics. Ann N Y Acad Sci. 1971 Jun 11;182:226–233. doi: 10.1111/j.1749-6632.1971.tb30659.x. [DOI] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau E., Maas W. K. Inhibition of replication of an F'lac episome in Hfr cells of Escherichia coli. J Bacteriol. 1968 Feb;95(2):531–539. doi: 10.1128/jb.95.2.531-539.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FALKOW S., CITARELLA R. V. MOLECULAR HOMOLOGY OF F-MEROGENOTE DNA. J Mol Biol. 1965 May;12:138–151. doi: 10.1016/s0022-2836(65)80288-1. [DOI] [PubMed] [Google Scholar]
- FALKOW S., WOHLHIETER J. A., CITARELLA R. V., BARON L. S. TRANSFER OF EPISOMIC ELEMENTS TO PROTEUS. I. TRANSFER OF F-LINKED CHROMOSOMAL DETERMINANTS. J Bacteriol. 1964 Jan;87:209–219. doi: 10.1128/jb.87.1.209-219.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FIERS W., SINSHEIMER R. L. The structure of the DNA of bacteriophage phi-X174. III. Ultracentrifugal evidence for a ring structure. J Mol Biol. 1962 Oct;5:424–434. doi: 10.1016/s0022-2836(62)80031-x. [DOI] [PubMed] [Google Scholar]
- FREDERICQ P. Transduction genetique des proprietes colicinogenes chez Escherichia coli et Shigella sonnei. C R Seances Soc Biol Fil. 1954 Feb;148(3-4):399–402. [PubMed] [Google Scholar]
- Falkow S., Citarella R. V., Wohlhieter J. A. The molecular nature of R-factors. J Mol Biol. 1966 May;17(1):102–116. doi: 10.1016/s0022-2836(66)80097-9. [DOI] [PubMed] [Google Scholar]
- Falkow S., Tompkins L. S., Silver R. P., Guerry P., Le Blanc D. J. The problems of drug-resistant pathogenic bacteria. The replication of R-factor DNA in Escherichia coli K-12 following conjugation. Ann N Y Acad Sci. 1971 Jun 11;182:153–171. doi: 10.1111/j.1749-6632.1971.tb30654.x. [DOI] [PubMed] [Google Scholar]
- Finnegan D. J., Willetts N. S. Two classes of Flac mutants insensitive to transfer inhibition by an F-like R factor. Mol Gen Genet. 1971;111(3):256–264. doi: 10.1007/BF00433110. [DOI] [PubMed] [Google Scholar]
- Foster T. J., Howe T. G. Recombination and complementation between R factors in Escheichia coli K 12. Genet Res. 1971 Dec;18(3):287–297. doi: 10.1017/s0016672300012696. [DOI] [PubMed] [Google Scholar]
- Freifelder D. R., Freifelder D. Studies on Escherichia coli sex factors. I. Specific labeling of F'Lac DNA. J Mol Biol. 1968 Feb 28;32(1):15–23. doi: 10.1016/0022-2836(68)90141-1. [DOI] [PubMed] [Google Scholar]
- Freifelder D. R., Freifelder D. Studies on Escherichia coli sex factors. II. Some physical properties of F'Lac and F DNA. J Mol Biol. 1968 Feb 28;32(1):25–35. doi: 10.1016/0022-2836(68)90142-3. [DOI] [PubMed] [Google Scholar]
- Freifelder D., Folkmanis A., Kirschner I. Studies on Escherichia coli sex factors: evidence that covalent circles exist within cells and the general problem of isolation of covalent circles. J Bacteriol. 1971 Mar;105(3):722–727. doi: 10.1128/jb.105.3.722-727.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freifelder D. Molecular weights of coliphages and coliphage DNA. IV. Molecular weights of DNA from bacteriophages T4, T5 and T7 and the general problem of determination of M. J Mol Biol. 1970 Dec 28;54(3):567–577. doi: 10.1016/0022-2836(70)90127-0. [DOI] [PubMed] [Google Scholar]
- Freifelder D. Studies on Escherichia coli sex factors. 3. Covalently closed F'Lac DNA molecules. J Mol Biol. 1968 May 28;34(1):31–38. doi: 10.1016/0022-2836(68)90232-5. [DOI] [PubMed] [Google Scholar]
- Freifelder D. Studies on Escherichia coli sex factors. IV. Molecular weights of the DNA of several F' elements. J Mol Biol. 1968 Jul 14;35(1):95–102. doi: 10.1016/s0022-2836(68)80039-7. [DOI] [PubMed] [Google Scholar]
- Frydman A., Meynell E. Interactions between de-repressed F-like R factors and wild type colicin B factors: superinfection immunity and repressor susceptibility. Genet Res. 1969 Dec;14(3):315–322. doi: 10.1017/s0016672300002135. [DOI] [PubMed] [Google Scholar]
- Fuke M., Inselburg J. Electron microscopic studies of replicating and catenated colicin factor E1 DNA isolated from minicells (DNA replication). Proc Natl Acad Sci U S A. 1972 Jan;69(1):89–92. doi: 10.1073/pnas.69.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gefter M. L., Hirota Y., Kornberg T., Wechsler J. A., Barnoux C. Analysis of DNA polymerases II and 3 in mutants of Escherichia coli thermosensitive for DNA synthesis. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3150–3153. doi: 10.1073/pnas.68.12.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert W., Dressler D. DNA replication: the rolling circle model. Cold Spring Harb Symp Quant Biol. 1968;33:473–484. doi: 10.1101/sqb.1968.033.01.055. [DOI] [PubMed] [Google Scholar]
- Godson G. N., Sinsheimer R. L. Lysis of Escherichia coli with a neutral detergent. Biochim Biophys Acta. 1967 Dec 19;149(2):476–488. doi: 10.1016/0005-2787(67)90175-x. [DOI] [PubMed] [Google Scholar]
- Goebel W. Replication of the DNA of the colicinogenic factor E 1 (Col E 1 ) at the restrictive temperature in a DNA replication mutant thermosensitive for DNA polymerase. 3. Nat New Biol. 1972 May 17;237(72):67–70. doi: 10.1038/newbio237067a0. [DOI] [PubMed] [Google Scholar]
- Goebel W. Studies on extrachromosomal DNA elements. Replication of the colicinogenic factor Col E1 in two temperature sensitive mutants of Escherichia coli defective in DNA replication. Eur J Biochem. 1970 Aug;15(2):311–320. doi: 10.1111/j.1432-1033.1970.tb01009.x. [DOI] [PubMed] [Google Scholar]
- Grindley J. N., Anderson E. S. I-like resistance factors with the fi+ character. Genet Res. 1971 Jun;17(3):267–271. doi: 10.1017/s0016672300012295. [DOI] [PubMed] [Google Scholar]
- Grubb W. B., O'Reilly R. J. Joint transduction of separate extrachromosomal drug resistance determinants in Staphylococcus aureus E169. Biochem Biophys Res Commun. 1971 Feb 5;42(3):377–383. doi: 10.1016/0006-291x(71)90381-0. [DOI] [PubMed] [Google Scholar]
- HARADA K., KAMEDA M., SUZUKI M., MITSUHASHI S. DRUG RESISTANCE OF ENTERIC BACTERIA. 3. ACQUISITION OF TRANSFERABILITY OF NONTRANSMISSIBLE R(TC) FACTOR IN COOPERATION WITH F FACTOR AND FORMATION OF FR(TC). J Bacteriol. 1964 Nov;88:1257–1265. doi: 10.1128/jb.88.5.1257-1265.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARADA K., KAMEDA M., SUZUKI M., MITSUHASHI S. DRUG RESISTANCE OF ENTERIC BACTERIA. II. TRANSDUCTION OF TRANSMISSIBLE DRUG-RESISTANCE (R) FACTORS WITH PHAGE EPSILON. J Bacteriol. 1963 Dec;86:1332–1338. doi: 10.1128/jb.86.6.1332-1338.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERSHEY A. D., BURGI E. COMPLEMENTARY STRUCTURE OF INTERACTING SITES AT THE ENDS OF LAMBDA DNA MOLECULES. Proc Natl Acad Sci U S A. 1965 Feb;53:325–328. doi: 10.1073/pnas.53.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haapala D. K., Falkow S. Physical studies of the drug-resistance transfer factor in Proteus. J Bacteriol. 1971 Apr;106(1):294–295. doi: 10.1128/jb.106.1.294-295.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn F. E., Ciak J. The problems of drug-resistant pathogenic bacteria. Elimination of bacterial episomes by DNA-complexing compounds. Ann N Y Acad Sci. 1971 Jun 11;182:295–304. doi: 10.1111/j.1749-6632.1971.tb30665.x. [DOI] [PubMed] [Google Scholar]
- Harada K., Kameda M., Suzuki M., Shigehara S., Mitsuhashi S. Drug resistance of enteric bacteria. 8. Chromosomal location of nontransferable R factor in Escherichia coli. J Bacteriol. 1967 Apr;93(4):1236–1241. doi: 10.1128/jb.93.4.1236-1241.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto H., Hirota Y. Gene recombination and segregation of resistance factor R in Escherichia coli. J Bacteriol. 1966 Jan;91(1):51–62. doi: 10.1128/jb.91.1.51-62.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann C., Clowes R. C. ColB2-K77, a fertility-repressed F-like factor. J Bacteriol. 1971 Sep;107(3):900–906. doi: 10.1128/jb.107.3.900-906.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helinski D. R., Clewell D. B. Circular DNA. Annu Rev Biochem. 1971;40:899–942. doi: 10.1146/annurev.bi.40.070171.004343. [DOI] [PubMed] [Google Scholar]
- Hickson F. T., Roth T. F., Helinski D. R. Circular DNA forms of a bacterial sex factor. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1731–1738. doi: 10.1073/pnas.58.4.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y., Mordoh J., Jacob F. On the process of cellular division in Escherichia coli. 3. Thermosensitive mutants of Escherichia coli altered in the process of DNA initiation. J Mol Biol. 1970 Nov 14;53(3):369–387. doi: 10.1016/0022-2836(70)90072-0. [DOI] [PubMed] [Google Scholar]
- Hirota Y., Ryter A., Jacob F. Thermosensitive mutants of E. coli affected in the processes of DNA synthesis and cellular division. Cold Spring Harb Symp Quant Biol. 1968;33:677–693. doi: 10.1101/sqb.1968.033.01.077. [DOI] [PubMed] [Google Scholar]
- Hirota Y. THE EFFECT OF ACRIDINE DYES ON MATING TYPE FACTORS IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1960 Jan;46(1):57–64. doi: 10.1073/pnas.46.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoar D. I. Fertility regulation in F-like resistance transfer factors. J Bacteriol. 1970 Mar;101(3):916–920. doi: 10.1128/jb.101.3.916-920.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn B., Korn D. Cosegregation of a sex factor with the Escherichia coli chromosome during curing by acridine orange. J Mol Biol. 1969 Oct 28;45(2):385–395. doi: 10.1016/0022-2836(69)90113-2. [DOI] [PubMed] [Google Scholar]
- Holloway B. W. Genetics of Pseudomonas. Bacteriol Rev. 1969 Sep;33(3):419–443. doi: 10.1128/br.33.3.419-443.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway B. W., Krishnapillai V., Stanisich V. Pseudomonas genetics. Annu Rev Genet. 1971;5:425–446. doi: 10.1146/annurev.ge.05.120171.002233. [DOI] [PubMed] [Google Scholar]
- Hudson B., Clayton D. A., Vinograd J. Complex mitochondrial DNA. Cold Spring Harb Symp Quant Biol. 1968;33:435–442. doi: 10.1101/sqb.1968.033.01.050. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Inuzuka M., Tomizawa J. I. P1-like plasmid in Escherichia coli 15. J Mol Biol. 1970 Jun 14;50(2):457–470. doi: 10.1016/0022-2836(70)90204-4. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Tomizawa J. Prophage P1, and extrachromosomal replication unit. Cold Spring Harb Symp Quant Biol. 1968;33:791–798. doi: 10.1101/sqb.1968.033.01.091. [DOI] [PubMed] [Google Scholar]
- Inselburg J., Fuke M. Isolation of catenated and replicating DNA molecules of colicin factor E1 from minicells. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2839–2842. doi: 10.1073/pnas.68.11.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inselburg J., Fuke M. Replicating DNA: structure of colicin factor E1. Science. 1970 Aug 7;169(3945):590–592. doi: 10.1126/science.169.3945.590. [DOI] [PubMed] [Google Scholar]
- Inselburg J. R factor deoxyribonucleic acid in chromosomeless progeny of Escherichia coli. J Bacteriol. 1971 Feb;105(2):620–628. doi: 10.1128/jb.105.2.620-628.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inselburg J. Segregation into and replication of plasmid deoxyribonucleic acid in chromosomeless segregants of Escherichia coli. J Bacteriol. 1970 Jun;102(3):642–647. doi: 10.1128/jb.102.3.642-647.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inuzuka N., Nakamura S., Inuzuka M., Tomoeda M. Specific action of sodium dodecyl sulfate on the sex factor of Escherichia coli K-12 Hfr strains. J Bacteriol. 1969 Nov;100(2):827–835. doi: 10.1128/jb.100.2.827-835.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ippen-Ihler K., Achtman M., Willetts N. Deletion map of the Escherichia coli K-12 sex factor F: the order of eleven transfer cistrons. J Bacteriol. 1972 Jun;110(3):857–863. doi: 10.1128/jb.110.3.857-863.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB F., WOLLMAN E. L. Les épisomes, éléments génétiques ajoutés. C R Hebd Seances Acad Sci. 1958 Jul 7;247(1):154–156. [PubMed] [Google Scholar]
- Johnston J. H., Richmond M. H. The increased rate of loss of penicillinase plasmids from Staphylococcus aureus in the presence of rifampicin. J Gen Microbiol. 1970 Jan;60(1):137–139. doi: 10.1099/00221287-60-1-137. [DOI] [PubMed] [Google Scholar]
- KAHN P., HELINSKI D. R. RELATIONSHIP BETWEEN COLICINOGENIC FACTORS E1 AND V AND AN F FACTOR IN ESCHERICHIA COLI. J Bacteriol. 1964 Dec;88:1573–1579. doi: 10.1128/jb.88.6.1573-1579.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasamatsu H., Rownd R. Replication of R-factors in Proteus mirabilis: replication under relaxed control. J Mol Biol. 1970 Aug;51(3):473–489. doi: 10.1016/0022-2836(70)90002-1. [DOI] [PubMed] [Google Scholar]
- Kass L. R., Yarmolinsky M. B. Segregation of functional sex factor into minicells. Proc Natl Acad Sci U S A. 1970 Jul;66(3):815–822. doi: 10.1073/pnas.66.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatoon H., Iyer R. V. Stable coexistence of Rfi factors in Escherichia coli. Can J Microbiol. 1971 May;17(5):669–675. doi: 10.1139/m71-108. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. T., Helinski D. R. DNA polymerase as a requirement for the maintenance of the bacterial plasmid colicinogenic factor E1. Biochem Biophys Res Commun. 1970 Dec 24;41(6):1538–1544. doi: 10.1016/0006-291x(70)90562-0. [DOI] [PubMed] [Google Scholar]
- Kline B. C., Helinski D. R. F 1 sex factor of Escherichia coli. Size and purification in the form of a strand-specific relaxation complex of supercoiled deoxyribonucleic acid and protein. Biochemistry. 1971 Dec 21;10(26):4975–4980. doi: 10.1021/bi00802a022. [DOI] [PubMed] [Google Scholar]
- Kontomichalou P., Mitani M., Clowes R. C. Circular R-factor molecules controlling penicillinase synthesis, replicating in Escherichia coli under either relaxed or stringent control. J Bacteriol. 1970 Oct;104(1):34–44. doi: 10.1128/jb.104.1.34-44.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecko D. J., Punch J. D. The problems of drug-resistant pathogenic bacteria. Regulation of R-factor replication in Proteus mirabilis. Ann N Y Acad Sci. 1971 Jun 11;182:201–216. doi: 10.1111/j.1749-6632.1971.tb30657.x. [DOI] [PubMed] [Google Scholar]
- LAVALLE R., JACOB F. [On the sensitivity of sexual and colicinogenic episomes of Escherichia coli K12 to the disintegration of radiophosphorus]. C R Hebd Seances Acad Sci. 1961 Mar 13;252:1678–1680. [PubMed] [Google Scholar]
- LEDERBERG J. Streptomycin resistance; a genetically recessive mutation. J Bacteriol. 1951 May;61(5):549–550. doi: 10.1128/jb.61.5.549-550.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUZZATI V., MATHIS A., MASSON F., WITZ J. SUTURE TRANSITIONS OBSERVED IN DNA AND POLY A IN SOLUTION AS A FUNCTION OF TEMPERATURE AND PH. J Mol Biol. 1964 Oct;10:28–41. doi: 10.1016/s0022-2836(64)80025-5. [DOI] [PubMed] [Google Scholar]
- Lang D., Bujard H., Wolff B., Russell D. Electron microscopy of size and shape of viral DNA in solutions of different ionic strengths. J Mol Biol. 1967 Jan 28;23(2):163–181. doi: 10.1016/s0022-2836(67)80024-x. [DOI] [PubMed] [Google Scholar]
- Lang D. Individual macromolecules: preparation and recent results with DNA. Philos Trans R Soc Lond B Biol Sci. 1971 May 27;261(837):151–158. doi: 10.1098/rstb.1971.0046. [DOI] [PubMed] [Google Scholar]
- Lang D., Mitani M. Simplified quantitative electron microscopy of biopolymers. Biopolymers. 1970;9(3):373–379. doi: 10.1002/bip.1970.360090310. [DOI] [PubMed] [Google Scholar]
- Lang D. Molecular weights of coliphages and coliphage DNA. 3. Contour length and molecular weight of DNA from bacteriophages T4, T5 and T7, and from bovine papilloma virus. J Mol Biol. 1970 Dec 28;54(3):557–565. doi: 10.1016/0022-2836(70)90126-9. [DOI] [PubMed] [Google Scholar]
- Lee C. S., Davidson N. Covalently closed minicircular DNA in Micrococcus lysodeikticus. Biochem Biophys Res Commun. 1968 Sep 6;32(5):757–762. doi: 10.1016/0006-291x(68)90304-5. [DOI] [PubMed] [Google Scholar]
- Levy S. B., Norman P. Segregation of transferable R factors into Escherichia coli minicells. Nature. 1970 Aug 8;227(5258):606–607. doi: 10.1038/227606a0. [DOI] [PubMed] [Google Scholar]
- Levy S. B. Physical and functional characteristics of R-factor deoxyribonucleic acid segregated into Escherichia coli minicells. J Bacteriol. 1971 Oct;108(1):300–308. doi: 10.1128/jb.108.1.300-308.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy S. B. Resistance of minicells to penicillin lysis: a method of obtaining large quantities of purified minicells. J Bacteriol. 1970 Sep;103(3):836–839. doi: 10.1128/jb.103.3.836-839.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy S. B. The problems of drug-resistant pathogenic bacteria. Studies on R factors segregated into E. coli minicells. Ann N Y Acad Sci. 1971 Jun 11;182:217–225. doi: 10.1111/j.1749-6632.1971.tb30658.x. [DOI] [PubMed] [Google Scholar]
- MACHATTIE L. A., BERNE K. I., THOMAS C. A., Jr ELECTRON MICROSCOPY OF DNA FROM HEMOPHILUS INFLUENZAE. J Mol Biol. 1965 Mar;11:648–649. doi: 10.1016/s0022-2836(65)80019-5. [DOI] [PubMed] [Google Scholar]
- MARMUR J., FALKOW S., MANDEL M. NEW APPROACHES TO BACTERIAL TAXONOMY. Annu Rev Microbiol. 1963;17:329–372. doi: 10.1146/annurev.mi.17.100163.001553. [DOI] [PubMed] [Google Scholar]
- MARMUR J., ROWND R., FALKOW S., BARON L. S., SCHILDKRAUT C., DOTY P. The nature of intergeneric episomal infection. Proc Natl Acad Sci U S A. 1961 Jul 15;47:972–979. doi: 10.1073/pnas.47.7.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONK M., CLOWES R. C. THE REGULATION OF COLICIN SYNTHESIS AND COLICIN FACTOR TRANSFER IN ESCHERICHIA COLI K12. J Gen Microbiol. 1964 Sep;36:385–392. doi: 10.1099/00221287-36-3-385. [DOI] [PubMed] [Google Scholar]
- MONK M., CLOWES R. C. TRANSFER OF THE COLICIN I FACTOR IN ESCHERICHIA COLI K12 AND ITS INTERACTION WITH THE F FERTILITY FACTOR. J Gen Microbiol. 1964 Sep;36:365–384. doi: 10.1099/00221287-36-3-365. [DOI] [PubMed] [Google Scholar]
- MacFarren A. C., Clowes R. C. A comparative study of two F-like colicin factors, ColV2 and ColV3, in Escherichia coli K-12. J Bacteriol. 1967 Aug;94(2):365–377. doi: 10.1128/jb.94.2.365-377.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara K., Kaiser A. D. Lambda dv: an autonomously replicating DNA fragment. Cold Spring Harb Symp Quant Biol. 1968;33:769–775. doi: 10.1101/sqb.1968.033.01.088. [DOI] [PubMed] [Google Scholar]
- Meynell E., Cooke M. Repressor-minus and operator-constitutive de-repressed mutants of F-like R factors: their effect on chromosomal transfer by HfrC. Genet Res. 1969 Dec;14(3):309–313. doi: 10.1017/s0016672300002123. [DOI] [PubMed] [Google Scholar]
- Meynell E., Meynell G. G., Datta N. Phylogenetic relationships of drug-resistance factors and other transmissible bacterial plasmids. Bacteriol Rev. 1968 Mar;32(1):55–83. doi: 10.1128/br.32.1.55-83.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuhashi S., Kameda M., Harada K., Suzuki M. Formation of recombinants between nontransmissible drug-resistance determinants and transfer factors. J Bacteriol. 1969 Mar;97(3):1520–1521. doi: 10.1128/jb.97.3.1520-1521.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuhashi S. The problems of drug-resistant pathogenic bacteria. Epidemiology and genetics of R factors. Ann N Y Acad Sci. 1971 Jun 11;182:141–152. doi: 10.1111/j.1749-6632.1971.tb30653.x. [DOI] [PubMed] [Google Scholar]
- Moody E. E., Runge R. The integration of autonomous transmissible plasmids into the chromosome of Escherichia coli K12. Genet Res. 1972 Apr;19(2):181–186. doi: 10.1017/s0016672300014427. [DOI] [PubMed] [Google Scholar]
- NAKAYA R., NAKAMURA A., MURATA Y. Resistance transfer agents in Shigella. Biochem Biophys Res Commun. 1960 Dec;3:654–659. doi: 10.1016/0006-291x(60)90081-4. [DOI] [PubMed] [Google Scholar]
- NOVICK R. P., RICHMOND M. H. NATURE AND INTERACTIONS OF THE GENETIC ELEMENTS GOVERNING PENICILLINASE SYNTHESIS IN STAPHYLOCOCCUS AUREUS. J Bacteriol. 1965 Aug;90:467–480. doi: 10.1128/jb.90.2.467-480.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NYGAARD A. P., HALL B. D. FORMATION AND PROPERTIES OF RNA-DNA COMPLEXES. J Mol Biol. 1964 Jul;9:125–142. doi: 10.1016/s0022-2836(64)80095-4. [DOI] [PubMed] [Google Scholar]
- Nishimura Y., Caro L., Berg C. M., Hirota Y. Chromosome replication in Escherichia coli. IV. Control of chromosome replication and cell division by an integrated episome. J Mol Biol. 1971 Feb 14;55(3):441–456. doi: 10.1016/0022-2836(71)90328-7. [DOI] [PubMed] [Google Scholar]
- Nisioka T., Mitani M., Clowes R. C. Molecular recombination between R-factor deoxyribonucleic acid molecules in Escherichia coli host cells. J Bacteriol. 1970 Jul;103(1):166–177. doi: 10.1128/jb.103.1.166-177.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisioka T., Mitani M., Clowes R. Composite circular forms of R factor deoxyribonucleic acid molecules. J Bacteriol. 1969 Jan;97(1):376–385. doi: 10.1128/jb.97.1.376-385.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Bouanchaud D. The problems of drug-resistant pathogenic bacteria. Extrachromosomal nature of drug resistance in Staphylococcus aureus. Ann N Y Acad Sci. 1971 Jun 11;182:279–294. doi: 10.1111/j.1749-6632.1971.tb30664.x. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Brodsky R. Studies on plasmid replication. I. Plasmid incompatibility and establishment in Staphylococcus aureus. J Mol Biol. 1972 Jul 21;68(2):285–302. doi: 10.1016/0022-2836(72)90214-8. [DOI] [PubMed] [Google Scholar]
- Novick R. P. Extrachromosomal inheritance in bacteria. Bacteriol Rev. 1969 Jun;33(2):210–263. doi: 10.1128/br.33.2.210-263.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OZEKI H., STOCKER B. A., SMITH S. M. Transmission of colicinogeny between strains of Salmonella typhimurium grown together. J Gen Microbiol. 1962 Sep;28:671–687. doi: 10.1099/00221287-28-4-671. [DOI] [PubMed] [Google Scholar]
- Ohki M., Tomizawa J. Asymmetric transfer of DNA strands in bacterial conjugation. Cold Spring Harb Symp Quant Biol. 1968;33:651–658. doi: 10.1101/sqb.1968.033.01.074. [DOI] [PubMed] [Google Scholar]
- Otsubo E., Nishimura Y., Hirota Y. Transfer-defective mutants of sex factors in Escherichia coli. I. Defective mutants and complementation analysis. Genetics. 1970 Feb;64(2):173–188. doi: 10.1093/genetics/64.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsubo E. Transfer-defective mutants of sex factors in Escherichia coli. II. Deletion mutants of an F-prime and deletion mapping of cistrons involved in genetic transfer. Genetics. 1970 Feb;64(2):189–197. doi: 10.1093/genetics/64.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou J. T., Anderson T. F. Role of pili in bacterial conjugation. J Bacteriol. 1970 Jun;102(3):648–654. doi: 10.1128/jb.102.3.648-654.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PUIG J., NAGELDEZWAIG R. ETUDE G'EN'ETIQUE D'UN FACTEUR COLICINOG'ENE B ET DE SON INFLUENCE SUR LA FERTILIT'E DES CROISEMENTS CHEZ ESCHERICHIA COLI K 12. Ann Inst Pasteur (Paris) 1964 Nov;107:SUPPL–SUPPL:131. [PubMed] [Google Scholar]
- Palchaudhuri S. R., Mazaitis A. J., Maas W. K., Kleinschmidt A. K. Characterization by electron microscopy of fused F-prime factors in Escherichia coli. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1873–1876. doi: 10.1073/pnas.69.7.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinney R. J., Smith J. T. R factor elimination during thymine starvation: effects of inhibition of protein synthesis and readdition of thymine. J Bacteriol. 1972 Aug;111(2):361–367. doi: 10.1128/jb.111.2.361-367.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punch J. D., Kopecko D. J. Positive and negative control of R-factor replication in Proteus mirabilis. J Bacteriol. 1972 Jan;109(1):336–349. doi: 10.1128/jb.109.1.336-349.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoades M., MacHattie L. A., Thomas C. A., Jr The P22 bacteriophage DNA molecule. I. The mature form. J Mol Biol. 1968 Oct 14;37(1):21–40. doi: 10.1016/0022-2836(68)90071-5. [DOI] [PubMed] [Google Scholar]
- Roozen K. J., Fenwick R. G., Jr, Curtiss R., 3rd Synthesis of ribonucleic acid and protein in plasmid-containing minicells of Escherichia coli K-12. J Bacteriol. 1971 Jul;107(1):21–33. doi: 10.1128/jb.107.1.21-33.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth T. F., Helinski D. R. Evidence for circular DNA forms of a bacterial plasmid. Proc Natl Acad Sci U S A. 1967 Aug;58(2):650–657. doi: 10.1073/pnas.58.2.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rownd R., Kasamatsu H., Mickel S. The molecular nature and replication of drug resistance factors of the Enterobacteriaceae. Ann N Y Acad Sci. 1971 Jun 11;182:188–206. doi: 10.1111/j.1749-6632.1971.tb30656.x. [DOI] [PubMed] [Google Scholar]
- Rownd R., Mickel S. Dissociation and reassociation of RTF and r-determinants of the R-factor NR1 in Proteus mirabilis. Nat New Biol. 1971 Nov 10;234(45):40–43. doi: 10.1038/newbio234040a0. [DOI] [PubMed] [Google Scholar]
- Rownd R., Nakaya R., Nakamura A. Molecular nature of the drug-resistance factors of the Enterobacteriaceae. J Mol Biol. 1966 Jun;17(2):376–393. doi: 10.1016/s0022-2836(66)80149-3. [DOI] [PubMed] [Google Scholar]
- Rownd R. Replication of a bacterial episome under relaxed control. J Mol Biol. 1969 Sep 28;44(3):387–402. doi: 10.1016/0022-2836(69)90368-4. [DOI] [PubMed] [Google Scholar]
- Rubin S. J., Rosenblum E. D. Effects of the recipient strain and ultraviolet irradiation on transduction kinetics of the penicillinase plasmid of Staphylococcus aureus. J Bacteriol. 1971 Dec;108(3):1192–1199. doi: 10.1128/jb.108.3.1192-1199.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp W. D., Ihler G. Strand selection during bacterial mating. Cold Spring Harb Symp Quant Biol. 1968;33:647–650. doi: 10.1101/sqb.1968.033.01.073. [DOI] [PubMed] [Google Scholar]
- Rush M. G., Gordon C. N., Novick R. P., Warner R. C. Penicillinase plasmid DNA from Staphylococcus aureus. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1304–1310. doi: 10.1073/pnas.63.4.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush M. G., Gordon C. N., Warner R. C. Circular deoxyribonucleic acid from Shigella dysenteriae Y6R. J Bacteriol. 1969 Nov;100(2):803–808. doi: 10.1128/jb.100.2.803-808.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- SILVER S., OZEKI H. Transfer of deoxyribonucleic acid accompanying the transmission of colicinogenic properties by cell mating. Nature. 1962 Sep 1;195:873–874. doi: 10.1038/195873a0. [DOI] [PubMed] [Google Scholar]
- Salisbury V., Hedges R. W., Datta N. Two modes of "curing" transmissible bacterial plasmids. J Gen Microbiol. 1972 May;70(3):443–452. doi: 10.1099/00221287-70-3-443. [DOI] [PubMed] [Google Scholar]
- Sanderson K. E. Revised linkage map of Salmonella typhimurium. Bacteriol Rev. 1967 Dec;31(4):354–372. doi: 10.1128/br.31.4.354-372.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaife J. Episomes. Annu Rev Microbiol. 1967;21:601–638. doi: 10.1146/annurev.mi.21.100167.003125. [DOI] [PubMed] [Google Scholar]
- Shaw W. V. The problems of drug-resistant pathogenic bacteria. Comparative enzymology of chloramphenicol resistance. Ann N Y Acad Sci. 1971 Jun 11;182:234–242. doi: 10.1111/j.1749-6632.1971.tb30660.x. [DOI] [PubMed] [Google Scholar]
- Shull F. W., Jr, Fralick J. A., Stratton L. P., Fisher W. D. Membrane association of conjugally transferred deoxyribonucleic acid in Escherichia coli minicells. J Bacteriol. 1971 May;106(2):626–633. doi: 10.1128/jb.106.2.626-633.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signer E. R. Plasmid formation: a new mode of lysogeny by phase lambda. Nature. 1969 Jul 12;223(5202):158–160. doi: 10.1038/223158a0. [DOI] [PubMed] [Google Scholar]
- Silver R. P., Falkow S. Specific labeling and physical characterization of R-factor deoxyribonucleic acid in Escherichia coli. J Bacteriol. 1970 Oct;104(1):331–339. doi: 10.1128/jb.104.1.331-339.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver R. P., Falkow S. Studies on resistance transfer factor deoxyribonucleic acid in Escherichia coli. J Bacteriol. 1970 Oct;104(1):340–344. doi: 10.1128/jb.104.1.340-344.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalka A., Burgi E., Hershey A. D. Segmental distribution of nucleotides in the DNA of bacteriophage lambda. J Mol Biol. 1968 May 28;34(1):1–16. doi: 10.1016/0022-2836(68)90230-1. [DOI] [PubMed] [Google Scholar]
- Smith H. W., Gyles C. L. The relationship between different transmissible plasmids introduced by F into the same strain of Escherichia coli K12. J Gen Microbiol. 1970 Aug;62(3):227–285. doi: 10.1099/00221287-62-3-277. [DOI] [PubMed] [Google Scholar]
- Smith H. W., Linggood M. A. Transfer factors in Escherichia coli with particular regard to their incidence in enteropathogenic strains. J Gen Microbiol. 1970 Aug;62(3):287–299. doi: 10.1099/00221287-62-3-287. [DOI] [PubMed] [Google Scholar]
- Sonstein S. A., Baldwin J. N. Loss of the penicillinase plasmid after treatment of Staphylococcus aureus with sodium dodecyl sulfate. J Bacteriol. 1972 Jan;109(1):262–265. doi: 10.1128/jb.109.1.262-265.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes R. B., Richmond M. H. Intergeneric transfer of a beta-lactamase gene between Ps. aeruginosa and E. coli. Nature. 1970 Jun 6;226(5249):952–954. doi: 10.1038/226952a0. [DOI] [PubMed] [Google Scholar]
- Taylor A. L. Current linkage map of Escherichia coli. Bacteriol Rev. 1970 Jun;34(2):155–175. doi: 10.1128/br.34.2.155-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay G. Y., Daniels M. J., Schaechter M. Isolation of a cell membrane-DNA-nascent RNA complex from bacteria. J Mol Biol. 1969 Feb 28;40(1):65–76. doi: 10.1016/0022-2836(69)90296-4. [DOI] [PubMed] [Google Scholar]
- Vapnek D., Lipman M. B., Rupp W. D. Physical properties and mechanism of transfer of R factors in Escherichia coli. J Bacteriol. 1971 Oct;108(1):508–514. doi: 10.1128/jb.108.1.508-514.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinograd J., Lebowitz J., Radloff R., Watson R., Laipis P. The twisted circular form of polyoma viral DNA. Proc Natl Acad Sci U S A. 1965 May;53(5):1104–1111. doi: 10.1073/pnas.53.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE T., FUKASAWA T. Episome-mediated transfer of drug resistance in Enterobacteriaceae. I. Transfer of resistance factors by conjugation. J Bacteriol. 1961 May;81:669–678. doi: 10.1128/jb.81.5.669-678.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE T., FUKASAWA T. Episome-mediated transfer of drug resistance in Enterobacteriaceae. II. Elimination of resistance factors with acridine dyes. J Bacteriol. 1961 May;81:679–683. doi: 10.1128/jb.81.5.679-683.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE T., FUKASAWA T. Episome-mediated transfer of drug resistance in Enterobacteriaceae. III. Transduotion of resistance factors. J Bacteriol. 1961 Aug;82:202–209. doi: 10.1128/jb.82.2.202-209.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE T., NISHIDA H., OGATA C., ARAI T., SATO S. EPISOME-MEDIATED TRANSFER OF DRUG RESISTANCE IN ENTEROBACTERIACEAE. VII. TWO TYPES OF NATURALLY OCCURRING R FACTORS. J Bacteriol. 1964 Sep;88:716–726. doi: 10.1128/jb.88.3.716-726.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE T., OGATA C., SATO S. EPISOME-MEDIATED TRANSFER OF DRUG RESISTANCE IN ENTEROBACTERIACEAE. 8. SIX-DRUG-RESISTANCE R FACTOR. J Bacteriol. 1964 Oct;88:922–928. doi: 10.1128/jb.88.4.922-928.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Furuse C., Sakaizumi S. Transduction of various R factors by phage P1 in Escherichia coli and by phage P22 in Salmonella typhimurium. J Bacteriol. 1968 Nov;96(5):1791–1795. doi: 10.1128/jb.96.5.1791-1795.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Ogata Y. Abortive transduction of resistance factor by bacteriophage P22 in Salmonella typhimurium. J Bacteriol. 1970 May;102(2):596–597. doi: 10.1128/jb.102.2.596-597.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Ogata Y. Genetic stability of various resistance factors in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1970 May;102(2):363–368. doi: 10.1128/jb.102.2.363-368.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T. The problems of drug-resistant pathogenic bacteria. The origin of R factors. Ann N Y Acad Sci. 1971 Jun 11;182:126–140. doi: 10.1111/j.1749-6632.1971.tb30652.x. [DOI] [PubMed] [Google Scholar]
- Wehrli W., Staehelin M. Actions of the rifamycins. Bacteriol Rev. 1971 Sep;35(3):290–309. doi: 10.1128/br.35.3.290-309.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisblum B., Davies J. Antibiotic inhibitors of the bacterial ribosome. Bacteriol Rev. 1968 Dec;32(4 Pt 2):493–528. [PMC free article] [PubMed] [Google Scholar]
- Willetts N. S. Plasmid specificity of two proteins required for conjugation in E. coli K12. Nat New Biol. 1971 Apr 7;230(14):183–185. doi: 10.1038/newbio230183a0. [DOI] [PubMed] [Google Scholar]
- Willetts N., Achtman M. Genetic analysis of transfer by the Escherichia coli sex factor F, using P1 transductional complementation. J Bacteriol. 1972 Jun;110(3):843–851. doi: 10.1128/jb.110.3.843-851.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlhieter J. A., Falkow S., Citarella R. V. Purification of episomal DNA with cellulose nitrate membrane filters. Biochim Biophys Acta. 1966 Dec 21;129(3):475–481. doi: 10.1016/0005-2787(66)90062-1. [DOI] [PubMed] [Google Scholar]
- Wolf B., Pato M. L., Ward C. B., Glaser D. A. On the origin and direction of replication of the E. coli chromosome. Cold Spring Harb Symp Quant Biol. 1968;33:575–584. doi: 10.1101/sqb.1968.033.01.064. [DOI] [PubMed] [Google Scholar]
- Zeuthen J., Pato M. L. Replication of the F'lac sex factor in the cell cycle of Escherichia coli. Mol Gen Genet. 1971;111(3):242–255. doi: 10.1007/BF00433109. [DOI] [PubMed] [Google Scholar]





